(3-Ammonio-2,2-dimethyl-propyl)carbamate Dihydrate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of the X-ray Crystal Structure
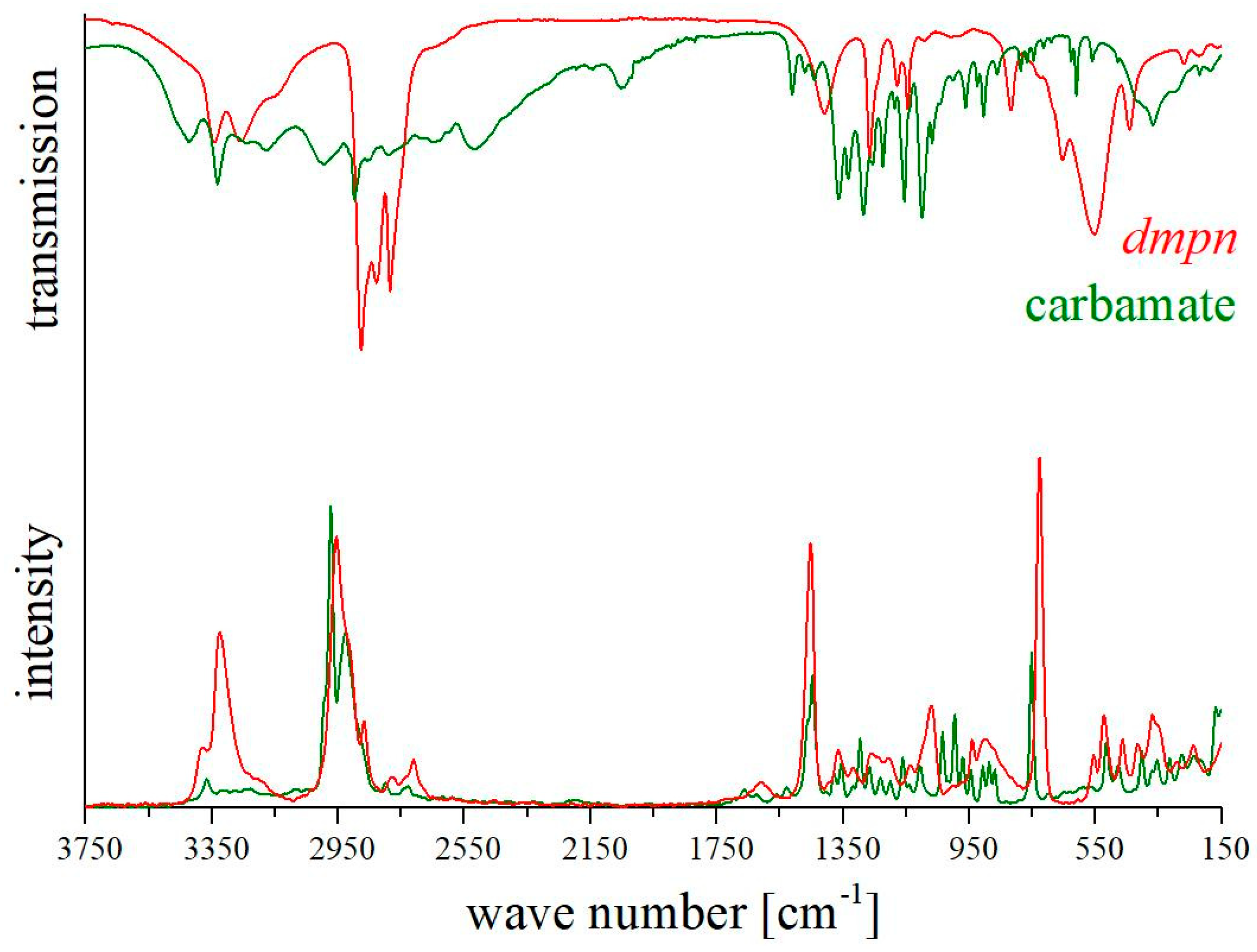
2.2. Spectroscopy
3. Materials and Methods
3.1. General Information
3.2. Synthesis of (3-Ammonio-2,2dimethylpropyl)carbamate Dihydrate
3.3. Spectra
3.4. X-ray Diffraction Studies
3.5. Elemental Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Park, H.; Jung, Y.M.; You, J.K.; Hong, W.H.; Kim, J.-N. Analysis of the CO2 and NH3 Reaction in an Aqueous Solution by 2D IR COS: Formation of bicarbonate and carbamate. J. Phys. Chem. A 2008, 112, 6558–6562. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Su, Y.; Tao, W.; Li, L.; Peng, Y. Post-combustion CO2 capture by aqueous ammonia: A state-of-the-art review. Int. J. Greenh. Gas Control 2012, 9, 355–371. [Google Scholar] [CrossRef]
- Jackson, P. Experimental and theoretical evidence suggests carbamate intermediates play a key role in CO2 sequestration catalysed by sterically hindered amines. Struct. Chem. 2014, 25, 1535–1546. [Google Scholar] [CrossRef]
- Caplow, M. Kinetics of carbamate formation and breakdown. J. Am. Chem. Soc. 1968, 90, 6795–6803. [Google Scholar] [CrossRef]
- George, M.; Weiss, R.G. Chemically reversible organogels: Aliphatic amines as “latent” gelators with carbon dioxide. J. Am. Chem. Soc. 2001, 123, 10393–10394. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.A.; Yamada, H.; Higashii, T.; Goto, K.; Onoda, M. CO2 capture by tertiary amine absorbents: A performance comparison study. Ind. Eng. Chem. Res. 2013, 52, 8323–8331. [Google Scholar] [CrossRef]
- Sogorb, M.A.; Vilanova, E. Enzymes involved in the detoxification of organophosphorus, carbamate and pyrethroid insecticides through hydrolysis. Toxicol. Lett. 2002, 128, 215–228. [Google Scholar] [CrossRef]
- Marrazza, G. Piezoelectric biosensors for organophosphate and carbamate pesticides: A review. Biosensors 2014, 4, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Powles, S.B. Resistance to AHAS inhibitor herbicides: Current understanding. Pest Manag. Sci. 2014, 70, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Latscha, H.P.; Kazmaier, U.; Klein, H.A. Organische Chemie: Chemie-Basiswissen II; Springer: Heidelberg, Germany, 2008. [Google Scholar]
- Vega, F.; Sanna, A.; Navarrete, B.; Maroto-Valer, M.M.; Cortés, V. Degradation of amine-based solvents in CO2 capture process by chemical absorption. Greenh. Gas Sci. Technol. 2014, 4, 707–733. [Google Scholar] [CrossRef]
- Spigarelli, B.P.; Kawatra, S.K. Opportunities and challenges in carbon dioxide capture. J. CO2 Util. 2013, 1, 69–87. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.H.; Hong, S.H. Carbon dioxide capture and use: Organic synthesis using carbon dioxide from exhaust gas. Angew. Chem. Int. Ed. 2014, 53, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Jayarathna, S.A.; Lie, B.; Melaaen, M.C. Amine based CO2 capture plant: Dynamic modeling and simulations. Int. J. Greenh. Gas Control 2013, 14, 282–290. [Google Scholar] [CrossRef]
- Goto, K.; Yogo, K.; Higashii, T. A review of efficiency penalty in a coal-fired power plant with post-combustion CO2 capture. Appl. Energy 2013, 111, 710–720. [Google Scholar] [CrossRef]
- Dutcher, B.; Fan, M.; Russell, A.G. Amine-based CO2 capture technology development from the beginning of 2013—A review. ACS Appl. Mater. Interfaces 2015, 7, 2137–2148. [Google Scholar] [CrossRef] [PubMed]
- Dibenedetto, A.; Angelini, A.; Stufano, P. Use of carbon dioxide as feedstock for chemicals and fuels: Homogeneous and heterogeneous catalysis. J. Chem. Technol. Biotechnol. 2014, 89, 334–353. [Google Scholar] [CrossRef]
- Brilman, W.; Alba, L.G.; Veneman, R. Capturing atmospheric CO2 using supported amine sorbents for microalgae cultivation. Biomass Bioenergy 2013, 53, 39–47. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.; Yan, J.; Tu, S.-T. CO2 Capture using amine solution mixed with ionic liquid. Ind. Eng. Chem. Res. 2014, 53, 2790–2799. [Google Scholar] [CrossRef]
- Niedermaier, I.; Bahlmann, M.; Papp, C.; Kolbeck, C.; Wei, W.; Calderón, S.K.; Grabau, M.; Schulz, P.S.; Wasserscheid, P.; Steinbrück, H.-P.; et al. Carbon dioxide capture by an amine functionalised ionic liquid: Fundamental differences of surface and bulk behavior. J. Am. Chem. Soc. 2014, 136, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Sculley, J.P.; Yuan, D.; Krishna, R.; Zhou, H.-C. Carbon dioxide capture from air using amine-grafted porous polymer networks. J. Phys. Chem. C 2013, 117, 4057–4061. [Google Scholar] [CrossRef]
- Chen, C.; Kim, J.; Ahn, W. CO2 capture by amine-functionalised nanoporous materials: A review. Korean J. Chem. Eng. 2014, 31, 1919–1934. [Google Scholar] [CrossRef]
- Salvatore, R.N.; Shin, S.I.; Nagle, A.S.; Jung, K.W. Efficient carbamate synthesis via a three-component coupling of an amine, CO2, and alkyl halides in the presence of Cs2CO3 and tetrabutylammonium iodide. J. Org. Chem. 2001, 66, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Sartori, G.; Savage, D.W. Sterically hindered amines for CO2, removal from gases. Ind. Eng. Chem. Fundam. 1983, 22, 239–249. [Google Scholar] [CrossRef]
- Hoerr, C.W.; Ralston, A.W. The solubilities of the normal saturated fatty acids. II. J. Org. Chem. 1944, 9, 329–337. [Google Scholar] [CrossRef]
- Aresta, M.; Quaranta, E. Role of the macrocyclic polyether in the synthesis of N-alkylcarbamate esters from primary amines, CO2 and alkyl halides in the presence of crown-ethers. Tetrahedron 1992, 48, 1515–1530. [Google Scholar] [CrossRef]
- Leibnitz, E.; Hager, W.; Gipp, S.; Bornemann, P. Studien über die Folgeprodukte der Paraffinoxydation. II. Inhaltsstoffe technischer Fettamingemische aus PO-Fettsäuren. J. Prakt. Chem. 1959, 9, 217–231. [Google Scholar] [CrossRef]
- Milner, P.J.; Siegelmann, R.L.; Forse, A.C.; Gonzalez, M.I.; Runcevski, T.; Martell, J.D.; Reimer, J.A.; Long, J.R. A diaminopropane-appended metal−organic framework enabling efficient CO2 capture from coal flue gas via a mixed adsorption mechanism. J. Am. Chem. Soc. 2017, 139, 13541–13553. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.-F.; Xu, T.-T.; Xu, X.-Y.; Niu, S.-R. (3-Ammonio-2-hydroxypropyl)carbamate-monohydrate. Acta Crystallogr. 2006, E62, 5191–5193. [Google Scholar] [CrossRef]
- Tiritirisa, I.; Kantlehner, W. Zur Fixierung von Kohlendioxid mit organischen Basen (Teil 1)—Reaktionen von Diaminen mit Kohlendioxid. Z. Naturforsch. 2011, 66B, 164–176. [Google Scholar]
- Grell, J.; Bernstein, J.; Tinhofer, G. Investigation of hydrogen bond patterns: A review of mathematical tools for the graph set approach. Crystallogr. Rev. 2002, 8, 1–56. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. 1990, B46, 256–262. [Google Scholar] [CrossRef]
- Jo, E.; Jhon, Y.H.; Choi, S.B.; Shim, J.-G.; Kim, J.-H.; Lee, J.H.; Lee, I.-Y.; Jang, K.-R.; Kim, J. Crystal structure and electronic properties of 2-amino-2-methyl-1-propanol (AMP) carbamate. Chem. Commun. 2010, 46, 9158–9160. [Google Scholar] [CrossRef] [PubMed]
- Frasco, D.L. Infrared spectra of ammonium carbamate and deuteroammonium carbamate. J. Chem. Phys. 1964, 41, 2134–2140. [Google Scholar] [CrossRef]
- Dell’Amico, D.B.; Calderazzo, F.; Labella, L.; Marchetti, F.; Pampaloni, G. Converting carbon dioxide into carbamato derivatives. Chem. Rev. 2003, 103, 3857–3898. [Google Scholar] [CrossRef] [PubMed]
- Ghazaryan, V.V.; Fleck, M.; Petrosyan, A.M. Mixed salts of amino acids: New analogs of the di-L-ornithinium(2+) chloride nitrate sulfate crystal. J. Cryst. Phys. Chem. 2011, 2, 7–16. [Google Scholar]
- Spectrum 10™; PerkinElmer Inc.: Waltham, MA, USA, 2008.
- Feustel, M. Grundlagen der ATR-Technik; Resultec Analytical Equipment: Illerkirchberg, Germany, 1999. [Google Scholar]
- Bruker. Opus, 6.5; Bruker: Billerica, MA, USA, 2009. [Google Scholar]
- Bruker. SAINT, APEX2, SADABS; Bruker AXS Inc.: Madison, WI, USA, 2011. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K. DIAMOND. Visual Crystal Structure Information System; Ver. 4.5.1.; Crystal Impact: Bonn, Germany, 2018. [Google Scholar]
- Reiss, G.J.; Engel, J.S. Hydrogen bonded 1,10-diammoniodecane—An example of an organo-template for the crystal engineering of polymeric polyiodides. CrystEngComm 2002, 4, 155–161. [Google Scholar] [CrossRef]
- Reiss, G.J.; Megen, M. Synthesis, structure and spectroscopy of a new polyiodide in the α,ω-diazaniumalkane iodide/iodine system. Z. Naturforsch. 2012, B67, 447–451. [Google Scholar] [CrossRef]
- Megen, M.; Reiss, G.J. I62− anion composed of two asymmetric triiodide moieties: A competition between halogen and hydrogen bond. Inorganics 2013, 1, 3–13. [Google Scholar] [CrossRef]
- Megen, M.; Jablonka, A.; Reiss, G.J. Synthesis, structure and thermal decomposition of a new iodine inclusion compound in the 2,2-dimethylpropane-1,3-diamine/HI/I2 System. Z. Naturforsch. 2014, B69, 753–760. [Google Scholar] [CrossRef]
Sample Availability: Smallsamples of the compounds (3-Ammonio-2,2-dimethylpropyl)carbamatedihydrateand2,2-dimethyl-1,3-diaminopropane are available from the authors. |

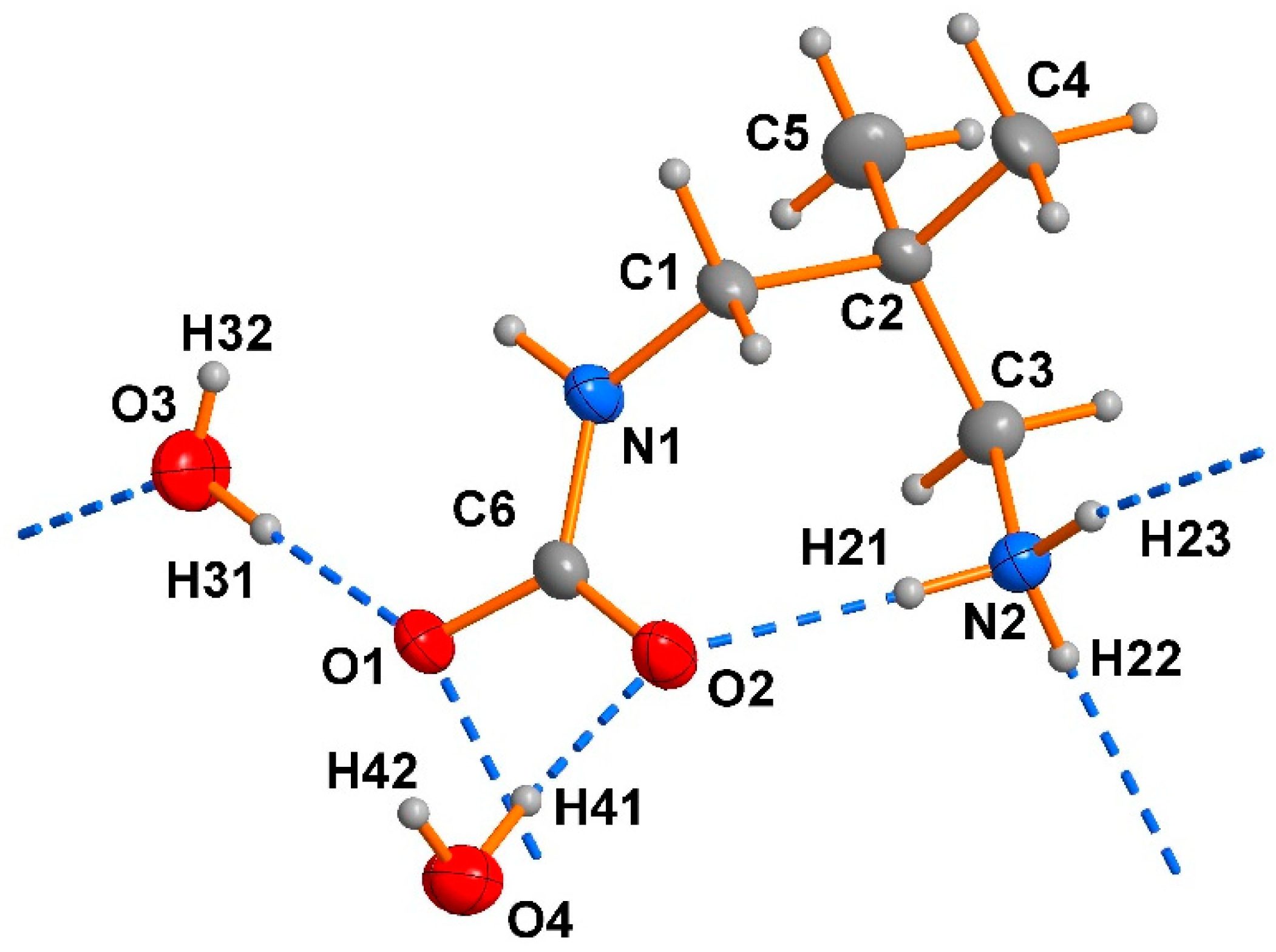
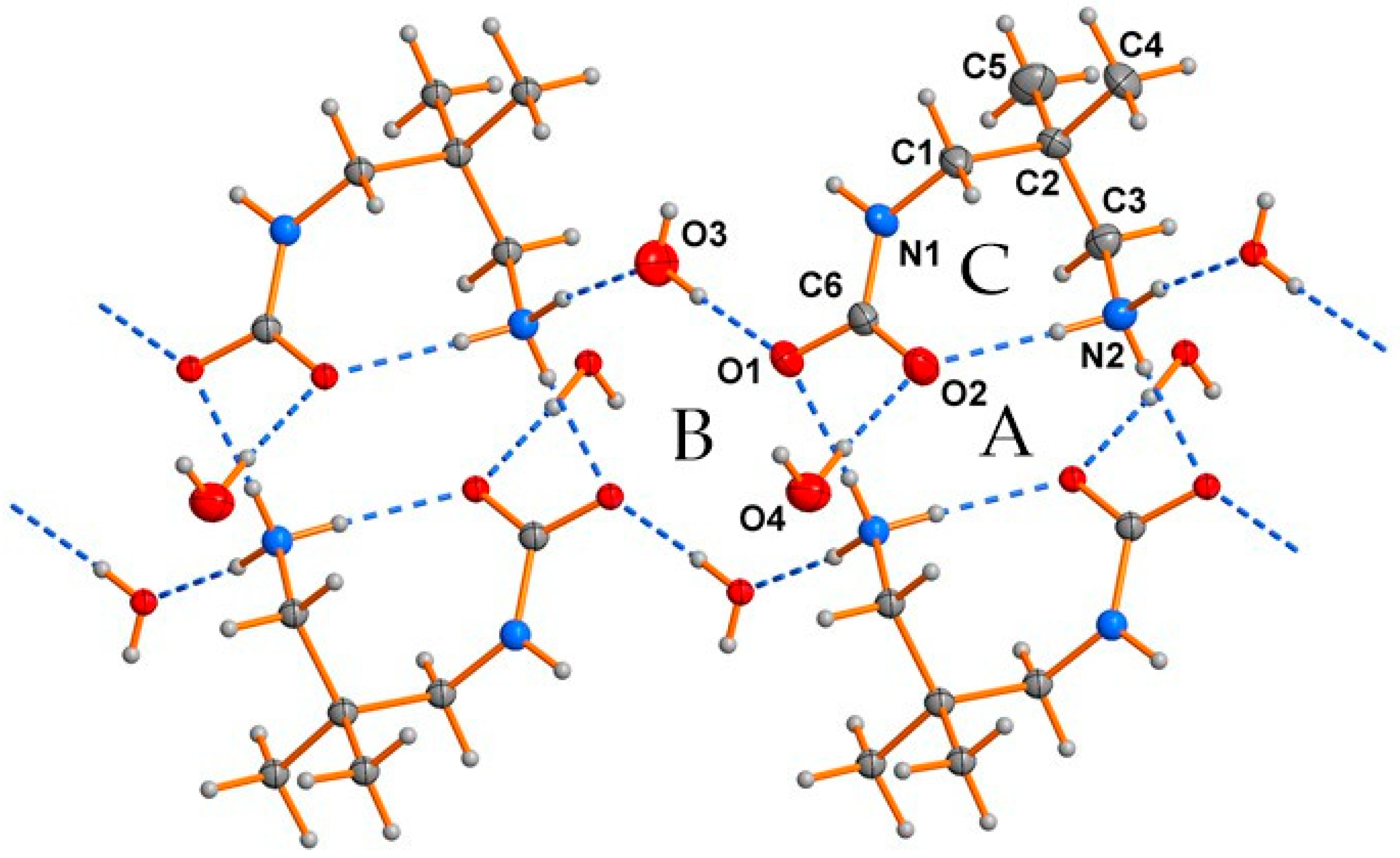
| Atoms | Bond Lengths [Å] | Atoms | Angles [°] |
|---|---|---|---|
| O1–C6 | 1.2782(9) | C1–N1–C6 | 123.43(7) |
| O2–C6 | 1.2639(10) | C1–C2–C3 | 111.74(6) |
| C1–C2 | 1.5358(11) | N1–C1–C2 | 115.43(7) |
| C2–C3 | 1.5290(11) | C2–C3–N2 | 114.74(7) |
| N1–C1 | 1.4466(10) | C1–C2–C3–N2 | −52.87(9) |
| N1–C6 | 1.3609(10) | C6–N1–C1–C2 | 103.29(9) |
| N2–C3 | 1.4901(11) | N1–C1–C2–C4 | −179.04(7) |
| Atoms | D–H [Å] | H ··· A [Å] | D ··· A [Å] | D–H ··· A [°] |
|---|---|---|---|---|
| N1–H11 ··· O4 ′ | 0.867(13) | 2.198(13) | 3.0455(11) | 165.8(11) |
| N2–H21 ··· O2 | 0.912(13) | 1.887(14) | 2.7747(10) | 163.9(12) |
| N2–H22 ··· O1 ″ | 0.915(13) | 1.870(14) | 2.7830(10) | 175.0(12) |
| N2–H23 ··· O3 ‴ | 0.893(13) | 2.001(13) | 2.8861(11) | 171.0(12) |
| O3–H31 ··· O1 | 0.838(16) | 1.963(16) | 2.7934(10) | 170.5(14) |
| O3–H32 ··· O4 ′ | 0.835(18) | 2.058(18) | 2.8764(11) | 166.5(16) |
| O4–H41 ··· O2 | 0.850(17) | 2.010(17) | 2.8583(10) | 175.3(15) |
| O4–H42 ··· O1 ′′′′ | 0.798(17) | 2.243(18) | 3.0037(11) | 159.4(16) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heimgert, J.; Neumann, D.; Reiss, G.J. (3-Ammonio-2,2-dimethyl-propyl)carbamate Dihydrate. Molbank 2018, 2018, M1015. https://doi.org/10.3390/M1015
Heimgert J, Neumann D, Reiss GJ. (3-Ammonio-2,2-dimethyl-propyl)carbamate Dihydrate. Molbank. 2018; 2018(3):M1015. https://doi.org/10.3390/M1015
Chicago/Turabian StyleHeimgert, Jaqueline, Dennis Neumann, and Guido J. Reiss. 2018. "(3-Ammonio-2,2-dimethyl-propyl)carbamate Dihydrate" Molbank 2018, no. 3: M1015. https://doi.org/10.3390/M1015
APA StyleHeimgert, J., Neumann, D., & Reiss, G. J. (2018). (3-Ammonio-2,2-dimethyl-propyl)carbamate Dihydrate. Molbank, 2018(3), M1015. https://doi.org/10.3390/M1015




