Polyethylene Glycol (PEG)-Based Wet-Adhesive Absorbable Bone Wax for Osseous Hemostasis and Repair
Abstract
1. Introduction
2. Results and Discussion
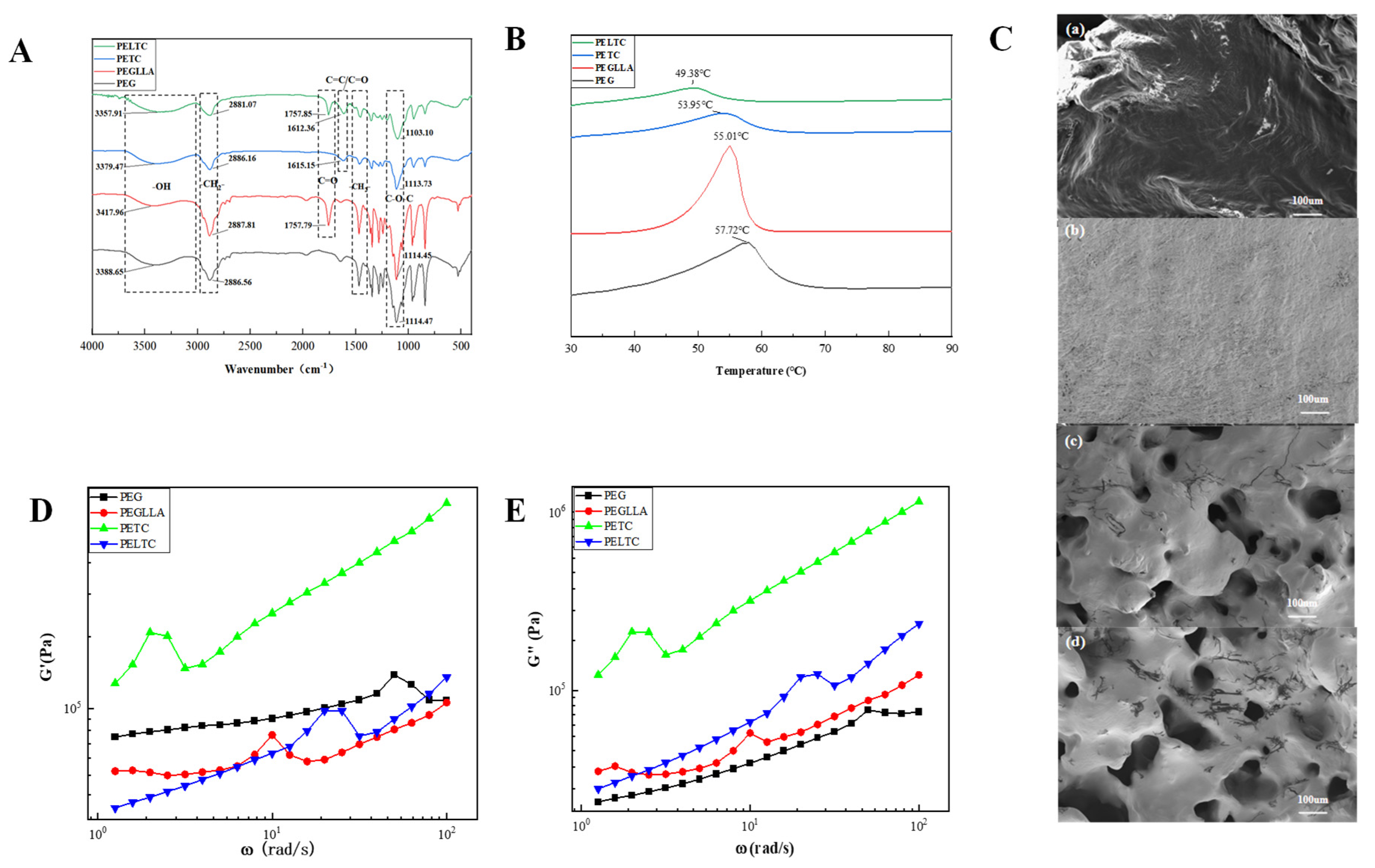
2.1. FTIR, DSC, SEM, and Rheology
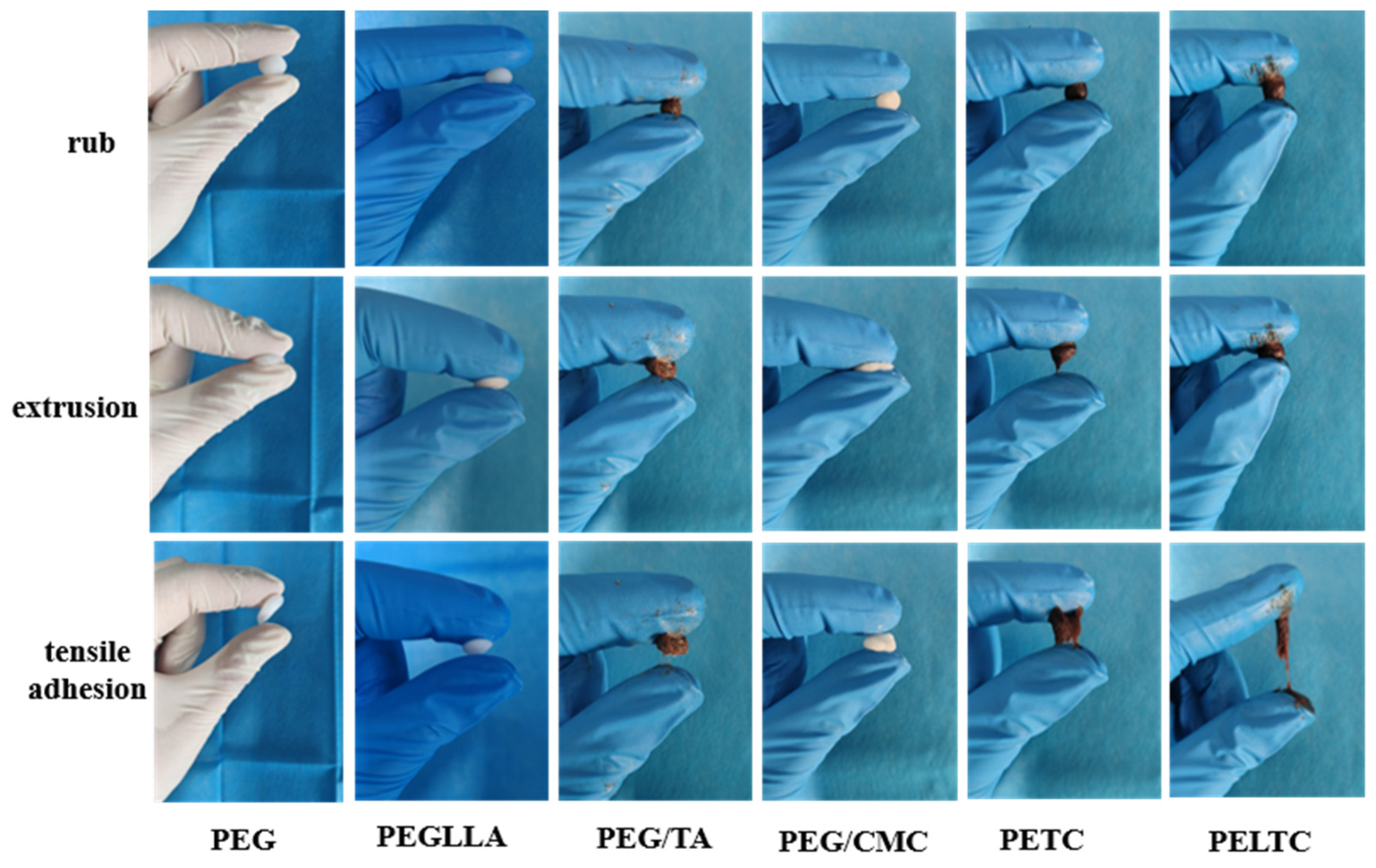
2.2. Plasticity
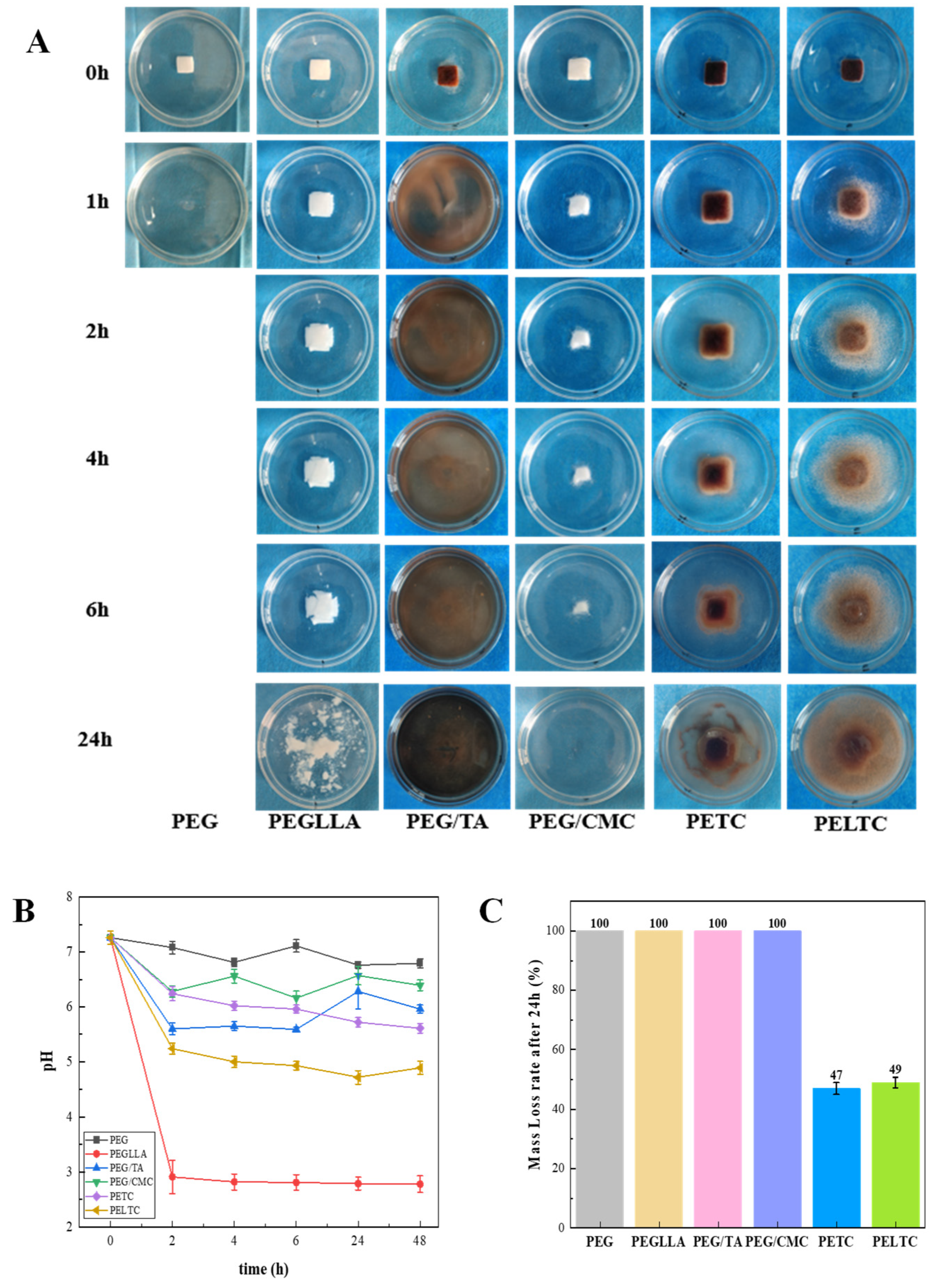
2.3. Dissolution Performance
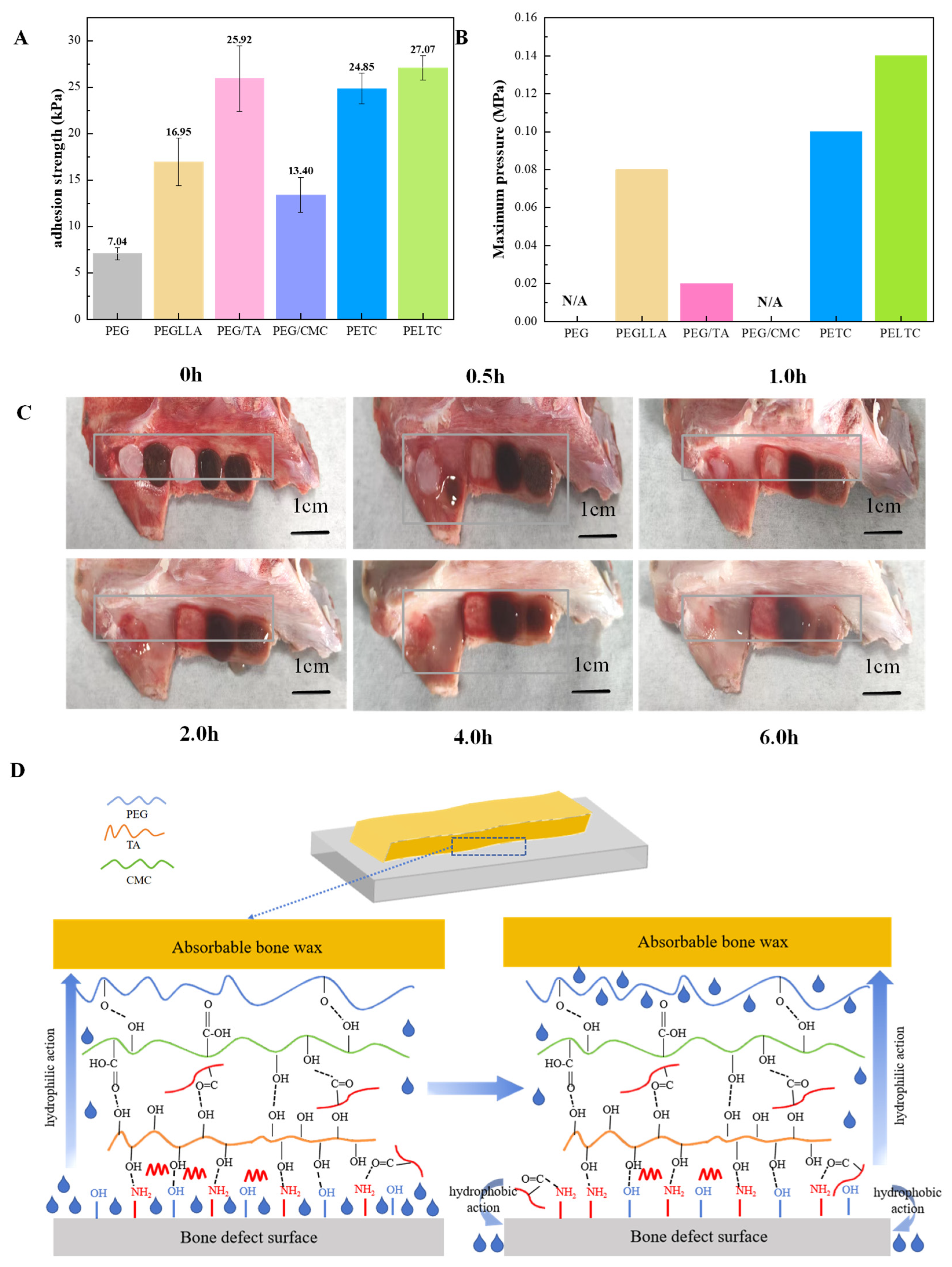
2.4. Adhesion
2.5. Cytotoxicity and Hemocompatibility
2.6. Assessment of Hemostasis Performance
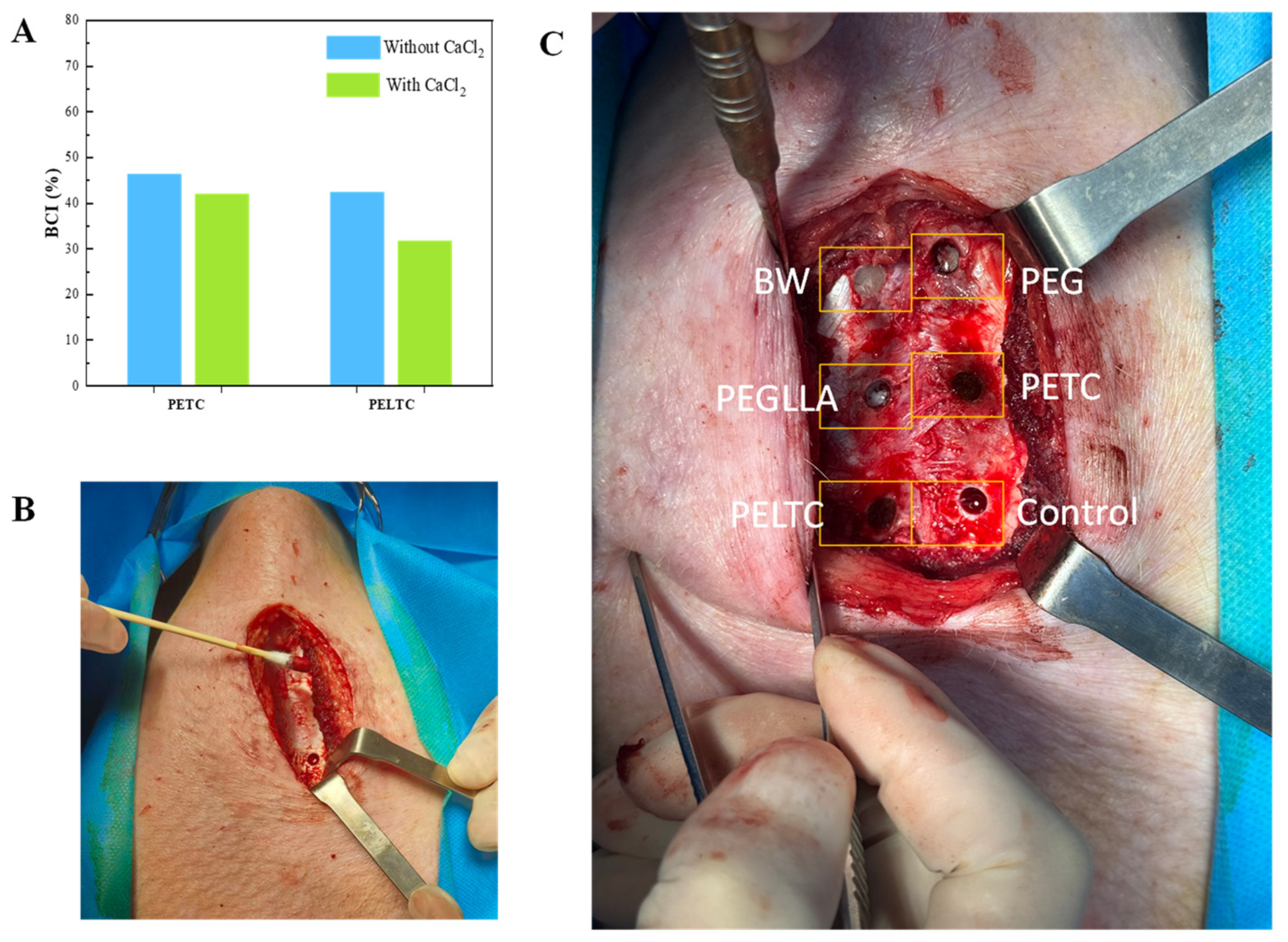
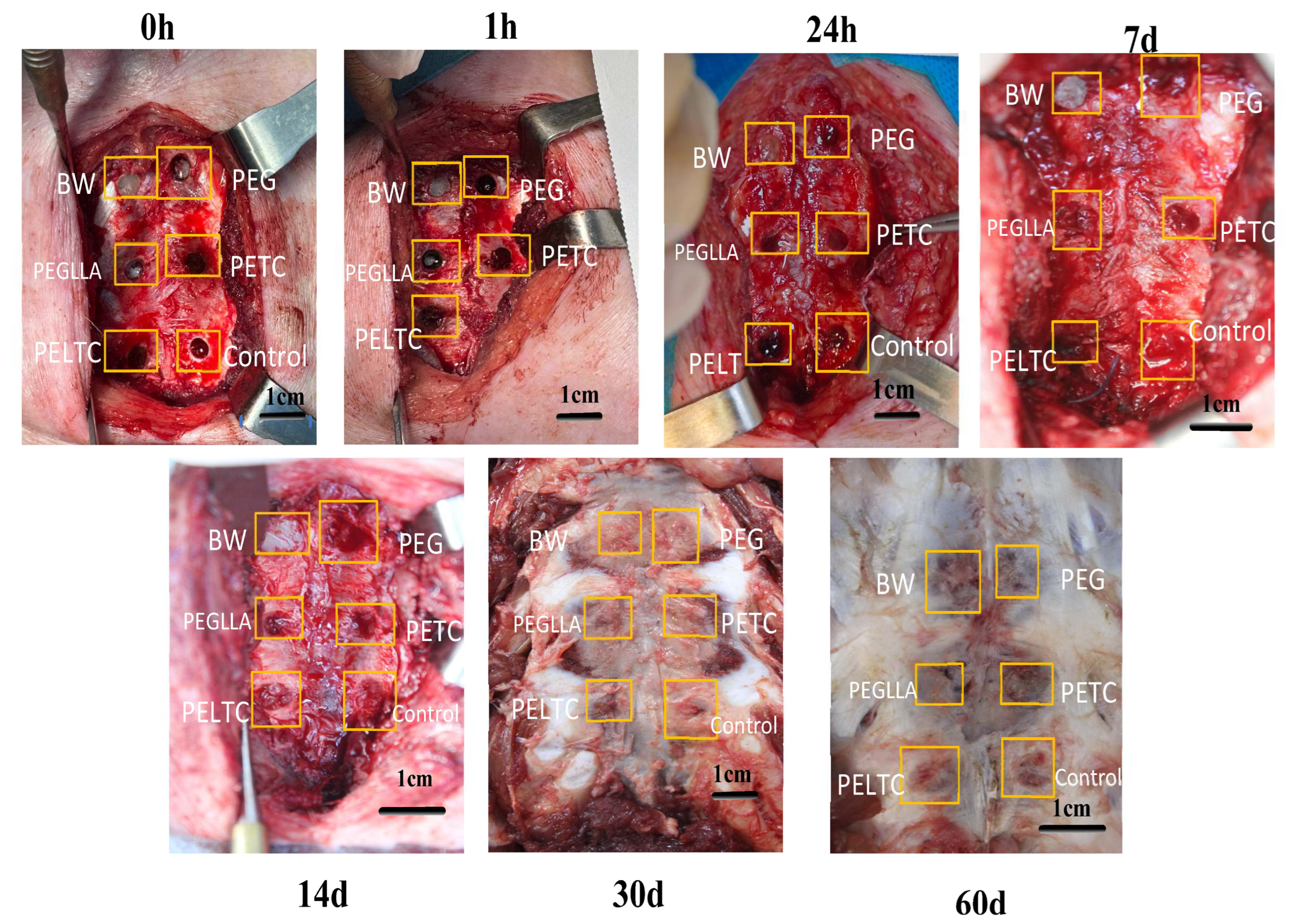
2.7. In Vivo Degradation of Absorbable Bone Wax
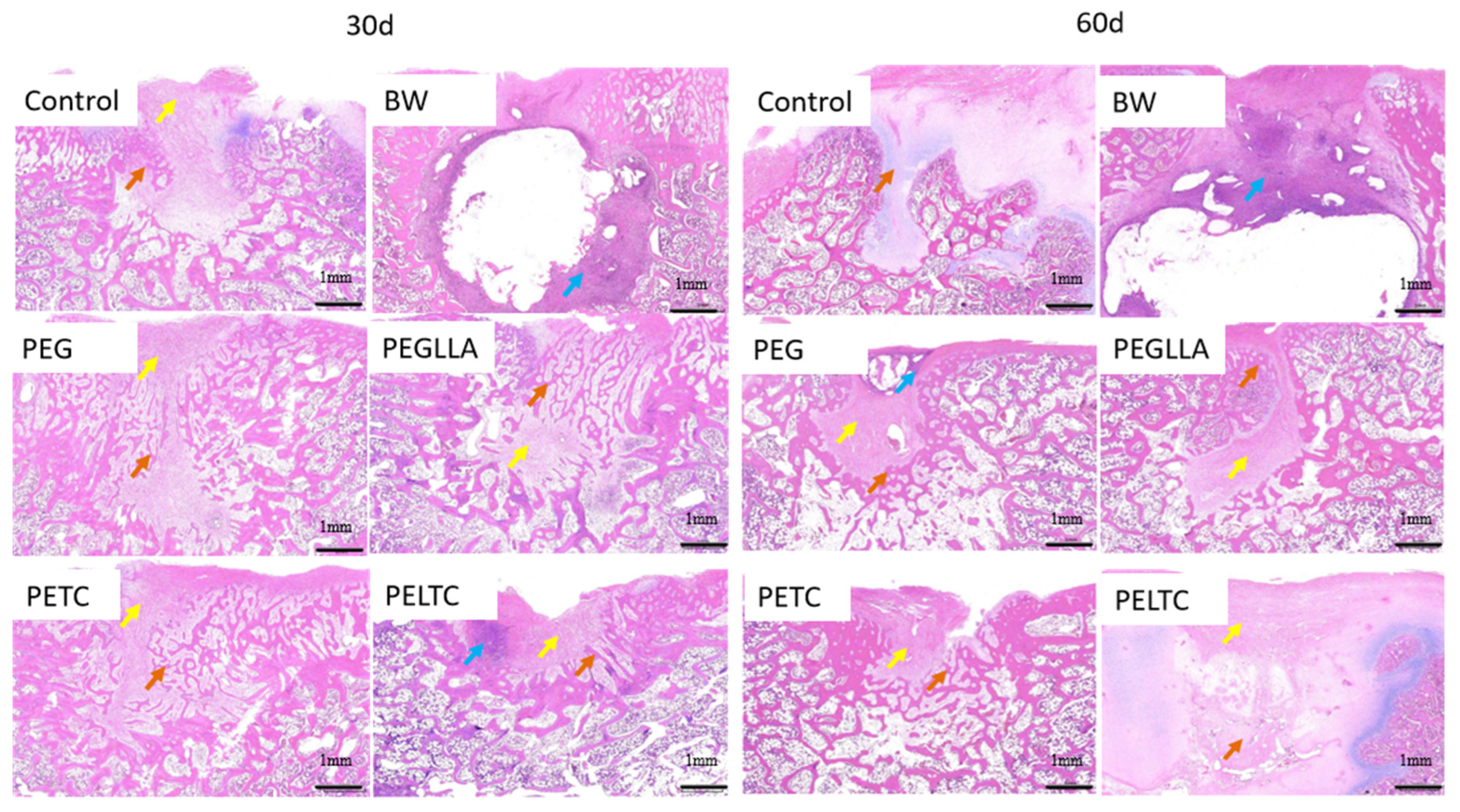
2.8. Bone Repair
3. Materials and Methods
3.1. Materials
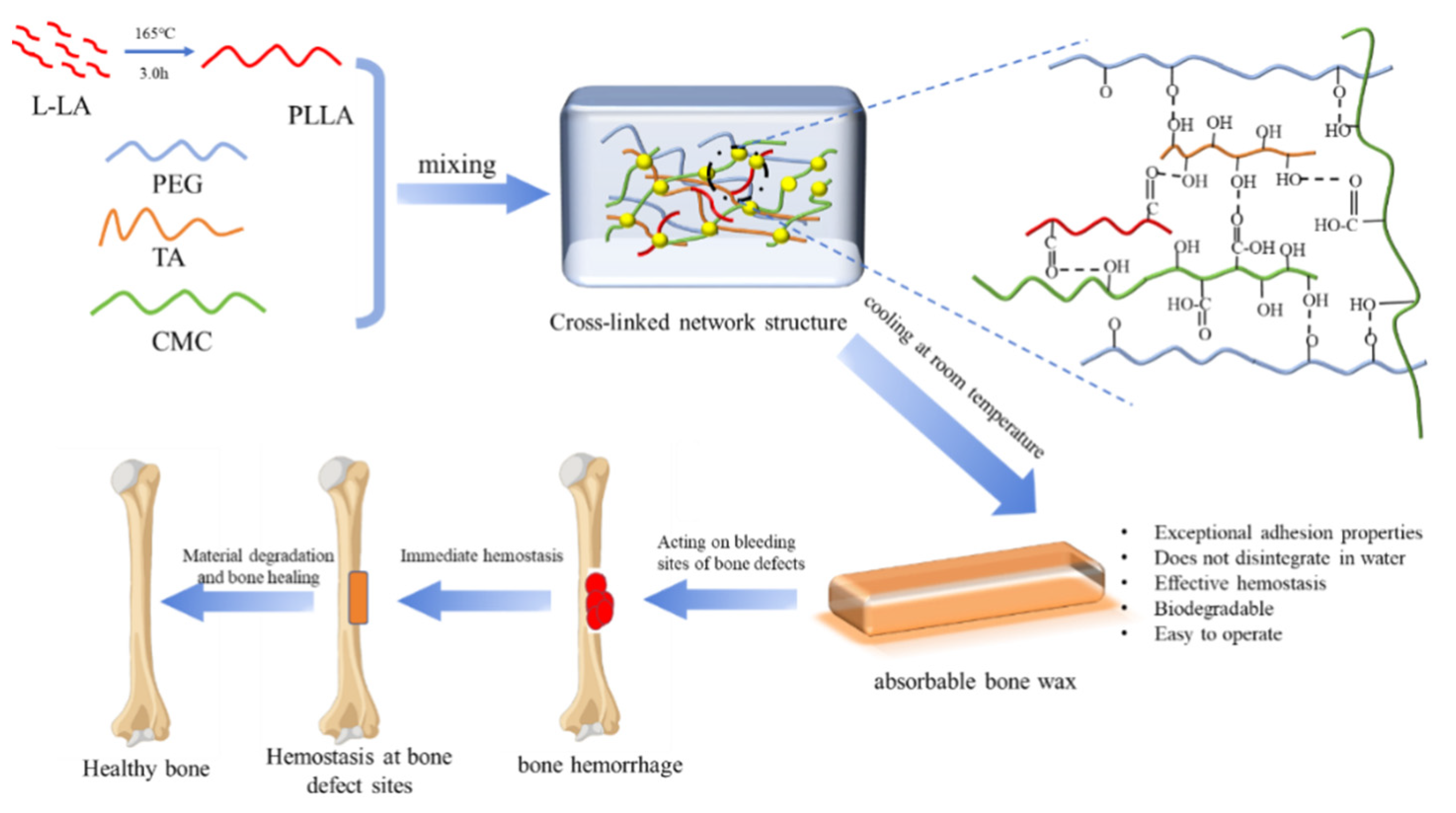
3.2. Preparation of Absorbable Bone Wax
3.3. Plasticity
3.4. Dissolution Performance Test
3.5. Adhesion Strength Test
3.6. Simulated Adhesion Experiment at Bleeding Sites
3.7. Sealing Performance Test
3.8. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.9. Differential Scanning Calorimetry (DSC) Analysis
3.10. Scanning Electron Microscopy (SEM) Characterization
3.11. Rotational Rheological Tests
3.12. Cytotoxicity Experiments
3.13. Hemolysis Rate
3.14. Whole Blood Clotting Test
3.15. In Vivo Hemostatic Performance and Dissolution Assessment
3.16. Bone Healing Status
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Biswas, L.; Chen, J.; De Angelis, J.; Singh, A.; Owen-Woods, C.; Ding, Z.; Pujol, J.M.; Kumar, N.; Zeng, F.; Ramasamy, S.K.; et al. Lymphatic vessels in bone support regeneration after injury. Cell 2023, 186, 382–397.e24. [Google Scholar] [CrossRef] [PubMed]
- Hickman, D.A.; Pawlowski, C.L.; Sekhon, U.D.S.; Marks, J.; Gupta, A.S. Biomaterials and Advanced Technologies for Hemostatic Management of Bleeding. Adv. Mater. 2017, 30, 1700859. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.M.J.; Razzaghof, M.; Ghadimi, E.; Seyedtabaei, S.M.M.; Ardakani, M.V.; Moharrami, A. The Efficacy of Bone Wax in Reduction of Perioperative Blood Loss in Total Hip Arthroplasty via Direct Anterior Approach A Prospective Randomized Clinical Trial. J. Bone Jt. Surg.-Am. Vol. 2022, 104, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.S.; Kozin, E.D.; Remenschneider, A.K.; Nakajima, H.H.; Lee, D.J. Characteristics of Wax Occlusion in the Surgical Repair of Superior Canal Dehiscence in Human Temporal Bone Specimens. Otol. Neurotol. 2016, 37, 83–88. [Google Scholar] [CrossRef]
- Zhou, H.; Ge, J.; Bai, Y.; Liang, C.; Yang, L. Translation of bone wax and its substitutes: History, clinical status and future directions. J. Orthop. Transl. 2019, 17, 64–72. [Google Scholar] [CrossRef]
- Sudmann, B.; Bang, G.; Sudmann, E. Histologically verified bone wax (beeswax) granuloma after median sternotomy in 17 of 18 autopsy cases. Pathology 2006, 38, 138–141. [Google Scholar] [CrossRef]
- Turgut, M.; Karademir, S.; Başaoğlu, H.K.; Tomruk, C.; Cetin, E.O.; Uyanikgil, Y.; Cengiz, A. Evaluation of the effects of Ankaferd haemostat application on bone regeneration in rats with calvarial defects: Histochemical, immunohistochemical and scintigraphic study. Folia Morphol. 2022, 81, 739–748. [Google Scholar] [CrossRef]
- Maki, Y.; Ishibashi, R.; Yamada, D.; Morita, T.; Chin, M.; Yamagata, S. Postoperative Ptosis and Diplopia Induced by the Intraoperative Application of Bone Wax. World Neurosurg. 2017, 103, 951.e1–951.e3. [Google Scholar] [CrossRef]
- Robicsek, F.; Masters, T.N.; Littman, L.; Born, G.V. The embolization of bone wax from sternotomy incisions. Ann. Thorac. Surg. 1981, 31, 357–359. [Google Scholar] [CrossRef]
- Magyar, C.E.; Aghaloo, T.L.; Atti, E.; Tetrad, S. Ostene, A New Alkylene Oxide Copolymer Bone Hemostatic Material, Does Not Inhibit Bone Healing. Neurosurgery 2008, 63, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Sun, D.; Fu, J.; Huang, K.; Yu, S.; Xie, Z. Comparison of PMMA bone cement and bone wax in treatment of iliac bone defects at the bone graft donor site. J. Third Mil. Med. Univ. 2017, 39, 1256–1261. [Google Scholar]
- Gibbs, L.; Kakis, A.; Weinstein, P.; Conte, J.E. Bone wax as a risk factor for surgical-site infection following neurospinal surgery. Infect. Control. Hosp. Epidemiol. 2004, 25, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Lv, M.; Zhang, T.; Zhang, Q.; Chen, Y.; Liu, Z.; Wei, R.; Cai, L. Copper-Loaded Biodegradable Bone Wax with Antibacterial and Angiogenic Properties in Early Bone Repair. ACS Biomater. Sci. Eng. 2021, 7, 663–671. [Google Scholar] [CrossRef]
- Brückner, T.; Schamel, M.; Kübler, A.C.; Groll, J.; Gbureck, U. Novel bone wax based on poly(ethylene glycol)-calcium phosphate cement mixtures. Acta Biomater. 2016, 33, 252–263. [Google Scholar] [CrossRef]
- Suvannapruk, W.; Thammarakcharoen, F.; Chokevivat, W.; Rukskul, P.; Suwanprateeb, J. Evaluation of PEG-PPG-PEG Copolymer Blends for Using As Resorbable Bone Wax. In Proceedings of the 4th International Conference on Multi-Functional Materials and Structures (MFMS 2013), King Mongkut’s University of Technology Thonburi, Bangkok, Thailand, 14–17 July 2013; Volume 747, p. 174. [Google Scholar]
- Suwanprateeb, J.; Suvannapruk, W.; Thammarakcharoen, F.; Chokevivat, W.; Rukskul, P. Preparation and characterization of PEG-PPG-PEG copolymer/pregelatinized starch blends for use as resorbable bone hemostatic wax. J. Mater. Sci.-Mater. Med. 2013, 24, 2881–2888. [Google Scholar] [CrossRef]
- Orgill, D.P.; Ehret, F.W.; Regan, J.F.; Glowacki, J.; Mulliken, J.B. Polyethylene glycol microfibrillar collagen composite as a new resorbable hemostatic bone wax. J. Biomed. Mater. Res. 1998, 39, 358–363. [Google Scholar] [CrossRef]
- Toriumi, D.M.; Bagal, A.A. Cyanoacrylate tissue adhesives for skin closure in the outpatient setting. Otolaryngol. Clin. N. Am. 2002, 35, 103–118. [Google Scholar] [CrossRef]
- Achneck, H.E.; Sileshi, B.; Jamiolkowski, R.M.; Albala, D.M.; Shapiro, M.L.; Lawson, J.H. A Comprehensive Review of Topical Hemostatic Agents Efficacy and Recommendations for Use. Ann. Surg. 2010, 251, 217–228. [Google Scholar] [CrossRef]
- Tomizawa, Y. Clinical benefits and risk analysis of topical hemostats: A review. J. Artif. Organs Off. J. Jpn. Soc. Artif. Organs 2005, 8, 137–142. [Google Scholar] [CrossRef]
- Brodbelt, A.R.; Miles, J.B.; Foy, P.M.; Broome, J.C. Intraspinal oxidised cellulose (Surgicel®) causing delayed paraplegia after thoracotomy: A report of three cases. Ann. R. Coll. Surg. Engl. 2002, 84, 97–99. [Google Scholar] [PubMed]
- Huang, W.; Cheng, S.; Wang, X.; Zhang, Y.; Chen, L.; Zhang, L. Noncompressible Hemostasis and Bone Regeneration Induced by an Absorbable Bioadhesive Self-Healing Hydrogel. Adv. Funct. Mater. 2021, 31, 2009189. [Google Scholar] [CrossRef]
- Cui, Z.K.; Kim, S.; Baljon, J.J.; Wu, B.M.; Aghaloo, T.; Lee, M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 2019, 10, 3523. [Google Scholar] [CrossRef] [PubMed]
- Wedmore, I.; McManus, J.G.; Pusateri, A.E.; Holcomb, J.B. A special report on the chitosan-based hemostatic dressing: Experience in current combat operations. J. Trauma-Inj. Infect. Crit. Care 2006, 60, 655–658. [Google Scholar] [CrossRef]
- Brown, M.A.; Daya, M.R.; Worley, J.A. Experience with Chitosan Dressings in a Civilian EMS System. J. Emerg. Med. 2009, 37, 1–7. [Google Scholar] [CrossRef]
- Chen, C.; Li, H.; Pan, J.; Yan, Z.; Yao, Z.; Fan, W.; Guo, C. Biodegradable composite scaffolds of bioactive glass/chitosan/carboxymethyl cellulose for hemostatic and bone regeneration. Biotechnol. Lett. 2015, 37, 457–465. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, M.; Ni, X.; Yang, L.; Kutty, M.G. Using calcium sulfate cement Hydroxypropyl methyl cellulose/sodium alginate composites as substitutes of bone wax. Int. J. Appl. Ceram. Technol. 2018, 15, 903–909. [Google Scholar] [CrossRef]
- Sarda, S.; Errassifi, F.; Marsan, O.; Geffre, A.; Trumel, C.; Drouet, C. Adsorption of tranexamic acid on hydroxyapatite: Toward the development of biomaterials with local hemostatic activity. Mater. Sci. Eng. C 2016, 66, 1–7. [Google Scholar] [CrossRef]
- Tsai, W.B.; Ahmed, I.N. The Impact of Polyethylene Glycol-Modified Chitosan Scaffolds on the Proliferation and Differentiation of Osteoblasts. Int. J. Biomater. 2023, 2023, 4864492. [Google Scholar] [CrossRef]
- Alcantar, N.A.; Aydil, E.S.; Israelachvili, J.N. Polyethylene glycol-coated biocompatible surfaces. J. Biomed. Mater. Res. 2000, 51, 343–351. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.M.; Spazierer, D.; Slezak, P.; Baumgartner, B.; Regenbogen, J.; Gulle, H. Swelling, sealing, and hemostatic ability of a novel biomaterial: A polyethylene glycol-coated collagen pad. J. Biomater. Appl. 2014, 29, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Buck, M.L.; Bulluss, K.J.; Smith, P.D. Wax on, wax off: A case report discussing a potential pitfall of dissolvable bone wax substitutes such as Ostene® in neurosurgery. Acta Neurochir. 2025, 167, 65. [Google Scholar] [CrossRef] [PubMed]
- Suwanprateeb, J.; Kiertkrittikhoon, S.; Kintarak, J.; Suvannapruk, W.; Thammarakcharoen, F.; Rukskul, P. In vivo assessment of new resorbable PEG–PPG–PEG copolymer/starch bone wax in bone healing and tissue reaction of bone defect in rabbit model. J. Mater. Sci. Mater. Med. 2014, 25, 2131–2139. [Google Scholar] [CrossRef]
- Zong, S.Y.; Lv, H.; Liu, C.J.; Zhu, L.W.; Duan, J.F.; Jiang, J.X. Mussel inspired Cu-tannic autocatalytic strategy for rapid self-polymerization of conductive and adhesive hydrogel sensors with extreme environmental tolerance. Chem. Eng. J. 2023, 465, 142831. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Zheng, D.T.; Huang, C.X.; Hu, Y.; Zheng, T.C.; An, J.J. Constructions of synergistic photothermal therapy antibacterial hydrogel based on polydopamine, tea polyphenols and polyvinyl alcohol and effects on wound healing in mouse. Colloids Surf. B-Biointerfaces 2022, 219, 112831. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, L.; Xiang, P.; Zhang, F.; Yang, Y.; Chao, L.; Liu, W.; Li, H.; Zhang, X. Aminated Lignin/Cellulose-Based Hydrogel with High Adhesion for Wearable Sensors. Langmuir 2025, 41, 15484–15493. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent crosslinked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef]
- Li, X.; Sun, S.; Feng, X.; Chen, Y.; Chen, S.; Ma, J.; Zhou, F. Tannic acid-crosslinked O-carboxymethyl chitosan hydrogels for enhanced antibacterial activity and rapid hemostasis. J. Biomater. Sci. Polym. Ed. 2023, 34, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Shi, G.; Wang, R.; Jiang, X.; Chen, Q.; Yu, A.; Lu, A. Bioinspired wet adhesive carboxymethyl cellulose-based hydrogel with rapid shape adaptability and antioxidant activity for diabetic wound repair. Carbohydr. Polym. 2024, 334, 122014. [Google Scholar] [CrossRef]
- El-Newehy, M.H.; El-Naggar, M.E.; Alotaiby, S.; El-Hamshary, H.; Moydeen, M.; Al-Deyab, S. Preparation of biocompatible system based on electrospun CMC/PVA nanofibers as controlled release carrier of diclofenac sodium. J. Macromol. Sci. Part A 2016, 53, 566–573. [Google Scholar] [CrossRef]
- Qi, X.-M.; Liu, S.-Y.; Chu, F.-B.; Pang, S.; Liang, Y.-R.; Guan, Y.; Peng, F.; Sun, R.-C. Preparation and Characterization of Blended Films from Quaternized Hemicelluloses and Carboxymethyl Cellulose. Materials 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Al-Harrasi, A.; Almohana, I.H.; Albayati, M.S.; Jawad, M.; Shah, Y.A.; Ullah, S.; Philip, A.K.; Halim, S.A.; Khan, A.; et al. The physicochemical properties and molecular docking study of plasticized amphotericin B loaded sodium alginate, carboxymethyl cellulose, and gelatin-based films. Heliyon 2024, 10, e24210. [Google Scholar] [CrossRef]
- Hu, X.; Xu, J.-Z.; Zhong, G.-J.; Luo, X.-L.; Li, Z.-M. Shear induced crystallization of poly(L-lactide) and poly(ethylene glycol) (PLLA-PEG-PLLA) copolymers with different block length. J. Polym. Res. 2011, 18, 675–680. [Google Scholar] [CrossRef]
- Banpean, A.; Takagi, H.; Shimizu, N.; Igarashi, N.; Sakurai, S. Small- and wide-angle X-ray scattering studies on confined crystallization of Poly(ethylene glycol) in Poly(L-lactic acid) spherulite in a PLLA/PEG blend. Polymer 2021, 229, 123971. [Google Scholar] [CrossRef]
- Lim, J.S.; Noda, I.; Im, S.S. Effect of hydrogen bonding on the crystallization behavior of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/silica hybrid composites. Polymer 2007, 48, 2745–2754. [Google Scholar] [CrossRef]
- Li, N.; Yang, X.; Liu, W.; Xi, G.; Wang, M.; Liang, B.; Ma, Z.; Feng, Y.; Chen, H.; Shi, C. Tannic Acid Cross-linked Polysaccharide-Based Multifunctional Hemostatic Microparticles for the Regulation of Rapid Wound Healing. Macromol. Biosci. 2018, 18, e1800209. [Google Scholar] [CrossRef]
- Guchait, A.; Saxena, A.; Chattopadhyay, S.; Mondal, T. Influence of Nanofillers on Adhesion Properties of Polymeric Composites. ACS Omega 2022, 7, 3844–3859. [Google Scholar] [CrossRef]
- Winter, H.H.; Chambon, F. Analysis of Linear Viscoelasticity of a Crosslinking Polymer at the Gel Point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Snoeijer, J.H.; Pandey, A.; Herrada, M.A.; Eggers, J. The relationship between viscoelasticity and elasticity. Proc. Math. Phys. Eng. Sci. 2020, 476, 20200419. [Google Scholar] [CrossRef] [PubMed]
- Ensing, B.; Tiwari, A.; Tros, M.; Hunger, J.; Domingos, S.R.; Pérez, C.; Smits, G.; Bonn, M.; Bonn, D.; Woutersen, S. On the origin of the extremely different solubilities of polyethers in water. Nat. Commun. 2019, 10, 2893. [Google Scholar] [CrossRef] [PubMed]
- Oyama, H.T.; Tanaka, Y.; Kadosaka, A. Rapid controlled hydrolytic degradation of poly(l-lactic acid) by blending with poly(aspartic acid-co-l-lactide). Polym. Degrad. Stab. 2009, 94, 1419–1426. [Google Scholar] [CrossRef]
- Codari, F.; Lazzari, S.; Soos, M.; Storti, G.; Morbidelli, M.; Moscatelli, D. Kinetics of the hydrolytic degradation of poly(lactic acid). Polym. Degrad. Stab. 2012, 97, 2460–2466. [Google Scholar] [CrossRef]
- Amsden, B. Solute Diffusion within Hydrogels. Mechanisms and Models. Macromolecules 1998, 31, 8382–8395. [Google Scholar] [CrossRef]
- Cass, C.A.; Burg, K.J. Tannic acid cross-linked collagen scaffolds and their anti-cancer potential in a tissue engineered breast implant. J. Biomater. Sci. Polym. Ed. 2012, 23, 281–298. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, W.; Lu, J.; Guo, X.; Xu, J.; Xu, W.; Chi, Y.; Takuya, N.; Wu, H.; Zhao, L. Tannic acid-based metal phenolic networks for bio-applications: A review. J. Mater. Chem. B 2021, 9, 4098–4110. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Yang, X.; Ma, Q. Tannic acid: A crosslinker leading to versatile functional polymeric networks: A review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, P.; Zhong, Q.-Z.; Liu, H.; Yu, Q.; Gao, N.; Hao, J.; Cui, J. Hydrogen Bonding-Driven Adaptive Coacervates as Protocells. ACS Appl. Mater. Interfaces 2025, 17, 6095–6102. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, Q.; Chen, Q.; Ma, Y.; Wang, Z.; Luo, L.; Ding, Q.; Li, H.; Tang, S. Carboxymethyl Chitosan/Tannic Acid Hydrogel with Antibacterial, Hemostasis, and Antioxidant Properties Promoting Skin Wound Repair. ACS Biomater. Sci. Eng. 2023, 9, 437–448. [Google Scholar] [CrossRef]
- Tang, B.; He, S.; Deng, Y.; Shan, Y.; Qin, H.; Noor, H.; Hou, X. Advanced binder with ultralow-content for high performance silicon anode. J. Power Sources 2023, 556, 232237. [Google Scholar] [CrossRef]
- Ham, A.S.; Klibanov, A.L.; Lawrence, M.B. Action at a Distance: Lengthening Adhesion Bonds with Poly(ethylene glycol) Spacers Enhances Mechanically Stressed Affinity for Improved Vascular Targeting of Microparticles. Langmuir 2009, 25, 10038–10044. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, Y.; Zhang, W.; Yu, M.; Chen, X.; Kong, M. Ternary Complex Coacervate of PEG/TA/Gelatin as Reinforced Bioadhesive for Skin Wound Repair. ACS Appl. Mater. Interfaces 2022, 14, 18097–18109. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, Z.; Li, Y.; Ding, X.; Li, D.; Shen, C.; Xu, F. Dual-Crosslinked Amorphous Polysaccharide Hydrogels Based on Chitosan/Alginate for Wound Healing Applications. Macromol. Rapid Commun. 2018, 39, e1800069. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Y.; Li, L.; Wang, C.; Wang, J.; Li, Y.; Chen, D.; Ding, X.; Shen, C.; Xu, F. Biomass-Derived Multilayer-Structured Microparticles for Accelerated Hemostasis and Bone Repair. Adv. Sci. 2020, 7, 2002243. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, C.; Zhou, H.; Liang, C.; Chen, W.; Bai, Y.; Ma, X.; Zhang, Y.; Yang, L. Moldable self-setting and bioactive bone wax for bone hemostasis and defect repair. J. Orthop. Transl. 2025, 50, 223–234. [Google Scholar] [CrossRef]
- Li, L.; Peng, H.; Du, Y.; Zheng, H.; Yang, A.; Lv, G.; Li, H. An antibacterial biomimetic adhesive with strong adhesion in both dry and underwater situations. J. Mater. Chem. B 2022, 10, 1063–1076. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, B.; Wu, H.; Liang, Y.; Ma, P.X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat. Commun. 2018, 9, 2784. [Google Scholar] [CrossRef]

| Sample | Clotting Time (min) | Bleeding Weight (mg) |
|---|---|---|
| Control | Over 60 | 2139 |
| BW | Immediate hemostasis | 0 |
| PEG | 2.15 | 36.5 |
| PEGLLA | 1.85 | 34.1 |
| PETC | Immediate hemostasis | 0 |
| PELTC | Immediate hemostasis | 0 |
| Sample | PEG2000/ wt% | PEG400/ wt% | PLLA/ wt% | TA/ wt% | CMC/wt% |
|---|---|---|---|---|---|
| PEG | 80 | 20 | / | / | / |
| PEGLLA | 90 | / | 10 | / | / |
| PEG/TA | 60 | 20 | / | 20 | / |
| PEG/CMC | 60 | 20 | / | / | 20 |
| PETC | 40 | 20 | / | 20 | 20 |
| PELTC | 30 | 20 | 10 | 20 | 20 |
| Grade | Degree of Lesion | Definition of Classification |
|---|---|---|
| 0 | Normal range | Under experimental conditions, considering factors such as age, sex, and strain, changes may occur, but under other circumstances, they might be considered deviations within the normal range. |
| 1 | Slight | The changes that occur are almost no more than the normal range of variations (i.e., minimal changes) |
| 2 | Mild | Lesions are easy to identify, but the severity is limited; the lesions may not cause any functional impairment; the affected tissue accounts for 11–20% of the examined tissue. |
| 3 | Moderate | The lesion is prominent and is likely to progress toward severity. It may cause limited dysfunction of tissues or organs; 21% to 40% of tissue is affected. |
| 4 | Severe | The lesions are severe and have developed into complete lesions, which are expected to cause significant tissue or organ dysfunction; the lesions involve 41–100% of the examined tissue area. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Cheng, H.; Yang, A.; Lv, G.; Zheng, H.; Li, H. Polyethylene Glycol (PEG)-Based Wet-Adhesive Absorbable Bone Wax for Osseous Hemostasis and Repair. Int. J. Mol. Sci. 2026, 27, 276. https://doi.org/10.3390/ijms27010276
Cheng H, Yang A, Lv G, Zheng H, Li H. Polyethylene Glycol (PEG)-Based Wet-Adhesive Absorbable Bone Wax for Osseous Hemostasis and Repair. International Journal of Molecular Sciences. 2026; 27(1):276. https://doi.org/10.3390/ijms27010276
Chicago/Turabian StyleCheng, Huiqiang, Aiping Yang, Guoyu Lv, Heng Zheng, and Hong Li. 2026. "Polyethylene Glycol (PEG)-Based Wet-Adhesive Absorbable Bone Wax for Osseous Hemostasis and Repair" International Journal of Molecular Sciences 27, no. 1: 276. https://doi.org/10.3390/ijms27010276
APA StyleCheng, H., Yang, A., Lv, G., Zheng, H., & Li, H. (2026). Polyethylene Glycol (PEG)-Based Wet-Adhesive Absorbable Bone Wax for Osseous Hemostasis and Repair. International Journal of Molecular Sciences, 27(1), 276. https://doi.org/10.3390/ijms27010276





