A Spatiotemporal Model of CXCL10 as a Master Regulator of Immune Evasion and Metastasis in Osteosarcoma
Abstract
1. Introduction
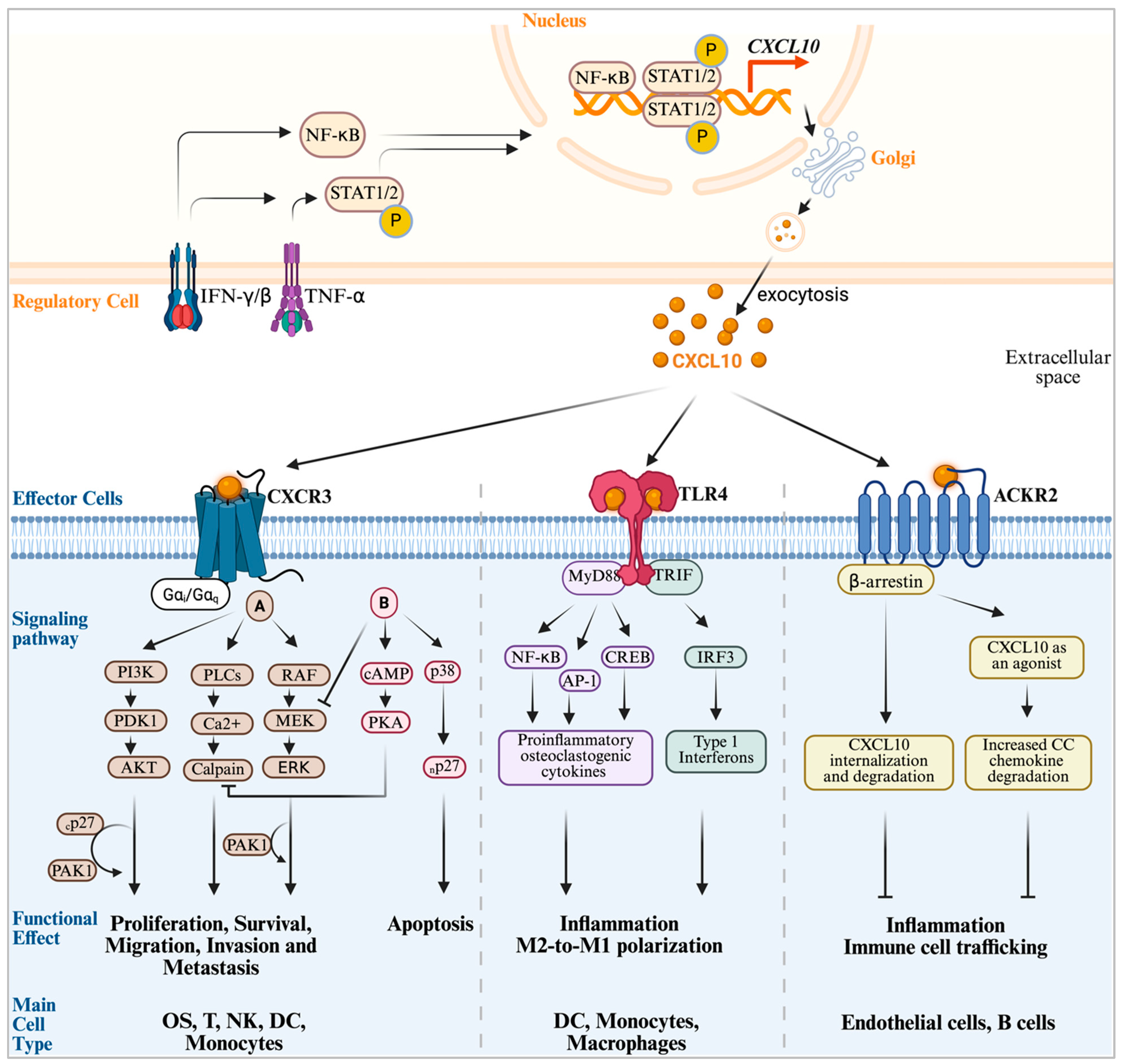
2. The CXCL10 Signaling Network
2.1. The CXCL10-CXCR3 Axis: The Canonical Signaling Pathway in Cancers, OS and Immune Modulation
2.2. The CXCL10-TLR4 Axis, a Non-Canonical Innate Immune Inflammatory Signaling Pathway
2.3. The CXCL0-ACKR2 Axis: An Atypical Scavenger and Chemokine Gradient Modulator
2.4. The CXCL10-CXCR3 Downstream Signaling Pathways in Cellular Activity Modulation
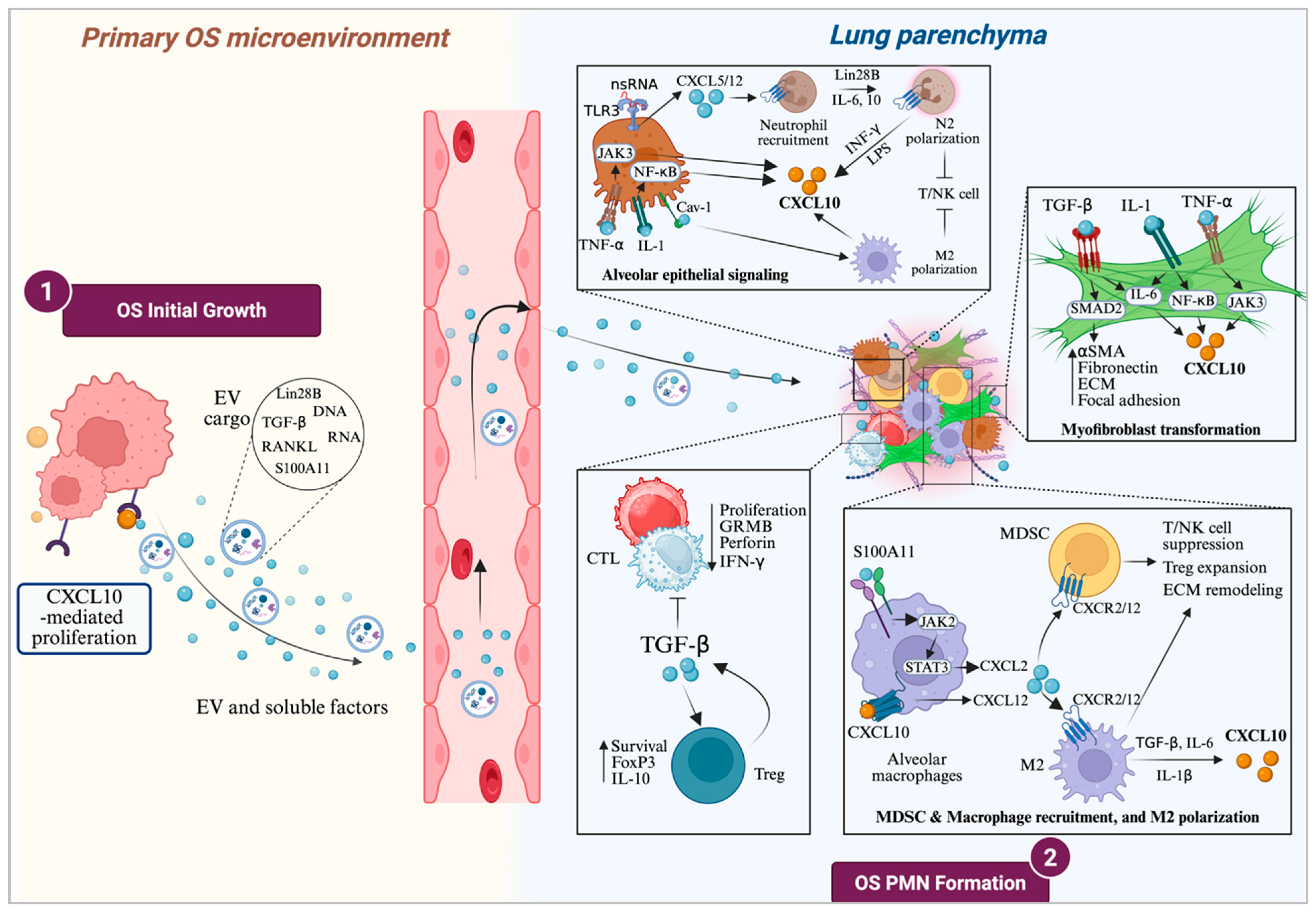
3. CXCL10’s Pro-Metastatic Arm: The Role of CXCL10 in OS Initiation, Growth and Early-Stage Metastasis
3.1. CXCL10 in OS Initiation
3.2. CXCL10 in OS Proliferation and Motility
| Cancer Type | Pro-Metastatic/Pro-Tumor Major Role | Ref. | Anti-Metastatic/Anti-Tumor Major Role | Ref. |
|---|---|---|---|---|
| Osteosarcoma | CXCR3-A autocrine signaling promotes cell migration, invasion, survival and lung metastasis through the AKT/PAK1 pathway | [71] | High intra-tumoral expression of CXCR3 correlates with dense infiltration of CD8+ T cells and NK cells, establishing an immune-hot microenvironment and improved prognosis. | [126] |
| Breast | Enhances tumor cell motility and promotes metastasis to the lungs and lymph nodes. | [127] | The CXCR3-B isoform has anti-proliferative and anti-angiogenic effects. High axis expression is associated with T-cell infiltration and a better response to therapy. | [69] |
| Melanoma | Autocrine CXCL10-CXCR3 signaling drives tumor cell proliferation and invasion, contributing to metastasis development and correlating with poor clinical outcomes. | [94] | High intra-tumoral expression of CXCL10 is a key biomarker for a T-cell-inflamed microenvironment and strongly predicts a positive clinical response to anti-PD-1 immunotherapy. | [128] |
| Colorectal | Promotes cancer cell proliferation, survival, and invasion through activation of the PI3K/AKT and Snail pathways. CXCR3 expression is linked to lymph node metastasis. | [119] | Mediates CD8+ T cell facilitation of vessel normalization and improved combinational immunotherapy | [129] |
| Renal Cell Carcinoma | High CXCR3 expression on tumor cells is associated with advanced tumor grade and metastatic progression. | [70] | In localized disease, high CXCR3 expression is linked to better survival, reflecting a strong, prognostically favorable immune cell infiltrate. | [130] |
| Pancreatic | Promotes tumor cell migration and contributes to perineural invasion. | [131] | Recruits and maintains an anti-tumor M1 macrophage in the TME. Blockade of the axis accelerates the progression of precancerous lesions. | [25] |
| Gastric | Promotes invasion and metastasis via the PI3K/AKT pathway | [95] | CXCR3 expression correlates with decreased infiltration of M2 macrophage and favorable outcome | [132] |
| Hepatocellular Carcinoma (HCC) | Blockade of CXCR3-B signaling increases tumor aggressiveness | [68] | High intra-tumoral expression of CXCL10 and CXCR3 is associated with increased CD8+ T-cell infiltration, reduced recurrence, and better overall survival. | [133] |
| Glioma | Tumoral CXCR3 promotes invasion and progression. Pharmacological antagonism inhibits tumor growth in preclinical models. | [134] | Recruits effector T cells and NK cells across the blood–brain barrier, a critical step for the efficacy of immunotherapies in this cold tumor. | [135] |
| Prostate | CXCL10-CXCR3 signaling promotes invasion and is associated with bone metastasis. | [122] | High CXCL10 expression within the tumor is associated with increased infiltration of cytotoxic T-cells, decreased Treg, and anti-tumor immunity. | [136] |
3.3. CXCL10 in OS Pre-Metastatic Niche (PMN) Formation
4. CXCL10’s Anti-Tumor Arm: The Role of CXCL10 in Immune Cell Modulation
4.1. CXCL10 in Immune Cell Trafficking
4.2. CXCL10 in Immune Cell Positioning and Function Modulation
4.3. CXCL10 in TME Immunosuppression Modulation
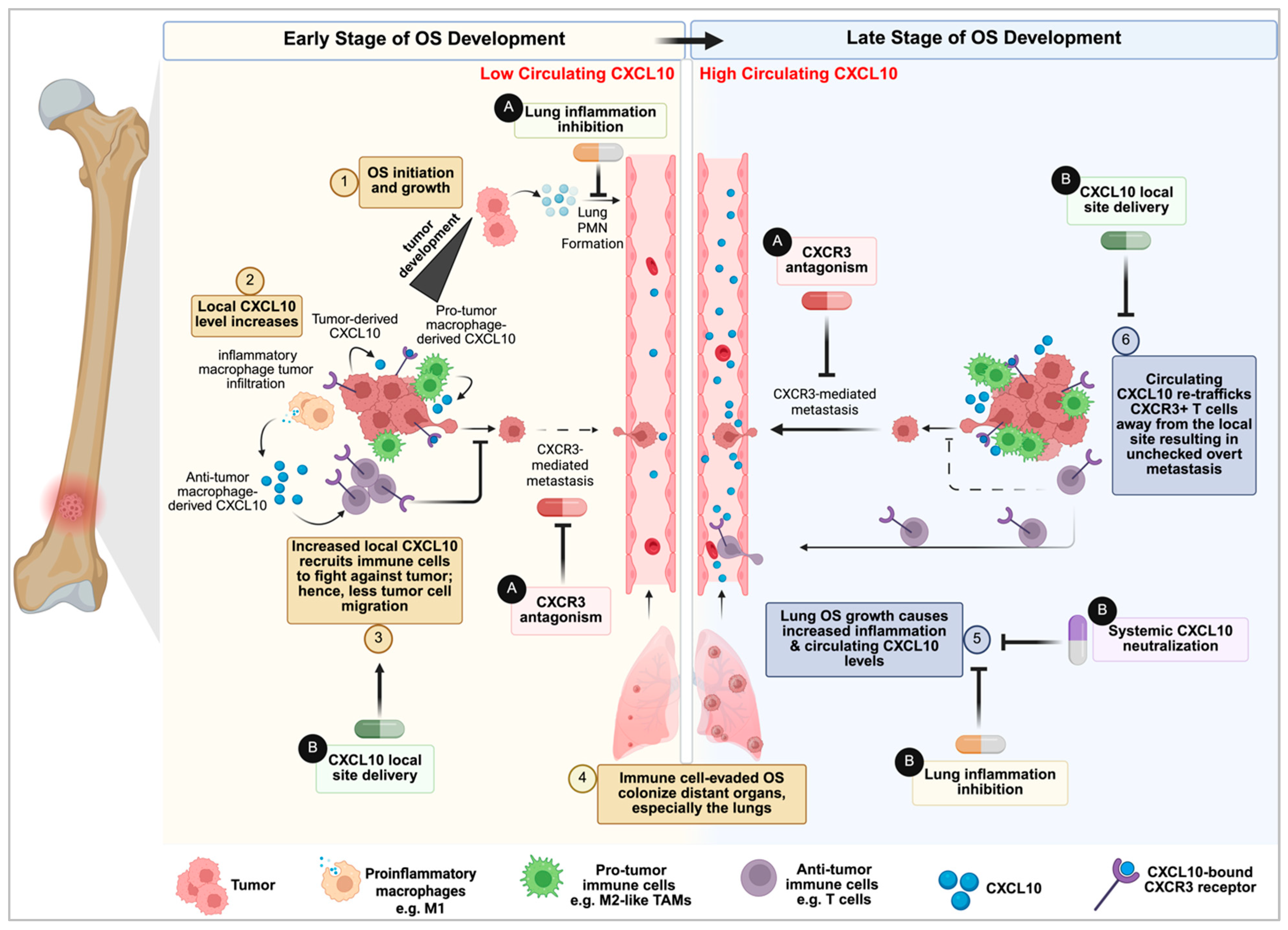
5. The Unifying Model: Circulating CXCL10 as a Tumor-Moderated Systemic Immune Decoy in Late-Stage Disease
6. Therapeutic Implications and Future Directions
6.1. Targeting the Pro-Metastatic Arm: CXCR3 Inhibition
6.1.1. CXCR3 Antagonism
6.1.2. CXCR3 Degradation
6.1.3. Dual CXCR3/CXCR4 Antagonism
6.2. Harnessing the Anti-Tumor Arm: Immunotherapy and Recruitment
6.2.1. Primary Site CXCL10 Upregulation
6.2.2. Circulating CXCL10 Neutralization
6.3. Maximizing the Efficacy of CXCR3 and CXCL10 Modulators
6.3.1. Combination Therapy
6.3.2. Targeted Delivery
6.4. Validation and Biomarkers
6.4.1. Validating the Spatiotemporal Model
6.4.2. Novel Biomarker Strategies: Beyond Serum CXCL10
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OS | Osteosarcoma |
| CXCL10 | C-X-C Motif Chemokine Ligand 10 |
| CXCR3 | C-X-C Motif Chemokine Receptor 3 |
| TLR4 | Toll-Like Receptor 4 |
| ACKR2 | Atypical Chemokine Receptor 2 |
| IFN-γ | Interferon-γ |
| TME | Tumor Microenvironment |
| ELR | Glutamic Acid-Leucine-Arginine |
| PMN | Pre-Metastatic Niche |
| MSC | Mesenchymal Stem Cells |
| TAM | Tumor-Associated Macrophages |
| MDSC | Myeloid-Derived Suppressor Cells |
| CAF | Cancer-Associated Fibroblasts |
| APC | Antigen-Presenting Cells |
| DC | Dendritic Cells |
| CTL | Cytotoxic T Lymphocytes |
| EMT | Epithelial–Mesenchymal Transition |
| EV | Extracellular Vesicles |
| ICB | Immune Checkpoint Blockade |
| ICI | Immune Checkpoint Inhibitors |
| NP | Nanoparticles |
| PAK1 | p21-Activated Kinase 1 |
| PROTAC | Proteolysis-Targeting Chimeras |
| RANKL | Receptor Activator of Nuclear factor-κB Ligand |
| TCE | T Cell Engagers |
| NKCE | Natural Killer-Cell Engagers |
References
- Gupta, G.P.; Massagué, J. Cancer Metastasis: Building a Framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef]
- Joyce, J.A.; Pollard, J.W. Microenvironmental Regulation of Metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef]
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of Deaths from Cancer Caused by Metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Wemer, M.; Winkelmann, W.; et al. Prognostic Factors in High-Grade Osteosarcoma of the Extremities or Trunk: An Analysis of 1,702 Patients Treated on Neoadjuvant Cooperative Osteosarcoma Study Group Protocols. J. Clin. Oncol. 2002, 20, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Kager, L.; Zoubek, A.; Pötschger, U.; Kastner, U.; Flege, S.; Kempf-Bielack, B.; Branscheid, D.; Kotz, R.; Salzer-Kuntschik, M.; Winkelmann, W.; et al. Primary Metastatic Osteosarcoma: Presentation and Outcome of Patients Treated on Neoadjuvant Cooperative Osteosarcoma Study Group Protocols. J. Clin. Oncol. 2003, 21, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Smeland, S.; Bielack, S.S.; Whelan, J.; Bernstein, M.; Hogendoorn, P.; Krailo, M.D.; Gorlick, R.; Janeway, K.A.; Ingleby, F.C.; Anninga, J.; et al. Survival and Prognosis with Osteosarcoma: Outcomes in More than 2000 Patients in the EURAMOS-1 (European and American Osteosarcoma Study) Cohort. Eur. J. Cancer 2019, 109, 36–50. [Google Scholar] [CrossRef]
- Pantel, K.; Brakenhoff, R.H. Dissecting the Metastatic Cascade. Nat. Rev. Cancer 2004, 4, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Yang, C.; Tian, Y.; Zhao, F.; Chen, Z.; Su, P.; Li, Y.; Qian, A. Bone Microenvironment and Osteosarcoma Metastasis. Int. J. Mol. Sci. 2020, 21, 6985. [Google Scholar] [CrossRef]
- Zhu, T.; Han, J.; Yang, L.; Cai, Z.; Sun, W.; Hua, Y.; Xu, J. Immune Microenvironment in Osteosarcoma: Components, Therapeutic Strategies and Clinical Applications. Front. Immunol. 2022, 13, 907550. [Google Scholar] [CrossRef]
- Du, X.; Wei, H.; Zhang, B.; Wang, B.; Li, Z.; Pang, L.K.; Zhao, R.; Yao, W. Molecular Mechanisms of Osteosarcoma Metastasis and Possible Treatment Opportunities. Front. Oncol. 2023, 13, 1117867. [Google Scholar] [CrossRef]
- Nirala, B.K.; Yamamichi, T.; Petrescu, D.I.; Shafin, T.N.; Yustein, J.T. Decoding the Impact of Tumor Microenvironment in Osteosarcoma Progression and Metastasis. Cancers 2023, 15, 5108. [Google Scholar] [CrossRef]
- Lei, Y.; Junxin, C.; Yongcan, H.; Xiaoguang, L.; Binsheng, Y. Role of MicroRNAs in the Crosstalk between Osteosarcoma Cells and the Tumour Microenvironment. J. Bone Oncol. 2020, 25, 100322. [Google Scholar] [CrossRef]
- Jin, J.; Cong, J.; Lei, S.; Zhang, Q.; Zhong, X.; Su, Y.; Lu, M.; Ma, Y.; Li, Z.; Wang, L.; et al. Cracking the Code: Deciphering the Role of the Tumor Microenvironment in Osteosarcoma Metastasis. Int. Immunopharmacol. 2023, 121, 110422. [Google Scholar] [CrossRef]
- Tatsuno, R.; Ichikawa, J.; Komohara, Y.; Pan, C.; Kawasaki, T.; Enomoto, A.; Aoki, K.; Hayakawa, K.; Iwata, S.; Jubashi, T.; et al. Pivotal Role of IL-8 Derived from the Interaction between Osteosarcoma and Tumor-Associated Macrophages in Osteosarcoma Growth and Metastasis via the FAK Pathway. Cell Death Dis. 2024, 15, 108, Erratum in Cell Death Dis. 2024, 15, 471. [Google Scholar] [CrossRef]
- Gyau, B.B.; Wang, J.; Wu, W.; Scull, B.; Major, A.M.; Jin, W.; Cates, J.M.M.; Hicks, J.; Man, T.K. Multiplex Imaging Mass Cytometry Reveals Prognostic Immunosuppressive Subpopulations and Macrophage-Driven Metastasis in Osteosarcoma. Cancers 2025, 17, 2780. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the Cancer Microenvironment and Their Relevance in Cancer Immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Flores, R.; Yu, A.; Okcu, M.F.; Murray, J.; Chintagumpala, M.; Hicks, J.; Lau, C.C.; Man, T.K. Elevated Expression of CXC Chemokines in Pediatric Osteosarcoma Patients. Cancer 2011, 117, 207–217. [Google Scholar] [CrossRef]
- Flores, R.J.; Kelly, A.J.; Li, Y.; Nakka, M.; Barkauskas, D.A.; Krailo, M.; Wang, L.L.; Perlaky, L.; Lau, C.C.; Hicks, M.J.; et al. A Novel Prognostic Model for Osteosarcoma Using Circulating CXCL10 and FLT3LG. Cancer 2017, 123, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Kastner, L.; Kandalaft, W.; Mahant, A.M.; Crimella, J.; Hakim, S.; Peng, X.P.; Isakoff, M.S.; Hayashi, M.; Loeb, D.M. Cytokine Profiling of Children, Adolescents, and Young Adults Newly Diagnosed with Sarcomas Demonstrates the Role of IL-1β in Osteosarcoma Metastasis. Cancers 2025, 17, 3009. [Google Scholar] [CrossRef]
- Wightman, S.C.; Uppal, A.; Pitroda, S.P.; Ganai, S.; Burnette, B.; Stack, M.; Oshima, G.; Khan, S.; Huang, X.; Posner, M.C.; et al. Oncogenic CXCL10 Signalling Drives Metastasis Development and Poor Clinical Outcome. Br. J. Cancer 2015, 113, 327–335. [Google Scholar] [CrossRef]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.J. CXCL9, CXCL10, CXCL11/CXCR3 Axis for Immune Activation—A Target for Novel Cancer Therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef]
- Eguchi, S.; Luo, W.; Zhu, H.; Hoang, H.M.; Xu, C.; Behbehani, G.K.; Tasneem, K.L.; Ayello, J.; Marcondes, M.; Lee, D.A.; et al. CXCL10-Induced Chemotaxis of Ex Vivo-Expanded Natural Killer Cells Combined with NKTR-255 Enhances Anti-Tumor Efficacy in Osteosarcoma. Mol. Ther. Oncol. 2025, 33, 201051. [Google Scholar] [CrossRef]
- Pandey, V.; Martinez, A.K.F.; Bastea, L.I.; Döppler, H.R.; Eisenhauer, J.; Le, T.; Edenfield, B.; Storz, P. Cxcl10/Cxcr3 Signaling Contributes to an Inflammatory Microenvironment and Its Blockade Enhances Progression of Murine Pancreatic Precancerous Lesions. eLife 2021, 10, e60646. [Google Scholar] [CrossRef]
- Yue, S.; Niu, D.; Ma, W.; Guan, Y.; Liu, Q.; Wang, X.; Xiao, Y.; Meng, J.; Ding, K.; Zhang, L.; et al. The CXCL10/CXCR3 Axis Regulates Th1 Cell Differentiation and Migration in Experimental Autoimmune Prostatitis through the PI3K/AKT Pathway. Andrology 2023, 12, 1408–1418. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, W.; Chen, R.; Xiang, B.; Zhou, S.; Lan, L. CXCL10 Is a Tumor Microenvironment and Immune Infiltration Related Prognostic Biomarker in Pancreatic Adenocarcinoma. Front. Mol. Biosci. 2021, 8, 611508. [Google Scholar] [CrossRef] [PubMed]
- Ardighieri, L.; Missale, F.; Bugatti, M.; Gatta, L.B.; Pezzali, I.; Monti, M.; Gottardi, S.; Zanotti, L.; Bignotti, E.; Ravaggi, A.; et al. Infiltration by CXCL10 Secreting Macrophages Is Associated With Antitumor Immunity and Response to Therapy in Ovarian Cancer Subtypes. Front. Immunol. 2021, 12, 690201. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Yan, T.; Guo, W.; Wang, W.; Zhao, Z.; Ren, T.; Huang, Y.; Zhang, H.; Yu, Y.; Liang, X. Identification of Potential Therapeutic Targets and Immune Cell Infiltration Characteristics in Osteosarcoma Using Bioinformatics Strategy. Front. Oncol. 2020, 10, 1628. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hu, Y.; Liu, T.; Wang, Y. Profiles of Immune Cell Infiltration and Immune-Related Genes in the Tumor Microenvironment of Osteosarcoma Cancer. BMC Cancer 2021, 21, 1345. [Google Scholar] [CrossRef]
- Wan, B.; Wang, R.; Nie, J.; Sun, Y.; Zhang, B.; Liu, W.; Chen, D. Analysis of Immune Gene Expression Subtypes Reveals Osteosarcoma Immune Heterogeneity. J. Oncol. 2021, 2021, 6649412. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, H.; Ren, T.; Huang, Y.; Liang, X.; Wang, W.; Niu, J.; Han, Y.; Guo, W. Development of a Prognostic Gene Signature Based on an Immunogenomic Infiltration Analysis of Osteosarcoma. J. Cell. Mol. Med. 2020, 24, 11230–11242. [Google Scholar] [CrossRef]
- Wu, T.; Yang, W.; Sun, A.; Wei, Z.; Lin, Q. The Role of CXC Chemokines in Cancer Progression. Cancers 2023, 15, 167. [Google Scholar] [CrossRef]
- Strieter, R.M.; Belperio, J.A.; Phillips, R.J.; Keane, M.P. CXC Chemokines in Angiogenesis of Cancer. Semin. Cancer Biol. 2004, 14, 195–200. [Google Scholar] [CrossRef]
- Strieter, R.M.; Burdick, M.D.; Mestas, J.; Gomperts, B.; Keane, M.P.; Belperio, J.A. Cancer CXC Chemokine Networks and Tumour Angiogenesis. Eur. J. Cancer 2006, 42, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hao, J.; Tan, Y.Q.; Zhu, T.; Pandey, V.; Lobie, P.E. CXC Chemokine Signaling in Progression of Epithelial Ovarian Cancer: Theranostic Perspectives. Int. J. Mol. Sci. 2022, 23, 2642. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, F.; Zhang, K.; Chen, Z. Comprehensive Analysis of Prognostic Value and Immune Infiltration of CXC Chemokines in Pancreatic Cancer. BMC Med. Genom. 2022, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wei, Y.; Sheng, F.; Xu, Y.; Liu, J.; Gao, L.; Yang, J.; Sun, X.; Huang, J.; Guo, Q. Comprehensive Analysis of the Prognosis and Immune Infiltration for CXC Chemokines in Colorectal Cancer. Aging 2021, 13, 17548–17567. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Gao, Y.; Ding, P.; Wu, T.; Ji, G. The Role of CXCL Family Members in Different Diseases. Cell Death Discov. 2023, 9, 212. [Google Scholar] [CrossRef]
- Yuan, M.; Zhu, H.; Xu, J.; Zheng, Y.; Cao, X.; Liu, Q. Tumor-Derived CXCL1 Promotes Lung Cancer Growth via Recruitment of Tumor-Associated Neutrophils. J. Immunol. Res. 2016, 2016, 6530410, Correction in J. Immunol. Res. 2020, 2020, 5106904. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Hou, M.F.; Kuo, P.L.; Huang, Y.F.; Tsai, E.M. Breast Tumor-Associated Osteoblast-Derived CXCL5 Increases Cancer Progression by ERK/MSK1/Elk-1/Snail Signaling Pathway. Oncogene 2013, 32, 4436–4447. [Google Scholar] [CrossRef]
- Song, Y.; Baba, T.; Li, Y.Y.; Furukawa, K.; Tanabe, Y.; Matsugo, S.; Sasaki, S.; Mukaida, N. Gemcitabine-Induced CXCL8 Expression Counteracts Its Actions by Inducing Tumor Neovascularization. Biochem. Biophys. Res. Commun. 2015, 458, 341–346. [Google Scholar] [CrossRef]
- Highfill, S.L.; Cui, Y.; Giles, A.J.; Smith, J.P.; Zhang, H.; Morse, E.; Kaplan, R.N.; Mackall, C.L. Disruption of CXCR2-Mediated MDSC Tumor Trafficking Enhances Anti-PD1 Efficacy. Sci. Transl. Med. 2014, 6, 237ra67. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.; Furth, E.E.; Vonderheide, R.H. CXCR2-Dependent Accumulation of Tumor-Associated Neutrophils Regulates T-Cell Immunity in Pancreatic Ductal Adenocarcinoma. Cancer Immunol. Res. 2016, 4, 968–982. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Jhurani, S.; Kwang, S.A.; Mastuo, Y.; Yi, T.; Guha, S.; Liu, M.; Aggarwal, B.B. Zerumbone Down-Regulates Chemokine Receptor CXCR4 Expression Leading to Inhibition of CXCL12-Induced Invasion of Breast and Pancreatic Tumor Cells. Cancer Res. 2008, 68, 8938–8944. [Google Scholar] [CrossRef]
- Yu, P.F.; Huang, Y.; Xu, C.L.; Lin, L.Y.; Han, Y.Y.; Sun, W.H.; Hu, G.H.; Rabson, A.B.; Wang, Y.; Shi, Y.F. Downregulation of CXCL12 in Mesenchymal Stromal Cells by TGFβ Promotes Breast Cancer Metastasis. Oncogene 2017, 36, 840–849. [Google Scholar] [CrossRef]
- Teicher, B.A.; Fricker, S.P. CXCL12 (SDF-1)/CXCR4 Pathway in Cancer. Clin. Cancer Res. 2010, 16, 2927–2931. [Google Scholar] [CrossRef]
- Chao, C.C.; Lee, C.W.; Chang, T.M.; Chen, P.C.; Liu, J.F. CXCL1/CXCR2 Paracrine Axis Contributes to Lung Metastasis in Osteosarcoma. Cancers 2020, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Chiang, Y.C.; Yu, P.A.; Peng, K.T.; Chi, M.C.; Lee, M.H.; Fang, M.L.; Lee, K.H.; Hsu, L.F.; Liu, J.F. A Role of CXCL1 Drives Osteosarcoma Lung Metastasis via VCAM-1 Production. Front. Oncol. 2021, 11, 735277. [Google Scholar] [CrossRef]
- Groom, J.R.; Luster, A.D. CXCR3 Ligands: Redundant, Collaborative and Antagonistic Functions. Immunol. Cell Biol. 2011, 89, 207–215. [Google Scholar] [CrossRef]
- Hirani, D.V.; Thielen, F.; Mansouri, S.; Danopoulos, S.; Vohlen, C.; Haznedar-Karakaya, P.; Mohr, J.; Wilke, R.; Selle, J.; Grosch, T.; et al. CXCL10 Deficiency Limits Macrophage Infiltration, Preserves Lung Matrix, and Enables Lung Growth in Bronchopulmonary Dysplasia. Inflamm. Regen. 2023, 43, 52. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, A.A.; Wang, S.; Agarwal, V.; Marcisak, E.F.; Zuo, A.; Jablonski, S.A.; Loth, M.; Fertig, E.J.; Macdougall, J.; Zhukovsky, E.; et al. DPP Inhibition Alters the CXCR3 Axis and Enhances NK and CD8+ T Cell Infiltration to Improve Anti-PD1 Efficacy in Murine Models of Pancreatic Ductal Adenocarcinoma. J. Immunother. Cancer 2021, 9, e002837. [Google Scholar] [CrossRef]
- Karin, N. CXCR3 Ligands in Cancer and Autoimmunity, Chemoattraction of Effector T Cells, and Beyond. Front. Immunol. 2020, 11, 976. [Google Scholar] [CrossRef]
- Karin, N.; Wildbaum, G.; Thelen, M. Biased Signaling Pathways via CXCR3 Control the Development and Function of CD4+ T Cell Subsets. J. Leukoc. Biol. 2016, 99, 857–862. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Wang, S.; Ni, H.; Zhao, P.; Chen, G.; Xu, B.; Yuan, L. The Role of CXCR3 and Its Ligands in Cancer. Front. Oncol. 2022, 12, 1022688. [Google Scholar] [CrossRef]
- Reynders, N.; Abboud, D.; Baragli, A.; Noman, M.Z.; Rogister, B.; Niclou, S.P.; Heveker, N.; Janji, B.; Hanson, J.; Szpakowska, M.; et al. The Distinct Roles of CXCR3 Variants and Their Ligands in the Tumor Microenvironment. Cells 2019, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, N.; Bonomini, S.; Romagnani, P.; Lazzaretti, M.; Morandi, F.; Colla, S.; Tagliaferri, S.; Lasagni, L.; Annunziato, F.; Crugnola, M.; et al. CXCR3 and Its Binding Chemokines in Myeloma Cells: Expression of Isoforms and Potential Relationships with Myeloma Cell Proliferation and Survival. Haematologica 2006, 91, 1489–1497. [Google Scholar]
- Duruisseaux, M.; Rabbe, N.; Antoine, M.; Vieira, T.; Poulot, V.; Cadranel, J.; Wislez, M. Pro-Tumoural CXCL10/CXCR3-A Autocrine Loop in Invasive Mucinous Lung Adenocarcinoma. ERJ Open Res. 2017, 3, 00047-2016. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Contreras, A.G.; Grimm, M.; Waaga-Gasser, A.M.; Briscoe, D.M.; Pal, S. Calcineurin Inhibitors Modulate CXCR3 Splice Variant Expression and Mediate Renal Cancer Progression. J. Am. Soc. Nephrol. 2008, 19, 2437–2446. [Google Scholar] [CrossRef]
- Loetscher, M.; Gerber, B.; Loetscher, P.; Jones, S.A.; Piali, L.; Clark-Lewis, I.; Baggiolini, M.; Moser, B. Chemokine Receptor Specific for IP10 and Mig: Structure, Function, and Expression in Activate T-Lymphocytes. J. Exp. Med. 1996, 184, 963–969. [Google Scholar] [CrossRef]
- Mikucki, M.E.; Fisher, D.T.; Matsuzaki, J.; Skitzki, J.J.; Gaulin, N.B.; Muhitch, J.B.; Ku, A.W.; Frelinger, J.G.; Odunsi, K.; Gajewski, T.F.; et al. Non-Redundant Requirement for CXCR3 Signalling during Tumoricidal T-Cell Trafficking across Tumour Vascular Checkpoints. Nat. Commun. 2015, 6, 7458. [Google Scholar] [CrossRef]
- Wendel, M.; Galani, I.E.; Suri-Payer, E.; Cerwenka, A. Natural Killer Cell Accumulation in Tumors Is Dependent on IFN-γ and CXCR3 Ligands. Cancer Res. 2008, 68, 8437–8445. [Google Scholar] [CrossRef]
- Khan, I.R.; Khurshid, S.; Almawash, S.; Kumar, R.; Akil, A.S.A.S.; Bhat, A.A.; Macha, M.A. G Protein-Coupled Receptor Signaling: Implications and Therapeutic Development Advances in Cancers. MedComm 2025, 6, e70375. [Google Scholar] [CrossRef]
- Dillemans, L.; Yu, K.; De Zutter, A.; Noppen, S.; Gouwy, M.; Berghmans, N.; Verhallen, L.; De Bondt, M.; Vanbrabant, L.; Brusselmans, S.; et al. Natural Carboxyterminal Truncation of Human CXCL10 Attenuates Glycosaminoglycan Binding, CXCR3A Signaling and Lymphocyte Chemotaxis, While Retaining Angiostatic Activity. Cell Commun. Signal. 2024, 22, 94. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Ge, S.; Zhang, M.; Mo, F.; Zhang, L.; Zhang, J.; Yang, C.; Tai, S.; Chen, X.; Zhang, L.; et al. Pathogenic Roles of CXCL10 in Experimental Autoimmune Prostatitis by Modulating Macrophage Chemotaxis and Cytokine Secretion. Front. Immunol. 2021, 12, 706027. [Google Scholar] [CrossRef]
- Yang, C.; Zheng, W.; Du, W. CXCR3A Contributes to the Invasion and Metastasis of Gastric Cancer Cells. Oncol. Rep. 2016, 36, 1686–1692. [Google Scholar] [CrossRef]
- Li, H.; Rong, S.; Chen, C.; Fan, Y.; Chen, T.; Wang, Y.; Chen, D.; Yang, C.; Yang, J. Disparate Roles of CXCR3A and CXCR3B in Regulating Progressive Properties of Colorectal Cancer Cells. Mol. Carcinog. 2019, 58, 171–184, Correction in Mol. Carcinog. 2021, 61, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Oh, S.; Kang, H.; Cho, H. Blocking CXCR3B Expression Increases Tumor Aggressiveness in Hepatocellular Carcinoma. Anticancer Res. 2024, 44, 5293–5301. [Google Scholar] [CrossRef]
- Li, Y.; Reader, J.C.; Ma, X.; Kundu, N.; Kochel, T.; Fulton, A.M. Divergent Roles of CXCR3 Isoforms in Promoting Cancer Stem-like Cell Survival and Metastasis. Breast Cancer Res. Treat. 2015, 149, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, T.; Suyama, T.; Imamura, Y.; Fuse, M.; Sakamoto, S.; Nihei, N.; Ueda, T.; Suzuki, H.; Seki, N.; Ichikawa, T. The Association of CXCR3 and Renal Cell Carcinoma Metastasis. J. Urol. 2014, 192, 567–574. [Google Scholar] [CrossRef]
- Gyau, B.B.; Wang, J.; Chen, X.; Clement, M.A.; Man, Z.D.; Major, A.M.; Weiser, M.C.; Xu, J.; Hicks, J.; Man, T.K. The Metastatic Role of the CXCL10-CXCR3 Axis and Its Therapeutic Potential in Osteosarcoma. J. Bone Oncol. 2025, 52, 100690. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Sun, Y.; Wang, Y.; Shi, H.; Han, X.; Mo, Y.; Wang, D.; Ke, Y.; Zeng, X. CXCL10 Conditions Alveolar Macrophages within the Premetastatic Niche to Promote Metastasis. Cancer Lett. 2022, 537, 215667. [Google Scholar] [CrossRef]
- Schulthess, F.T.; Paroni, F.; Sauter, N.S.; Shu, L.; Ribaux, P.; Haataja, L.; Strieter, R.M.; Oberholzer, J.; King, C.C.; Maedler, K. CXCL10 Impairs β Cell Function and Viability in Diabetes through TLR4 Signaling. Cell Metab. 2009, 9, 125–139. [Google Scholar] [CrossRef]
- Liu, H.; Ling, C.C.; Yeung, W.H.O.; Pang, L.; Liu, J.; Zhou, J.; Zhang, W.Y.; Liu, X.B.; Ng, T.P.K.; Yang, X.X.; et al. Monocytic MDSC Mobilization Promotes Tumor Recurrence after Liver Transplantation via CXCL10/TLR4/MMP14 Signaling. Cell Death Dis. 2021, 12, 489. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, B.; Jin, W.J.; Kim, H.H.; Ha, H.; Lee, Z.H. Pathogenic Roles of CXCL10 Signaling through CXCR3 and TLR4 in Macrophages and T Cells: Relevance for Arthritis. Arthritis Res. Ther. 2017, 19, 163. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Brouchet, A.; Gilhodes, J.; Van Acker, N.; Brion, R.; Bouvier, C.; Asse-Mat, P.; Gaspar, N.; Aubert, S.; Guinebretiere, J.M.; Marie, B.; et al. Characterization of Macrophages and Osteoclasts in the Osteosarcoma Tumor Microenvironment at Diagnosis: New Perspective for Osteosarcoma Treatment? Cancers 2021, 13, 423. [Google Scholar] [CrossRef]
- Richert, I.; Berchard, P.; Abbes, L.; Novikov, A.; Chettab, K.; Vandermoeten, A.; Dumontet, C.; Karanian, M.; Kerzerho, J.; Caroff, M.; et al. A TLR4 Agonist Induces Osteosarcoma Regression by Inducing an Antitumor Immune Response and Reprogramming M2 Macrophages to M1 Macrophages. Cancers 2023, 15, 4635. [Google Scholar] [CrossRef]
- Bachelerie, F.; Ben-Baruch, A.; Burkhardt, A.M.; Combadiere, C.; Farber, J.M.; Graham, G.J.; Horuk, R.; Sparre-Ulrich, A.H.; Locati, M.; Luster, A.D.; et al. International Union of Basic and Clinical Pharmacology. LXXXIX. Update on the Extended Family of Chemokine Receptors and Introducing a New Nomenclature for Atypical Chemokine Receptors. Pharmacol. Rev. 2014, 66, 1–79, Correction in Pharmacol. Rev. 2014, 66, 467. [Google Scholar] [CrossRef]
- Chevigné, A.; Janji, B.; Meyrath, M.; Reynders, N.; D’uonnolo, G.; Uchański, T.; Xiao, M.; Berchem, G.; Ollert, M.; Kwon, Y.J.; et al. Cxcl10 Is an Agonist of the Cc Family Chemokine Scavenger Receptor Ackr2/D6. Cancers 2021, 13, 1054. [Google Scholar] [CrossRef]
- Mantovani, A. Wandering Pathways in the Regulation of Innate Immunity and Inflammation. J. Autoimmun. 2017, 85, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hansell, C.A.H.; Fraser, A.R.; Hayes, A.J.; Pingen, M.; Burt, C.L.; Lee, K.M.; Medina-Ruiz, L.; Brownlie, D.; Macleod, M.K.L.; Burgoyne, P.; et al. The Atypical Chemokine Receptor Ackr2 Constrains NK Cell Migratory Activity and Promotes Metastasis. J. Immunol. 2018, 201, 2510–2519. [Google Scholar] [CrossRef]
- Albano, F.; Mollica Poeta, V.; Zotti, L.; Castagna, A.; Felicetta, A.; Mesaglio, A.; Zaghen, E.; Sironi, M.; Capucetti, A.; Di Donato, R.; et al. Selective Expression and Significance of ACKR2 in Lung Aerocytes. J. Immunother. Cancer 2025, 13, e009467. [Google Scholar] [CrossRef]
- Bonecchi, R.; Graham, G.J. Atypical Chemokine Receptors and Their Roles in the Resolution of the Inflammatory Response. Front. Immunol. 2016, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Bonavita, O.; Poeta, V.M.; Setten, E.; Massara, M.; Bonecchi, R. ACKR2: An Atypical Chemokine Receptor Regulating Lymphatic Biology. Front. Immunol. 2016, 7, 691, Correction in Front. Immunol. 2017, 8, 520. [Google Scholar] [CrossRef] [PubMed]
- Nibbs, R.J.B.; Graham, G.J. Immune Regulation by Atypical Chemokine Receptors. Nat. Rev. Immunol. 2013, 13, 815–829. [Google Scholar] [CrossRef]
- Benoit, A.; Lequeux, A.; Harter, P.; Berchem, G.; Janji, B. Atypical Chemokine Receptor 2 Expression Is Directly Regulated by Hypoxia Inducible Factor-1 Alpha in Cancer Cells under Hypoxia. Sci. Rep. 2024, 14, 26589. [Google Scholar] [CrossRef]
- Xu, H.; Lin, S.; Zhou, Z.; Li, D.; Zhang, X.; Yu, M.; Zhao, R.; Wang, Y.; Qian, J.; Li, X.; et al. New Genetic and Epigenetic Insights into the Chemokine System: The Latest Discoveries Aiding Progression toward Precision Medicine. Cell. Mol. Immunol. 2023, 20, 739–776. [Google Scholar] [CrossRef]
- Crawley, A.M.; Vranjkovic, A.; Faller, E.; McGuinty, M.; Busca, A.; Burke, S.C.; Cousineau, S.; Kumar, A.; MacPherson, P.A.; Angel, J.B. Jak/STAT and PI3K Signaling Pathways Have Both Common and Distinct Roles in IL-7-Mediated Activities in Human CD8+ T Cells. J. Leukoc. Biol. 2013, 95, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Sánchez, M.C.; Rodríguez-Serrano, C.; Almeida, J.; San Segundo, L.; Inogés, S.; Santos-Briz, Á.; García-Briñón, J.; Corchete, L.A.; San Miguel, J.F.; Del Cañizo, C.; et al. Targeting of PI3K/AKT/MTOR Pathway to Inhibit T Cell Activation and Prevent Graft-versus-Host Disease Development. J. Hematol. Oncol. 2016, 9, 113. [Google Scholar] [CrossRef]
- Leonard, M.R.; Jones, D.M.; Read, K.A.; Pokhrel, S.; Tuazon, J.A.; Warren, R.T.; Yount, J.S.; Oestreich, K.J. Aiolos Promotes CXCR3 Expression on Th1 Cells via Positive Regulation of IFN-γ/STAT1 Signaling. JCI Insight 2024, 10, e180287. [Google Scholar] [CrossRef]
- Wang, Z.; Ao, X.; Shen, Z.; Ao, L.; Wu, X.; Pu, C.; Guo, W.; Xing, W.; He, M.; Yuan, H.; et al. Tnf-α Augments Cxcl10/Cxcr3 Axis Activity to Induce Epithelial-Mesenchymal Transition in Colon Cancer Cell. Int. J. Biol. Sci. 2021, 17, 2683–2702. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Wang, C.; Wang, Y.; Xu, Y.; Li, X.; Johnson, N.A.; Mukherji, A.; Lo, U.G.; Xu, L.; Gonzalez, J.; et al. Ectopic JAK–STAT Activation Enables the Transition to a Stem-like and Multilineage State Conferring AR-Targeted Therapy Resistance. Nat. Cancer 2022, 3, 1071–1087, Erratum in Nat. Cancer 2022, 3, 1271. [Google Scholar] [CrossRef]
- He, L.F.; Xu, H.W.; Chen, M.; Xian, Z.R.; Wen, X.F.; Chen, M.N.; Du, C.W.; Huang, W.H.; Wu, J.D.; Zhang, G.J. Activated-PAK4 Predicts Worse Prognosis in Breast Cancer and Promotes Tumorigenesis through Activation of PI3K/AKT Signaling. Oncotarget 2017, 8, 17573–17585. [Google Scholar] [CrossRef]
- Antonicelli, F.; Lorin, J.; Kurdykowski, S.; Gangloff, S.C.; Le Naour, R.; Sallenave, J.M.; Hornebeck, W.; Grange, F.; Bernard, P. CXCL10 Reduces Melanoma Proliferation and Invasiveness in Vitro and in Vivo. Br. J. Dermatol. 2011, 164, 720–728. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, J.; Wang, T.; Zhang, X.; Liu, D. CXCL10/CXCR3 Axis Promotes the Invasion of Gastric Cancer via PI3K/AKT Pathway-Dependent MMPs Production. Biomed. Pharmacother. 2016, 82, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.A.; Kiezun, A.; Tonzi, P.; Van Allen, E.M.; Carter, S.L.; Baca, S.C.; Cowley, G.S.; Bhatt, A.S.; Rheinbay, E.; Pedamallu, C.S.; et al. Complementary Genomic Approaches Highlight the PI3K/MTOR Pathway as a Common Vulnerability in Osteosarcoma. Proc. Natl. Acad. Sci. USA 2014, 111, E5564–E5573. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, X.; Zhou, Y.; Chen, X.; Peng, K.; Jiang, R.; Liu, Z.; Song, X.; Xia, H. LINC01060 Knockdown Inhibits Osteosarcoma Cell Malignant Behaviors in Vitro and Tumor Growth and Metastasis in Vivo through the PI3K/Akt Signaling. Cancer Biol. Ther. 2023, 24, 2198904. [Google Scholar] [CrossRef]
- Yu, Y.; Luk, F.; Yang, J.L.; Walsh, W.R. Ras/Raf/MEK/ERK Pathway Is Associated with Lung Metastasis of Osteosarcoma in an Orthotopic Mouse Model. Anticancer Res. 2011, 31, 1147–1152. [Google Scholar]
- Bagheri-Yarmand, R.; Mandal, M.; Taludker, A.H.; Wang, R.A.; Vadlamudi, R.K.; Kung, H.J.; Kumar, R. Etk/Bmx Tyrosine Kinase Activates Pak1 and Regulates Tumorigenicity of Breast Cancer Cells. J. Biol. Chem. 2001, 276, 29403–29409. [Google Scholar] [CrossRef]
- Qu, Y.; Lin, Z.; Qi, Y.; Qi, Y.; Chen, Y.; Zhou, Q.; Zeng, H.; Liu, Z.; Wang, Z.; Wang, J.; et al. PAK1 Expression Determines Poor Prognosis and Immune Evasion in Metastatic Renal Cell Carcinoma Patients. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.G.; Youn, H.S.; Kwon, T.W.; Son, B.; Kang, J.H.; Yang, H.J.; Seong, K.M.; Kim, W.; Youn, B.H. PAK1 Tyrosine Phosphorylation Is Required to Induce Epithelial-Mesenchymal Transition and Radioresistance in Lung Cancer Cells. Cancer Res. 2014, 74, 5520–5531. [Google Scholar] [CrossRef]
- Chen, X.; Cates, J.M.M.; Du, Y.C.; Jain, A.; Jung, S.Y.; Li, X.N.; Hicks, J.M.; Man, T.K. Mislocalized Cytoplasmic P27 Activates PAK1-Mediated Metastasis and Is a Prognostic Factor in Osteosarcoma. Mol. Oncol. 2020, 14, 846–864. [Google Scholar] [CrossRef]
- Wang, J.; Gyau, B.B.; Xu, J.; Major, A.M.; Hicks, J.; Man, T.-K. CRISPR-Mediated Analysis of P27 and PAK1 Phosphorylation Reveals Complex Regulation of Osteosarcoma Metastasis. Onco 2025, 5, 40. [Google Scholar] [CrossRef]
- Liang, J.; Zubovitz, J.; Petrocelli, T.; Kotchetkov, R.; Connor, M.K.; Han, K.; Lee, J.-H.; Ciarallo, S.; Catzavelos, C.; Beniston, R.; et al. PKB/Akt Phosphorylates P27, Impairs Nuclear Import of P27 and Opposes P27-Mediated G1 Arrest. Nat. Med. 2002, 8, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jonasch, E.; Alexander, A.; Short, J.D.; Cai, S.; Wen, S.; Tsavachidou, D.; Tamboli, P.; Czerniak, B.A.; Do, K.A.; et al. Cytoplasmic Sequestration of P27 via AKT Phosphorylation in Renal Cell Carcinoma. Clin. Cancer Res. 2009, 15, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.H.; Cheong, J.W.; Kim, J.Y.; Eom, J.I.; Lee, S.T.; Sook Hahn, J.; Ko, Y.W.; Lee, M.H. Cytoplasmic Mislocalization of P27Kip1 Protein Is Associated with Constitutive Phosphorylation of Akt or Protein Kinase B and Poor Prognosis in Acute Myelogenous Leukemia. Cancer Res. 2004, 64, 5225–5231. [Google Scholar] [CrossRef]
- Shin, I.; Yakes, F.M.; Rojo, F.; Shin, N.-Y.; Bakin, A.V.; Baselga, J.; Arteaga, C.L. PKB/Akt Mediates Cell-Cycle Progression by Phosphorylation of P27Kip1 at Threonine 157 and Modulation of Its Cellular Localization. Nat. Med. 2002, 8, 1145–1152. [Google Scholar] [CrossRef]
- Gasper, N.A.; Petty, C.C.; Schrum, L.W.; Marriott, I.; Bost, K.L. Bacterium-Induced CXCL10 Secretion by Osteoblasts Can Be Mediated in Part through Toll-Like Receptor 4. Infect. Immun. 2002, 70, 4075–4082. [Google Scholar] [CrossRef]
- Miura, M.; Kitaura, H.; Ohori, F.; Narita, K.; Ren, J.; Noguchi, T.; Marahleh, A.; Ma, J.; Lin, A.; Fan, Z.; et al. Role of CXCL10 Released from Osteocytes in Response to TNF-α Stimulation on Osteoclasts. Sci. Rep. 2025, 15, 3040. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.N.; Kim, K.O.; Jin, W.J.; Lee, S.; Kim, H.H.; Ha, H.; Lee, Z.H. CXCL10 Promotes Osteolytic Bone Metastasis by Enhancing Cancer Outgrowth and Osteoclastogenesis. Cancer Res. 2012, 72, 3175–3186. [Google Scholar] [CrossRef]
- Dong, Y.; Song, C.; Wang, Y.; Lei, Z.; Xu, F.; Guan, H.; Chen, A.; Li, F. Inhibition of PRMT5 Suppresses Osteoclast Differentiation and Partially Protects against Ovariectomy-Induced Bone Loss through Downregulation of CXCL10 and RSAD2. Cell. Signal. 2017, 34, 55–65. [Google Scholar] [CrossRef]
- Lee, E.Y.; Lee, Z.H.; Song, Y.W. The Interaction between CXCL10 and Cytokines in Chronic Inflammatory Arthritis. Autoimmun. Rev. 2013, 12, 554–557. [Google Scholar] [CrossRef]
- Lee, M.N.; Hwang, H.S.; Oh, S.H.; Roshanzadeh, A.; Kim, J.W.; Song, J.H.; Kim, E.S.; Koh, J.T. Elevated Extracellular Calcium Ions Promote Proliferation and Migration of Mesenchymal Stem Cells via Increasing Osteopontin Expression. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.; Hornicek, F.; MacConaill, L.; Harmon, D.; Tariq, Z.; Garraway, L.; Duan, Z. High-Throughput Genotyping in Osteosarcoma Identifies Multiple Mutations in Phosphoinositide-3-Kinase and Other Oncogenes. Cancer 2012, 118, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Vega, O.A.; Lucero, C.M.J.; Araya, H.F.; Jerez, S.; Tapia, J.C.; Antonelli, M.; Salazar-Onfray, F.; Las Heras, F.; Thaler, R.; Riester, S.M.; et al. Wnt/β-Catenin Signaling Activates Expression of the Bone-Related Transcription Factor RUNX2 in Select Human Osteosarcoma Cell Types. J. Cell. Biochem. 2017, 118, 3662–3674. [Google Scholar] [CrossRef]
- Hilton, M.J.; Tu, X.; Wu, X.; Bai, S.; Zhao, H.; Kobayashi, T.; Kronenberg, H.M.; Teitelbaum, S.L.; Ross, F.P.; Kopan, R.; et al. Notch Signaling Maintains Bone Marrow Mesenchymal Progenitors by Suppressing Osteoblast Differentiation. Nat. Med. 2008, 14, 306–314. [Google Scholar] [CrossRef]
- Liu, F.; Sun, X.; Deng, S.; Wu, Y.; Liu, X.; Wu, C.; Huang, K.; Li, Y.; Dong, Z.; Xiao, W.; et al. Cxcl10 and Cxcr3 Regulate Self-Renewal and Differentiation of Hematopoietic Stem Cells. Stem Cell Res. Ther. 2024, 15, 248. [Google Scholar] [CrossRef] [PubMed]
- Pradelli, E.; Karimdjee-Soilihi, B.; Michiels, J.F.; Ricci, J.E.; Millet, M.A.; Vandenbos, F.; Sullivan, T.J.; Collins, T.L.; Johnson, M.G.; Medina, J.C.; et al. Antagonism of Chemokine Receptor CXCR3 Inhibits Osteosarcoma Metastasis to Lungs. Int. J. Cancer 2009, 125, 2586–2594. [Google Scholar] [CrossRef]
- Zipin-Roitman, A.; Meshel, T.; Sagi-Assif, O.; Shalmon, B.; Avivi, C.; Pfeffer, R.M.; Witz, I.P.; Ben-Baruch, A. CXCL10 Promotes Invasion-Related Properties in Human Colorectal Carcinoma Cells. Cancer Res. 2007, 67, 3396–3405. [Google Scholar] [CrossRef]
- Walser, T.C.; Rifat, S.; Ma, X.; Kundu, N.; Ward, C.; Goloubeva, O.; Johnson, M.G.; Medina, J.C.; Collins, T.L.; Fulton, A.M. Antagonism of CXCR3 Inhibits Lung Metastasis in a Murine Model of Metastatic Breast Cancer. Cancer Res. 2006, 66, 7701–7707. [Google Scholar] [CrossRef]
- Kawada, K.; Sonoshita, M.; Sakashita, H.; Takabayashi, A.; Yamaoka, Y.; Manabe, T.; Inaba, K.; Minato, N.; Oshima, M.; Taketo, M.M. Pivotal Role of CXCR3 in Melanoma Cell Metastasis to Lymph Nodes. Cancer Res. 2004, 64, 4010–4017. [Google Scholar] [CrossRef]
- Shen, D.; Cao, X. Potential Role of CXCR3 in Proliferation and Invasion of Prostate Cancer Cells. Int. J. Clin. Exp. Pathol. 2015, 8, 8091–8098. [Google Scholar]
- Sun, Y.; Mo, Y.; Jiang, S.; Shang, C.; Feng, Y.; Zeng, X. CXC Chemokine Ligand-10 Promotes the Accumulation of Monocyte-like Myeloid-Derived Suppressor Cells by Activating P38 MAPK Signaling under Tumor Conditions. Cancer Sci. 2023, 114, 142–151. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Yang, Q.; Lv, X.; Huang, W.; Zhou, Z.; Wang, Y.; Zhang, Z.; Yuan, T.; Ding, X.; et al. Single-Cell RNA Landscape of Intratumoral Heterogeneity and Immunosuppressive Microenvironment in Advanced Osteosarcoma. Nat. Commun. 2020, 11, 6322, Correction in Nat. Commun. 2021, 12, 2567. [Google Scholar] [CrossRef]
- Petrovic-Djergovic, D.; Popovic, M.; Chittiprol, S.; Cortado, H.; Ransom, R.F.; Partida-Sánchez, S. CXCL10 Induces the Recruitment of Monocyte-Derived Macrophages into Kidney, Which Aggravate Puromycin Aminonucleoside Nephrosis. Clin. Exp. Immunol. 2015, 180, 305–315. [Google Scholar] [CrossRef]
- Tang, Y.; Gu, Z.; Fu, Y.; Wang, J. CXCR3 from Chemokine Receptor Family Correlates with Immune Infiltration and Predicts Poor Survival in Osteosarcoma. Biosci. Rep. 2019, 39, BSR20192134. [Google Scholar] [CrossRef]
- Goldberg-Bittman, L.; Neumark, E.; Sagi-Assif, O.; Azenshtein, E.; Meshel, T.; Witz, I.P.; Ben-Baruch, A. The Expression of the Chemokine Receptor CXCR3 and Its Ligand, CXCL10, in Human Breast Adenocarcinoma Cell Lines. Immunol. Lett. 2004, 92, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Reschke, R.; Yu, J.; Flood, B.; Higgs, E.F.; Hatogai, K.; Gajewski, T.F. Immune Cell and Tumor Cell-Derived CXCL10 Is Indicative of Immunotherapy Response in Metastatic Melanoma. J. Immunother. Cancer 2021, 9, e003521, Correction in J. Immunother. Cancer 2021, 9, e003521corr1. [Google Scholar] [CrossRef]
- Yan, W.; Qiu, L.; Yang, M.; Xu, A.; Ma, M.; Yuan, Q.; Ma, X.; Liang, W.; Li, X.; Lu, Y. CXCL10 Mediates CD8+ T Cells to Facilitate Vessel Normalization and Improve the Efficacy of Cetuximab Combined with PD-1 Checkpoint Inhibitors in Colorectal Cancer. Cancer Lett. 2023, 567, 216263. [Google Scholar] [CrossRef] [PubMed]
- Lindner, A.K.; Martowicz, A.; Untergasser, G.; Haybaeck, J.; Compérat, E.; Kocher, F.; Seeber, A.; Thurnher, M.; Pichler, R. CXCR3 Expression Is Associated with Advanced Tumor Stage and Grade Influencing Survival after Surgery of Localised Renal Cell Carcinoma. Cancers 2023, 15, 1001. [Google Scholar] [CrossRef] [PubMed]
- Hirth, M.; Gandla, J.; Höper, C.; Gaida, M.M.; Agarwal, N.; Simonetti, M.; Demir, A.; Xie, Y.; Weiss, C.; Michalski, C.W.; et al. CXCL10 and CCL21 Promote Migration of Pancreatic Cancer Cells Toward Sensory Neurons and Neural Remodeling in Tumors in Mice, Associated With Pain in Patients. Gastroenterology 2020, 159, 665–681.e13. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, J.; Yan, H.; Liu, H.; Yin, S. Chemokine Receptor CXCR3 Correlates with Decreased M2 Macrophage Infiltration and Favorable Prognosis in Gastric Cancer. BioMed Res. Int. 2019, 2019, 6832867. [Google Scholar] [CrossRef]
- Xiao, W.; Huang, H.; Zheng, P.; Liu, Y.; Chen, Y.; Chen, J.; Zheng, X.; Chen, L.; Jiang, J. The CXCL10/CXCR3 Pathway Contributes to the Synergy of Thermal Ablation and PD-1 Blockade Therapy against Tumors. Cancers 2023, 15, 1427. [Google Scholar] [CrossRef]
- Liu, C.; Luo, D.; Reynolds, B.A.; Meher, G.; Katritzky, A.R.; Lu, B.; Gerard, C.J.; Bhadha, C.P.; Harrison, J.K. Chemokine Receptor CXCR3 Promotes Growth of Glioma. Carcinogenesis 2011, 32, 129–137. [Google Scholar] [CrossRef]
- Brown, M.; Yang, Y.; Mckay, Z.; Bigner, D.; Ashley, D.; Gromeier, M.; Nair, S. IMMU-43. CXCR3 Signaling Engages Glioma Infiltrating T Cells and Is Required for the Antitumor Efficacy of Innate Stimulating Immunotherapy. Neuro Oncol. 2022, 24, vii140–vii141. [Google Scholar] [CrossRef]
- Muthuswamy, R.; Corman, J.M.; Dahl, K.; Chatta, G.S.; Kalinski, P. Functional Reprogramming of Human Prostate Cancer to Promote Local Attraction of Effector CD8+ T Cells. Prostate 2016, 76, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.N.; Rafii, S.; Lyden, D. Preparing the “Soil”: The Premetastatic Niche. Cancer Res. 2006, 66, 11089–11093. [Google Scholar] [CrossRef]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-Metastatic Niches: Organ-Specific Homes for Metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Y.; Pan, Y.; Lan, X.; Song, F.; Sun, J.; Zhou, K.; Liu, X.; Ren, X.; Wang, F.; et al. Cancer-Derived Exosomal MiR-25-3p Promotes Pre-Metastatic Niche Formation by Inducing Vascular Permeability and Angiogenesis. Nat. Commun. 2018, 9, 5395. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Xie, L.; Lu, C.; Gu, C.; Xia, Y.; Lv, J.; Xuan, Z.; Fang, L.; Yang, J.; Zhang, L.; et al. Gastric Cancer-Derived Exosomal MiR-519a-3p Promotes Liver Metastasis by Inducing Intrahepatic M2-like Macrophage-Mediated Angiogenesis. J. Exp. Clin. Cancer Res. 2022, 41, 296. [Google Scholar] [CrossRef]
- Pein, M.; Insua-Rodríguez, J.; Hongu, T.; Riedel, A.; Meier, J.; Wiedmann, L.; Decker, K.; Essers, M.A.G.; Sinn, H.P.; Spaich, S.; et al. Metastasis-Initiating Cells Induce and Exploit a Fibroblast Niche to Fuel Malignant Colonization of the Lungs. Nat. Commun. 2020, 11, 1494. [Google Scholar] [CrossRef]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Sun, D.; Zhai, X.; Chen, F.; Niu, J.; Zhu, H. Macrophages in the Premetastatic and Metastatic Niche: Key Functions and Therapeutic Directions. J. Transl Med. 2025, 23, 602. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Xu, Y.; Chen, H.; Zhu, X.; Huang, L.; Chen, Z.; Xu, H.; Song, G.; Lu, J.; Huang, W.; et al. Extracellular-Vesicle-Packaged S100A11 from Osteosarcoma Cells Mediates Lung Premetastatic Niche Formation by Recruiting GMDSCs. Cell Rep. 2024, 43, 113751. [Google Scholar] [CrossRef]
- Garimella, R.; Washington, L.; Isaacson, J.; Vallejo, J.; Spence, M.; Tawfik, O.; Rowe, P.; Brotto, M.; Perez, R. Extracellular Membrane Vesicle Derived from 143b Osteosarcom Cells Contain Pro-Osteoclastogenic Cargo: A Nove Communication Mechanism in Osteosarcoma Bone Microenvironment. Transl. Oncol. 2014, 7, 331–340. [Google Scholar] [CrossRef]
- Mazumdar, A.; Urdinez, J.; Boro, A.; Migliavacca, J.; Arlt, M.J.E.; Muff, R.; Fuchs, B.; Snedeker, J.G.; Gvozdenovic, A. Osteosarcoma-Derived Extracellular Vesicles Induce Lung Fibroblast Reprogramming. Int. J. Mol. Sci. 2020, 21, 5451. [Google Scholar] [CrossRef]
- Baglio, S.R.; Lagerweij, T.; Pérez-Lanzón, M.; Ho, X.D.; Léveillé, N.; Melo, S.A.; Cleton-Jansen, A.M.; Jordanova, E.S.; Roncuzzi, L.; Greco, M.; et al. Blocking Tumor-Educated MSC Paracrine Activity Halts Osteosarcoma Progression. Clin. Cancer Res. 2017, 23, 3721–3733, Correction in Clin. Cancer Res. 2018, 24, 724. [Google Scholar] [CrossRef]
- Elias, J.A.; Lentz, V.; Cummings, P.J. Transforming Growth Factor-Beta Regulation of IL-6 Production by Unstimulated and IL-1-Stimulated Human Fibroblasts. J. Immunol. 1991, 146, 3437–3443. [Google Scholar] [CrossRef]
- Yao, Z.; Fenoglio, S.; Gao, D.C.; Camiolo, M.; Stiles, B.; Lindsted, T.; Schlederer, M.; Johns, C.; Altorki, N.; Mittal, V.; et al. TGF-β IL-6 Axis Mediates Selective and Adaptive Mechanisms of Resistance to Molecular Targeted Therapy in Lung Cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 15535–15540. [Google Scholar] [CrossRef]
- Prapiadou, S.; Živković, L.; Thorand, B.; George, M.J.; Van Der Laan, S.W.; Malik, R.; Herder, C.; Koenig, W.; Ueland, T.; Kleveland, O.; et al. Proteogenomic Data Integration Reveals CXCL10 as a Potentially Downstream Causal Mediator for IL-6 Signaling on Atherosclerosis. Circulation 2024, 149, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Chiovato, L.; Rotondi, M. Interleukin-6, CXCL10 and Infiltrating Macrophages in COVID-19-Related Cytokine Storm: Not One for All But All for One! Front. Immunol. 2021, 12, 668507. [Google Scholar] [CrossRef]
- Jiang, K.; Li, Y.; Xiang, C.; Xiong, Y.; Jia, J. TGF-Β3 Regulates Adhesion Formation through the JNK/c-Jun Pathway during Flexor Tendon Healing. BMC Musculoskelet. Disord. 2021, 22, 843. [Google Scholar] [CrossRef] [PubMed]
- Ventura, J.J.; Kennedy, N.J.; Flavell, R.A.; Davis, R.J. JNK Regulates Autocrine Expression of TGF-Β1. Mol. Cell 2004, 15, 269–278. [Google Scholar] [CrossRef]
- Feng, W.; Liu, H.; Luo, T.; Liu, D.; Du, J.; Sun, J.; Wang, W.; Han, X.; Yang, K.; Guo, J.; et al. Combination of IL-6 and SIL-6R Differentially Regulate Varying Levels of RANKL-Induced Osteoclastogenesis through NF-ΚB, ERK and JNK Signaling Pathways. Sci. Rep. 2017, 7, 41411, Correction in Sci. Rep. 2022, 12, 3746. [Google Scholar] [CrossRef]
- Ishijima, T.; Nakajima, K. Inflammatory Cytokines TNFα, IL-1β, and IL-6 Are Induced in Endotoxin- Stimulated Microglia through Different Signaling Cascades. Sci. Prog. 2021, 104. [Google Scholar] [CrossRef]
- Luster, A.D.; Ravetch, J. V Genomic Characterization of a Gamma-Interferon-Inducible Gene (IP-10) and Identification of an Interferon-Inducible Hypersensitive Site. Mol. Cell. Biol. 1987, 7, 3723–3731. [Google Scholar] [CrossRef] [PubMed]
- Loetscher, M.; Loetscher, P.; Brass, N.; Meese, E.; Moser, B. Lymphocyte-Specific Chemokine Receptor CXCR3: Regulation, Chemokine Binding and Gene Localization. Eur. J. Immunol. 1998, 28, 3696–3705. [Google Scholar] [CrossRef]
- Qin, S.; Rottman, J.B.; Myers, P.; Kassam, N.; Weinblatt, M.; Loetscher, M.; Koch, A.E.; Moser, B.; Mackay, C.R. The Chemokine Receptors CXCR3 and CCR5 Mark Subsets of T Cells Associated with Certain Inflammatory Reactions. J. Clin. Investig. 1998, 101, 746–754. [Google Scholar] [CrossRef]
- Ali, A.; Canaday, L.M.; Feldman, H.A.; Cevik, H.; Moran, M.T.; Rajaram, S.; Lakes, N.; Tuazon, J.A.; Seelamneni, H.; Krishnamurthy, D.; et al. Natural Killer Cell Immunosuppressive Function Requires CXCR3-Dependent Redistribution within Lymphoid Tissues. J. Clin. Investig. 2021, 131, e146686. [Google Scholar] [CrossRef]
- Spranger, S.; Dai, D.; Horton, B.; Gajewski, T.F. Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 2017, 31, 711–723.e4. [Google Scholar] [CrossRef]
- Jhaveri, N.; Ben Cheikh, B.; Nikulina, N.; Ma, N.; Klymyshyn, D.; DeRosa, J.; Mihani, R.; Pratapa, A.; Kassim, Y.; Bommakanti, S.; et al. Mapping the Spatial Proteome of Head and Neck Tumors: Key Immune Mediators and Metabolic Determinants in the Tumor Microenvironment. GEN Biotechnol. 2023, 2, 418–434. [Google Scholar] [CrossRef]
- House, I.G.; Savas, P.; Lai, J.; Chen, A.X.Y.; Oliver, A.J.; Teo, Z.L.; Todd, K.L.; Henderson, M.A.; Giuffrida, L.; Petley, E.V.; et al. Macrophage-Derived CXCL9 and CXCL10 Are Required for Antitumor Immune Responses Following Immune Checkpoint Blockade. Clin. Cancer Res. 2020, 26, 487–504. [Google Scholar] [CrossRef]
- Dong, M.; Lu, L.; Xu, H.; Ruan, Z. DC-Derived CXCL10 Promotes CTL Activation to Suppress Ovarian Cancer. Transl. Res. 2024, 272, 126–139. [Google Scholar] [CrossRef]
- Peng, D.; Kryczek, I.; Nagarsheth, N.; Zhao, L.; Wei, S.; Wang, W.; Sun, Y.; Zhao, E.; Vatan, L.; Szeliga, W.; et al. Epigenetic Silencing of TH1-Type Chemokines Shapes Tumour Immunity and Immunotherapy. Nature 2015, 527, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.J.; Salehi-Rad, R.; Tran, L.M.; Oh, M.S.; Dumitras, C.; Crosson, W.P.; Li, R.; Patel, T.S.; Man, S.; Yean, C.E.; et al. CXCL9/10-Engineered Dendritic Cells Promote T Cell Activation and Enhance Immune Checkpoint Blockade for Lung Cancer. Cell Rep. Med. 2024, 5, 101479. [Google Scholar] [CrossRef]
- Yoneyama, H.; Narumi, S.; Zhang, Y.; Murai, M.; Baggiolini, M.; Lanzavecchia, A.; Ichida, T.; Asakura, H.; Matsushima, K. Pivotal Role of Dendritic Cell-Derived CXCL10 in the Retention of T Helper Cell 1 Lymphocytes in Secondary Lymph Nodes. J. Exp. Med. 2002, 195, 1257–1266. [Google Scholar] [CrossRef]
- Ligon, J.A.; Choi, W.; Cojocaru, G.; Fu, W.; Hsiue, E.H.C.; Oke, T.F.; Siegel, N.; Fong, M.H.; Ladle, B.; Pratilas, C.A.; et al. Pathways of Immune Exclusion in Metastatic Osteosarcoma Are Associated with Inferior Patient Outcomes. J. Immunother. Cancer 2021, 9, e001772. [Google Scholar] [CrossRef] [PubMed]
- Lacinski, R.A.; Dziadowicz, S.A.; Melemai, V.K.; Fitzpatrick, B.; Pisquiy, J.J.; Heim, T.; Lohse, I.; Schoedel, K.E.; Llosa, N.J.; Weiss, K.R.; et al. Spatial Multiplexed Immunofluorescence Analysis Reveals Coordinated Cellular Networks Associated with Overall Survival in Metastatic Osteosarcoma. Bone Res. 2024, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Chen, H.; Yuan, Q.; Wang, J.; Niu, M.; Hou, L.; Gu, J.; Zhang, J. MyD88 in Hepatic Stellate Cells Enhances Liver Fibrosis via Promoting Macrophage M1 Polarization. Cell Death Dis. 2022, 13, 411. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, W.; Shen, L.; Yao, Y.; Xia, W.; Ni, C. Tumor Battlefield within Inflamed, Excluded or Desert Immune Phenotypes: The Mechanisms and Strategies. Exp. Hematol. Oncol. 2024, 13, 80. [Google Scholar] [CrossRef]
- Cambien, B.; Karimdjee, B.F.; Richard-Fiardo, P.; Bziouech, H.; Barthel, R.; Millet, M.A.; Martini, V.; Birnbaum, D.; Scoazec, J.Y.; Abello, J.; et al. Organ-Specific Inhibition of Metastatic Colon Carcinoma by CXCR3 Antagonism. Br. J. Cancer 2009, 100, 1755–1764. [Google Scholar] [CrossRef]
- Chow, M.T.; Ozga, A.J.; Servis, R.L.; Frederick, D.T.; Lo, J.A.; Fisher, D.E.; Freeman, G.J.; Boland, G.M.; Luster, A.D. Intratumoral Activity of the CXCR3 Chemokine System Is Required for the Efficacy of Anti-PD-1 Therapy. Immunity 2019, 50, 1498–1512.e5. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, L.; Zhang, Y.; Li, F.F. A Comprehensive Analysis of Immune Infiltration in the Tumor Microenvironment of Osteosarcoma. Cancer Med. 2021, 10, 5696–5711. [Google Scholar] [CrossRef]
- Wijtmans, M.; Verzijl, D.; Leurs, R.; De Esch, I.J.P.; Smit, M.J. Towards Small-Molecule CXCR3 Ligands with Clinical Potential. ChemMedChem 2008, 3, 861–872. [Google Scholar] [CrossRef]
- Saftescu, S.; Vornicu, V.-N.; Popovici, D.-I.; Dragomir, R.-D.; Nagy, D.-S.; Sandu, D.-L.; Dulan, A.; Negru, Ș.-M.; Negru, A.-G. Decoding Treatment Failures in Metastatic Renal Cell Carcinoma: Predictors Across Immunotherapy and Targeted Therapies from a Retrospective Real-World Analysis. J. Clin. Med. 2025, 14, 5271. [Google Scholar] [CrossRef] [PubMed]
- Balan, M.; Pal, S. A Novel CXCR3-B Chemokine Receptor-Induced Growth-Inhibitory Signal in Cancer Cells Is Mediated through the Regulation of Bach-1 Protein and Nrf2 Protein Nuclear Translocation. J. Biol. Chem. 2014, 289, 3126–3137, Correction in J. Biol. Chem. 2020, 295, 10509. [Google Scholar] [CrossRef]
- Huo, R.; Jiang, Y.; Zhang, L.; Du, S.; Zhou, D. CXCR3 Inhibitors for Therapeutic Interventions: Current Status and Perspectives. Front. Pharmacol. 2025, 16, 1556196. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.A.; Äänismaa, P.; Ertel, E.A.; Hühn, E.; Strasser, D.S.; Rey, M.; Murphy, M.J.; Martinic, M.M.; Pouzol, L.; Froidevaux, S.; et al. Discovery of Clinical Candidate ACT-777991, a Potent CXCR3 Antagonist for Antigen-Driven and Inflammatory Pathologies. J. Med. Chem. 2023, 66, 4179–4196. [Google Scholar] [CrossRef]
- Christen, U.; Pouzol, L.; Tunis, M.; Sassi, A.; Tondello, C.; Bayer, M.; Hintermann, E.; Strasser, D.S.; Schuldes, S.; Mentzel, U.; et al. Combination Treatment of a Novel CXCR3 Antagonist ACT-777991 with an Anti-CD3 Antibody Synergistically Increases Persistent Remission in Experimental Models of Type 1 Diabetes. Clin. Exp. Immunol. 2023, 214, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Boof, M.L.; Géhin, M.; Voors-Pette, C.; Hsin, C.H.; Sippel, V.; Strasser, D.S.; Dingemanse, J. Pharmacokinetics, Pharmacodynamics and Safety of the Novel C-X-C Chemokine Receptor 3 Antagonist ACT-777991: Results from the First-in-Human Study in Healthy Adults. Br. J. Clin. Pharmacol. 2024, 90, 588–599. [Google Scholar] [CrossRef]
- Li, X.; Song, Y. Proteolysis-Targeting Chimera (PROTAC) for Targeted Protein Degradation and Cancer Therapy. J. Hematol. Oncol. 2020, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Zhang, C.; Li, X.; Wang, J.; Zhang, H.; Feng, Y.; Xu, N.; Li, H.; Tan, C.; Jiang, Y.; et al. MRNA PROTACs: Engineering PROTACs for High-Efficiency Targeted Protein Degradation. MedComm 2024, 5, e478. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, L.; Zhao, S.; Sun, B.; Cheng, L.; Tang, Y.; Luo, Z.; Lin, Z.; Zhu, J.; Zhu, W.; et al. CXCR4-Mediated Osteosarcoma Growth and Pulmonary Metastasis Is Suppressed by MicroRNA-613. Cancer Sci. 2018, 109, 2412–2422. [Google Scholar] [CrossRef]
- Laverdiere, C.; Hoang, B.H.; Yang, R.; Sowers, R.; Qin, J.; Meyers, P.A.; Huvos, A.G.; Healey, J.H.; Gorlick, R. Messenger RNA Expression Levels of CXCR4 Correlate with Metastatic Behavior and Outcome in Patients with Osteosarcoma. Clin. Cancer Res. 2005, 11, 2561–2567. [Google Scholar] [CrossRef]
- Watts, A.O.; Van Lipzig, M.M.H.; Jaeger, W.C.; Seeber, R.M.; Van Zwam, M.; Vinet, J.; Van Der Lee, M.M.C.; Siderius, M.; Zaman, G.J.R.; Boddeke, H.W.G.M.; et al. Identification and Profiling of CXCR3-CXCR4 Chemokine Receptor Heteromer Complexes. Br. J. Pharmacol. 2013, 168, 1662–1674. [Google Scholar] [CrossRef]
- Singh, A.K.; Arya, R.K.; Trivedi, A.K.; Sanyal, S.; Baral, R.; Dormond, O.; Briscoe, D.M.; Datta, D. Chemokine Receptor Trio: CXCR3, CXCR4 and CXCR7 Crosstalk via CXCL11 and CXCL12. Cytokine Growth Factor Rev. 2013, 24, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhang, Z.; Wang, H.; Zhan, Y.; Li, G.; Yang, H.; Fei, Z.; Xu, Y.; Li, W. CXCR3 Expression in Colorectal Cancer Cells Enhanced Invasion through Preventing CXCR4 Internalization. Exp. Cell Res. 2018, 371, 162–174. [Google Scholar] [CrossRef]
- Li, X.; Lu, M.; Yuan, M.; Ye, J.; Zhang, W.; Xu, L.; Wu, X.; Hui, B.; Yang, Y.; Wei, B.; et al. CXCL10-Armed Oncolytic Adenovirus Promotes Tumor-Infiltrating T-Cell Chemotaxis to Enhance Anti-PD-1 Therapy. Oncoimmunology 2022, 11, 2118210. [Google Scholar] [CrossRef] [PubMed]
- Fenis, A.; Demaria, O.; Gauthier, L.; Vivier, E.; Narni-Mancinelli, E. New Immune Cell Engagers for Cancer Immunotherapy. Nat. Rev. Immunol. 2024, 24, 471–486. [Google Scholar] [CrossRef]
- Tapia-Galisteo, A.; Álvarez-Vallina, L.; Sanz, L. Bi- and Trispecific Immune Cell Engagers for Immunotherapy of Hematological Malignancies. J. Hematol. Oncol. 2023, 16, 83. [Google Scholar] [CrossRef]
- Fucà, G.; Spagnoletti, A.; Ambrosini, M.; de Braud, F.; Di Nicola, M. Immune Cell Engagers in Solid Tumors: Promises and Challenges of the next Generation Immunotherapy. ESMO Open 2021, 6, 100046. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; Blanchard-Alvarez, A.; Grondin, G.; Trichard, S.; Cesari, C.; Sapet, M.; Bosco, F.; et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 2019, 177, 1701–1713.e16. [Google Scholar] [CrossRef]
- Yellin, M.; Paliienko, I.; Balanescu, A.; Ter-Vartanian, S.; Tseluyko, V.; Xu, L.A.; Tao, X.; Cardarelli, P.M.; Leblanc, H.; Nichol, G.; et al. A Phase II, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Efficacy and Safety of MDX-1100, a Fully Human Anti-CXCL10 Monoclonal Antibody, in Combination with Methotrexate in Patients with Rheumatoid Arthritis. Arthritis Rheum. 2012, 64, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Ottolino-Perry, K.; Diallo, J.S.; Lichty, B.D.; Bell, J.C.; Andrea McCart, J. Intelligent Design: Combination Therapy with Oncolytic Viruses. Mol. Ther. 2010, 18, 251–263. [Google Scholar] [CrossRef]
- Simon, G.; Subbiah, V.; Rosen, L.; Lenz, H.-J.; Park, H.; Patel, M.; Miles, D.; Wallis, S.; Evilevitch, V.; Krige, D.; et al. 762 First-in-Human Phase 1a Study of NG-641, a Tumour-Selective Vector Expressing a FAP-TAc Bispecific Antibody and Immune Enhancer Module, in Patients with Metastatic/Advanced Epithelial Tumours (STAR). J. ImmunoTher. Cancer 2022, 10, A794. [Google Scholar]
- Lillie, T.; Parkes, E.; Ottensmeier, C.; Krige, D.; Ravanfar, B.; Evilevitch, V.; Thomas, M.; Rosen, L. Abstract CT214: A Multicenter Phase 1a/b Study of NG-641, a Tumor-Selective Transgene-Expressing Adenoviral Vector, and Nivolumab in Patients with Metastatic or Advanced Epithelial Tumors (NEBULA). Cancer Res. 2022, 82, CT214. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.L.; Zhao, H.Y.; Zhang, F.C.; Jiang, X.B. A Novel Recombinant Protein of IP10-EGFRvIIIscFv and CD8+ Cytotoxic T Lymphocytes Synergistically Inhibits the Growth of Implanted Glioma in Mice. Cancer Immunol. Immunother. 2013, 62, 1261–1272. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune Checkpoint Blockade Therapy for Cancer: An Overview of FDA-Approved Immune Checkpoint Inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Mundekkad, D.; Cho, W.C. Nanoparticles in Clinical Translation for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Lari, S.; Hiyari, S.; de Araújo Silva, D.N.; de Brito Bezerra, B.; Ishii, M.; Monajemzadeh, S.; Cui, Z.K.; Tetradis, S.; Lee, M.; Pirih, F.Q. Local Delivery of a CXCR3 Antagonist Decreases the Progression of Bone Resorption Induced by LPS Injection in a Murine Model. Clin. Oral Investig. 2022, 26, 5163–5169. [Google Scholar] [CrossRef]
- Liang, J.; Wang, H.; Ding, W.; Huang, J.; Zhou, X.; Wang, H.; Dong, X.; Li, G.; Chen, E.; Zhou, F.; et al. Nanoparticle-Enhanced Chemo-Immunotherapy to Trigger Robust Antitumor Immunity. Sci. Adv. 2020, 6, eabc3646. [Google Scholar] [CrossRef]
- Kar, U.K.; Srivastava, M.K.; Andersson, Å.; Baratelli, F.; Huang, M.; Kickhoefer, V.A.; Dubinett, S.M.; Rome, L.H.; Sharma, S. Novel Ccl21-Vault Nanocapsule Intratumoral Delivery Inhibits Lung Cancer Growth. PLoS ONE 2011, 6, e18758. [Google Scholar] [CrossRef]
- Seynhaeve, A.L.B.; Liu, H.; Priester, M.I.; Valentijn, M.; van Holten-Neelen, C.; Brouwer, R.W.W.; van Brakel, M.; Dik, W.A.; van IJcken, W.F.J.; Debets, R.; et al. CXCL10 Secreted by Pericytes Mediates TNFα-Induced Vascular Leakage in Tumors and Enhances Extravasation of Nanoparticle-Based Chemotherapeutics. Cancer Res. 2025, 85, 1596–1610. [Google Scholar] [CrossRef] [PubMed]
- Tomura, M. New Tools for Imaging of Immune Systems: Visualization of Cell Cycle, Cell Death, and Cell Movement by Using the Mice Lines Expressing Fucci, SCAT3.1, and Kaede and KikGR. In Intravital Imaging of Dynamic Bone and Immune Systems; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1763. [Google Scholar]
- Tomura, M.; Yoshida, N.; Tanaka, J.; Karasawa, S.; Miwa, Y.; Miyawaki, A.; Kanagawa, O. Monitoring Cellular Movement in Vivo with Photoconvertible Fluorescence Protein “Kaede” Transgenic Mice. Proc. Natl. Acad. Sci. USA 2008, 105, 10871–10876. [Google Scholar] [CrossRef]
- Du, Y.; Ding, X.; Ye, Y. The Spatial Multi-Omics Revolution in Cancer Therapy: Precision Redefined. Cell Rep. Med. 2024, 5, 101740. [Google Scholar] [CrossRef] [PubMed]
- Ptacek, J.; Locke, D.; Finck, R.; Cvijic, M.E.; Li, Z.; Tarolli, J.G.; Aksoy, M.; Sigal, Y.; Zhang, Y.; Newgren, M.; et al. Multiplexed Ion Beam Imaging (MIBI) for Characterization of the Tumor Microenvironment across Tumor Types. Lab. Investig. 2020, 100, 1111–1123. [Google Scholar] [CrossRef]
- Tan, W.C.C.; Nerurkar, S.N.; Cai, H.Y.; Ng, H.H.M.; Wu, D.; Wee, Y.T.F.; Lim, J.C.T.; Yeong, J.; Lim, T.K.H. Overview of Multiplex Immunohistochemistry/Immunofluorescence Techniques in the Era of Cancer Immunotherapy. Cancer Commun. 2020, 40, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Chen, X.; Ba, Y. CXCL10/CXCR3 Overexpression as a Biomarker of Poor Prognosis in Patients with Stage II Colorectal Cancer. Mol. Clin. Oncol. 2016, 4, 23–30. [Google Scholar] [CrossRef]
| Feature | CXCR3-A | CXCR3-B |
|---|---|---|
| Primary Function | Pro-tumorigenic, Pro-metastatic, Chemotaxis | Anti-tumorigenic, Angiostatic, Pro-apoptotic |
| Signaling Pathways | Gαi-dependent; PI3K/AKT, MAPK/ERK, Ca2+ mobilization | Gαs-dependent; cAMP/PKA, p38 MAPK |
| Key Cellular Targets | Tumor Cells: Promotes survival, proliferation, invasion. T/NK Cells: Drives chemotaxis/recruitment | Tumor Cells: Induces growth arrest/apoptosis. Endothelial Cells: Inhibits migration/tube formation. |
| Role in OS | Overexpressed in metastatic OS; drives lung colonization via PAK1 activation. | Often downregulated in metastatic OS; restoration inhibits growth. |
| Ligand Affinity | High affinity for CXCL9, CXCL10, CXCL11 | High affinity for CXCL4 (PF-4), CXCL9, CXCL10, CXCL11 |
| Therapeutic Implications | Target for antagonism (inhibit metastasis) | Target for agonism (restore angiostasis/apoptosis) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Gyau, B.B.; Man, T.-K. A Spatiotemporal Model of CXCL10 as a Master Regulator of Immune Evasion and Metastasis in Osteosarcoma. Int. J. Mol. Sci. 2026, 27, 319. https://doi.org/10.3390/ijms27010319
Gyau BB, Man T-K. A Spatiotemporal Model of CXCL10 as a Master Regulator of Immune Evasion and Metastasis in Osteosarcoma. International Journal of Molecular Sciences. 2026; 27(1):319. https://doi.org/10.3390/ijms27010319
Chicago/Turabian StyleGyau, Benjamin B., and Tsz-Kwong Man. 2026. "A Spatiotemporal Model of CXCL10 as a Master Regulator of Immune Evasion and Metastasis in Osteosarcoma" International Journal of Molecular Sciences 27, no. 1: 319. https://doi.org/10.3390/ijms27010319
APA StyleGyau, B. B., & Man, T.-K. (2026). A Spatiotemporal Model of CXCL10 as a Master Regulator of Immune Evasion and Metastasis in Osteosarcoma. International Journal of Molecular Sciences, 27(1), 319. https://doi.org/10.3390/ijms27010319





