Abstract
The title compounds – N-(3-acetyl-2-thienyl)-2-bromoacetamide and N-(3-acetyl-2-thienyl)-2-phthalimidoacetamide – were synthesized in one step from 3-acetylthiophen-2-amine and the corresponding acetyl halogenides. Detailed spectroscopic data (1H NMR, 13C NMR, 15N NMR, MS, IR) for these compounds are presented.
Recently, we have investigated a modified Gewald reaction [1] for the preparation of 3-acetyl-2-aminothiophenes [2]. We here report the synthesis of two acetamides derived from 3-acetylthiophen-2-amine (1) (Scheme 1). These molecules are expected to be versatile intermediates for advanced investigations regarding the chemistry of 3-acetylthiophenes of type 1.
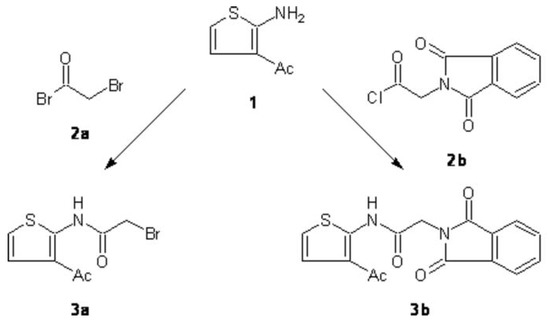
Scheme 1.
Preparation of the title compounds 3a and 3b
N-(3-Acetyl-2-thienyl)-2-bromoacetamide (3a):
Under stirring at room temperature, to 4.23 g (30 mmol) thiophenamine 1 [2] in 70 mL of dry 1,4-dioxane were added dropwise 6.06 g (30 mmol) of bromoacetyl bromide (2a) in 20 mL of 1,4-dioxane. After 3 h the reaction mixture was poured into ice-cold H2O (ca. 300 mL), the resulting precipitate was filtered off, washed with H2O, and dried under reduced pressure to afford pure 3a (4.72 g, 60%) as a beige powder.
The compound slowly decomposes in DMSO- or MeOH-solution.
Melting point: 96–97°C.
IR (KBr) [3]: 1660, 1640 cm–1.
1H NMR (300 MHz, DMSO-d6) [4]: δ (ppm) 12.18 (s, 1H, NH), 7.43 (d, 3J(H4,H5) = 5.8 Hz, 1H, H4), 7.06 (d, 3J(H4,H5) = 5.8 Hz, 1H, H5), 4.43 (s, 2H, CH2), 2.52 (s, 3H, CH3).
1H NMR (500 MHz, CDCl3) [5]: δ (ppm) 12.66 (s, 1H, NH), 7.23 (d, 3J(H4,H5) = 5.8 Hz, 1H, H4), 6.81 (d, 3J(H4,H5) = 5.8 Hz, 1H, H5), 4.09 (s, 2H, CH2), 2.55 (s, 3H, CH3).
13C NMR (75 MHz, DMSO-d6) [4]: δ (ppm) 195.8 (COCH3), 164.6 (NCO, 2J(NCO,CH2) = 4.3 Hz, 2J(NCO,NH) = 4.3 Hz), 147.0 (C2), 125.2 (C4, 1J = 170.2 Hz, 2J(C4,H5) = 4.2 Hz), 121.8 (C3), 117.1 (C5, 1J = 189.3 Hz, 2J(C5,H4) = 6.0 Hz), 29.0 (CH2, 1J = 155.6 Hz), 28.8 (CH3, 1J = 127.7 Hz).
13C NMR (125 MHz, CDCl3) [5]: δ (ppm) 196.0 (COCH3, 2J(COCH3,CH3) = 5.9 Hz, 3J(CO,H4) = 0.9 Hz), 164.1 (NCO, 2J(NCO,CH2) = 4.5 Hz), 148.3 (C2, 2J(C2,NH) = 2.1 Hz, 3J(C2,H4) = 10.0 Hz, 3J(C2,H5) = 7.6 Hz), 124.4 (C4, 1J = 168.8 Hz, 2J(C4,H5) = 3.6 Hz), 122.0 (C3, 2J(C3,H4) = 5.8 Hz, 3J(C3,H5) = 9.1 Hz), 3J(C3,CH3) = 1.3 Hz), 116.9 (C5, 1J = 187.7 Hz, 2J(C5,H4) = 5.0 Hz), 28.7 (CH3, 1J = 127.9 Hz), 27.9 (CH2, 1J = 153.9 Hz).
15N NMR (50 MHz, CDCl3) [6]: δ (ppm) –248.9 (NH).
MS (m/z, %) [7]: 263 (M+, 27), 261 (M+, 25), 141 (100), 126 (75), 43 (59).
Elemental Analysis: Calculated for C8H8BrNO2S (262.12) · 0.1 H2O: C, 36.41%; H, 3.13%; N, 5.31%.
Found: C, 36.15%; H, 2.92%; N, 5.03%.
N-(3-Acetyl-2-thienyl)-2-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetamide (3b):
At room temperature, to 2.12 g (15 mmol) of thiophenamine 1 [2] in 20 mL of dry 1,4-dioxane were added dropwise 3.35 g (15 mmol) of phthalimidoacetyl chloride (2b) [8] in 20 mL of 1,4-dioxane. The reaction mixture was stirred overnight and then poured into H2O (ca. 100 mL). Upon neutralization with solid NaHCO3 a yellowish precipitate was formed which was filtered off, washed with H2O, and dried under reduced pressure to afford pure 3b (4.33 g, 88%) as a yellowish powder.
Melting point: 208–212 °C.
IR (KBr) [3]: 1773, 1719, 1693, 1635 cm–1.
1H NMR (500 MHz, CDCl3) [5]: δ (ppm) 12.25 (s, 1H, NH), 7.91 (m, 2H, Phth-H3,6), 7.76 (m, 2H, Phth-H4,5), 7.17 (d, 3J(Th-H4,Th-H5) = 5.8 Hz, 1H, Th-H4), 6.75 (d, 3J(Th-H5,Th-H4) = 5.8 Hz, 1H, Th-H5), 4.65 (s, 2H, CH2), 2.49 (s, 3H, CH3).
13C NMR (125 MHz, CDCl3) [5]: δ (ppm) 196.1 (COCH3), 167.4 (Phth-CO), 164.1 (HNCO), 148.5 (Th-C2), 134.4 (Phth-C4,5), 131.9 (Phth-C1,2), 124.2 (Th-C4), 123.8 (Phth-C3,6), 121.5 (Th-C3), 116.6 (Th-C5), 40.7 (CH2), 28.6 (CH3).
15N NMR (50 MHz, CDCl3) [6]: δ (ppm) –228.8 (NCH2), –252.7 (NH).
MS (m/z, %) [7]: 328 (M+, 17), 168 (17), 160 (100), 141 (27).
Elemental Analysis: Calculated for C16H12N2O4S (328.34) · 0.1 H2O: C, 58.21%; H, 3.72%; N, 8.49%.
Found: C, 57.86%; H, 3.87%; N, 8.40%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6References and Notes
- Gewald, K. Chem. Ber. 1965, 98, 3571–3577. [Green Version]
- Eller, G. A.; Holzer, W. Molecules 2006, 11, 371–376. [PubMed][Green Version]
- The spectrum was obtained on a Perkin-Elmer FTIR 1605 spectrophotometer.
- The spectrum was obtained on a Varian UnityPlus 300 spectrometer (299.95 MHz for 1H, 75.43 MHz for 13C) at 28 °C. The center of the solvent signal was used as an internal standard which was related to TMS with δ 2.49 ppm (1H NMR) and δ 39.5 ppm (13C NMR).
- The spectrum was obtained on a Bruker Avance 500 spectrometer (500.13 MHz for 1H, 125.77 MHz for 13C) at 294 K. The center of the solvent signal was used as an internal standard which was related to TMS with δ 7.26 ppm (1H NMR) and δ 77.0 ppm (13C NMR).
- The spectrum was obtained on a Bruker Avance 500 spectrometer (50.68 MHz for 15N) and was referenced against neat, external nitromethane (coaxial capillary).
- The spectrum was obtained on a Shimadzu QP 1000 instrument (EI, 70eV).
- Usifoh, C. O.; Lambert, D. M.; Wouters, J.; Scriba, G. K. E. Arch. Pharm. (Weinheim, Ger.) 2001, 334, 323–331. [Green Version]
© 2006 MDPI. All rights reserved.