Dicyclohexylammonium O,O’-Diphenyl Phosphate, [(C6H11)2NH2][(C6H5O)2P(O)(O)]: Spectroscopic Study, Crystal Structure, and Hirshfeld Surface Analysis
Abstract
:1. Introduction
2. Results and Discussion
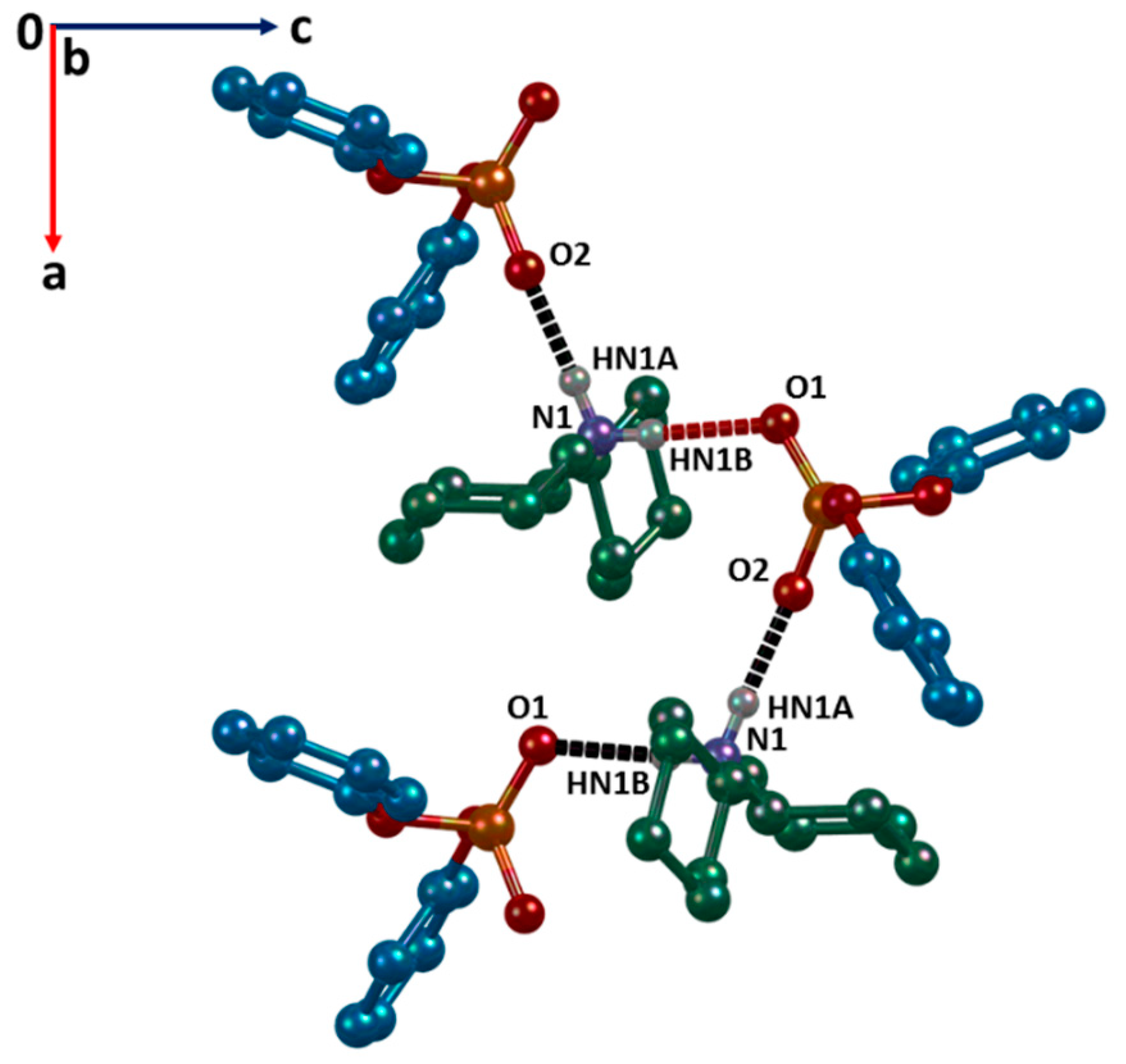
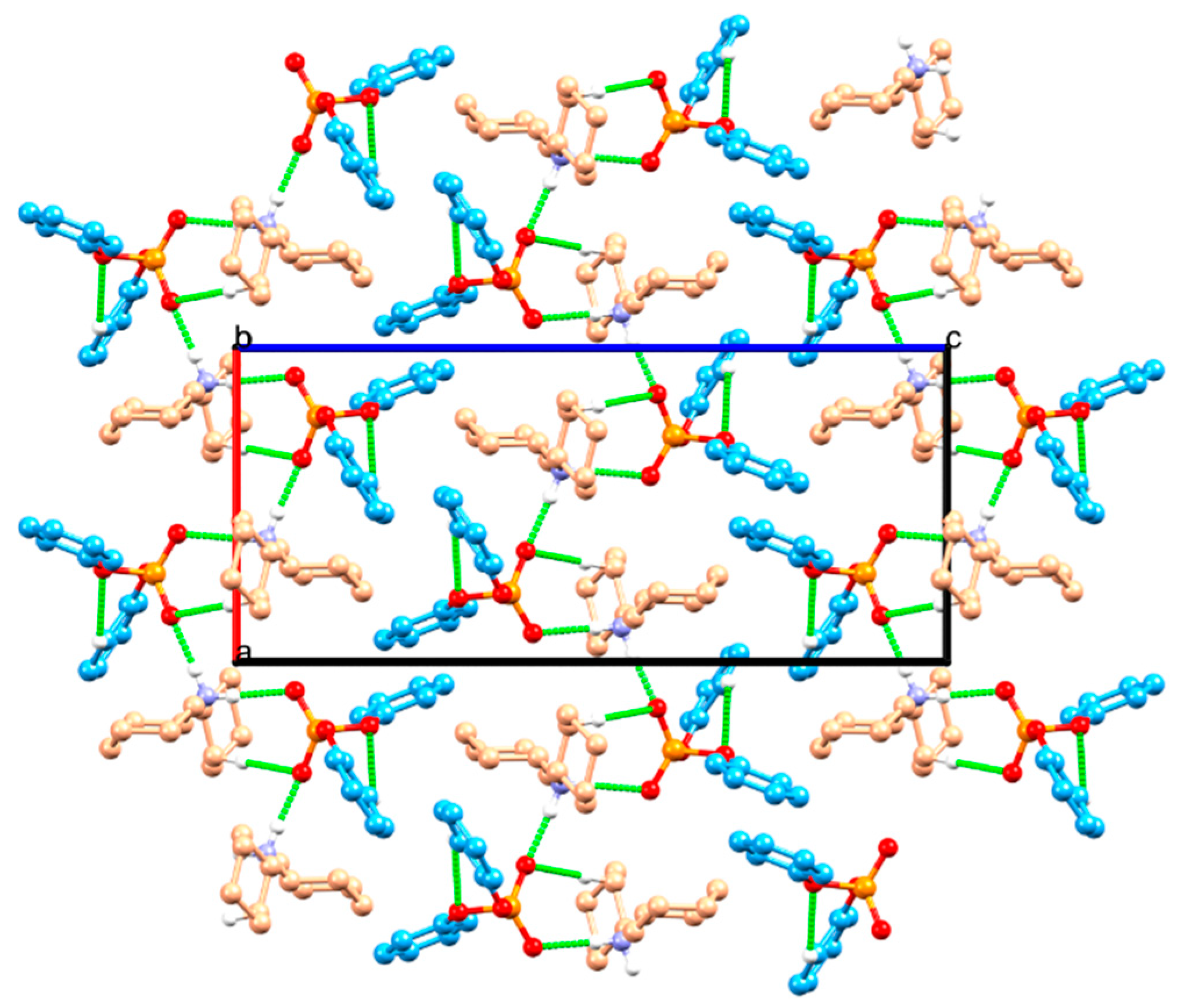
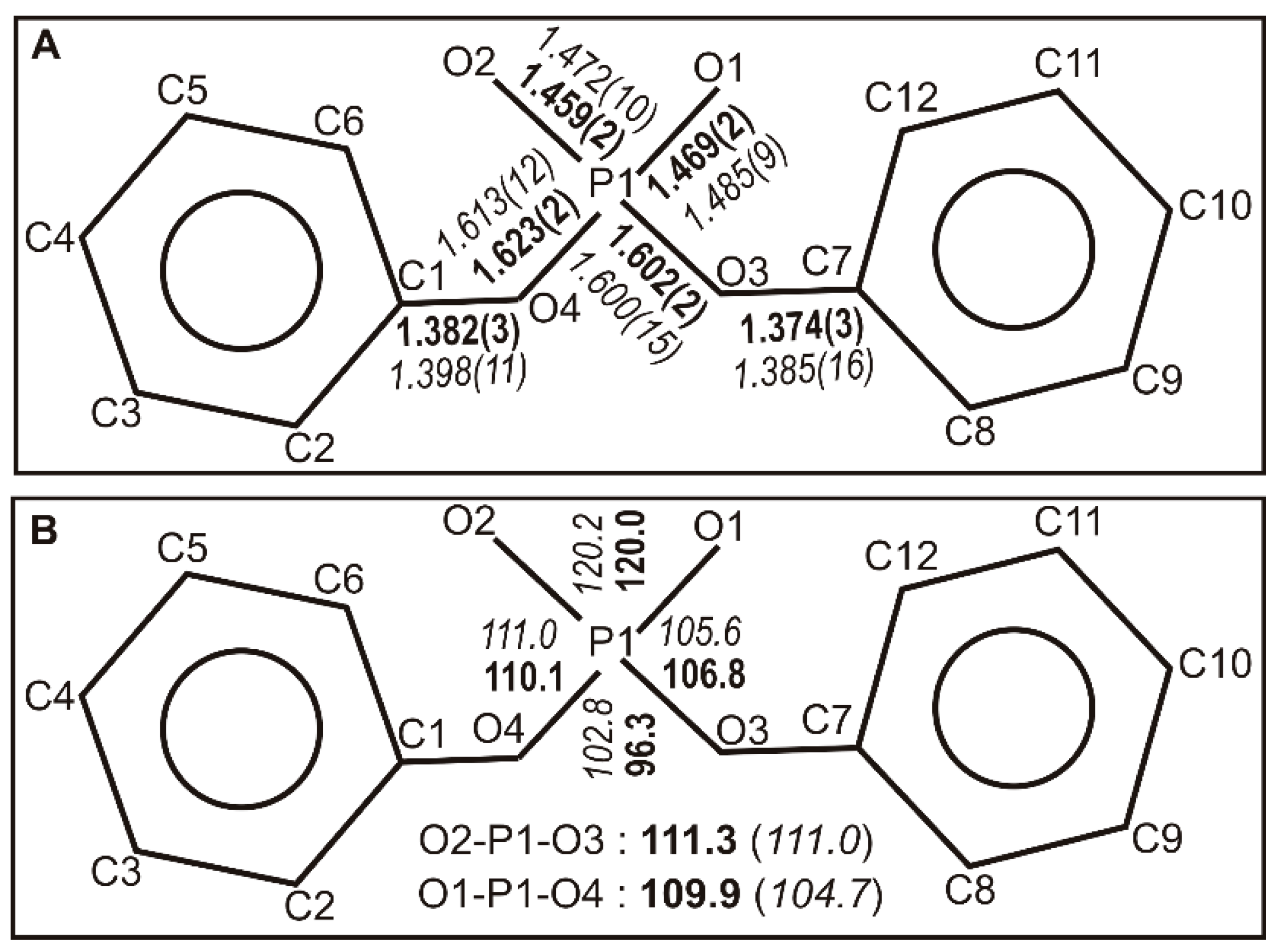
2.1. Structure Description
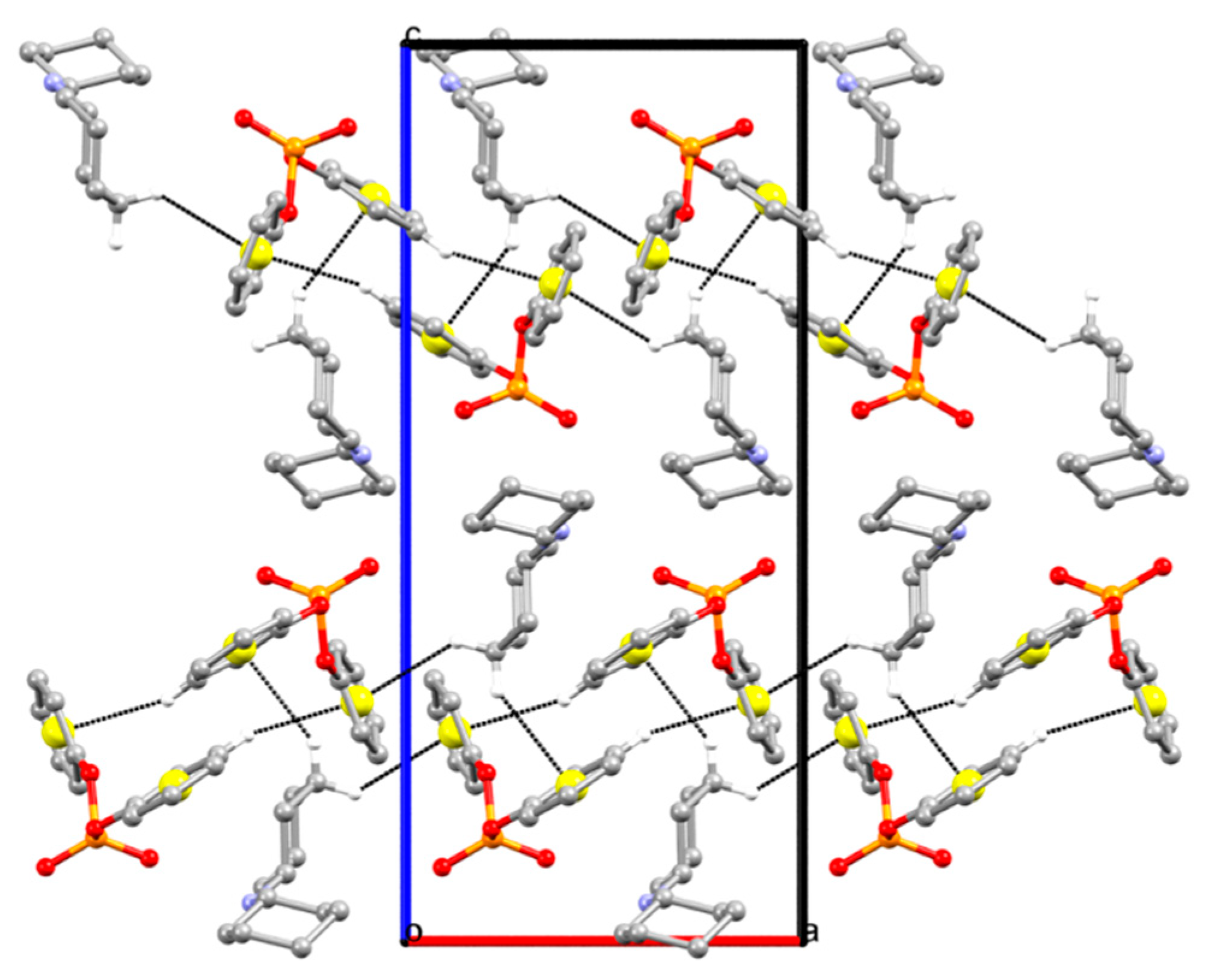
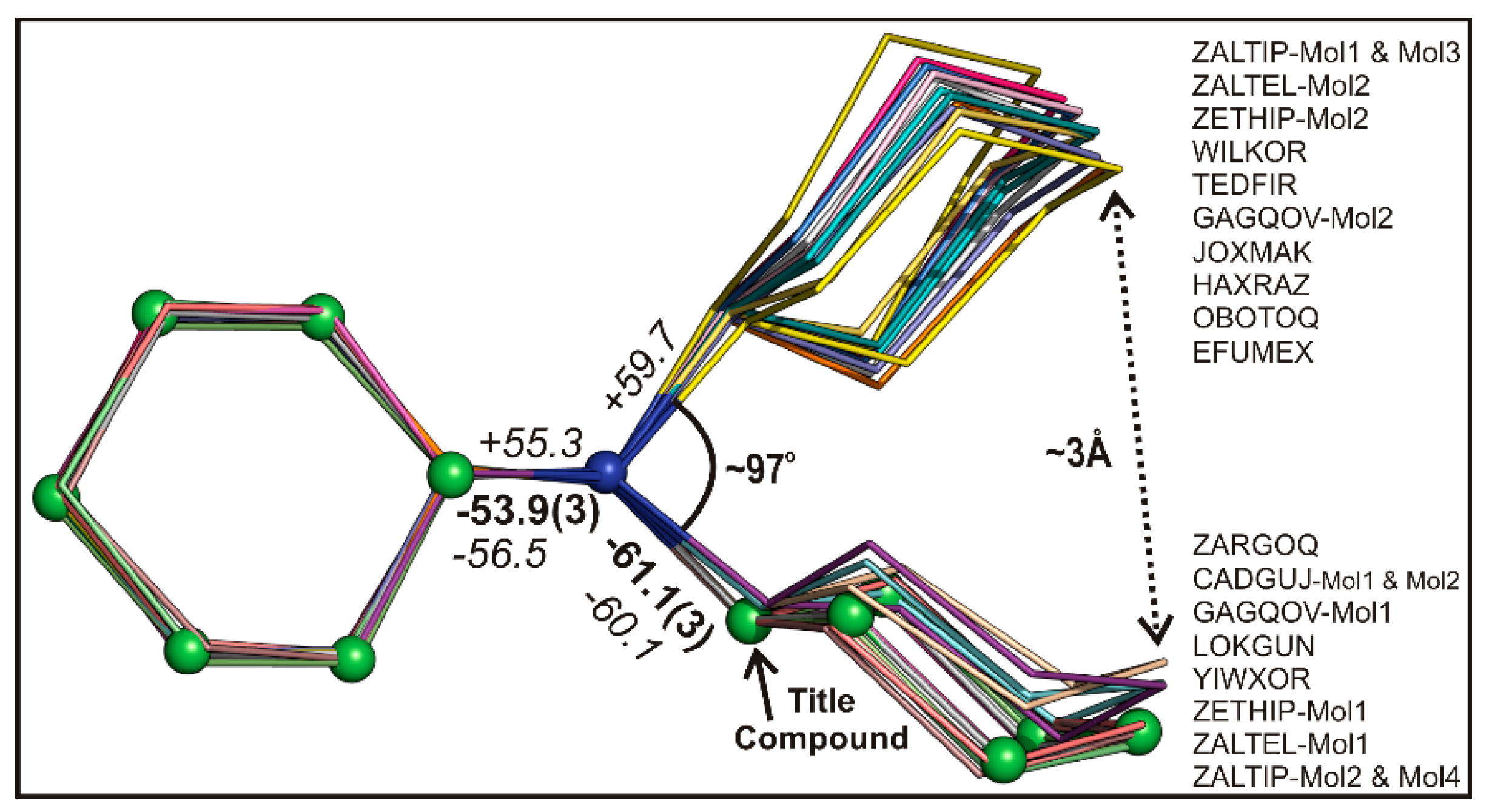
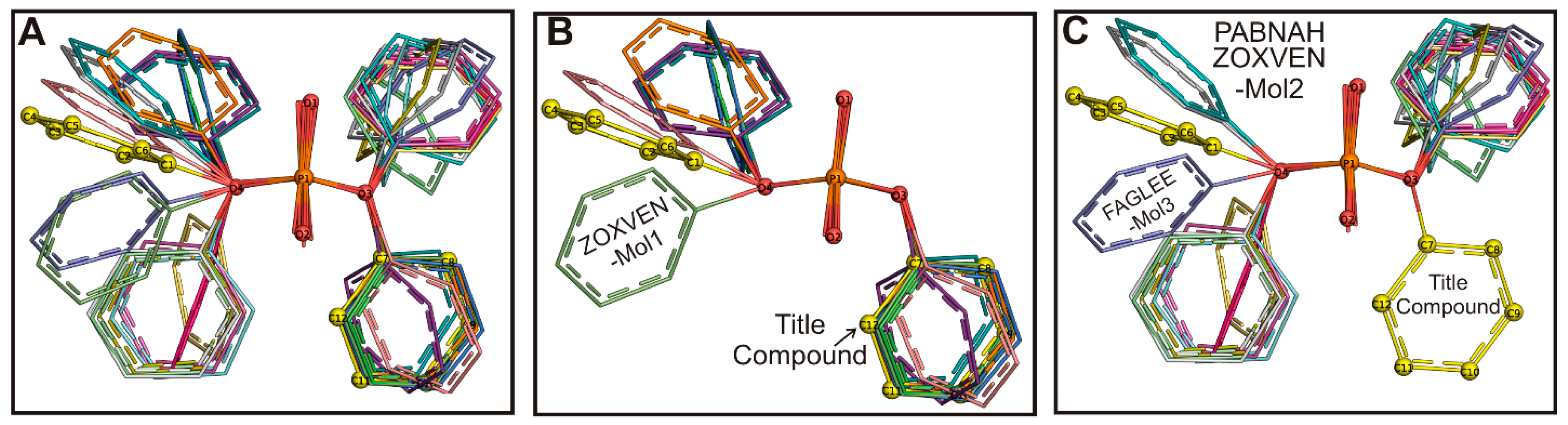
2.2. Structural Comparison
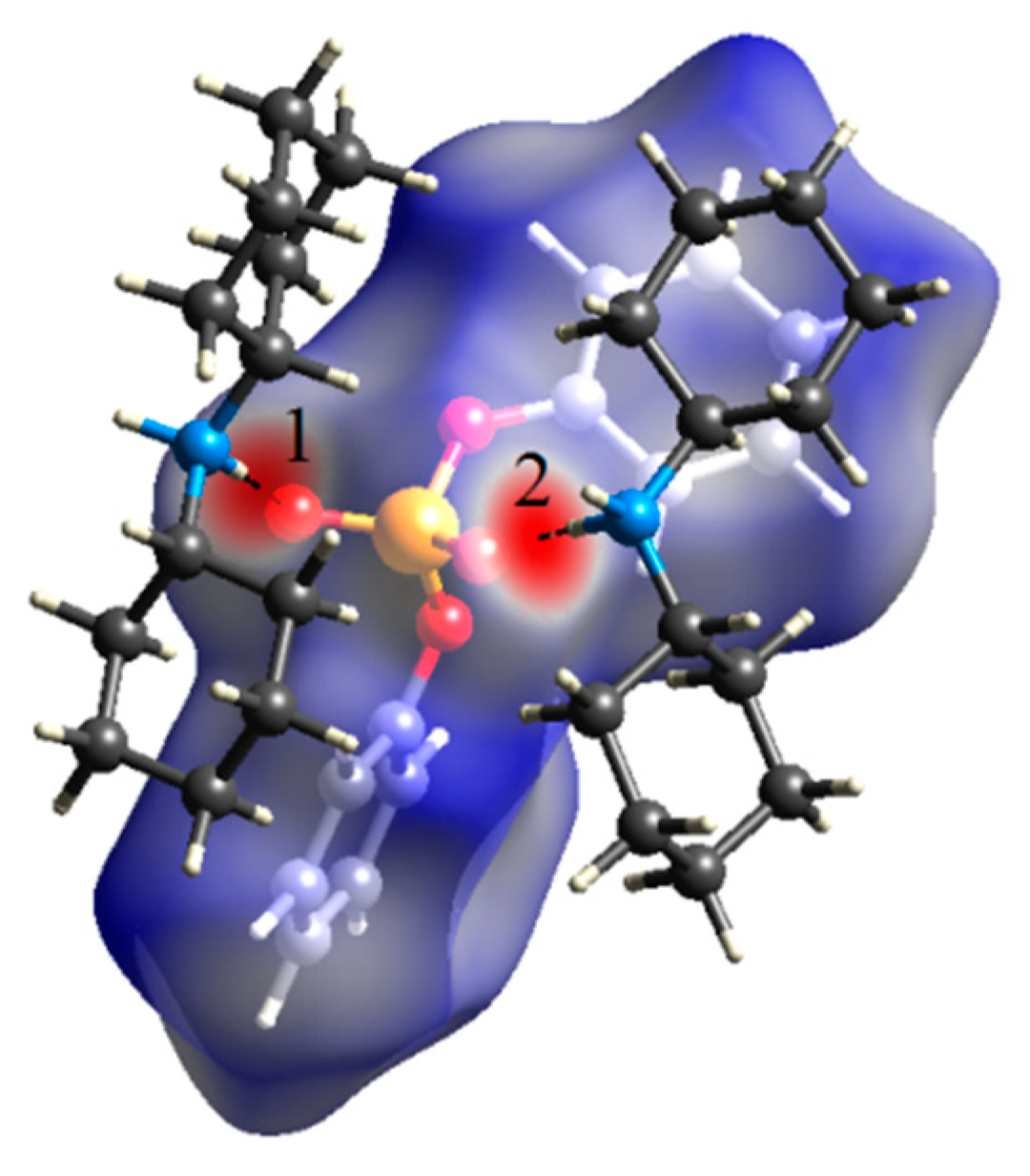
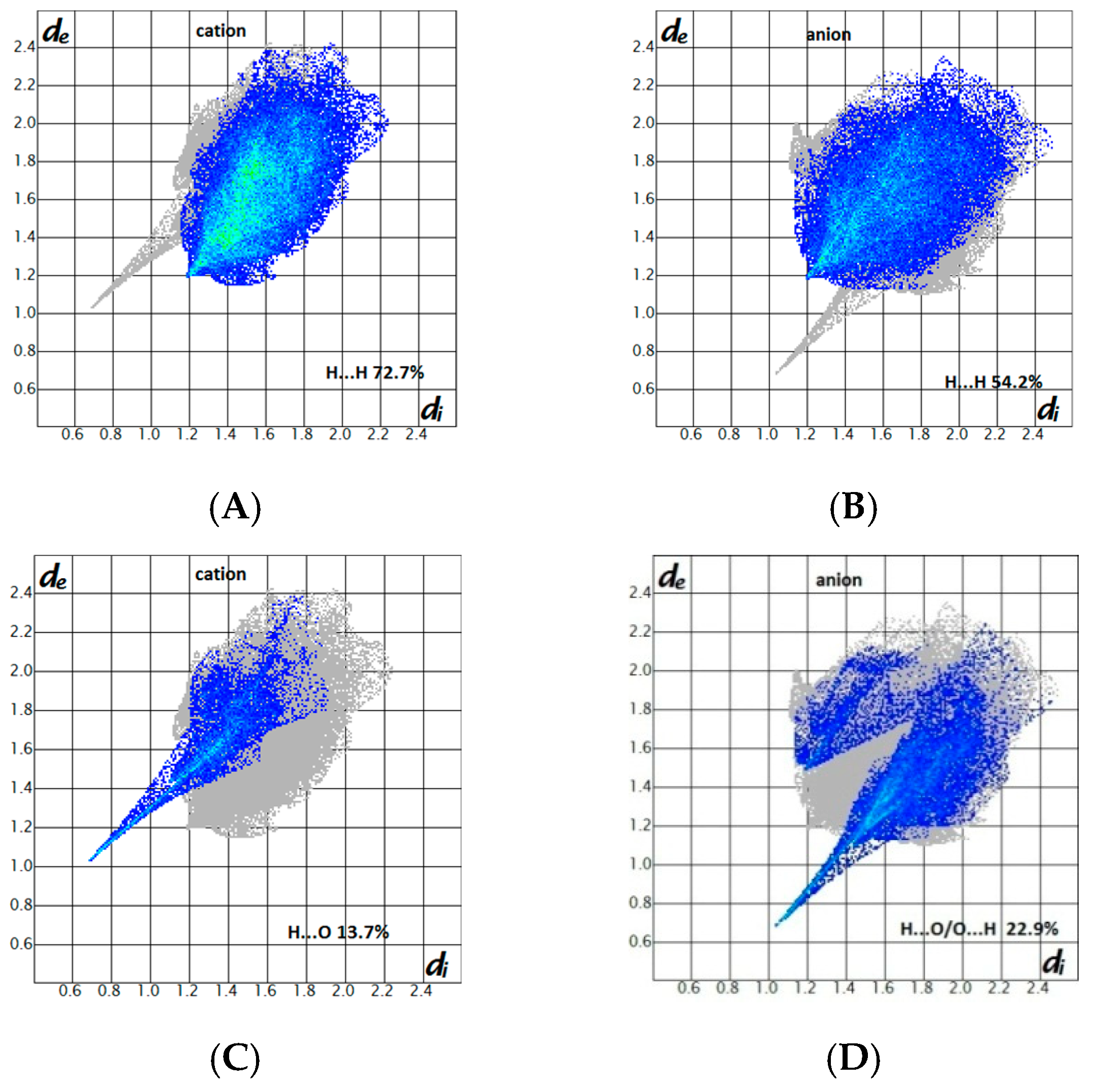
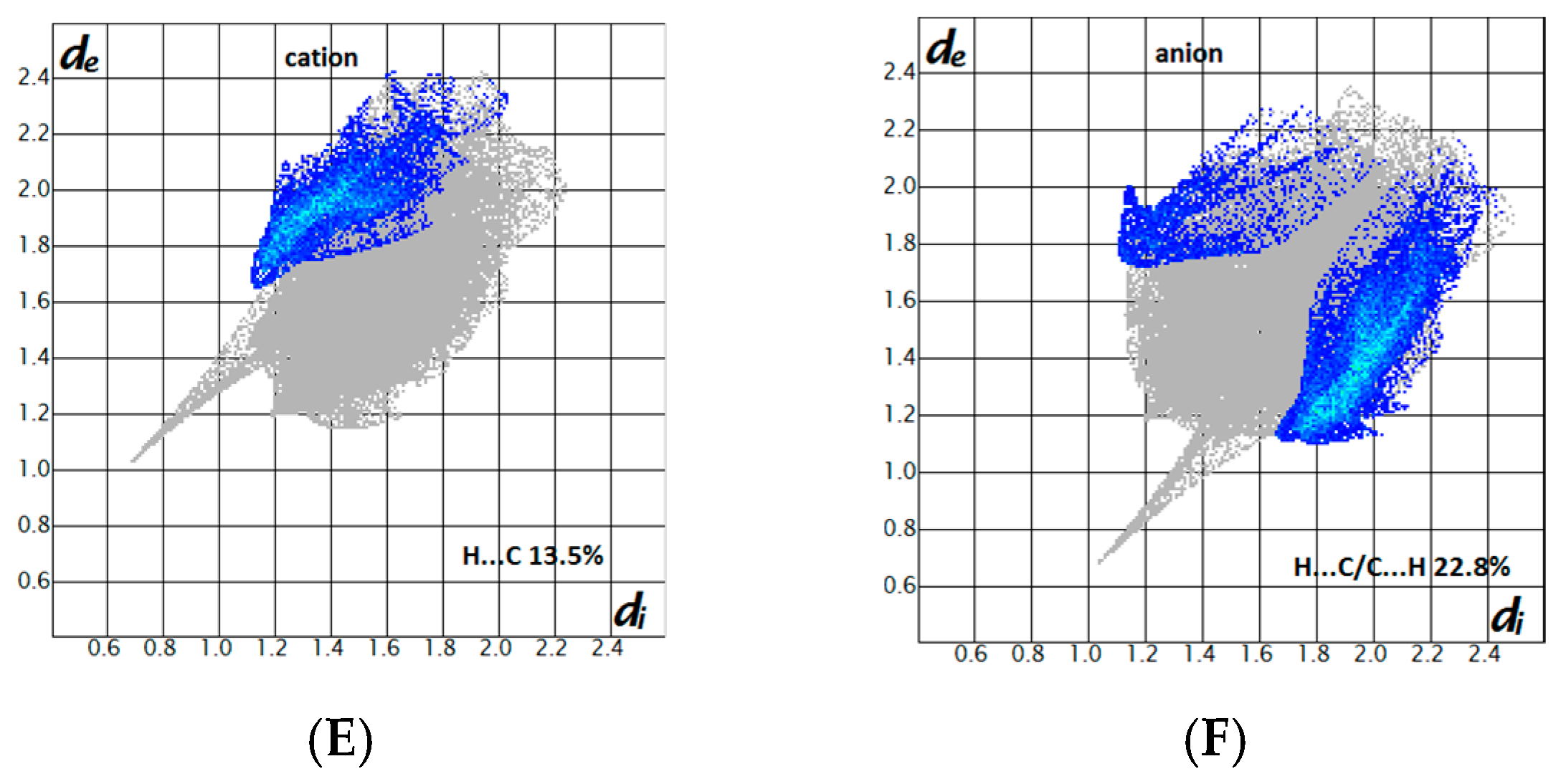
2.3. Hirshfeld Surface Analysis and Fingerprint Plots
2.4. Spectroscopic Study
3. Materials and Methods
3.1. Synthesis of [(C6H11)2NH2][(C6H5O)2P(O)(O)]
3.2. X-ray Data of Crystal Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, H.-J.; Tan, Q.-Y.; Pang, Y.-J. (2,4-Dihydroxybenzylidene)dimethyl-ammonium dichlorophosphinate. Acta Cryst. E 2009, 65, o353. [Google Scholar] [CrossRef] [PubMed]
- Krzyżanowska, B.; Stec, W.J.; Wieczorek, M.W.; Błaszczyk, J. Synthesis and assignment of absolute configuration of enantiomeric dicyclohexylammonium 2-oxo-2-thioxo-1,3,2-oxathiaphospholanes. Heteroat. Chem. 1994, 5, 533–539. [Google Scholar] [CrossRef]
- Sabbaghi, F.; Pourayoubi, M.; Dušek, M.; Eigner, V.; Bayat, S.; Damodaran, K.; Nečas, M.; Kučeráková, M. Analysis of P–O–C, P–S–C and P–O–P angles: A database survey completed with four new X-ray crystal structures. Struct. Chem. 2016, 27, 1831–1844. [Google Scholar] [CrossRef]
- Hamzehee, F.; Pourayoubi, M.; Nečas, M.; Choquesillo-Lazarte, D. Extensive analysis of N–H···O hydrogen bonding in four classes of phosphorus compounds: A combined experimental and database study. Acta Cryst. C 2017, 73, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Eghbali Toularoud, M.; Pourayoubi, M.; Dušek, M.; Eigner, V.; Damodaran, K. Chiral one-dimensional hydrogen-bonded architectures constructed from single-enantiomer phosphoric triamides. Acta Cryst. C 2018, 74, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Torabi Farkhani, E.; Pourayoubi, M.; Izadyar, M.; Andreev, P.V.; Shchegravina, E.S. Evaluation of N–H···S and N–H···π interactions in O,O’-diethyl N-(2,4,6-trimethylphenyl)thiophosphate: A combination of X-ray crystallographic and theoretical studies. Acta Cryst. C 2018, 74, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Pidcock, E. The relevance of chirality in space group analysis: A database study of common hydrogen-bonding motifs and their symmetry preferences. CrystEngComm 2008, 10, 1258–1264. [Google Scholar] [CrossRef]
- Corbridge, D.E.C. Phosphorus, Chemistry, Biochemistry & Technology; Elsevier: Amsterdam, The Netherlands, 2000; pp. 69–74. [Google Scholar]
- Diop, T.; Diop, L.; Maris, T.; Stoeckli-Evans, H. Dicyclohexylammonium hydrogen phenylphosphonate. Acta Cryst. E 2012, 68, o1432. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.R.; Day, R.O.; Yoshida, Y.; Holmes, J.M. Hydrogen-bonded phosphate esters. Synthesis and structure of imidazole-containing salts of diphenyl phosphate and (trichloromethyl)phosphonic acid. J. Am. Chem. Soc. 1992, 114, 1771–1778. [Google Scholar] [CrossRef]
- Bartczak, T.J. Structure of a racemic compound: The dicyclohexylammonium salt of (2R,4R,2S,4S)-cis-4-methyl-2-oxido-1,3,2-dioxaphosphorinane 2-sulphide, C12H24N+.C4H8O3PS−−. Acta Cryst. C 1983, 39, 1059–1062. [Google Scholar] [CrossRef]
- Kamenecka, T.M.; Overman, L.E.; Ly Sakata, S.K. Construction of substituted cyclohexanones by reductive cyclization of 7-oxo-2,8-alkadienyl esters. Org. Lett. 2002, 4, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, H.; Śliwiński, M.; Wolf, W.M.; Bodalski, R. Diastereo- and enantioselective synthesis of bicyclic α-methylene-δ-valero-lactones by asymmetric michael reaction. Synlett 2004, 2004, 1995–1999. [Google Scholar] [CrossRef]
- Harger, M.J.P.; Sreedharan-Menon, R. Stereochemistry of the methoxide induced rearrangement of an α-bromophosphonamidate: Cleavage of the P–N and P–C bonds in the azaphosphiridine oxide intermediate 1. J. Chem. Soc. Perkin Trans. 1 1997, 4, 527–532. [Google Scholar] [CrossRef]
- Yamamoto, J.H.; Udachin, K.A.; Enright, G.D.; Carty, A.J. Phosphorus monoxide as a quadruply bridging ligand: Syntheses and X-ray crystal structures of Ru5(CO)15(μ4-PF) and [H2NCy2][Ru5(CO)15(μ4-PO)]. Chem. Commun. 1998, 20, 2259–2260. [Google Scholar] [CrossRef]
- Mazurek, J.; Lis, T. Crystal and molecular structure of potassium, ammonium and dicyclohexylammonium salts of (2-oxopropyl) phosphonic acid in monoionized state. J. Mol. Struct. 1999, 474, 143–155. [Google Scholar] [CrossRef]
- Diop, T.; Diop, L.; Diop, C.A.K.; Molloy, K.C.; Kociok-Köhn, G. Dicyclohexylammonium trimethylbis(hydrogen phenylphosphonato)stannate(IV). Acta Cryst. E 2011, 67, m1872–m1873. [Google Scholar] [CrossRef] [PubMed]
- Nifantev, E.E.; Kukhareva, T.S.; Popkova, T.N.; Dyachenko, V.I.; Kolomiets, A.F.; Magomedova, N.S.; Belskii, V.K. Synthesis and transformations of benzo-1,3,2-dioxaphosphor (V) inanes based on fluorinated derivatives of orthooxybenzyl alcohol. Zhurnal Obs. Khimii 1996, 66, 61–67. [Google Scholar]
- Bodalski, R.; Jankowski, S.; Glówka, M.L.; Filipiak, T.; Quin, L.D. Anchimeric participation of a methoxy group in a reaction of a metathiophosphate. J. Org. Chem. 1994, 59, 5173–5178. [Google Scholar] [CrossRef]
- Mikolajczyk, M.; Luczak, J.; Wieczorek, M.W.; Blaszczyk, J. Stereochemistry of organophosphorus cyclic compounds. Part 17. Synthesis of N-methyl-2-methoxy-1,3,2-oxazaphosphorinane-2-thione (III) and dicyclohexylammonium salt of N-methyl-2-oxo-2-thio-1,3,2-oxazaphosphorinane (V) and their crystal and molecular structures. Pol. J. Chem. 1993, 67, 1087–1097. [Google Scholar]
- Kashemirov, B.A.; Ju, J.-Y.; Bau, R.; McKenna, C.E. “Troika Acids”: Synthesis, structure, and fragmentation pathways of novel α-(hydroxyimino)phosphonoacetic acids. J. Am. Chem. Soc. 1995, 117, 7285–7286. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphic System, Open-Source Version 1.5, Schrödinger, LLC. Available online: www.pymol.org (accessed on 27 February 2019).
- Głowiak, T.; Wnek, I. Crystal and molecular structure of glycinium diphenylphosphate. J. Crystallogr. Spectrosc. Res. 1985, 15, 157–171. [Google Scholar] [CrossRef]
- Głowiak, T.; Podgórska, I. X-ray, spectroscopic and magnetic studies of hexaaquacopper(II) di(diphenylphosphate) diglycine. Inorg. Chim. Acta 1986, 125, 83–88. [Google Scholar] [CrossRef]
- Stockland, R.A., Jr.; Levine, A.M.; Giovine, M.T.; Guzei, I.A.; Cannistra, J.C. Reductive elimination from metal phosphonate complexes: Circumvention of competing protonolysis reactions. Organometallics 2004, 23, 647–656. [Google Scholar] [CrossRef]
- Toney, J.H.; Brock, C.P.; Marks, T.J. Aqueous coordination chemistry of vanadocene dichloride with nucleotides and phosphoesters. Mechanistic implications for a new class of antitumor agents. J. Am. Chem. Soc. 1986, 108, 7263–7274. [Google Scholar] [CrossRef]
- Głowiak, T.; Podgórska, I. Structure of tetraaqua-bis[μ-(glycylglycinato)-NOO’]-dicopper(II) bis(diphenyl phosphate) dihydrate. Acta Cryst. C 1987, 43, 53–57. [Google Scholar] [CrossRef]
- Małecka, M.; Rybarczyk-Pirek, A.; Grabowski, S.J.; Malinowska, K.; Ochocki, J. 3,5-Dimethyl-2H-pyrazol-1-ium diphenylphosphate. Acta Cryst. E 2002, 58, o1113–o1115. [Google Scholar] [CrossRef]
- Safin, D.A.; Babashkina, M.G.; Robeyns, K.; Mitoraj, M.P.; Kubisiak, P.; Brela, M.; Garcia, Y. Experimental and theoretical investigations of the NiII complex with N-phosphorylated thiourea iPrNHC(S)NHP(O)(OPh)2. CrystEngComm 2013, 15, 7845–7851. [Google Scholar] [CrossRef]
- Babashkina, M.G.; Robeyns, K.; Filinchuk, Y.; Safin, D.A. Detailed studies of the interaction of 3-chloroaniline with O,O’-diphenylphosphorylisothiocyanate. New J. Chem. 2016, 40, 1230–1236. [Google Scholar] [CrossRef]
- Dixon, R.P.; Geib, S.J.; Hamilton, A.D. Molecular recognition: Bis-acylguanidiniums provide a simple family of receptors for phosphodiesters. J. Am. Chem. Soc. 1992, 114, 365–366. [Google Scholar] [CrossRef]
- Král, V.; Furuta, H.; Shreder, K.; Lynch, V.; Sessler, J.L. Protonated sapphyrins. Highly effective phosphate receptors. J. Am. Chem. Soc. 1996, 118, 1595–1607. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer; University of Western Australia: Perth, Australia, 2007. [Google Scholar]
- Sabbaghi, F.; Pourayoubi, M.; Nečas, M.; Damodaran, K. Two single-enantiomer amidophosphoesters: A database study on the chirality of (O)2P(O)N-based structures. Acta Cryst. C 2019, 75, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Aranburu Leiva, A.I.; Kaur, M.; Benjamin, S.L.; Jones, A.M.; Langley, S.K.; Mewis, R.E. 1,8-bis(2-hydroxy-3,5-di-tert-butylbenzyl)-4,11-dibenzyl-1,4,8,11-tetraazacyclotetradecane. Molbank 2017, 2017, M963. [Google Scholar] [CrossRef]
- Osborne, D.W. The pyridinolysis of diaryl methyl phosphates. J. Org. Chem. 1964, 29, 3570–3574. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—new features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- CCDC. Available online: http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 27 January 2019).



| Bond Distances | |||
| O1–P1 | 1.469(2) | C1–O4 | 1.382(3) |
| P1–O2 | 1.459(2) | C7–O3 | 1.374(3) |
| O3–P1 | 1.602(2) | C13–N1 | 1.499(4) |
| O4–P1 | 1.623(2) | C19–N1 | 1.506(4) |
| Angles | |||
| O2–P1–O1 | 119.96(12) | O3–P1–O4 | 96.31(11) |
| O1–P1–O3 | 106.78(12) | C7–O3–P1 | 126.80(18) |
| O2–P1–O3 | 111.27(13) | C1–O4–P1 | 124.5(2) |
| O1–P1–O4 | 109.86(12) | C13–N1–C19 | 118.1(2) |
| O2–P1–O4 | 110.05(15) | C2–C1–O4 | 115.1(3) |
| D–H…A | D–H (Å) | H…A (Å) | D…A (Å) | ∠D–H…A (°) |
|---|---|---|---|---|
| N1–HN1B…O1#1 | 0.83(3) | 1.97(3) | 2.806(3) | 177(3) |
| N1–HN1A…O2#2 | 0.90(3) | 1.84(3) | 2.730(3) | 176(3) |
| C12–H12…O4 | 0.93 | 2.62 | 3.139(4) | 115.8 |
| C24–H24B…O2#1 | 0.97 | 2.65 | 3.548(4) | 154.9 |
| C11–H11…Cg1#3 | 0.93 | 2.80 | 3.629(3) | 149 |
| C15–H15B…Cg1#4 | 0.97 | 2.97 | 3.664(4) | 130 |
| C15–H15A…Cg2#4 | 0.97 | 2.95 | 3.765(4) | 142 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moghaddam, S.N.; Shabari, A.R.; Sabbaghi, F.; Pourayoubi, M.; Salam, A.A.A. Dicyclohexylammonium O,O’-Diphenyl Phosphate, [(C6H11)2NH2][(C6H5O)2P(O)(O)]: Spectroscopic Study, Crystal Structure, and Hirshfeld Surface Analysis. Molbank 2019, 2019, M1051. https://doi.org/10.3390/M1051
Moghaddam SN, Shabari AR, Sabbaghi F, Pourayoubi M, Salam AAA. Dicyclohexylammonium O,O’-Diphenyl Phosphate, [(C6H11)2NH2][(C6H5O)2P(O)(O)]: Spectroscopic Study, Crystal Structure, and Hirshfeld Surface Analysis. Molbank. 2019; 2019(1):M1051. https://doi.org/10.3390/M1051
Chicago/Turabian StyleMoghaddam, Samira Nazari, Akbar Raissi Shabari, Fahimeh Sabbaghi, Mehrdad Pourayoubi, and Abdul Ajees Abdul Salam. 2019. "Dicyclohexylammonium O,O’-Diphenyl Phosphate, [(C6H11)2NH2][(C6H5O)2P(O)(O)]: Spectroscopic Study, Crystal Structure, and Hirshfeld Surface Analysis" Molbank 2019, no. 1: M1051. https://doi.org/10.3390/M1051
APA StyleMoghaddam, S. N., Shabari, A. R., Sabbaghi, F., Pourayoubi, M., & Salam, A. A. A. (2019). Dicyclohexylammonium O,O’-Diphenyl Phosphate, [(C6H11)2NH2][(C6H5O)2P(O)(O)]: Spectroscopic Study, Crystal Structure, and Hirshfeld Surface Analysis. Molbank, 2019(1), M1051. https://doi.org/10.3390/M1051





