(1R,5S)-6-(4-Methyl-2-oxo-2,5-dihydrofuran-3-yl)-3-phenyl-4-oxa-2,6-diazabicyclo[3.2.0]hept-2-en-7-one
Abstract
1. Introduction
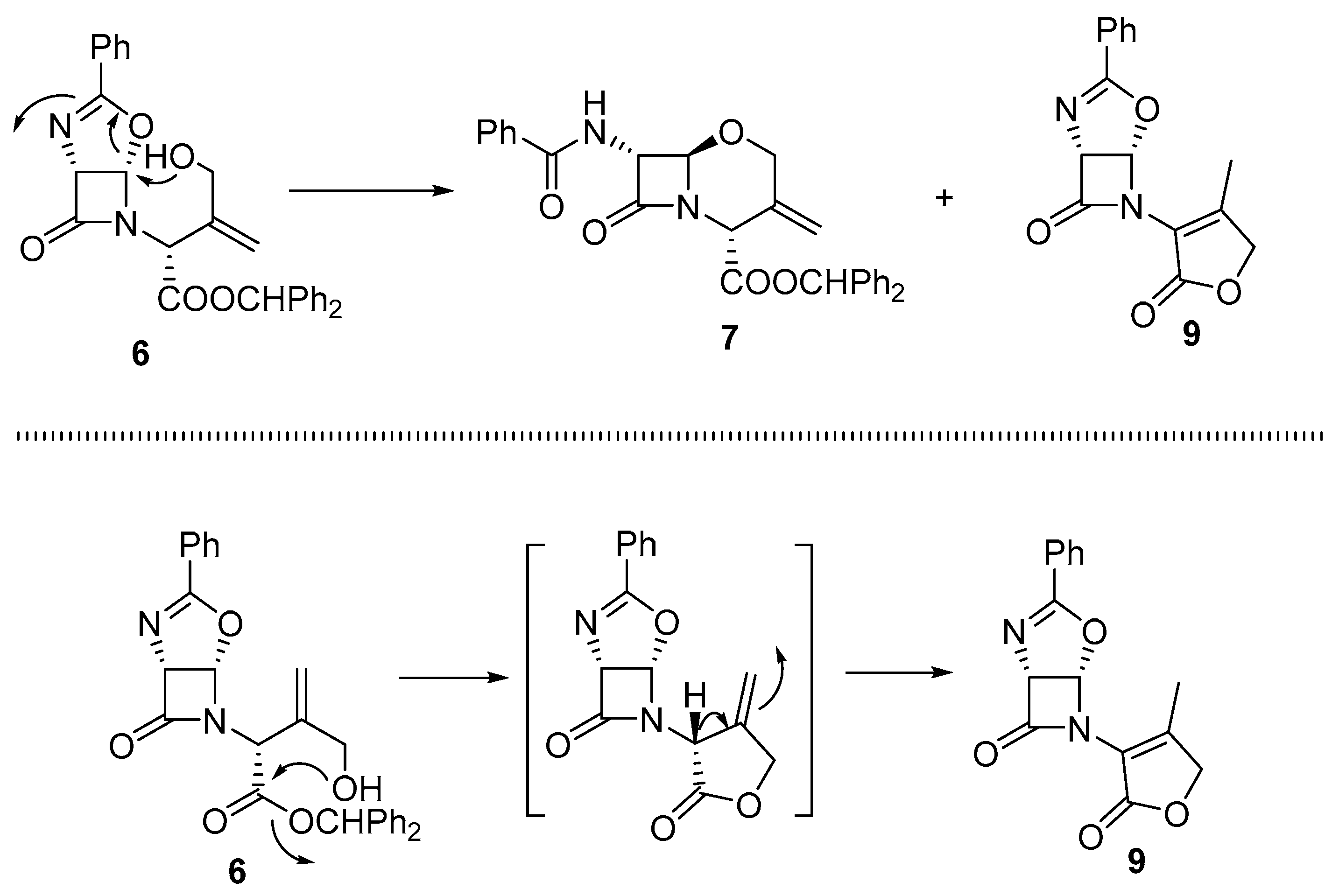
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of (1R,5S)-6-(4-Methyl-2-oxo-2,5-dihydrofuran-3-yl)-3-phenyl-4-oxa-2,6-diazabicyclo[3.2.0]hept-2-en-7-one (9)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.H.; Zhang, X.X.; Jin, L.L.; Yang, S.; Lei, P.S. Synthesis and antibacterial activity of novel ketolides with 11,12-quinoylalkyl side chains. Bioorg. Med. Chem. Lett. 2018, 28, 2358–2363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, L.; Yi, L.; Wang, X.; Zhang, Y.; Liu, J.; Guo, X.; Liu, L.; Shao, C.; Xin, L. A novel antimicrobial substance produced by Lactobacillus rhamnous LS8. Food Control 2016, 73, 754–760. [Google Scholar] [CrossRef]
- Journal, E.; Pathology, P. Biological control of grapevine crown gall: Purification and partial characterisation of an antibacterial substance. Eur. J. Plant. Pathol. 2013, 124, 427–437. [Google Scholar]
- Hakimelahi, G.H.; Li, P.C.; Moosavimovahedi, A.A.; Chamani, J.; Khodarahmi, G.A.; Ly, T.W.; Valiyev, F.; Leong, M.K.; Hakimelahi, S.; Shia, K.S. Application of the Barton photochemical reaction in the synthesis of 1-dethia-3-aza-1-carba-2-oxacephem: A novel agent against resistant pathogenic microorganisms. Org. Biomol. Chem. 2003, 1, 2461–2467. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Doi, M.; Nagata, H.; Kubota, T.; Kume, M.; Murakami, K. The 7α-methoxy substituent in cephem or oxacephem antibiotics enhances in vivo anti-Helicobacter felis activity in mice after oral administration. J. Antimicrob. Chemother. 2000, 45, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Tombor, Z.; Greff, Z.; Nyitrai, J.; Kajtár-Peredy, M. Simple and condensed β-lactams, XIX. Synthesis of some new 7-acylamino-2-iso-oxacephem-4-carboxylic acids. Eur. J. Org. Chem. 2010, 1995, 825–835. [Google Scholar] [CrossRef]
- Lee, C.H.; Chen, I.L.; Li, C.C.; Chien, C.C. Clinical benefit of ertapenem compared to flomoxef for the treatment of cefotaxime-resistant Enterobacteriaceae bacteremia. Infect. Drug Resist. 2018, 11, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.R. Moxalactam is not more active on extended spectrum β-lactamase (ESBL) producing bacteria than on non-ESBL producers. Infect. Drug Resist. 2018, 11, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, S.; Okonogi, T.; Murai, Y.; Kudo, T.; Yoshida, T.; Kondo, S.; Christensen, B.G. Synthesis of a novel 2-beta-methyl-1-oxacephalosporin, OCP-9-176. J. Antibiot. 1988, 41, 1154–1157. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Nagata, W.; Yoshioka, M.; Narisada, M.; Yoshida, T.; Harada, Y.; Yamada, H. Discovery and development of Moxalactam (6059-S): The chemistry and biology of 1-oxacephems. Med. Res. Rev. 1981, 1, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Tsuji, T.; Uyeo, S.; Yamamoto, S.; Aoki, T.; Nishitani, Y.; Mori, S.; Satoh, H.; Hamada, Y.; Ishitobi, H.; et al. Stereocontrolled, straightforward synthesis of 3-substituted methyl 7α-methoxy-1-oxacephems. Tetrahedron Lett. 1980, 21, 351–354. [Google Scholar] [CrossRef]


| Entry | Lewis Acid a | Temperature | Solvent | Yields of 7 b | Yields of 9 b |
|---|---|---|---|---|---|
| 1 | BF3·Et2O | 25 °C | EtOAc | 90% | 1% |
| 2 | LiCl | 25 °C | EtOAc | 33% | 60% |
| 3 | LiCl | 25 °C | EtOH | 1% | 92% |
| 4 | ZnCl2 | 25 °C | EtOAc | 29% | 65% |
| 5 | FeCl3 | 25 °C | EtOAc | 46% | 42% |
| 6 | Yb(OTf)3 | 25 °C | EtOAc | 56% | 36% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, D.-J.; Pham, V.; Tippin, M.-A.; Song, L.; Zi, X.; Zhang, E.; Liu, H.-M. (1R,5S)-6-(4-Methyl-2-oxo-2,5-dihydrofuran-3-yl)-3-phenyl-4-oxa-2,6-diazabicyclo[3.2.0]hept-2-en-7-one. Molbank 2018, 2018, M1016. https://doi.org/10.3390/M1016
Fu D-J, Pham V, Tippin M-A, Song L, Zi X, Zhang E, Liu H-M. (1R,5S)-6-(4-Methyl-2-oxo-2,5-dihydrofuran-3-yl)-3-phenyl-4-oxa-2,6-diazabicyclo[3.2.0]hept-2-en-7-one. Molbank. 2018; 2018(3):M1016. https://doi.org/10.3390/M1016
Chicago/Turabian StyleFu, Dong-Jun, Victor Pham, Matthew-Alexander Tippin, Liankun Song, Xiaolin Zi, En Zhang, and Hong-Min Liu. 2018. "(1R,5S)-6-(4-Methyl-2-oxo-2,5-dihydrofuran-3-yl)-3-phenyl-4-oxa-2,6-diazabicyclo[3.2.0]hept-2-en-7-one" Molbank 2018, no. 3: M1016. https://doi.org/10.3390/M1016
APA StyleFu, D.-J., Pham, V., Tippin, M.-A., Song, L., Zi, X., Zhang, E., & Liu, H.-M. (2018). (1R,5S)-6-(4-Methyl-2-oxo-2,5-dihydrofuran-3-yl)-3-phenyl-4-oxa-2,6-diazabicyclo[3.2.0]hept-2-en-7-one. Molbank, 2018(3), M1016. https://doi.org/10.3390/M1016




