Transcriptomic Analysis of High-Intensity Interval Training in High-Fat-Diet-Induced Spontaneous Hypertensive Rats’ Brains
Abstract
1. Introduction
2. Results
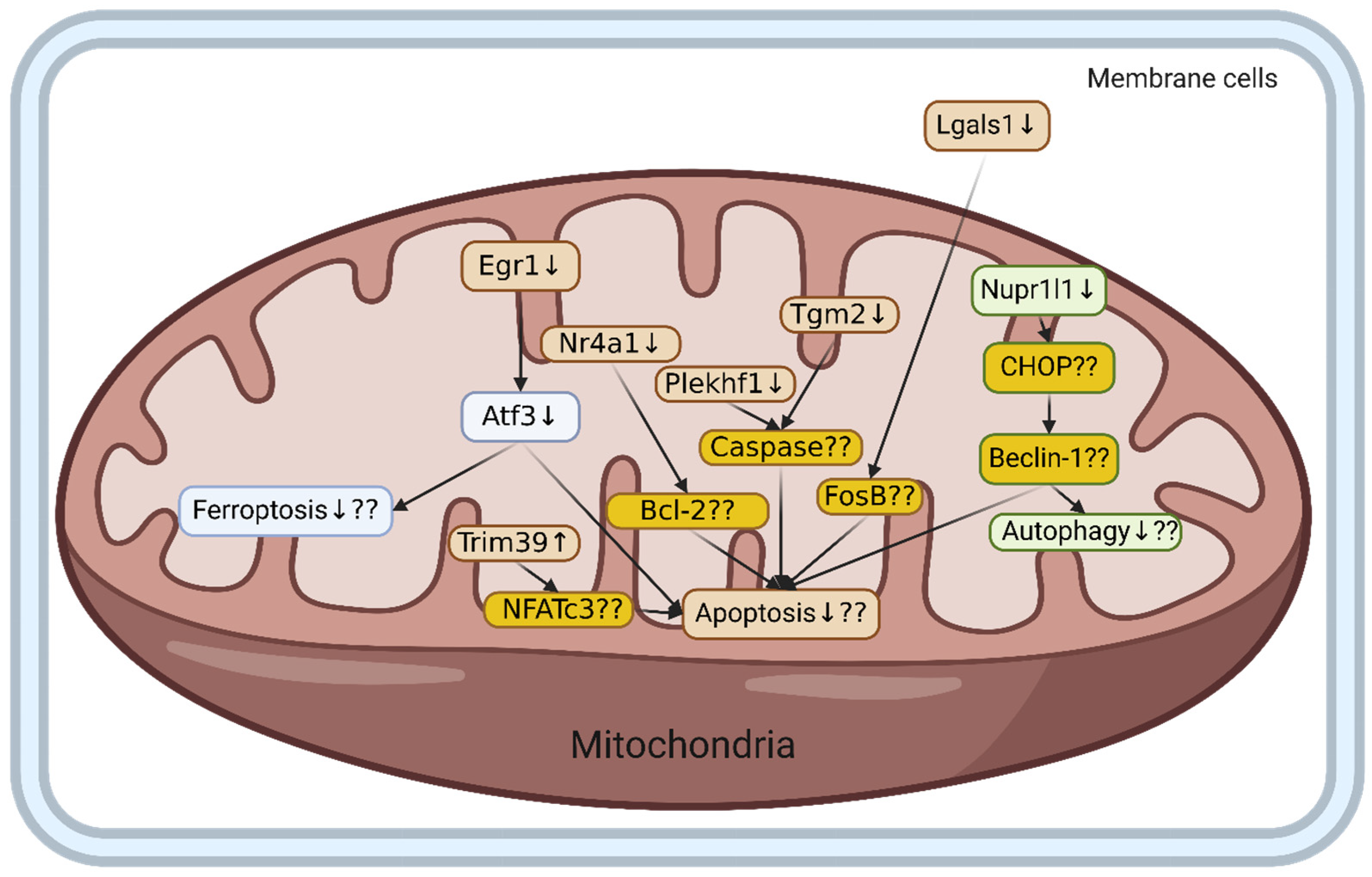
Differentially Expressed Gene Pathway via Functional Enrichment Analysis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Exercise Procedure
4.3. RNA Isolation
4.4. Transcriptomic Analysis
4.5. RNA-Sequencing and Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabbatini, M.; Tomassoni, D.; Amenta, F. Hypertensive brain damage: Comparative evaluation of protective effect of treatment with dihydropyridine derivatives in spontaneously hypertensive rats. Mech. Ageing Dev. 2001, 122, 2085–2105. [Google Scholar] [CrossRef]
- Haile, D.G.; Taddess, N.; Mekuria, A.D.; Abebe, A.M.; Mezemir, Y. Prevalence of hypertension and associated factors among adults in Debre Berhan Town, North Shoa Zone, Ethiopia, 2020. Vasc. Health Risk Manag. 2021, 17, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, A.S.; Seux, M.L.; Staessen, J.A.; Birkenhäger, W.H.; Forette, F. Cerebral complications of hypertension. J. Hum. Hypertens. 2000, 14, 605–616. [Google Scholar] [CrossRef]
- Ye, Z.; Zeng, Q.; Ning, L.; Huang, W.; Su, Q. Systolic blood pressure is associated with abnormal alterations in brain cortical structure: Evidence from a Mendelian randomization study. Eur. J. Intern. Med. 2024, 120, 92–98. [Google Scholar] [CrossRef]
- Ben-Shabat, M.; Awad-Igbaria, Y.; Sela, S.; Gross, B.; Yagil, Y.; Yagil, C.; Palzur, E. Predisposition to cortical neurodegenerative changes in brains of hypertension prone rats. J. Transl. Med. 2023, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Jama, H.A.; Muralitharan, R.R.; Xu, C.; O’Donnell, J.A.; Bertagnolli, M.; Broughton, B.R.S.; Head, G.A.; Marques, F.Z. Rodent models of hypertension. Br. J. Pharmacol. 2022, 179, 918–937. [Google Scholar] [CrossRef]
- Cholerton, B.; Baker, L.D.; Craft, S. Insulin, cognition, and dementia. Eur. J. Pharmacol. 2013, 719, 170–179. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Oxidative stress modulates Sir2α in rat hippocampus and cerebral cortex. Eur. J. Neurosci. 2006, 23, 2573–2580. [Google Scholar] [CrossRef]
- Meireles, M.; Rodríguez-Alcalá, L.M.; Marques, C.; Norberto, S.; Freitas, J.; Fernandes, I.; Mateus, N.; Gomes, A.; Faria, A.; Calhau, C. Effect of chronic consumption of blackberry extract on high-fat induced obesity in rats and its correlation with metabolic and brain outcomes. Food Funct. 2016, 7, 127–139. [Google Scholar] [CrossRef]
- Chaar, L.J.; Coelho, A.; Silva, N.M.; Festuccia, W.L.; Antunes, V.R. High-fat diet-induced hypertension and autonomic imbalance are associated with an upregulation of CART in the dorsomedial hypothalamus of mice. Physiol. Rep. 2016, 4, e12811. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-q.; Chen, Z.; Chen, L.-X. Endoplasmic reticulum stress: A novel mechanism and therapeutic target for cardiovascular diseases. Acta Pharmacol. Sin. 2016, 37, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Jeon, B.; Baek, J.; Yun, Y.; Kim, D.; Chang, B.; Kim, S.; Kim, S. High fat diet-induced brain damaging effects through autophagy-mediated senescence, inflammation and apoptosis mitigated by ginsenoside F1-enhanced mixture. J. Ginseng Res. 2022, 46, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Poulet, R.; Gentile, M.T.; Vecchione, C.; Distaso, M.; Aretini, A.; Fratta, L.; Russo, G.; Echart, C.; Maffei, A.; De Simoni, M.G.; et al. Acute hypertension induces oxidative stress in brain tissues. J. Cereb. Blood Flow Metab. 2006, 26, 253–262. [Google Scholar] [CrossRef]
- Yang, J.; Sun, P.; Xu, X.; Liu, X.; Lan, L.; Yi, M.; Xiao, C.; Ni, R.; Fan, Y. TAK1 improves cognitive function via suppressing RIPK1-driven neuronal apoptosis and necroptosis in rats with chronic hypertension. Aging Dis. 2023, 14, 1799. [Google Scholar] [CrossRef]
- Chen, F.; Yi, W.-M.; Wang, S.-Y.; Yuan, M.-H.; Wen, J.; Li, H.-Y.; Zou, Q.; Liu, S.; Cai, Z.-Y. A long-term high-fat diet influences brain damage and is linked to the activation of HIF-1α/AMPK/mTOR/p70S6K signalling. Front. Neurosci. 2022, 16, 978431. [Google Scholar] [CrossRef]
- Herting, M.M.; Chu, X. Exercise, cognition, and the adolescent brain. Birth Defects Res. 2017, 109, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.M.; Solomon, S.D. Influence of physical activity on hypertension and cardiac structure and function. Curr. Hypertens. Rep. 2015, 17, 77. [Google Scholar] [CrossRef]
- Suk, M.; Shin, Y. Effect of high-intensity exercise and high-fat diet on lipid metabolism in the liver of rats. J. Exerc. Nutr. Biochem. 2015, 19, 289. [Google Scholar] [CrossRef]
- Huang, W.-C.; Xu, J.-W.; Li, S.; Ng, X.E.; Tung, Y.-T. Effects of exercise on high-fat diet–induced non-alcoholic fatty liver disease and lipid metabolism in ApoE knockout mice. Nutr. Metab. 2022, 19, 10. [Google Scholar] [CrossRef]
- Peri-Okonny, P.; Fu, Q.; Zhang, R.; Vongpatanasin, W. Exercise, the brain, and hypertension. Curr. Hypertens. Rep. 2015, 17, 82. [Google Scholar] [CrossRef]
- Costa, E.C.; Hay, J.L.; Kehler, D.S.; Boreskie, K.F.; Arora, R.C.; Umpierre, D.; Szwajcer, A.; Duhamel, T.A. Effects of High-Intensity Interval Training Versus Moderate-Intensity Continuous Training on Blood Pressure in Adults with Pre- to Established Hypertension: A Systematic Review and Meta-Analysis of Randomized Trials. Sports Med. 2018, 48, 2127–2142. [Google Scholar] [CrossRef] [PubMed]
- de Souza Mesquita, F.O.; Gambassi, B.B.; Silva, M.d.O.; Moreira, S.R.; Neves, V.R.; Gomes-Neto, M.; Schwingel, P.A. Effect of High-Intensity Interval Training on Exercise Capacity, Blood Pressure, and Autonomic Responses in Patients with Hypertension: A Systematic Review and Meta-analysis. Sports Health 2023, 15, 571–578. [Google Scholar] [CrossRef]
- Lopes, S.; Mesquita-Bastos, J.; Alves, A.J.; Ribeiro, F. Exercise as a tool for hypertension and resistant 1. hypertension management: Current insights. Integr. Blood Press. Control 2018, 11, 65–67. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Hargreaves, M. Exercise adaptations: Molecular mechanisms and potential targets for therapeutic benefit. Nat. Rev. Endocrinol. 2020, 16, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, H.; Wang, S.; Weng, H.; Luo, Z.; Ou, G.; Chen, Y.; Xu, L.; So, K.F.; Deng, L.; et al. Regular exercise ameliorates high-fat diet-induced depressive-like behaviors by activating hippocampal neuronal autophagy and enhancing synaptic plasticity. Cell Death Dis. 2024, 15, 737. [Google Scholar] [CrossRef]
- Marcourt, C.; Rivera, C.; Tuvikene, J.; Langeard, A.; Esvald, E.-E.; Cabrera-Cabrera, F.; Timmusk, T.; Temprado, J.-J.; Laurin, J. High-intensity interval and moderate-intensity continuous training on cerebral energy metabolism in older rats. GeroScience, 2025; Online ahead of print. [Google Scholar]
- Khoramipour, K.; Bejeshk, M.A.; Rajizadeh, M.A.; Najafipour, H.; Dehghan, P.; Farahmand, F. High-Intensity Interval Training Ameliorates Molecular Changes in the Hippocampus of Male Rats with the Diabetic Brain: The Role of Adiponectin. Mol. Neurobiol. 2023, 60, 3486–3495. [Google Scholar] [CrossRef]
- Ebrahimnezhad, N.; Nayebifar, S.; Soltani, Z.; Khoramipour, K. High-intensity interval training reduced oxidative stress and apoptosis in the hippocampus of male rats with type 2 diabetes: The role of the PGC1α-Keap1-Nrf2 signaling pathway. Iran. J. Basic Med. Sci. 2023, 26, 1313. [Google Scholar]
- Marques Neto, S.R.; Castiglione, R.C.; da Silva, T.C.B.; Paes, L.d.S.; Pontes, A.; Oliveira, D.F.; Ferraz, E.B.; Caldas, C.C.A.; Nascimento, J.H.M.; Bouskela, E. Effects of high intensity interval training on neuro-cardiovascular dynamic changes and mitochondrial dysfunction induced by high-fat diet in rats. PLoS ONE 2020, 15, e0240060. [Google Scholar] [CrossRef]
- Laggner, M.; Oberndorfer, F.; Golabi, B.; Bauer, J.; Zuckermann, A.; Hacker, P.; Lang, I.; Skoro-Sajer, N.; Gerges, C.; Taghavi, S.; et al. EGR1 is implicated in right ventricular cardiac remodeling associated with pulmonary hypertension. Biology 2022, 11, 677. [Google Scholar] [CrossRef]
- Lehman, C.W.; Smith, A.; Kelly, J.; Jacobs, J.L.; Dinman, J.D.; Kehn-Hall, K. EGR1 upregulation during encephalitic viral infections contributes to inflammation and cell death. Viruses 2022, 14, 1210. [Google Scholar] [CrossRef]
- Nyunt, T.; Britton, M.; Wanichthanarak, K.; Budamagunta, M.; Voss, J.C.; Wilson, D.W.; Rutledge, J.C.; Aung, H.H. Mitochondrial oxidative stress-induced transcript variants of ATF3 mediate lipotoxic brain microvascular injury. Free. Radic. Biol. Med. 2019, 143, 25–46. [Google Scholar] [CrossRef]
- Kang, L.; Piao, M.; Liu, N.; Gu, W.; Feng, C. Sevoflurane exposure induces neuronal cell ferroptosis initiated by increase of intracellular hydrogen peroxide in the developing brain via ER stress ATF3 activation. Mol. Neurobiol. 2024, 61, 2313–2335. [Google Scholar] [CrossRef]
- Basu-Shrivastava, M.; Mojsa, B.; Mora, S.; Robbins, I.; Bossis, G.; Lassot, I.; Desagher, S. Trim39 regulates neuronal apoptosis by acting as a SUMO-targeted E3 ubiquitin-ligase for the transcription factor NFATc3. Cell Death Differ. 2022, 29, 2107–2122. [Google Scholar] [CrossRef]
- Moriyama, K.; Horino, A.; Kohyama, K.; Nishito, Y.; Morio, T.; Sakuma, H. Oxygen–glucose deprivation increases NR4A1 expression and promotes its extranuclear translocation in mouse astrocytes. Brain Sci. 2024, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.S.; Dickerson, E.E.; Zhang, L.X.; Richendrfer, H.; Karamchedu, P.N.; Badger, G.J.; Schmidt, T.A.; Fredericks, A.M.; Elsaid, K.A.; Jay, G.D. Quadruped gait and regulation of apoptotic factors in Tibiofemoral joints following intra-articular rhPRG4 injection in Prg4 null mice. Int. J. Mol. Sci. 2022, 23, 4245. [Google Scholar] [CrossRef] [PubMed]
- Tatsukawa, H.; Hitomi, K. Role of transglutaminase 2 in cell death, survival, and fibrosis. Cells 2021, 10, 1842. [Google Scholar] [CrossRef]
- Abel, W.F.; Funk, C.R.; Blenda, A.V. Galectins in the pathogenesis of cerebrovascular accidents: An overview. J. Exp. Neurosci. 2019, 13, 1179069519836794. [Google Scholar] [CrossRef]
- Sadeghi, S.; Delphan, M.; Shams, M.; Esmaeili, F.; Shanaki-Bavarsad, M.; Shanaki, M. The high-intensity interval training (HIIT) and curcumin supplementation can positively regulate the autophagy pathway in myocardial cells of STZ-induced diabetic rats. BMC Res. Notes 2023, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Mirakabad, F.S.T.; Khoramgah, M.S.; Abdollahifar, M.-A.; Tehrani, A.S.; Rezaei-Tavirani, M.; Niknazar, S.; Tahmasebinia, F.; Mahmoudiasl, G.-R.; Khoshsirat, S.; Abbaszadeh, H.A. NUPR1-CHOP experssion, autophagosome formation and apoptosis in the postmortem striatum of chronic methamphetamine user. J. Chem. Neuroanat. 2021, 114, 101942. [Google Scholar] [CrossRef]
- Kim, S.H.; Yu, H.S.; Park, H.G.; Ahn, Y.M.; Kim, Y.S.; Lee, Y.H.; Ha, K.; Shin, S.Y. Egr1 regulates lithium-induced transcription of the Period 2 (PER2) gene. Biochim. Biophys. Acta 2013, 1832, 1969–1979. [Google Scholar] [CrossRef]
- Sasaki, H.; Hattori, Y.; Ikeda, Y.; Kamagata, M.; Iwami, S.; Yasuda, S.; Tahara, Y.; Shibata, S. Forced rather than voluntary exercise entrains peripheral clocks via a corticosterone/noradrenaline increase in PER2::LUC mice. Sci. Rep. 2016, 6, 27607. [Google Scholar] [CrossRef]
- Woodruff, E.R.; Chun, L.E.; Hinds, L.R.; Spencer, R.L. Diurnal Corticosterone Presence and Phase Modulate Clock Gene Expression in the Male Rat Prefrontal Cortex. Endocrinology 2016, 157, 1522–1534. [Google Scholar] [CrossRef]
- Narain, P.; Petković, A.; Šušić, M.; Haniffa, S.; Anwar, M.; Arnoux, M.; Drou, N.; Antonio-Saldi, G.; Chaudhury, D. Nighttime-specific differential gene expression in suprachiasmatic nucleus and habenula is associated with resilience to chronic social stress. Transl. Psychiatry 2024, 14, 407. [Google Scholar] [CrossRef]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, J.S.; Branecky, K.L.; Yang, W.; Ellacott, K.L.J.; Niswender, K.D.; Yamazaki, S. High-fat diet acutely affects circadian organisation and eating behavior. Eur. J. Neurosci. 2013, 37, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.J.; Chen, S.S.; Wang, D.C.; Hung, H.S. High-fat diet reduces novelty-induced expression of activity-regulated cytoskeleton-associated protein. J. Cell. Physiol. 2020, 235, 1065–1075. [Google Scholar] [CrossRef]
- Gallo, F.T.; Katche, C.; Morici, J.F.; Medina, J.H.; Weisstaub, N.V. Immediate Early Genes, Memory and Psychiatric Disorders: Focus on c-Fos, Egr1 and Arc. Front. Behav. Neurosci. 2018, 12, 79. [Google Scholar] [CrossRef]
- Chen, J.; Ding, Q.; An, L.; Wang, H. Ca2+-stimulated adenylyl cyclases as therapeutic targets for psychiatric and neurodevelopmental disorders. Front. Pharmacol. 2022, 13, 949384. [Google Scholar] [CrossRef] [PubMed]
- Karampour, S.; Ravasi, A.A.; Choobineh, S. The Effect of High-Intensity Interval Training with and Without Caloric Restriction on Spatial Learning and Long-Term Memory of Obese Rats. Middle East J. Rehabil. Health Stud. 2019, 6, e96740. [Google Scholar] [CrossRef]
- Rahmi, U.; Goenawan, H.; Sylviana, N.; Setiawan, I.; Putri, S.T.; Andriyani, S.; Fitriana, L.A. Exercise induction at expression immediate early gene (c-Fos, ARC, EGR-1) in the hippocampus: A systematic review. Dement. Neuropsychol. 2024, 18, e20230015. [Google Scholar] [CrossRef]
- Cruz-Mendoza, F.; Jauregui-Huerta, F.; Aguilar-Delgadillo, A.; García-Estrada, J.; Luquin, S. Immediate Early Gene c-fos in the Brain: Focus on Glial Cells. Brain Sci. 2022, 12, 687. [Google Scholar] [CrossRef]
- Abe, T.; Kitaoka, Y.; Kikuchi, D.M.; Takeda, K.; Numata, O.; Takemasa, T. High-intensity interval training-induced metabolic adaptation coupled with an increase in Hif-1α and glycolytic protein expression. J. Appl. Physiol. 2015, 119, 1297–1302. [Google Scholar] [CrossRef]
- Yazdani, F.; Shahidi, F.; Karimi, P. The effect of 8 weeks of high-intensity interval training and moderate-intensity continuous training on cardiac angiogenesis factor in diabetic male rats. J. Physiol. Biochem. 2020, 76, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Rahim, H.A.; Damirchi, A.; Babaei, P. Comparison of HIIT and MICT and further detraining on metabolic syndrome and asprosin signaling pathway in metabolic syndrome model of rats. Sci. Rep. 2024, 14, 11313. [Google Scholar] [CrossRef]
- Pengam, M.; Goanvec, C.; Moisan, C.; Simon, B.; Albacète, G.; Féray, A.; Guernec, A.; Amérand, A. Moderate intensity continuous versus high intensity interval training: Metabolic responses of slow and fast skeletal muscles in rat. PLoS ONE 2023, 18, e0292225. [Google Scholar] [CrossRef] [PubMed]
- Felipe, S.M.d.S.; Pacheco, C.; Martins, J.E.R.; de Freitas, R.M.; de Oliveira, P.E.G.; Mendes, S.V.D.; Alves, J.O.; Ceccatto, V.M. Optimization of RNA Extraction Protocol for Rat Skeletal Muscle Samples. J. Appl. Life Sci. Int. 2023, 26, 10–16. [Google Scholar] [CrossRef]
- Manzoor, F.; Tsurgeon, C.A.; Gupta, V. Exploring RNA-Seq Data Analysis Through Visualization Techniques and Tools: A Systematic Review of Opportunities and Limitations for Clinical Applications. Bioengineering 2025, 12, 56. [Google Scholar] [CrossRef]
- Available online: https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/samplepreps_truseq/truseq-stranded-mrna-workflow/truseq-stranded-mrna-workflow-reference-1000000040498-00.pdf (accessed on 31 August 2023).
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
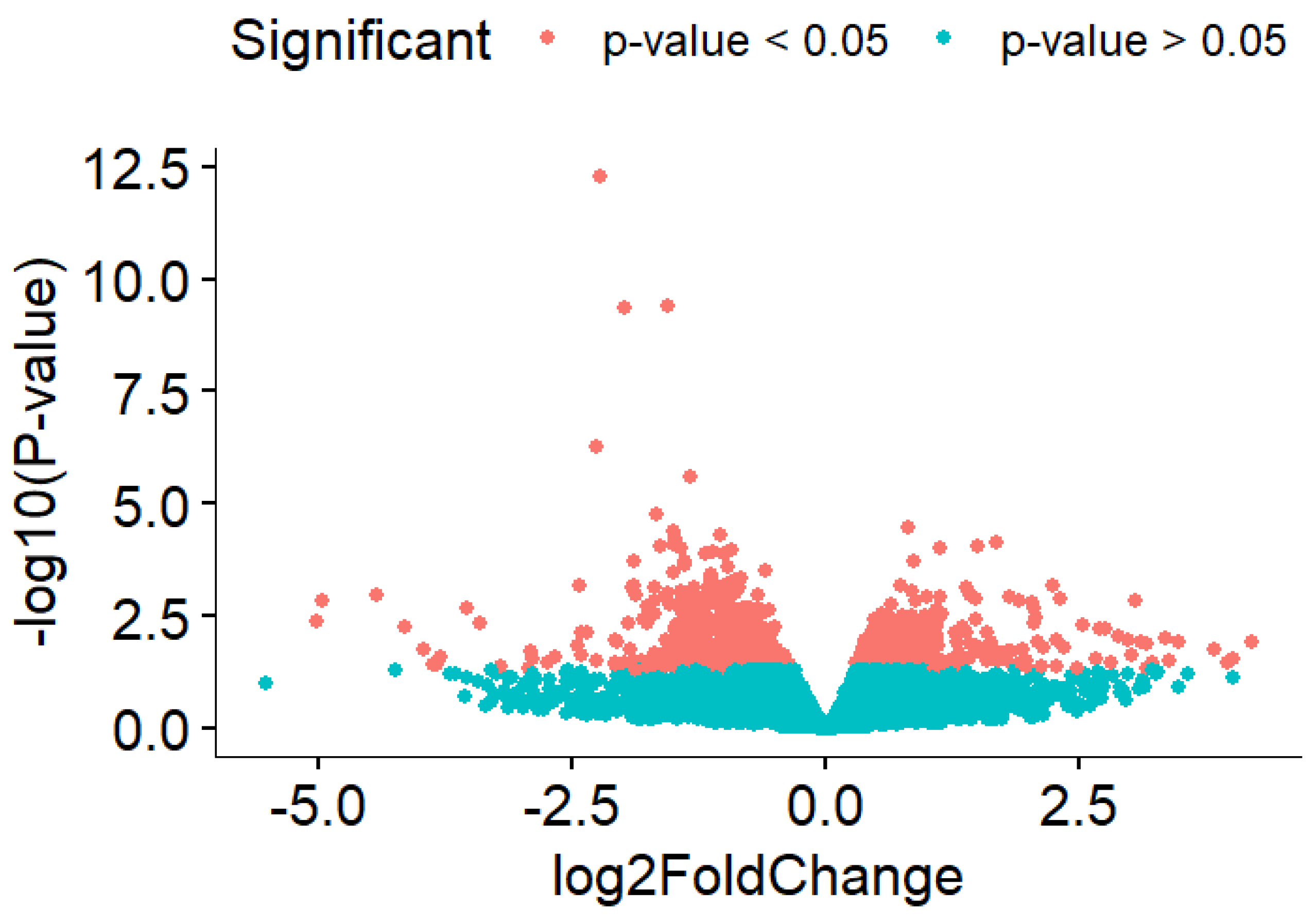


| # | Symbol | log2FC | p Value |
|---|---|---|---|
| 1 | Egr1 | −0.845 | <0.001 |
| 2 | Atf3 | −1.683 | 0.001 |
| 3 | Tgm2 | −0.965 | 0.001 |
| 4 | Lgals1 | −1.085 | 0.001 |
| 5 | Nr4a1 | −1.423 | <0.001 |
| 6 | Plekhf1 | −0.891 | 0.001 |
| 7 | Trim39 | 1.496 | <0.001 |
| 8 | Nupr1l1 | −1.504 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Sadiq, A.; Shah, I.A.; Wu, B.-T.; Lin, Y.-Y.; Su, Y.-A.; Yang, A.-L.; Lee, S.-D. Transcriptomic Analysis of High-Intensity Interval Training in High-Fat-Diet-Induced Spontaneous Hypertensive Rats’ Brains. Int. J. Mol. Sci. 2026, 27, 304. https://doi.org/10.3390/ijms27010304
Sadiq A, Shah IA, Wu B-T, Lin Y-Y, Su Y-A, Yang A-L, Lee S-D. Transcriptomic Analysis of High-Intensity Interval Training in High-Fat-Diet-Induced Spontaneous Hypertensive Rats’ Brains. International Journal of Molecular Sciences. 2026; 27(1):304. https://doi.org/10.3390/ijms27010304
Chicago/Turabian StyleSadiq, Arslan, Iqbal Ali Shah, Bor-Tsang Wu, Yi-Yuan Lin, Yi-An Su, Ai-Lun Yang, and Shin-Da Lee. 2026. "Transcriptomic Analysis of High-Intensity Interval Training in High-Fat-Diet-Induced Spontaneous Hypertensive Rats’ Brains" International Journal of Molecular Sciences 27, no. 1: 304. https://doi.org/10.3390/ijms27010304
APA StyleSadiq, A., Shah, I. A., Wu, B.-T., Lin, Y.-Y., Su, Y.-A., Yang, A.-L., & Lee, S.-D. (2026). Transcriptomic Analysis of High-Intensity Interval Training in High-Fat-Diet-Induced Spontaneous Hypertensive Rats’ Brains. International Journal of Molecular Sciences, 27(1), 304. https://doi.org/10.3390/ijms27010304








