Abstract
1,3-Dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one has been synthesized through a multi-step pathway starting from commercially available 2-nitroaniline. A structure characterization of this new substituted pyrrolo[1,2-a]quinoxaline compound was achieved by using FT-IR, 1H-NMR, 13C-NMR, X-Ray and HRMS spectral analysis. This new pyrroloquinoxaline derivative shows an interesting cytotoxic potential against several human leukemia cell lines (HL60, K562 and U937 cells).
1. Introduction
Many heterocyclic compounds have attracted a lot of attention, due to their large-spread biological and pharmaceutical activities. Among them, the pyrrolo[1,2-a]quinoxalines were common motifs, with a broad range of pharmacological properties, and could be considered as a privileged substructure for drug design [1,2]. Such nitrogen-containing valuable heterocyclic compounds have been previously described as antipsychotic agents [3], antiviral agents [4], adenosine receptor modulators [5], antituberculosis agents [6,7], antiprotozoal agents [8,9,10,11,12,13,14] and anticancer agents [15,16,17,18,19]. The discovery of novel and original anti-cancer derivatives is one of the most important goals in pharmaceutical chemistry. In the course of our work, that has been devoted to discovering new heterocyclic pyrroloquinoxaline compounds employed in cancer chemotherapy, we previously identified the 1,3-dihydro-1-{1-[4-(3-phenylpyrrolo[1,2-a]quinoxalin-4-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one JG576 as being endowed with interesting activity towards various human leukemia cells [18]. This anti-leukemic pyrroloquinoxaline derivative JG576 was previously reported as a new structural analogue of derivative A6730 (Figure 1), a reference Protein kinase B (PKB, also known as Akt) inhibitor that presents antiproliferative activity against several human leukemia cell lines [15,16,17,18,19].
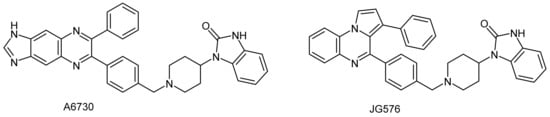
Figure 1.
Chemical structures of compounds A6730 and JG576.
In this context, and as an extension of our research work on the development of novel anticancer pyrrolo[1,2-a]quinoxaline heterocyclic drugs, we decided to further substitute our valuable nitrogen heterocyclic pharmacophore. Thus, taking into account our experience in the field of the synthesis of new bioactive heterocyclic derivatives based on this pyrrolo[1,2-a]quinoxaline heterocyclic scaffold [8,9,10,11,12,13,14,15,16,17,18,19], we use our previously described anti-leukemic JG576 moiety (Figure 1) [18] as a template for the design and synthesis of a new compound in which the benzylpiperidinyl benzimidazolone and phenyl substituents were inverted between the positions 3 and 4 of the pyrrolo[1,2-a]quinoxaline moiety. Consequently, we report herein on the synthesis and structural identification of the 1,3-dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one 9. This original substituted pyrrolo[1,2-a]quinoxalines 9 is then tested against three leukemia cell lines, namely: K562, U937 and HL60.
2. Results and Discussion
2.1. 1,3-Dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one
The synthesis of the 1,3-dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one 9 has been accomplished in eight steps, starting from the commercially available 2-nitroaniline 1 according to the sequence depicted in Scheme 1. The Clauson–Kaas reaction of commercially available 2-nitroaniline 1 with 2,5-dimethoxytetrahydrofuran (DMTHF) in refluxing acetic acid gave the phenylpyrrole 2, which was then reduced using a NaBH4-CuSO4 system to provide the attempted 1-(2-aminophenyl)pyrrole 3. The reaction of aminophenylpyrrole 3 with triphosgene in toluene led to the lactam 4, which was subsequently chlorodehydroxylated with phosphorous oxychloride (POCl3), leading to the 4-chloropyrrolo[1,2-a]quinoxaline 5 [8,9,10,11,12,13,14,17,18,19]. The Suzuki–Miyaura cross-coupling reaction of this synthesized 4-chloropyrroloquinoxaline 5 with phenylboronic acid performed in the presence of Pd(PPh3)4 as a catalyst, and in the presence of sodium carbonate used as the base, gave the 4-phenylpyrrolo[1,2-a]quinoxaline 6. In the next step, the C3-H iodination of the 4-phenylpyrrolo[1,2-a]quinoxaline 6 was then realized with iodine sources according to two previously described methods [20]. At first, this C3-H iodination of the pyrroloquinoxaline skeleton was achieved with I2 under the action of p-toluenesulfonic acid monohydrate (PTSA.H2O) in DMSO as the solvent at 100 °C, leading to the 3-iodo-4-phenylpyrrolo[1,2-a]quinoxaline 7 in 35% yield. Next, by using tetrabutylammonium iodide (TBAI) as the source of iodine, the reaction was realized in the presence of 4-methylbenzenesulfonohydrazide (TsNHNH2) and tert-butyl hydroperoxide (TBHP) in water at 100 °C, and the 3-iodo-4-phenylpyrrolo[1,2-a]quinoxaline 7 was obtained in 47% yield. The characterization of compounds 3–6 [8,9] and 7 [20] were in accordance with those previously described in the literature. The Suzuki–Miyaura cross-coupling reaction of halogenated pyrroloquinoxaline 7 with 4-formylphenylboronic acid using Pd(PPh3)4 as a catalyst, sodium carbonate as the base and dimethoxyethane (DME) as the solvent system led to the aldehyde 8. This new aldehyde 8 was then engaged in a reductive amination with NaBH3CN and 4-(2-ketobenzimidazolin-1-yl)piperidine to give the pyrroloquinoxalines 9 [17,18,19]. The structure of this new synthesized pyrroloquinoxaline derivative 9 was then confirmed by FTIR, 1H/13C-NMR, X-ray and ESI-MS analysis (see Supplementary Materials, Figures S1–S4). The 3D structural determination of the substituted pyrrolo[1,2-a]quinoxaline 9 was established by X-ray crystallography (Figure 2) [21] and confirmed the structure in the solid state as anticipated on the basis of NMR data.
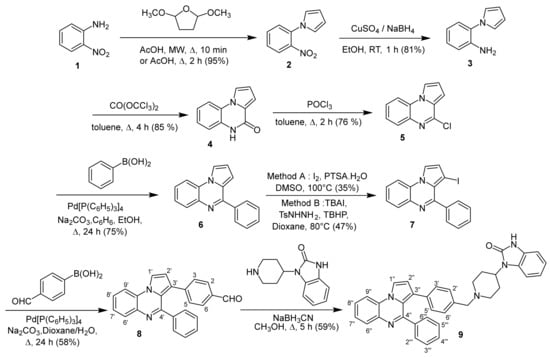
Scheme 1.
Synthesis of 1,3-dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one (9).
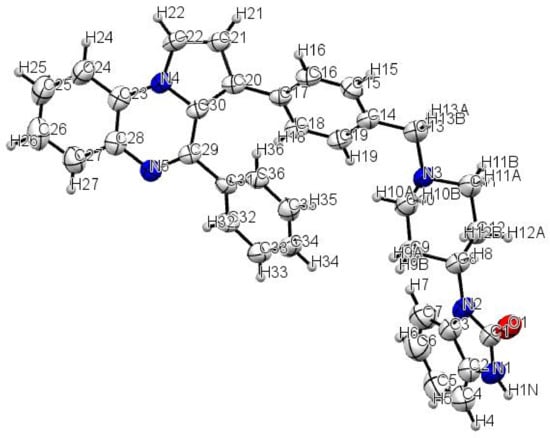
Figure 2.
The ORTEP (Oak Ridge Thermal Ellipsoid Plot) drawing of the 1,3-dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one (9) with thermal ellipsoids at a 30% level.
2.2. Cytotoxic Activity
The cytotoxic activity of this new 1,3-dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one 9 was evaluated against the following human leukemia U937, K562 and HL60 cell lines with the MTS assay, using compounds A6730 and JG576 as our reference standard drugs [15,16]. As listed in Table 1, the IC50 values of 1,3-dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one 9 were noticed in the same range as those observed for our reference drug A6730 and the previously synthesized JG576. At first, the antiproliferative activity of our new pyrroloquinoxaline derivative 9 was evaluated against the human myeloid leukemia cell lines K562, U937 and HL60. Against the human K562 chronic myeloid leukemia cell line, this new substituted compound 9 showed a moderate antiproliferative activity with an IC50 of 17 μM, similar to that of the reference compound, A6730 (IC50 = 17 μM), but slightly better than that of our previously described compound, JG576 (IC50 = 19 μM). Surprisingly, against the HL60 human acute myeloblastic leukemia cell line, our new tested compound 9 was noticed to be much less active than the reference derivative A6730; i.e., IC50 = 31 μM for 9 versus 5.5 μM for A6730.

Table 1.
In vitro activity of compounds 9, JG576 and A6730 on K562, HL60 and U937 cells.
Against the human acute myeloblastic leukemia U937 cell line, derivative 9 showed an antiproliferative activity of 8 μM in the same range as the one observed for A6730 (IC50 = 8 μM), whereas an IC50 of 12 μM was noticed for JG576 against this same leukemia cell line.
3. Materials and Methods
Commercial reagents were used as received without additional purification. The melting points were determined with an SM-LUX-POL Leitz hot-stage microscope (Leitz GMBH, Midland, ON, USA) and are uncorrected. IR spectra were recorded on an NICOLET 380FT-IR spectrophotometer (Thermo Electron Scientific Instruments LLC, Madison, WI, USA). NMR spectra were recorded with tetramethylsilane as an internal standard using a BRUKER AVANCE 300 spectrometer (Bruker BioSpin, Wissembourg, France). The splitting patterns have been reported as follows: s = singlet; bs = broad singlet; d = doublet; t = triplet; q = quartet; dd = double doublet; ddd = double double doublet; dt = double triplet; m = multiplet. 2D-NMR experiments have been used for resonance assignments. Analytical TLC were carried out on 0.25 precoated silica gel plates (POLYGRAM SIL G/UV254, Macherey-Nagel, Allentown, PA, USA) and the visualization of compounds after UV light irradiation. Silica gel 60 (70–230 mesh) was used for column chromatography. High-resolution mass spectra (electrospray in positive mode, ESI+) were recorded on a Waters Q-TOF Ultima apparatus (Bruker Daltonics, Bremen, Germany) [13,14].
3.1. 4-(4-Phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzaldehyde (8)
A mixture of the 3-iodo-4-phenylpyrrolo[1,2-a]quinoxaline 7 (3.5 mmol), 4-formylphenylboronic acid (7.0 mmol), Pd(PPh3)4 (0.17 mmol) and Na2CO3 (7.0 mmol) in dioxane/H2O (4:1, 40 mL) was stirred and heated at 80 °C under nitrogen for 5 h. It was then cooled and transferred to a separating funnel, and the reaction flask was washed out with water (15 mL) and dichloromethane (20 mL), the washings being added to the separating funnel. The organic layer was separated, and the aqueous phase extracted with dichloromethane (2 × 20 mL). The combined organic extracts were then washed with water (50 mL), dried over Na2SO4 and filtered, and the filtrate evaporated under reduced pressure. The crude residue was triturated in ethanol. The resulting precipitate was filtered, washed with ethanol and then with diethyl ether, and dried. A column chromatography of this precipitate on silica gel using dichloromethane as eluent gave the pure product 8 (58%). m.p. 219–221 °C; IR (KBr) 1695 (CO), 1602 (C=N). 1H-NMR (δ, ppm, CDCl3, 300 MHz): 9.92 (s, 1H, HC=O), 8.12 (d, 1H, J = 2.85 Hz, H-1′), 8.10 (dd, 1H, J = 8.10 and 1.50 Hz, H-9′), 7.97 (dd, 1H, J = 8.10 and 1.50 Hz, H-6′), 7.60 (ddd, 1H, J = 8.10, 7.85 and 1.50 Hz, H-8′), 7.53 (ddd, 1H, J = 8.10, 7.85 and 1.50 Hz, H-7′), 7.52 (d, 2H, J = 8.40 Hz, H-2 and H-6), 7.38–7.34 (m, 2H, H phenyl), 7.23–7.16 (m, 1H, H phenyl), 7.11 (d, 2H, J = 8.40 Hz, H-3 and H-5), 7.08–7.04 (m, 2H, H phenyl), 7.00 (d, 1H, J = 2.85 Hz, H-2′). 1HR-MS m/z [M + H]+ Calcd for C24H17N2O: 349.1341, found: 349.1340.
3.2. 1,3-Dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one (9)
The pH of a solution of the aldehyde 8 (0.72 mmol) and 4-(2-ketobenzimidazolin-1-yl)piperidine (0.86 mmol) in 17 mL methanol was adjusted to 6 by the dropwise addition of glacial acetic acid. Powered sodium cyanoborohydride (2.0 mmol) was then added to the previous solution, and the resultant mixture was refluxed for 5 h. After the removal of the methanol by rotary evaporation, the residue was triturated in water and extracted with dichloromethane. The organic layer was washed with water, dried over magnesium sulfate and evaporated to dryness. The obtained solid was then triturated with isopropanol, filtered, washed with diethyl ether and dried under reduced pressure to the crude product. A column chromatography of this precipitate on silica gel using ethyl acetate-cyclohexane (1/1) and then chloroform-methanol (9/1) as eluents gave the pure product 9 (59%). Pale-yellow crystals, m.p. 150–152 °C; IR (KBr) 3435 (NH), 1692 (CO). 1H-NMR (δ, ppm, CDCl3, 300 MHz): 10.20 (s, 1H, NH imid.), 8.10 (d, 1H, J = 3.00 Hz, H-1″), 8.07 (dd, 1H, J = 8.25 and 1.35 Hz, H-9″), 7.95 (dd, 1H, J = 8.25 and 1.35 Hz, H-6″), 7.57 (ddd, 1H, J = 8.25, 7.80 and 1.35 Hz, H-8″), 7.47 (ddd, 1H, J = 8.25, 7.80 and 1.35 Hz, H-7″), 7.40–7.33 (m, 3H, H-2‴, H-6‴ and H benzimid.), 7.16–6.94 (m, 11H, H-2′, H-6′, H-3′, H-5′, H-3‴, H-4‴, H-5‴, H-2′ and 3 H benzimid.), 4.50–4.41 (m, 1H, CH pip.), 3.51 (s, 2H, CH2N), 3.07–3.02 (m, 2H, NCH2 pip.), 2.61–2.49 (m, 2H, NCH2 pip.), 2.22–2.14 (m, 2H, CH2 pip.), 1.91–1.85 (m, 2H, CH2 pip.). 13C-NMR (δ, ppm, CDCl3, 500 MHz): 155.6 (C=O imid.), 155.1 (C-4″), 138.4 (C-3a″), 135.9 (C-5a″), 130.1 (C-3‴ and C-5‴), 129.6 (C-2‴ and C-6‴), 129.2 (C-1′), 129.1 (C-2′ and C-6′), 128.4 (C-7″), 128.3 (C-8″), 128.0 (C-4′ and C-benzimid.), 127.7 (C-6‴), 127.6 (C-3′ and C-5′), 127.1 (C-1‴ and C-benzimid.), 125.3 (C-9″), 125.0 (C-9a″), 121.2 (C-4‴), 121.1 (C-benzimid.), 121.0 (C-3″), 116.0 (C-benzimid.), 114.0 (C-benzimid.), 113.5 (C-benzimid.), 109.9 (C-1″), 109.7 (C-2″), 62.5 (NCH2), 52.9 (2NCH2 pip.), 50.8 (CH pip.), 29.3 (2CH2 pip.). HR-MS m/z [M + H]+ Calcd for C36H32N5O: 550.2607, found: 550.2609.
3.3. X-ray Data
The structure of compound 9 was established by X-ray crystallography (Figure 1). The pale-yellow single crystal of 9 was obtained by slow evaporation from a methanol/chloroform solution (v/v: 10/90): triclinic, space group P-1, a = 7.8563(8) Å, b = 9.2517(10) Å, c = 21.803(2) Å, α = 95.923(3)°, β = 98.136(2)°, γ = 96.538(3)°, V = 1547.1(3) Å3, Z = 2, δ(calcd) = 1.180 Mg.m−3, FW = 549.66 for C36H31N5O, F(000) = 580. The full crystallographic results have been deposited at the Cambridge Crystallographic Data Centre (CCDC-2141829), UK, as supplementary material [21]. The data were corrected for Lorentz and polarization effects and for empirical absorption correction [22]. The structure was solved by direct methods Shelx 2013 [23] and refined using the Shelx 2013 [23] suite of programs.
3.4. Cytotoxic Activity
The MTS proliferation tests on the human leukemic cell lines U937, K562 and HL60 were performed as previously described by our team [15,16,24].
4. Conclusions
Taking into account our previous research using the valuable and biological active pyrrolo[1,2-a]quinoxaline scaffold, we designed and synthesized a new 1,3-dihydro-1-{1-[4-(4-phenylpyrrolo[1,2-a]quinoxalin-3-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-one 9 and then evaluated its antileukemic activity on several human leukemic cell lines, such as U937, K562 and HL60. This pyrrolo[1,2-a]quinoxaline inhibitor 9 exhibiting interesting antileukemia properties (IC50 ranging from 8 to 31 μM) may become a potential candidate for further pharmacomodulations and pharmacological investigations.
Supplementary Materials
FTIR, 1H-NMR, 13C-NMR and HRMS spectra of title compound 9 are available online. Figure S1: 1H-NMR spectrum of compound 9; Figure S2: 13C-NMR spectrum of compound 9; Figure S3: FT-IR spectrum of compound 9; Figure S4: HRMS data for compound 9.
Author Contributions
J.G. and S.M. did the synthesis and prepared and revised the manuscript; S.S. carried out the experiments; S.A.-R. helped in the analysis of the compounds; V.D. conducted the in vitro tests; N.P. carried out the crystallographic experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was supported by a grant from Ligue Contre le Cancer (Comité Aquitaine-Charentes, Bordeaux, France).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kalinin, A.A.; Islamova, L.N.; Fazleeva, G.M. New achievements in the synthesis of pyrrolo[1,2-a]quinoxaline. Chem. Heterocycl. Compd. 2019, 55, 584–597. [Google Scholar] [CrossRef]
- Huang, A.; Ma, C. Recent progress in biological activities and synthetic methodologies of pyrroloquinoxalines. Mini-Rev. Med. Chem. 2013, 13, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Campiani, G.; Butini, S.; Fattorusso, C.; Trotta, F.; Franceschina, S.; De Angelis, M.; Nielsen, K.S. Novel Aryl Piperazine Derivatives with Medical Utility. 2006, p. WO2006072608. Available online: https://worldwide.espacenet.com/publicationDetails/originalDocument?CC=WO&NR=2006072608A2&KC=A2&FT=D&ND=3&date=20060713&DB=&locale=fr_EP (accessed on 13 January 2022).
- Campiani, G.; Aiello, F.; Fabbrini, M.; Morelli, E.; Ramunno, A.; Armaroli, S.; Nacci, V.; Garofalo, A.; Greco, G.; Novellino, E.; et al. Quinoxalinylethylpyridylthioureas (QXPTs) as potent non-nucleoside HIV-1 reverse transcriptase (RT) inhibitors. Further SAR studies and identification of a novel orally bioavailable hydrazine-based antiviral agent. J. Med. Chem. 2001, 44, 305–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schann, S.; Mayer, S.; Gardan, S. Pyrrolo[1,2-a]quinoxaline Derivatives as Adenosine A3 Receptor Modulators and Uses Thereof. 2007, p. EP1798233. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?II=0&ND=3&adjacent=true&locale=fr_EP&FT=D&date=20070620&CC=EP&NR=1798233A1&KC=A1 (accessed on 13 January 2022).
- Wang, T.; Tang, Y.; Yang, Y.; An, Q.; Sang, Z.; Yang, T.; Liu, P.; Zhang, T.; Deng, Y.; Luo, Y. Discovery of novel anti-tuberculosis agents with pyrrolo[1,2-a]quinoxaline-base scaffold. Bioorg. Med. Chem. Lett. 2018, 28, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Makane, V.B.; Vamshi Krishna, E.; Karale, U.B.; Babar, D.A.; Kalari, S.; Rekha, E.M.; Shukla, M.; Kaul, G.; Sriram, D.; Chopra, S.; et al. Synthesis of novel4,5-dihydropyrrolo[1,2-a]quinoxalines, pyrrolo[1,2-a]quinoxalin-2-ones and their antituberculosis and anticancer activity. Arch. Pharma. 2020, 353, e2000192. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Grellier, P.; Labaied, M.; Sonnet, P.; Léger, J.-M.; Déprez-Poulain, R.; Forfar-Bares, I.; Dallemagne, P.; Lemaître, N.; Péhourcq, F.; et al. Synthesis, antimalarial activity and molecular modeling of new pyrrolo[1,2-a]quinoxalines, bispyrrolo[1,2-a]quinoxalines, bispyrido[3,2-e]pyrrolo[1,2-a]pyrazines and bispyrrolo[1,2-a]thieno[3,2-e]pyrazines. J. Med. Chem. 2004, 47, 1997–2009. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Forfar, I.; Mamani-Matsuda, M.; Desplat, V.; Saliège, M.; Thiolat, D.; Massip, S.; Tabourier, A.; Léger, J.-M.; Dufaure, B.; et al. Synthesis, Analytical Behaviour and Biological Evaluation of New 4-Substituted Pyrrolo[1,2-a]quinoxalines as Antileishmanial Agents. Bioorg. Med. Chem. 2007, 15, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Moreau, S.; Ronga, L.; Basmaciyan, L.; Cohen, A.; Rubio, S.; Bentzinger, G.; Azas, N.; Mullié, C.; Sonnet, P. Design, Synthesis and Antimalarial Activity of Some New Aminoalcohol-pyrrolo[1,2-a]quinoxaline Derivatives. Lett. Drug Des. Discov. 2016, 13, 932–942. [Google Scholar] [CrossRef]
- Guillon, J.; Moreau, S.; Mouray, E.; Sinou, V.; Forfar, I.; Belisle-Fabre, S.; Desplat, V.; Millet, P.; Parzy, D.; Jarry, C.; et al. New Ferrocenic Pyrrolo[1,2-a]quinoxaline Derivatives: Synthesis, and In Vitro Antimalarial Activity. Bioorg. Med. Chem. 2008, 16, 9133–9144. [Google Scholar] [CrossRef]
- Guillon, J.; Mouray, E.; Moreau, S.; Mullié, C.; Forfar, I.; Desplat, V.; Belisle-Fabre, S.; Ravanello, F.; Le-Naour, A.; Pinaud, N.; et al. New Ferrocenic Pyrrolo[1,2-a]quinoxaline Derivatives: Synthesis, and in Vitro Antimalarial Activity—Part II. Eur. J. Med. Chem. 2011, 46, 2310–2326. [Google Scholar] [CrossRef] [PubMed]
- Ronga, L.; Del Favero, M.; Cohen, A.; Soum, C.; Le Pape, P.; Savrimoutou, S.; Pinaud, N.; Mullié, C.; Daulouede, S.; Vincendeau, P.; et al. Design, Synthesis and Biological Evaluation of Novel 4-Alkapolyenylpyrrolo[1,2-a]quinoxalines as Antileishmanial Agents—Part III. Eur. J. Med. Chem. 2014, 81, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Cohen, A.; Gueddouda, N.M.; Das, R.N.; Moreau, S.; Ronga, L.; Savrimoutou, S.; Basmaciyan, L.; Monnier, A.; Monget, M.; et al. Design, synthesis and antimalarial activity of novel bis{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}amine derivatives. J. Enzyme Inhib. Med. Chem. 2017, 32, 547–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Pinaud, N.; Lesbordes, J.; Peyrilles, E.; Marchivie, M.; Routier, S.; et al. Synthesis and evaluation of the cytotoxic activity of novel ethyl 4-[4-(4-substitutedpiperidin-1-yl)]benzyl-phenylpyrrolo[1,2-a]quinoxaline-carboxylate derivatives in myeloid and lymphoid leukemia cell lines. Eur. J. Med. Chem. 2016, 113, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Rubio, S.; Pinaud, N.; Bigat, D.; Enriquez, E.; Marchivie, M.; et al. Synthesis and Antiproliferative Effect of Ethyl 4-[4-(4-Substituted Piperidin-1-yl)]benzylpyrrolo[1,2-a]quinoxalinecarboxylate Derivatives on Human Leukemia Cells. ChemMedChem 2017, 12, 940–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desplat, V.; Geneste, A.; Begorre, M.-A.; Belisle-Fabre, S.; Brajot, S.; Massip, S.; Thiolat, D.; Mossalayi, D.; Jarry, C.; Guillon, J. Synthesis of New Pyrrolo[1,2-a]quinoxaline Derivatives as Potential Inhibitors of Akt Kinase. J. Enzym. Inhib. Med. Chem. 2008, 23, 648–658. [Google Scholar] [CrossRef] [Green Version]
- Desplat Moreau, S.; Gay, A.; Belisle-Fabre, S.; Thiolat, D.; Massip, S.; Macky, G.; Godde, F.; Mossalayi, D.; Jarry, C.; Guillon, J. Synthesis and evaluation of the antiproliferative ctivity of novel pyrrolo[1,2-a]quinoxaline derivatives, potential inhibitors of Akt Kinase. Part II. J. Enzyme Inhib. Med. Chem. 2010, 25, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Savrimoutou, S.; Rubio, S.; Moreau, S.; Pinaud, N.; Marchivie, M.; Desplat, V. 1-Phenyl-8-[[4-(pyrrolo[1,2-a]quinoxalin-4-yl)phenyl]methyl]-1,3,8-triazaspiro[4.5]decan-4-one: Synthesis, Crystal Structure and Anti-leukemic Activity. Molbank 2020, 2020, M1113. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wei, Y.; Yang, Z.; Li, Y.; Liu, Y.; Liu, P. Highly selective C3-H iodination of pyrrolo[1,2-a]quinoxalines. Org. Biomol. Chem. 2021, 19, 5191–5196. [Google Scholar] [CrossRef] [PubMed]
- Supplementary X-ray Crystallographic Data: Cambridge Crystallographic Data Centre, University Chemical Lab, Lensfield Road, Cambridge, CB2 1EW, UK. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 13 January 2022).
- Sheldrick, G.M. SADABS. University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillon, J.; Savrimoutou, S.; Rubio, S.; Desplat, V. 1-Methyl-3-{4-[(4-(2-oxo-2,3-dihydro-1H-benzimidazol-1-yl)piperidin-1-yl)benzyl]}-2-phenylindole. Molbank 2018, 2018, M1023. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).