Cytoprotective and Immunomodulatory Properties of Mesenchymal Stem Cell Secretome and Its Effect on Organotypic Hippocampal Cultures in Mouse Model of Temporal Lobe Epilepsy
Abstract
1. Introduction
2. Results
2.1. Characterization of Pilocarpine-Induced Mouse Model of Temporal Lobe Epilepsy
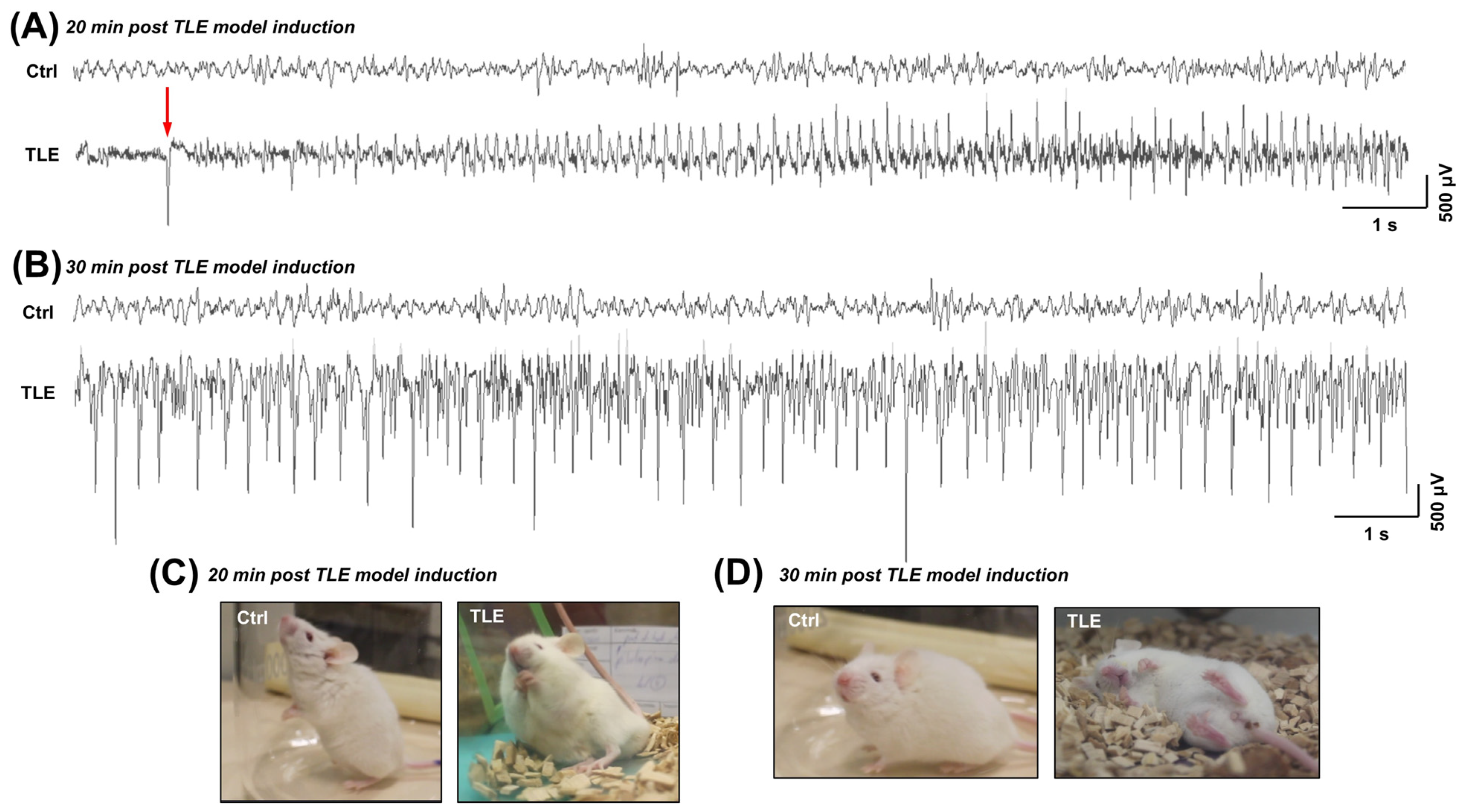
2.1.1. Electroencephalographic (EEG) Alterations in the Acute Phase of the TLE Model (Observed Within the First Hour Following Pilocarpine Administration)
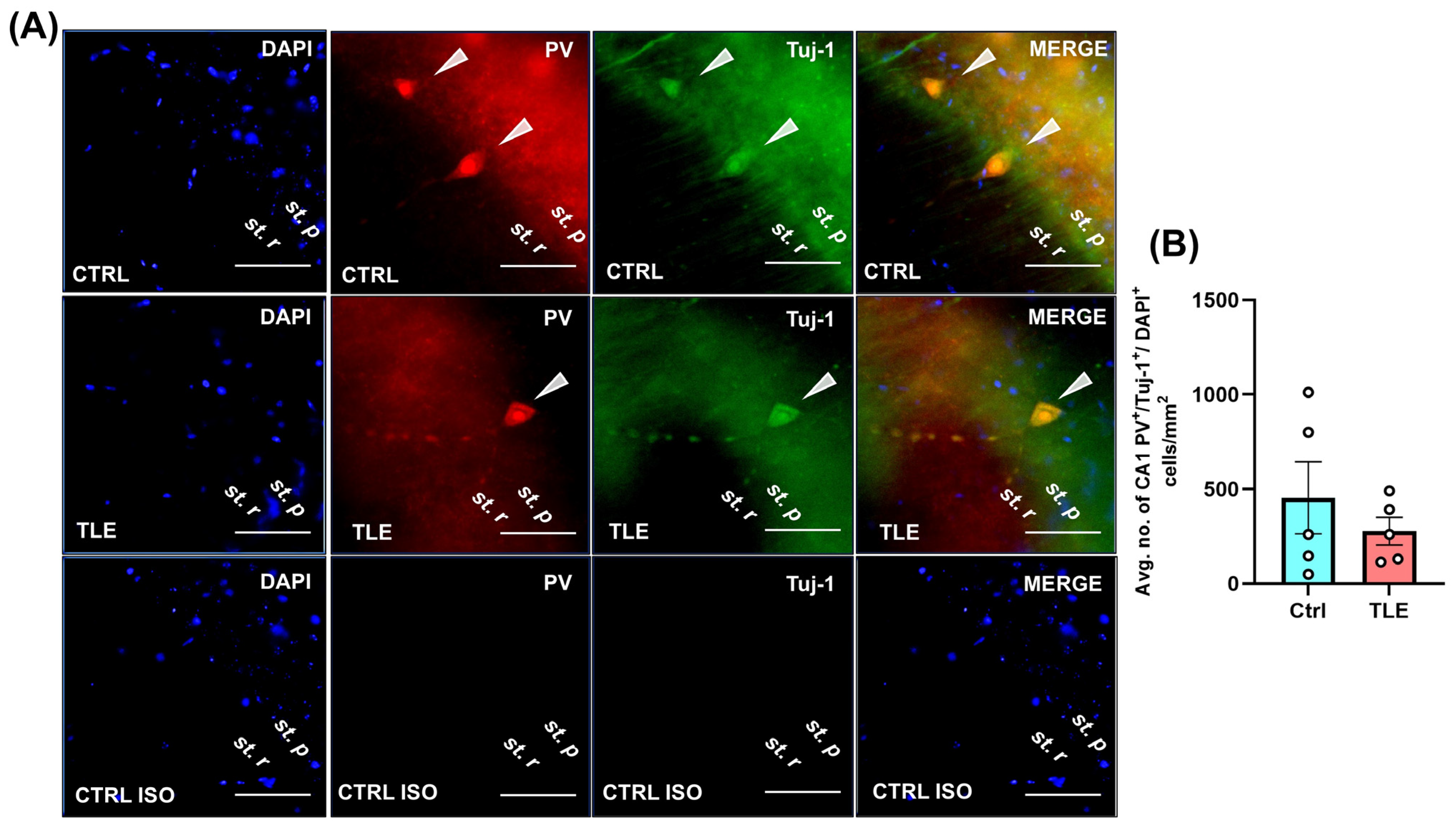
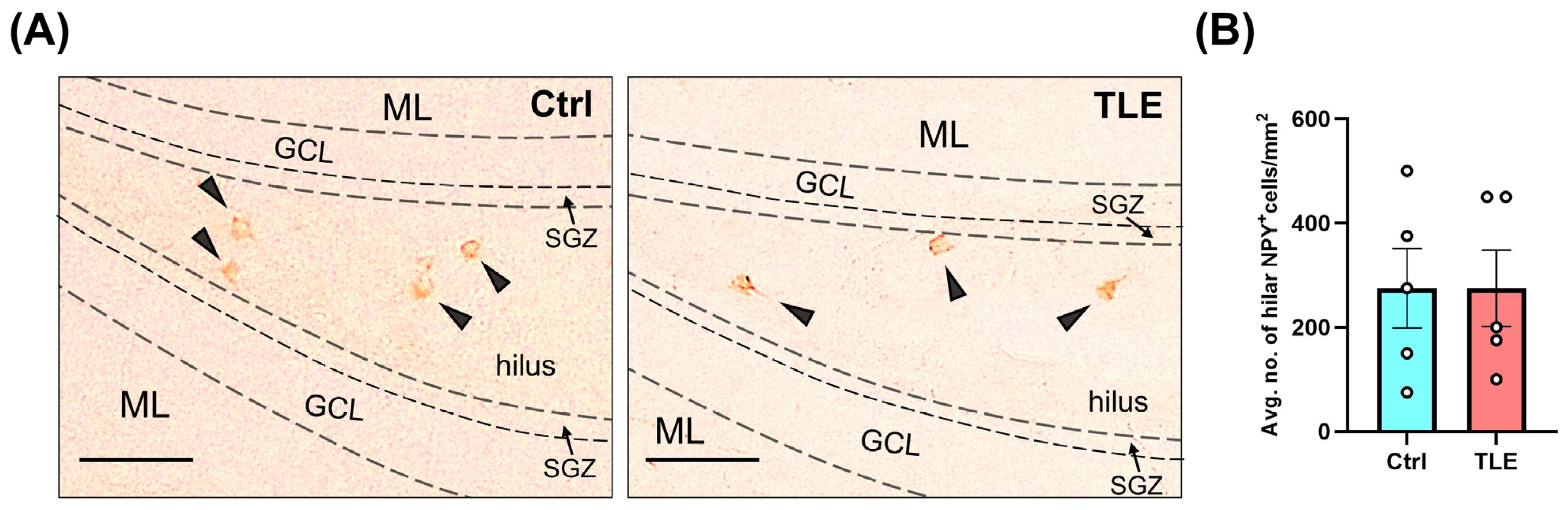
2.1.2. Profile of Hippocampal Parvalbumin (PV)- and Neuropeptide Y (NPY)-Positive Cells in the Latent Phase of the TLE Model (10 Days Post Model Induction)
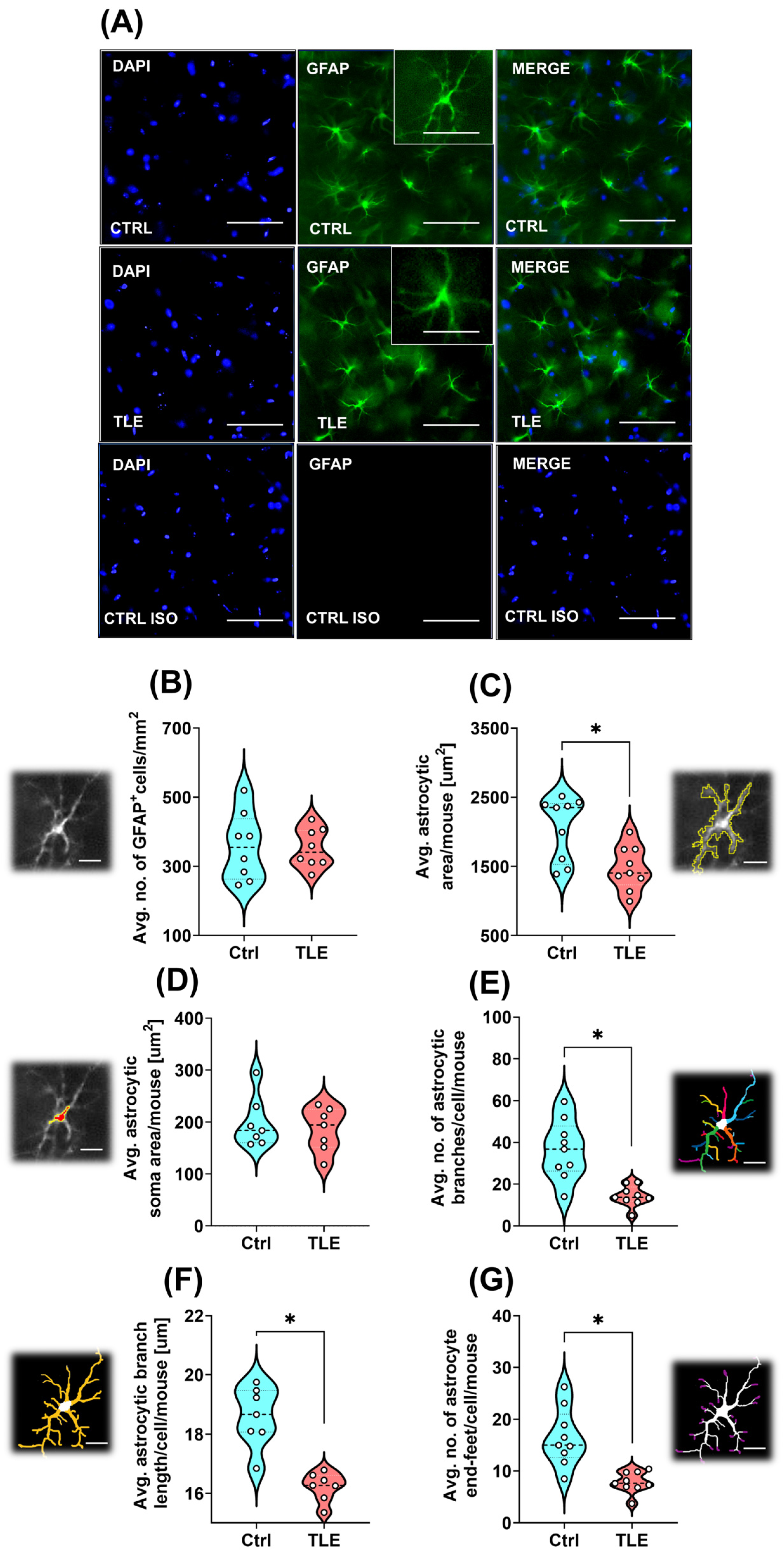
2.1.3. Astrocyte Morphology Changes Under Epileptogenesis in the Latent Phase of the TLE Model (10 Days Post Model Induction)
2.2. Optimization of Organotypic Hippocampal Slice Culture Method
2.3. Characterization of Mesenchymal Stem Cells Secretome
2.4. Assessment of Cytoprotective and Immunomodulatory Properties of the Mesenchymal Stem Cells Secretome in Organotypic Hippocampal Cultures Derived from Temporal-Lobe Epilepsy Mouse Model
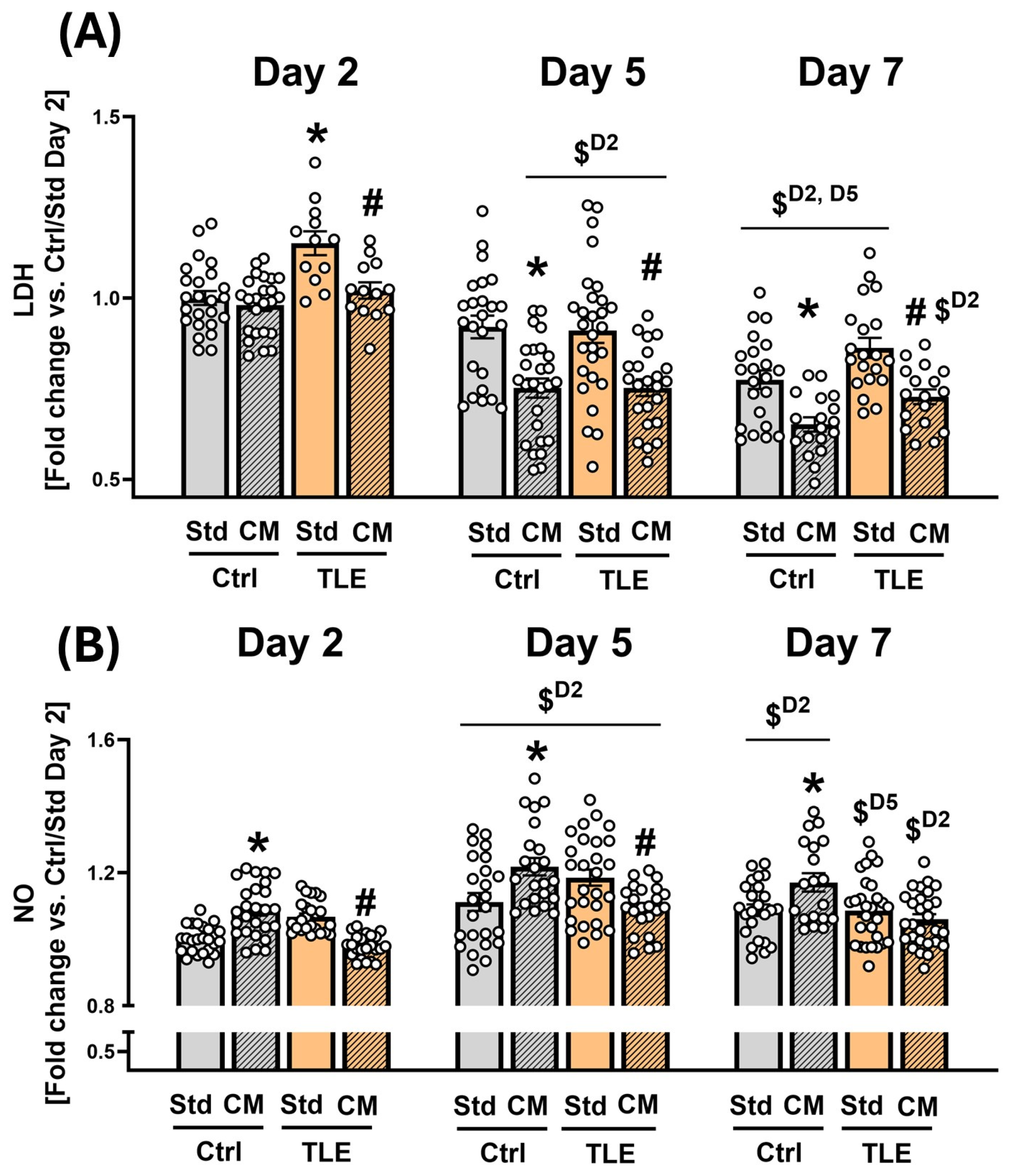
2.4.1. Initial Assessment of Cytoprotective and Immunomodulatory Effects of MSC-Conditioned Culture Medium on Isolated Hippocampal Tissue
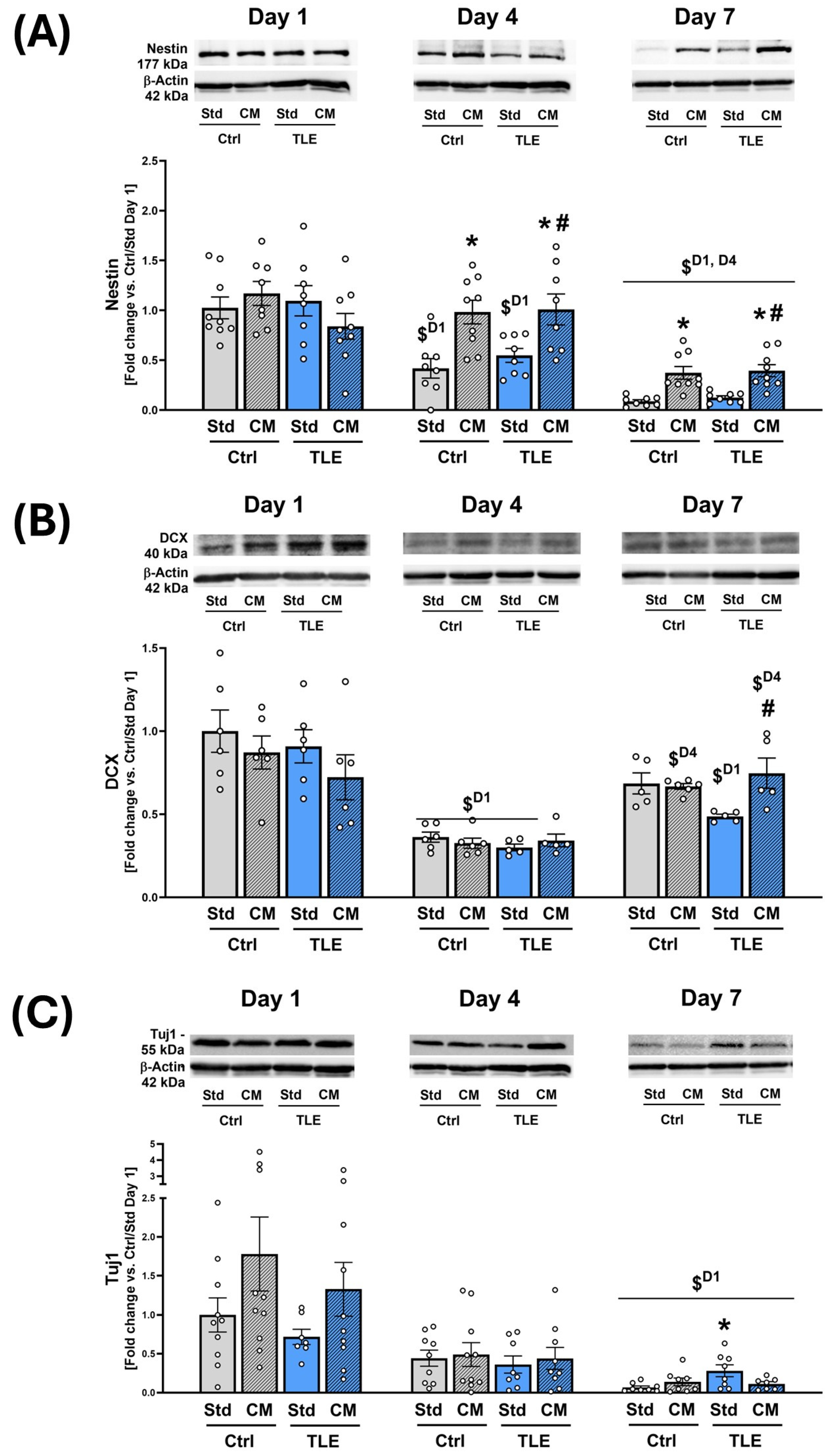
2.4.2. Investigation into the Protective Effects of Mesenchymal Stem Cell Secretome on Neural Progenitor Markers in Isolated Hippocampal Slices
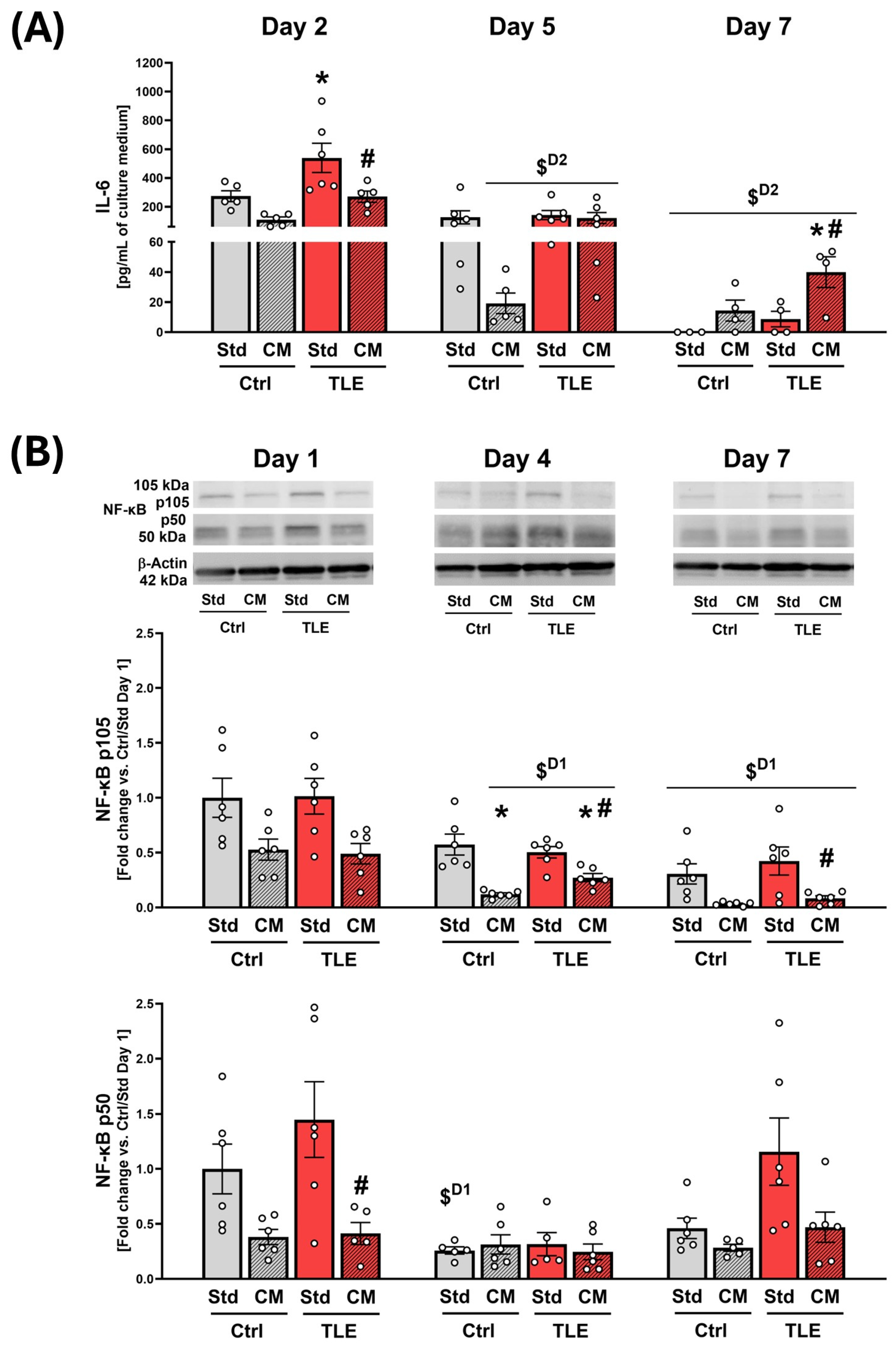
2.4.3. Evaluation of Immunomodulatory Action of Mesenchymal Stem Cell Secretome in Organotypic Hippocampal Cultures
3. Discussion
3.1. Characterization of Pilocarpine-Induced Temporal Lobe Epilepsy Mouse Model and Organotypic Hippocampal Slice Cultures
3.2. The Role of Growth Factors of Mesenchymal Stem Cells Secretome in Epilepsy
3.3. The Potential of the Mesenchymal Stem Cell Secretome to Preserve Neural Progenitor Populations and Its Immunomodulatory Properties in Organotypic Hippocampal Cultures from Temporal Lobe Epilepsy Mouse Model
3.4. Limitations and Future Directions
4. Materials and Methods
4.1. Animals
4.2. Housing Conditions
4.3. Implantations of Transmitters
4.4. Induction of Seizures in Mouse Model of Pilocarpine-Induced TLE
4.5. Radiotelemetry EEG Recording
4.6. Immunofluorescence Using CLARITY
4.7. Immunohistochemistry
4.8. Establishment of OHCs
4.9. Preparation of MSC-CM for OHC Experiments
4.10. Evaluation of LDH Activity and NO Level in the Culture Media of OHCs
4.11. LUMINEX® Multiplex Assays
4.12. Western Blot Analysis
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANGPT-1 | angiopoietin-1 |
| BDNF | brain derived neurotrophic factor |
| bFGF | basic fibroblast growth factor |
| BMP-4 | bone morphogenetic protein 4 |
| DCX | doublecortin |
| GDNF | glial-derived neurotrophic factor |
| HGF | hepatocyte growth factor |
| IL-4 | interleukin-4 |
| IL-6 | interleukin-6 |
| LDH | lactate dehydrogenase |
| MSCs | Mesenchymal Stem Cells |
| MSC-CM | Mesenchymal Stem Cell-conditioned medium |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | nitric oxide |
| NRG1 β1 | neuregulin-1 β1 |
| OHCs | Organotypic Hippocampal Cultures |
| TLE | Temporal Lobe Epilepsy |
| Tuj1 | β-tubulin III |
References
- Berg, A.T.; Scheffer, I.E. New concepts in classification of the epilepsies: Entering the 21st century: New Concepts in Classification. Epilepsia 2011, 52, 1058–1062. [Google Scholar] [CrossRef]
- Falco-Walter, J. Epilepsy—Definition, Classification, Pathophysiology, and Epidemiology. Semin. Neurol. 2020, 40, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, A.; Lukasiuk, K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011, 10, 173–186. [Google Scholar] [CrossRef]
- Schartz, N.D.; Herr, S.A.; Madsen, L.; Butts, S.J.; Torres, C.; Mendez, L.B.; Brewster, A.L. Spatiotemporal profile of Map2 and microglial changes in the hippocampal CA1 region following pilocarpine-induced status epilepticus. Sci. Rep. 2016, 6, 24988. [Google Scholar] [CrossRef] [PubMed]
- Swartz, B.E.; Houser, C.R.; Tomiyasu, U.; Walsh, G.O.; DeSalles, A.; Rich, J.R.; Delgado-Escueta, A. Hippocampal Cell Loss in Posttraumatic Human Epilepsy. Epilepsia 2006, 47, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Campo, P.; Garrido, M.I.; Moran, R.J.; García-Morales, I.; Poch, C.; Toledano, R.; Gil-Nagel, A.; Dolan, R.J.; Friston, K.J. Network reconfiguration and working memory impairment in mesial temporal lobe epilepsy. NeuroImage 2013, 72, 48–54. [Google Scholar] [CrossRef]
- Akyuz, E.; Polat, A.K.; Eroglu, E.; Kullu, I.; Angelopoulou, E.; Paudel, Y.N. Revisiting the role of neurotransmitters in epilepsy: An updated review. Life Sci. 2021, 265, 118826. [Google Scholar] [CrossRef]
- Cano, A.; Fonseca, E.; Ettcheto, M.; Sánchez-López, E.; De Rojas, I.; Alonso-Lana, S.; Morató, X.; Souto, E.B.; Toledo, M.; Boada, M.; et al. Epilepsy in Neurodegenerative Diseases: Related Drugs and Molecular Pathways. Pharmaceuticals 2021, 14, 1057. [Google Scholar] [CrossRef]
- Engel, J. A Proposed Diagnostic Scheme for People with Epileptic Seizures and with Epilepsy: Report of the ILAE Task Force on Classification and Terminology. Epilepsia 2001, 42, 796–803. [Google Scholar] [CrossRef]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef]
- Riazi, K.; Galic, M.A.; Pittman, Q.J. Contributions of peripheral inflammation to seizure susceptibility: Cytokines and brain excitability. Epilepsy Res. 2010, 89, 34–42. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Androsova, G.; Krause, R.; Borghei, M.; Wassenaar, M.; Auce, P.; Avbersek, A.; Becker, F.; Berghuis, B.; Campbell, E.; Coppola, A.; et al. Comparative effectiveness of antiepileptic drugs in patients with mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 2017, 58, 1734–1741. [Google Scholar] [CrossRef]
- Badyra, B.; Sułkowski, M.; Milczarek, O.; Majka, M. Mesenchymal stem cells as a multimodal treatment for nervous system diseases. Stem Cells Transl. Med. 2020, 9, 1174–1189. [Google Scholar] [CrossRef]
- Milczarek, O.; Jarocha, D.; Starowicz–Filip, A.; Kwiatkowski, S.; Badyra, B.; Majka, M. Multiple Autologous Bone Marrow-Derived CD271+ Mesenchymal Stem Cell Transplantation Overcomes Drug-Resistant Epilepsy in Children. Stem Cells Transl. Med. 2018, 7, 20–33. [Google Scholar] [CrossRef]
- Milczarek, O.; Jarocha, D.; Starowicz–Filip, A.; Kasprzycki, M.; Kijowski, J.; Mordel, A.; Kwiatkowski, S.; Majka, M. Bone Marrow Nucleated Cells and Bone Marrow-Derived CD271+ Mesenchymal Stem Cell in Treatment of Encephalopathy and Drug-Resistant Epilepsy. Stem Cell Rev. Rep. 2024, 20, 1015–1025. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, K.-S.; Bae, S.; Son, H.K.; Myung, P.-K.; Hong, H.J.; Kim, H. Cytokine Secretion Profiling of Human Mesenchymal Stem Cells by Antibody Array. Int. J. Stem Cells 2009, 2, 59–68. [Google Scholar] [CrossRef]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef]
- Strzelec, M.; Detka, J.; Mieszczak, P.; Sobocińska, M.K.; Majka, M. Immunomodulation—A general review of the current state-of-the-art and new therapeutic strategies for targeting the immune system. Front. Immunol. 2023, 14, 1127704. [Google Scholar] [CrossRef]
- Long, Q.; Upadhya, D.; Hattiangady, B.; Kim, D.-K.; An, S.Y.; Shuai, B.; Prockop, D.J.; Shetty, A.K. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. USA 2017, 114, E3536–E3545. [Google Scholar] [CrossRef]
- Fukumura, S.; Sasaki, M.; Kataoka-Sasaki, Y.; Oka, S.; Nakazaki, M.; Nagahama, H.; Morita, T.; Sakai, T.; Tsutsumi, H.; Kocsis, J.D.; et al. Intravenous infusion of mesenchymal stem cells reduces epileptogenesis in a rat model of status epilepticus. Epilepsy Res. 2018, 141, 56–63. [Google Scholar] [CrossRef]
- Huang, P.-Y.; Shih, Y.-H.; Tseng, Y.-j.; Ko, T.-L.; Fu, Y.-S.; Lin, Y.-Y. Xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly as a potential therapy for rat pilocarpine-induced epilepsy. Brain Behav. Immun. 2016, 54, 45–58. [Google Scholar] [CrossRef]
- Turski, W.A.; Cavalheiro, E.A.; Bortolotto, Z.A.; Mello, L.M.; Schwarz, M.; Turski, L. Seizures produced by pilocarpine in mice: A behavioral, electroencephalographic and morphological analysis. Brain Res. 1984, 321, 237–253. [Google Scholar] [CrossRef]
- Cavalheiro, E.A.; Leite, J.P.; Bortolotto, Z.A.; Turski, W.A.; Ikonomidou, C.; Turski, L. Long-Term Effects of Pilocarpine in Rats: Structural Damage of the Brain Triggers Kindling and Spontaneous I Recurrent Seizures. Epilepsia 1991, 32, 778–782. [Google Scholar] [CrossRef]
- Leite, J.P.; Bortolotto, Z.A.; Cavalheiro, E.A. Spontaneous recurrent seizures in rats: An experimental model of partial epilepsy. Neurosci. Biobehav. Rev. 1990, 14, 511–517. [Google Scholar] [CrossRef]
- Henderson, K.W.; Gupta, J.; Tagliatela, S.; Litvina, E.; Zheng, X.; Van Zandt, M.A.; Woods, N.; Grund, E.; Lin, D.; Royston, S.; et al. Long-Term Seizure Suppression and Optogenetic Analyses of Synaptic Connectivity in Epileptic Mice with Hippocampal Grafts of GABAergic Interneurons. J. Neurosci. 2014, 34, 13492–13504, Correction in J. Neurosci. 2016, 36, 5427–5428. [Google Scholar] [CrossRef]
- Scharfman, H.E.; Goodman, J.H.; Sollas, A.L. Granule-Like Neurons at the Hilar/CA3 Border after Status Epilepticus and Their Synchrony with Area CA3 Pyramidal Cells: Functional Implications of Seizure-Induced Neurogenesis. J. Neurosci. 2000, 20, 6144–6158. [Google Scholar] [CrossRef]
- Parent, J.M.; Yu, T.W.; Leibowitz, R.T.; Geschwind, D.H.; Sloviter, R.S.; Lowenstein, D.H. Dentate Granule Cell Neurogenesis Is Increased by Seizures and Contributes to Aberrant Network Reorganization in the Adult Rat Hippocampus. J. Neurosci. 1997, 17, 3727–3738. [Google Scholar] [CrossRef]
- Glien, M.; Brandt, C.; Potschka, H.; Löscher, W. Effects of the Novel Antiepileptic Drug Levetiracetam on Spontaneous Recurrent Seizures in the Rat Pilocarpine Model of Temporal Lobe Epilepsy. Epilepsia 2002, 43, 350–357. [Google Scholar] [CrossRef]
- Chakir, A.; Fabene, P.F.; Ouazzani, R.; Bentivoglio, M. Drug resistance and hippocampal damage after delayed treatment of pilocarpine-induced epilepsy in the rat. Brain Res. Bull. 2006, 71, 127–138. [Google Scholar] [CrossRef]
- Lybrand, Z.R.; Goswami, S.; Zhu, J.; Jarzabek, V.; Merlock, N.; Aktar, M.; Smith, C.; Zhang, L.; Varma, P.; Cho, K.-O.; et al. A critical period of neuronal activity results in aberrant neurogenesis rewiring hippocampal circuitry in a mouse model of epilepsy. Nat. Commun. 2021, 12, 1423. [Google Scholar] [CrossRef]
- Walter, C.; Murphy, B.L.; Pun, R.Y.K.; Spieles-Engemann, A.L.; Danzer, S.C. Pilocarpine-Induced Seizures Cause Selective Time-Dependent Changes to Adult-Generated Hippocampal Dentate Granule Cells. J. Neurosci. 2007, 27, 7541–7552. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Y.; Hu, H.; Wang, J.; Liu, Z.; Gao, F. FDG-PET and NeuN-GFAP Immunohistochemistry of Hippocampus at Different Phases of the Pilocarpine Model of Temporal Lobe Epilepsy. Int. J. Med. Sci. 2015, 12, 288–294. [Google Scholar] [CrossRef]
- Humpel, C. Organotypic brain slice cultures: A review. Neuroscience 2015, 305, 86–98. [Google Scholar] [CrossRef]
- Magalhães, D.M.; Pereira, N.; Rombo, D.M.; Beltrão-Cavacas, C.; Sebastião, A.M.; Valente, C.A. Ex vivo model of epilepsy in organotypic slices—A new tool for drug screening. J. Neuroinflamm. 2018, 15, 203. [Google Scholar] [CrossRef]
- De Simoni, A.; Griesinger, C.B.; Edwards, F.A. Development of Rat CA1 Neurones in Acute Versus Organotypic Slices: Role of Experience in Synaptic Morphology and Activity. J. Physiol. 2003, 550, 135–147. [Google Scholar] [CrossRef]
- Drexel, M.; Sperk, G. Seizure-induced overexpression of NPY induces epileptic tolerance in a mouse model of spontaneous recurrent seizures. Front. Mol. Neurosci. 2022, 15, 974784. [Google Scholar] [CrossRef]
- Schwaller, B.; Tetko, I.V.; Tandon, P.; Silveira, D.C.; Vreugdenhil, M.; Henzi, T.; Potier, M.-C.; Celio, M.R.; Villa, A.E.P. Parvalbumin deficiency affects network properties resulting in increased susceptibility to epileptic seizures. Mol. Cell. Neurosci. 2004, 25, 650–663. [Google Scholar] [CrossRef]
- Upadhya, D.; Attaluri, S.; Liu, Y.; Hattiangady, B.; Castro, O.W.; Shuai, B.; Dong, Y.; Zhang, S.-C.; Shetty, A.K. Grafted hPSC-derived GABA-ergic interneurons regulate seizures and specific cognitive function in temporal lobe epilepsy. npj Regen. Med. 2022, 7, 38. [Google Scholar] [CrossRef]
- Mátyás, A.; Borbély, E.; Mihály, A. Hippocampal Sclerosis in Pilocarpine Epilepsy: Survival of Peptide-Containing Neurons and Learning and Memory Disturbances in the Adult NMRI Strain Mouse. Int. J. Mol. Sci. 2021, 23, 204. [Google Scholar] [CrossRef]
- Plata, A.; Lebedeva, A.; Denisov, P.; Nosova, O.; Postnikova, T.Y.; Pimashkin, A.; Brazhe, A.; Zaitsev, A.V.; Rusakov, D.A.; Semyanov, A. Astrocytic Atrophy Following Status Epilepticus Parallels Reduced Ca2+ Activity and Impaired Synaptic Plasticity in the Rat Hippocampus. Front. Mol. Neurosci. 2018, 11, 215. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Tian, G.-F.; Han, X.; Peng, W.; Takano, T.; Ransom, B.; Nedergaard, M. Loss of Astrocytic Domain Organization in the Epileptic Brain. J. Neurosci. 2008, 28, 3264–3276. [Google Scholar] [CrossRef]
- Bedner, P.; Dupper, A.; Hüttmann, K.; Müller, J.; Herde, M.K.; Dublin, P.; Deshpande, T.; Schramm, J.; Häussler, U.; Haas, C.A.; et al. Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain 2015, 138, 1208–1222. [Google Scholar] [CrossRef]
- Eid, T.; Thomas, M.; Spencer, D.; Rundén-Pran, E.; Lai, J.; Malthankar, G.; Kim, J.; Danbolt, N.; Ottersen, O.; De Lanerolle, N. Loss of glutamine synthetase in the human epileptogenic hippocampus: Possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet 2004, 363, 28–37. [Google Scholar] [CrossRef]
- Marcus, H.J.; Carpenter, K.L.H.; Price, S.J.; Hutchinson, P.J. In vivo assessment of high-grade glioma biochemistry using microdialysis: A study of energy-related molecules, growth factors and cytokines. J. Neurooncol. 2010, 97, 11–23. [Google Scholar]
- Lee, M.K.; Tuttle, J.B.; Rebhun, L.I.; Cleveland, D.W.; Frankfurter, A. The expression and posttranslational modification of a neuron-specific β-tubulin isotype during chick embryogenesis. Cell Motil. Cytoskelet. 1990, 17, 118–132. [Google Scholar] [CrossRef]
- Koh, S.-H.; Kim, K.S.; Choi, M.R.; Jung, K.H.; Park, K.S.; Chai, Y.G.; Roh, W.; Hwang, S.J.; Ko, H.-J.; Huh, Y.-M.; et al. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 2008, 1229, 233–248. [Google Scholar] [CrossRef]
- Ribeiro, C.A.; Fraga, J.S.; Grãos, M.; Neves, N.M.; Reis, R.L.; Gimble, J.M.; Sousa, N.; Salgado, A.J. The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res. Ther. 2012, 3, 18. [Google Scholar] [CrossRef]
- Cai, M.; Lin, W. The Function of NF-Kappa B During Epilepsy, a Potential Therapeutic Target. Front. Neurosci. 2022, 16, 851394. [Google Scholar] [CrossRef]
- Aronica, E.; Boer, K.; Van Vliet, E.A.; Redeker, S.; Baayen, J.C.; Spliet, W.G.M.; Van Rijen, P.C.; Troost, D.; Lopes Da Silva, F.H.; Wadman, W.J.; et al. Complement activation in experimental and human temporal lobe epilepsy. Neurobiol. Dis. 2007, 26, 497–511. [Google Scholar] [CrossRef]
- Takahashi, D.K.; Vargas, J.R.; Wilcox, K.S. Increased coupling and altered glutamate transport currents in astrocytes following kainic-acid-induced status epilepticus. Neurobiol. Dis. 2010, 40, 573–585. [Google Scholar]
- Vezzani, A.; Conti, M.; De Luigi, A.; Ravizza, T.; Moneta, D.; Marchesi, F.; De Simoni, M.G. Interleukin-1β Immunoreactivity and Microglia Are Enhanced in the Rat Hippocampus by Focal Kainate Application: Functional Evidence for Enhancement of Electrographic Seizures. J. Neurosci. 1999, 19, 5054–5065. [Google Scholar] [CrossRef]
- Wyeth, M.S.; Zhang, N.; Mody, I.; Houser, C.R. Selective Reduction of Cholecystokinin-Positive Basket Cell Innervation in a Model of Temporal Lobe Epilepsy. J. Neurosci. 2010, 30, 8993–9006. [Google Scholar] [CrossRef]
- Dinocourt, C.; Petanjek, Z.; Freund, T.F.; Ben-Ari, Y.; Esclapez, M. Loss of interneurons innervating pyramidal cell dendrites and axon initial segments in the CA1 region of the hippocampus following pilocarpine-induced seizures. J. Comp. Neurol. 2003, 459, 407–425. [Google Scholar] [CrossRef]
- Kobayashi, M.; Buckmaster, P.S. Reduced Inhibition of Dentate Granule Cells in a Model of Temporal Lobe Epilepsy. J. Neurosci. 2003, 23, 2440–2452. [Google Scholar] [CrossRef]
- Lurton, D.; Cavalheiro, E.A. Neuropeptide-Y immunoreactivity in the pilocarpine model of temporal lobe epilepsy. Exp. Brain Res. 1997, 116, 186–190. [Google Scholar] [CrossRef]
- Sperk, G.; Marksteiner, J.; Gruber, B.; Bellmann, R.; Mahata, M.; Ortler, M. Functional changes in neuropeptide Y- and somatostatin-containing neurons induced by limbic seizures in the rat. Neuroscience 1992, 50, 831–846. [Google Scholar] [CrossRef]
- Sierra, A.; Martín-Suárez, S.; Valcárcel-Martín, R.; Pascual-Brazo, J.; Aelvoet, S.-A.; Abiega, O.; Deudero, J.J.; Brewster, A.L.; Bernales, I.; Anderson, A.E.; et al. Neuronal Hyperactivity Accelerates Depletion of Neural Stem Cells and Impairs Hippocampal Neurogenesis. Cell Stem Cell 2015, 16, 488–503. [Google Scholar] [CrossRef]
- Baldassarro, V.A.; Perut, F.; Cescatti, M.; Pinto, V.; Fazio, N.; Alastra, G.; Parziale, V.; Bassotti, A.; Fernandez, M.; Giardino, L.; et al. Intra-individual variability in the neuroprotective and promyelinating properties of conditioned culture medium obtained from human adipose mesenchymal stromal cells. Stem Cell Res. Ther. 2023, 14, 128. [Google Scholar] [CrossRef]
- Fierro, F.A.; Kalomoiris, S.; Sondergaard, C.S.; Nolta, J.A. Effects on Proliferation and Differentiation of Multipotent Bone Marrow Stromal Cells Engineered to Express Growth Factors for Combined Cell and Gene Therapy. Stem Cells 2011, 29, 1727–1737. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, W.; Deng, Q.; Zhang, X.; Zhang, J. Protective Effects of BDNF Overexpression Bone Marrow Stromal Cell Transplantation in Rat Models of Traumatic Brain Injury. J. Mol. Neurosci. 2013, 49, 409–416. [Google Scholar] [CrossRef]
- Nie, W.-B.; Zhang, D.; Wang, L.-S. Growth Factor Gene-Modified Mesenchymal Stem Cells in Tissue Regeneration. Drug Des. Dev. Ther. 2020, 14, 1241–1256. [Google Scholar] [CrossRef]
- Pillai, A.; Kale, A.; Joshi, S.; Naphade, N.; Raju, M.S.V.K.; Nasrallah, H.; Mahadik, S.P. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: Correlation with plasma BDNF and psychopathology. Int. J. Neuropsychopharm. 2010, 13, 535–539. [Google Scholar] [CrossRef]
- Kern, M.A.; Bamborschke, S.; Nekic, M.; Schubert, D.; Rydin, C.; Lindholm, D.; Schirmacher, P. Concentrations of Hepatocyte Growth Factor in Cerebrospinal Fluid Under Normal and Different Pathological Conditions. Cytokine 2001, 14, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.M.; Jerónimo-Santos, A.; Sebastião, A.M.; Diógenes, M.J. Adenosine A2A Receptors as novel upstream regulators of BDNF-mediated attenuation of hippocampal Long-Term Depression (LTD). Neuropharmacology 2014, 79, 389–398. [Google Scholar]
- Akimoto, M.; Baba, A.; Ikeda-Matsuo, Y.; Yamada, M.K.; Itamura, R.; Nishiyama, N.; Ikegaya, Y.; Matsuki, N. Hepatocyte growth factor as an enhancer of nmda currents and synaptic plasticity in the hippocampus. Neuroscience 2004, 128, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Lu, Y.; Yang, F.; Shen, W.; Tang, T.T.-T.; Feng, L.; Duan, S.; Lu, B. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat. Neurosci. 2010, 13, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, S.; Sypecka, J.; Jablonska, A.; Strojek, L.; Wielgos, M.; Domanska-Janik, K.; Sarnowska, A. Neuroprotective Potential and Paracrine Activity of Stromal Vs. Culture-Expanded hMSC Derived from Wharton Jelly under Co-Cultured with Hippocampal Organotypic Slices. Mol. Neurobiol. 2018, 55, 6021–6036. [Google Scholar] [CrossRef]
- Bae, M.H.; Bissonette, G.B.; Mars, W.M.; Michalopoulos, G.K.; Achim, C.L.; Depireux, D.A.; Powell, E.M. Hepatocyte growth factor (HGF) modulates GABAergic inhibition and seizure susceptibility. Exp. Neurol. 2010, 221, 129–135. [Google Scholar] [CrossRef]
- Haghani, S.; Jamali-Raeufy, N.; Zeinivand, M.; Mehrabi, S.; Aryan, L.; Fahanik-Babaei, J. Hepatocyte Growth Factor Attenuates the Severity of Status Epilepticus in Kainic Acid-induced Model of Temporal Lobe Epilepsy by Targeting Apoptosis and Astrogliosis. Basic Clin. Neurosci. 2021, 12, 805–816. [Google Scholar] [CrossRef]
- Kanter-Schlifke, I.; Fjord-Larsen, L.; Kusk, P.; Ängehagen, M.; Wahlberg, L.; Kokaia, M. GDNF released from encapsulated cells suppresses seizure activity in the epileptic hippocampus. Exp. Neurol. 2009, 216, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Nanobashvili, A.; Melin, E.; Emerich, D.; Tornøe, J.; Simonato, M.; Wahlberg, L.; Kokaia, M. Unilateral ex vivo gene therapy by GDNF in epileptic rats. Gene Ther. 2019, 26, 65–74. [Google Scholar] [CrossRef]
- Paolone, G.; Falcicchia, C.; Lovisari, F.; Kokaia, M.; Bell, W.J.; Fradet, T.; Barbieri, M.; Wahlberg, L.U.; Emerich, D.F.; Simonato, M. Long-Term, Targeted Delivery of GDNF from Encapsulated Cells Is Neuroprotective and Reduces Seizures in the Pilocarpine Model of Epilepsy. J. Neurosci. 2019, 39, 2144–2156. [Google Scholar] [CrossRef]
- Mikroulis, A.; Waloschková, E.; Bengzon, J.; Woldbye, D.; Pinborg, L.H.; Jespersen, B.; Avila, A.S.; Laszlo, Z.I.; Henstridge, C.; Ledri, M.; et al. GDNF Increases Inhibitory Synaptic Drive on Principal Neurons in the Hippocampus via Activation of the Ret Pathway. Int. J. Mol. Sci. 2022, 23, 13190. [Google Scholar] [CrossRef]
- Shin, H.Y.; Lee, Y.J.; Kim, H.J.; Park, C.; Kim, J.H.; Wang, K.C.; Kim, D.G.; Koh, G.Y.; Paek, S.H. Protective role of COMP-Ang1 in ischemic rat brain. J. Neurosci. Res. 2010, 88, 1052–1063. [Google Scholar] [CrossRef]
- Sabirzhanov, B.; Faden, A.I.; Aubrecht, T.; Henry, R.; Glaser, E.; Stoica, B.A. MicroRNA-711–Induced Downregulation of Angiopoietin-1 Mediates Neuronal Cell Death. J. Neurotrauma 2018, 35, 2462–2481. [Google Scholar] [CrossRef] [PubMed]
- Kosacka, J.; Nowicki, M.; Kacza, J.; Borlak, J.; Engele, J.; Spanel-Borowski, K. Adipocyte-derived angiopoietin-1 supports neurite outgrowth and synaptogenesis of sensory neurons. J. Neurosci. Res. 2006, 83, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Berry, M.; Logan, A.; Ahmed, Z. Activation of the BMP4/Smad1 Pathway Promotes Retinal Ganglion Cell Survival and Axon Regeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1748–1759. [Google Scholar] [CrossRef]
- Farrukh, F.; Davies, E.; Berry, M.; Logan, A.; Ahmed, Z. BMP4/Smad1 Signalling Promotes Spinal Dorsal Column Axon Regeneration and Functional Recovery After Injury. Mol. Neurobiol. 2019, 56, 6807–6819. [Google Scholar] [CrossRef]
- Xu, H.; Huang, W.; Wang, Y.; Sun, W.; Tang, J.; Li, D.; Xu, P.; Guo, L.; Yin, Z.Q.; Fan, X. The function of BMP4 during neurogenesis in the adult hippocampus in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 157–164. [Google Scholar] [CrossRef]
- Kataria, H.; Alizadeh, A.; Karimi-Abdolrezaee, S. Neuregulin-1/ErbB network: An emerging modulator of nervous system injury and repair. Prog. Neurobiol. 2019, 180, 101643. [Google Scholar] [CrossRef]
- Shi, L.; Bergson, C.M. Neuregulin 1: An intriguing therapeutic target for neurodevelopmental disorders. Transl. Psychiatry 2020, 10, 190. [Google Scholar] [CrossRef]
- Tan, G.-H.; Liu, Y.-Y.; Hu, X.-L.; Yin, D.-M.; Mei, L.; Xiong, Z.-Q. Neuregulin 1 represses limbic epileptogenesis through ErbB4 in parvalbumin-expressing interneurons. Nat. Neurosci. 2012, 15, 258–266. [Google Scholar] [CrossRef]
- Gao, R.; Ji, M.-H.; Gao, D.-P.; Yang, R.-H.; Zhang, S.-G.; Yang, J.-J.; Shen, J.-C. Neuroinflammation-Induced Downregulation of Hippocampacal Neuregulin 1-ErbB4 Signaling in the Parvalbumin Interneurons Might Contribute to Cognitive Impairment in a Mouse Model of Sepsis-Associated Encephalopathy. Inflammation 2017, 40, 387–400. [Google Scholar] [CrossRef]
- AlRuwaili, R.; Al-kuraishy, H.M.; Al-Gareeb, A.I.; Ali, N.H.; Alexiou, A.; Papadakis, M.; Saad, H.M.; Batiha, G.E.-S. The Possible Role of Brain-derived Neurotrophic Factor in Epilepsy. Neurochem. Res. 2024, 49, 533–547, Correction in Neurochem. Res. 2024, 49, 1417–1418. [Google Scholar] [CrossRef]
- Biagini, G.; Avoli, M.; Marcinkiewicz, J.; Marcinkiewicz, M. Brain-derived neurotrophic factor superinduction parallels anti-epileptic−neuroprotective treatment in the pilocarpine epilepsy model. J. Neurochem. 2001, 76, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, B.; Zucchini, S.; Su, T.; Bovolenta, R.; Berto, E.; Marconi, P.; Marzola, A.; Mora, G.N.; Fabene, P.F.; Simonato, M. Localized overexpression of FGF-2 and BDNF in hippocampus reduces mossy fiber sprouting and spontaneous seizures up to 4 weeks after pilocarpine-induced status epilepticus: Attenuation of Mossy Fiber Sprouting. Epilepsia 2011, 52, 572–578. [Google Scholar] [CrossRef]
- Kuramoto, S.; Yasuhara, T.; Agari, T.; Kondo, A.; Jing, M.; Kikuchi, Y.; Shinko, A.; Wakamori, T.; Kameda, M.; Wang, F.; et al. BDNF-secreting capsule exerts neuroprotective effects on epilepsy model of rats. Brain Res. 2011, 1368, 281–289. [Google Scholar] [CrossRef]
- Falcicchia, C.; Paolone, G.; Emerich, D.F.; Lovisari, F.; Bell, W.J.; Fradet, T.; Wahlberg, L.U.; Simonato, M. Seizure-Suppressant and Neuroprotective Effects of Encapsulated BDNF-Producing Cells in a Rat Model of Temporal Lobe Epilepsy. Mol. Ther.-Methods Clin. Dev. 2018, 9, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Kokaia, M.; Ernfors, P.; Kokaia, Z.; Elmer, E.; Jaenisch, R.; Lindvall, O. Suppressed epileptogenesis in BDNF mutant mice. Exp. Neurol. 1995, 133, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Croll, S.D.; Suri, C.; Compton, D.L.; Simmons, M.V.; Yancopoulos, G.D.; Lindsay, R.M.; Wiegand, S.J.; Rudge, J.S.; Scharfman, H.E. Brain-derived neurotrophic factor transgenic mice exhibit passive avoidance deficits, increased seizure severity and in vitro hyperexcitability in the hippocampus and entorhinal cortex. Neuroscience 1999, 93, 1491–1506. [Google Scholar] [CrossRef]
- Mathern, G.W.; Babb, T.L.; Micevych, P.E.; Blanco, C.E.; Pretorius, J.K. Granule cell mRNA levels for BDNF, NGF, and NT-3 correlate with neuron losses or supragranular mossy fiber sprouting in the chronically damaged and epileptic human hippocampus. Mol. Chem. Neuropathol. 1997, 30, 53–76. [Google Scholar] [CrossRef]
- Murray, K.D.; Isackson, P.J.; Eskin, T.A.; King, M.A.; Montesinos, S.P.; Abraham, L.A.; Roper, S.N. Altered mRNA expression for brain-derived neurotrophic factor and type II calcium/Calmodulin-dependent protein kinase in the hippocampus of patients with intractable temporal lobe epilepsy. J. Comp. Neurol. 2000, 418, 411–422. [Google Scholar] [CrossRef]
- Takahashi, M.; Hayashi, S.; Kakita, A.; Wakabayashi, K.; Fukuda, M.; Kameyama, S.; Tanaka, R.; Takahashi, H.; Nawa, H. Patients with temporal lobe epilepsy show an increase in brain-derived neurotrophic factor protein and its correlation with neuropeptide Y. Brain Res. 1999, 818, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jiang, J. COX-2/PGE2 axis regulates hippocampal BDNF/TrkB signaling via EP2 receptor after prolonged seizures. Epilepsia Open 2020, 5, 418–431. [Google Scholar] [CrossRef]
- Zucchini, S.; Barbieri, M.; Simonato, M. Alterations in Seizure Susceptibility and in Seizure-induced Plasticity after Pharmacologic and Genetic Manipulation of the Fibroblast Growth Factor-2 System. Epilepsia 2005, 46, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Huang, B.; Wang, Y.; Zhang, Y.; Ma, Q.; Ren, Y. Bone marrow mesenchymal stem cells-derived exosomes improve injury of hippocampal neurons in rats with depression by upregulating microRNA-26a expression. Int. Immunopharmacol. 2020, 82, 106285. [Google Scholar] [CrossRef] [PubMed]
- Tilotta, V.; Vadalà, G.; Ambrosio, L.; Cicione, C.; Di Giacomo, G.; Russo, F.; Papalia, R.; Denaro, V. Mesenchymal stem cell-derived secretome enhances nucleus pulposus cell metabolism and modulates extracellular matrix gene expression in vitro. Front. Bioeng. Biotechnol. 2023, 11, 1152207. [Google Scholar] [CrossRef]
- Haspolat, S.; Mihçi, E.; Coşkun, M.; Gümüslü, S.; Özbenm, T.; Yegin, O. Interleukin-1β, Tumor Necrosis Factor-α, and Nitrite Levels in Febrile Seizures. J. Child Neurol. 2002, 17, 749–751. [Google Scholar] [CrossRef]
- Kovács, R.; Rabanus, A.; Otáhal, J.; Patzak, A.; Kardos, J.; Albus, K.; Heinemann, U.; Kann, O. Endogenous nitric oxide is a key promoting factor for initiation of seizure-like events in hippocampal and entorhinal cortex slices. J. Neurosci. 2009, 29, 8565–8577. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef] [PubMed]
- Salari, V.; Mengoni, F.; Del Gallo, F.; Bertini, G.; Fabene, P.F. The Anti-Inflammatory Properties of Mesenchymal Stem Cells in Epilepsy: Possible Treatments and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 9683. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liao, R.; Li, X.; Zhang, C.; Huo, S.; Qin, L.; Xiong, Y.; He, T.; Xiao, G.; Zhang, T. Mesenchymal stem cells in treating human diseases: Molecular mechanisms and clinical studies. Signal Transduct. Target. Ther. 2025, 10, 262. [Google Scholar] [CrossRef]
- Lendahl, U.; Zimmerman, L.B.; McKay, R.D.G. CNS stem cells express a new class of intermediate filament protein. Cell 1990, 60, 585–595. [Google Scholar] [CrossRef]
- Mignone, J.L.; Kukekov, V.; Chiang, A.; Steindler, D.; Enikolopov, G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J. Comp. Neurol. 2004, 469, 311–324. [Google Scholar] [CrossRef]
- Hattiangady, B.; Rao, M.; Shetty, A. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol. Dis. 2004, 17, 473–490. [Google Scholar] [CrossRef]
- D’Alessio, L.; Konopka, H.; López, E.M.; Seoane, E.; Consalvo, D.; Oddo, S.; Kochen, S.; López-Costa, J.J. Doublecortin (DCX) immunoreactivity in hippocampus of chronic refractory temporal lobe epilepsy patients with hippocampal sclerosis. Seizure 2010, 19, 567–572. [Google Scholar] [CrossRef]
- Liu, J.Y.W.; Matarin, M.; Reeves, C.; McEvoy, A.W.; Miserocchi, A.; Thompson, P.; Sisodiya, S.M.; Thom, M. Doublecortin-expressing cell types in temporal lobe epilepsy. Acta Neuropathol. Commun. 2018, 6, 60. [Google Scholar] [CrossRef]
- Houser, C.R. Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Res. 1990, 535, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Kuruba, R.; Hattiangady, B.; Shetty, A.K. Hippocampal neurogenesis and neural stem cells in temporal lobe epilepsy. Epilepsy Behav. 2009, 14, 65–73. [Google Scholar] [CrossRef]
- Ciryam, P.; Gerzanich, V.; Simard, J.M. Interleukin-6 in Traumatic Brain Injury: A Janus-Faced Player in Damage and Repair. J. Neurotrauma 2023, 40, 2249–2269. [Google Scholar] [CrossRef]
- Liimatainen, S.; Fallah, M.; Kharazmi, E.; Peltola, M.; Peltola, J. Interleukin-6 levels are increased in temporal lobe epilepsy but not in extra-temporal lobe epilepsy. J. Neurol. 2009, 256, 796–802. [Google Scholar] [CrossRef]
- Li, W.; Wu, J.; Zeng, Y.; Zheng, W. Neuroinflammation in epileptogenesis: From pathophysiology to therapeutic strategies. Front. Immunol. 2023, 14, 1269241. [Google Scholar] [CrossRef]
- Kummer, K.K.; Zeidler, M.; Kalpachidou, T.; Kress, M. Role of IL-6 in the regulation of neuronal development, survival and function. Cytokine 2021, 144, 155582. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Echevarria, F.D.; Sousa, J.L.; Konlian, D.O.; Dallas, G.; Formichella, C.R.; Sankaran, P.; Goralski, P.J.; Gustafson, J.R.; Sappington, R.M. Interleukin-6 promotes microtubule stability in axons via Stat3 protein–protein interactions. iScience 2021, 24, 103141. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, L.; Lu, D.; Wu, Z.; Han, Y.; Xu, P.; Chang, L.; Wu, Q. Interleukin 4 Affects Epilepsy by Regulating Glial Cells: Potential and Possible Mechanism. Front. Mol. Neurosci. 2020, 13, 554547. [Google Scholar] [CrossRef]
- Gadani, S.P.; Cronk, J.C.; Norris, G.T.; Kipnis, J. IL-4 in the Brain: A Cytokine to Remember. J. Immunol. 2012, 189, 4213–4219. [Google Scholar] [CrossRef]
- Zuiderwijk-Sick, E.A.; Van Der Putten, C.; Timmerman, R.; Veth, J.; Pasini, E.M.; Van Straalen, L.; Van Der Valk, P.; Amor, S.; Bajramovic, J.J. Exposure of Microglia to Interleukin-4 Represses NF-κB-Dependent Transcription of Toll-Like Receptor-Induced Cytokines. Front. Immunol. 2021, 12, 771453. [Google Scholar] [CrossRef]
- Derecki, N.C.; Cardani, A.N.; Yang, C.H.; Quinnies, K.M.; Crihfield, A.; Lynch, K.R.; Kipnis, J. Regulation of learning and memory by meningeal immunity: A key role for IL-4. J. Exp. Med. 2010, 207, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Jiang, Q.; Ding, W.; Yue, P.; Wang, J.; Zhao, K.; Zhang, H. Interleukin 4 inhibits high mobility group box-1 protein-mediated NLRP3 inflammasome formation by activating peroxisome proliferator-activated receptor-γ in astrocytes. Biochem. Biophys. Res. Commun. 2019, 509, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, T.V.; Zabrodskaya, Y.M.; Litovchenko, A.V.; Paramonova, N.M.; Kasumov, V.R.; Kravtsova, S.V.; Skiteva, E.N.; Sitovskaya, D.A.; Bazhanova, E.D. Relationship between Neuroglial Apoptosis and Neuroinflammation in the Epileptic Focus of the Brain and in the Blood of Patients with Drug-Resistant Epilepsy. Int. J. Mol. Sci. 2022, 23, 12561. [Google Scholar] [CrossRef]
- Somade, O.T.; Ajayi, B.O.; Adeyi, O.E.; Aina, B.O.; David, B.O.; Sodiya, I.D. Activation of NF-kB mediates up-regulation of cerebellar and hypothalamic pro-inflammatory chemokines (RANTES and MCP-1) and cytokines (TNF-α, IL-1β, IL-6) in acute edible camphor administration. Sci. Afr. 2019, 5, e00114. [Google Scholar] [CrossRef]
- Koh, G.Y. Orchestral actions of angiopoietin-1 in vascular regeneration. Trends Mol. Med. 2013, 19, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Alawo, D.O.A.; Tahir, T.A.; Fischer, M.; Bates, D.G.; Amirova, S.R.; Brindle, N.P.J. Regulation of Angiopoietin Signalling by Soluble Tie2 Ectodomain and Engineered Ligand Trap. Sci. Rep. 2017, 7, 3658. [Google Scholar] [CrossRef]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef]
- Cunningham, M.; Cho, J.-H.; Leung, A.; Savvidis, G.; Ahn, S.; Moon, M.; Lee, P.K.J.; Han, J.J.; Azimi, N.; Kim, K.-S.; et al. hPSC-Derived Maturing GABAergic Interneurons Ameliorate Seizures and Abnormal Behavior in Epileptic Mice. Cell Stem Cell 2014, 15, 559–573. [Google Scholar] [CrossRef]
- Curia, G.; Longo, D.; Biagini, G.; Jones, R.S.G.; Avoli, M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 2008, 172, 143–157. [Google Scholar] [CrossRef]
- Zhou, H.; Lin, Z.; Voges, K.; Ju, C.; Gao, Z.; Bosman, L.W.; Ruigrok, T.J.; Hoebeek, F.E.; De Zeeuw, C.I.; Schonewille, M. Cerebellar modules operate at different frequencies. eLife 2014, 3, e02536. [Google Scholar] [CrossRef]
- Straub, J.; Vitko, I.; Gaykema, R.P.; Perez-Reyes, E. Preparation and Implantation of Electrodes for Electrically Kindling VGAT-Cre Mice to Generate a Model for Temporal Lobe Epilepsy. J. Vis. Exp. JoVE 2021. [Google Scholar] [CrossRef]
- Englot, D.J.; Mishra, A.M.; Mansuripur, P.K.; Herman, P.; Hyder, F.; Blumenfeld, H. Remote Effects of Focal Hippocampal Seizures on the Rat Neocortex. J. Neurosci. 2008, 28, 9066–9081. [Google Scholar] [CrossRef] [PubMed]
- Krestel, H.E.; Shimshek, D.R.; Jensen, V.; Nevian, T.; Kim, J.; Geng, Y.; Bast, T.; Depaulis, A.; Schonig, K.; Schwenk, F.; et al. A Genetic Switch for Epilepsy in Adult Mice. J. Neurosci. 2004, 24, 10568–10578. [Google Scholar] [CrossRef]
- Lundt, A.; Wormuth, C.; Siwek, M.E.; Müller, R.; Ehninger, D.; Henseler, C.; Broich, K.; Papazoglou, A.; Weiergräber, M. EEG Radiotelemetry in Small Laboratory Rodents: A Powerful State-of-the Art Approach in Neuropsychiatric, Neurodegenerative, and Epilepsy Research. Neural Plast. 2016, 2016, 8213878. [Google Scholar]
- Weiergräber, M.; Henry, M.; Hescheler, J.; Smyth, N.; Schneider, T. Electrocorticographic and deep intracerebral EEG recording in mice using a telemetry system. Brain Res. Protoc. 2005, 14, 154–164. [Google Scholar] [CrossRef]
- Beamer, E.; Otahal, J.; Sills, G.J.; Thippeswamy, T. Nw-Propyl-l-arginine (L-NPA) reduces status epilepticus and early epileptogenic events in a mouse model of epilepsy: Behavioural, EEG and immunohistochemical analyses. Eur. J. Neurosci. 2012, 36, 3194–3203. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.F.; Girskis, K.M.; Rubenstein, J.L.; Alvarez-Buylla, A.; Baraban, S.C. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat. Neurosci. 2013, 16, 692–697. [Google Scholar] [CrossRef]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Celio, M.R.; Heizmann, C.W. Calcium-binding protein parvalbumin as a neuronal marker. Nature 1981, 293, 300–302. [Google Scholar] [CrossRef]
- Kapuscinski, J. DAPI: A DNA-Specific Fluorescent Probe. Biotech. Histochem. 1995, 70, 220–233. [Google Scholar] [CrossRef]
- Mikkelsen, J.D.; O’Hare, M.M.T. An immunohistochemical and chromatographic analysis of the distribution and processing of proneuropeptide Y in the rat suprachiasmatic nucleus. Peptides 1991, 12, 177–185. [Google Scholar] [CrossRef]
- Rowan, S.; Todd, A.J.; Spike, R.C. Evidence that neuropeptide Y is present in GABAergic neurons in the superficial dorsal horn of the rat spinal cord. Neuroscience 1993, 53, 537–545. [Google Scholar] [CrossRef]
- Dalby, N.O.; Mody, I. The process of epileptogenesis: A pathophysiological approach. Curr. Opin. Neurol. 2001, 14, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, A.; Sutula, T.P. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002, 1, 173–181. [Google Scholar] [CrossRef]
- Arshad, M.; Naegele, J. Induction of Temporal Lobe Epilepsy in Mice with Pilocarpine. Bio-Protocol 2020, 10, e3533. [Google Scholar] [CrossRef] [PubMed]
- Goffin, K.; Nissinen, J.; Van Laere, K.; Pitkänen, A. Cyclicity of spontaneous recurrent seizures in pilocarpine model of temporal lobe epilepsy in rat. Exp. Neurol. 2007, 205, 501–505. [Google Scholar] [CrossRef]
- Gogolla, N.; Galimberti, I.; DePaola, V.; Caroni, P. Preparation of organotypic hippocampal slice cultures for long-term live imaging. Nat. Protoc. 2006, 1, 1165–1171. [Google Scholar] [CrossRef]
- Legrand, C.; Bour, J.M.; Jacob, C.; Capiaumont, J.; Martial, A.; Marc, A.; Wudtke, M.; Kretzmer, G.; Demangel, C.; Duval, D.; et al. Lactate dehydrogenase (LDH) activity of the number of dead cells in the medium of cultured eukaryotic cells as marker. J. Biotechnol. 1992, 25, 231–243, Erratum in J. Biotechnol. 1993, 31, 234. [Google Scholar] [CrossRef] [PubMed]
- Liy, P.M.; Puzi, N.N.A.; Jose, S.; Vidyadaran, S. Nitric oxide modulation in neuroinflammation and the role of mesenchymal stem cells. Exp. Biol. Med. 2021, 246, 2399–2406. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
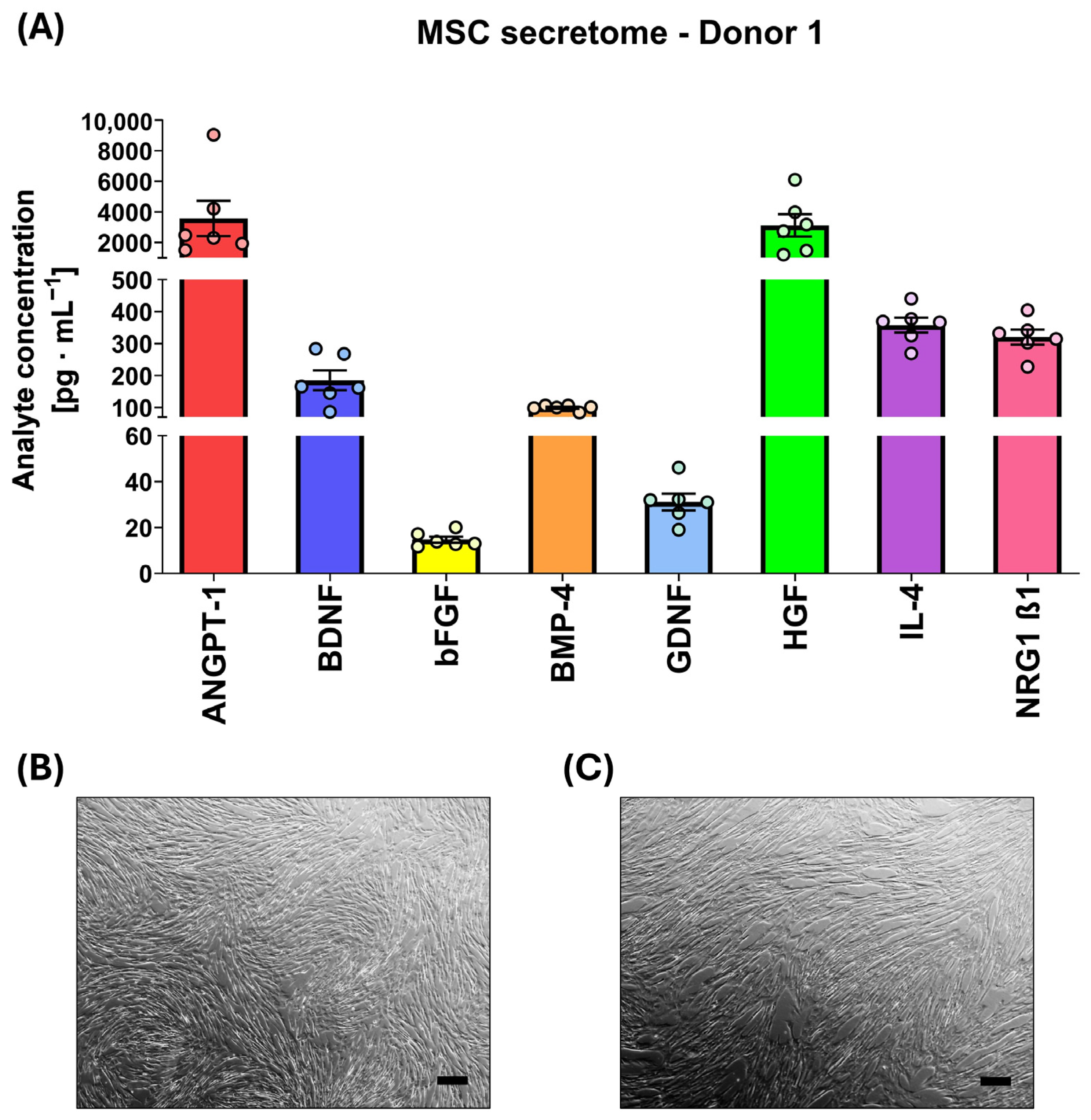
| MSC | Analyte Concentration [pg·mL−1] M ± SD | |||||||
|---|---|---|---|---|---|---|---|---|
| ANGPT-1 | BDNF | bFGF | BMP-4 | GDNF | HGF | IL-4 | NRG1 β1 | |
| Donor 1 | 3580.2 ± 903.7 | 185.1 ± 53.9 | 14.7 ± 0.1 | 99.9 ± 3.0 | 31.1 ± 3.8 | 3118.0 ± 1306.7 | 357.9 ± 36.7 | 320.4 ± 29.0 |
| Donor 2 | 1380.5 ± 228.1 | 199.3 ± 32.5 | 18.4 ± 4.5 | 83.1 ± 12.9 | 42.1 ± 10.4 | 2028.9 ± 775.0 | 264.3 ± 39.1 | 238.0 ± 36.1 |
| Donor 3 | 1650.8 ± 454.2 | 290.8 ± 78.9 | 31.0 ± 12.3 | 98.3 ± 0.3 | 87.5 ± 6.7 | 3112.0 ± 1119.3 | 371.5 ± 35.8 | 350.9 ± 31.9 |
| Donor 4 | 2403.9 ± 742.5 | 341.6 ± 128.6 | 40.0 ± 7.6 | 128.7 ± 0.0 | 75.9 ± 16.5 | 3247.8 ± 164.4 | 462.2 ± 105.1 | 393.3 ± 74.3 |
| Mean | 2253.9 ± 582.1 | 254.2 ± 73.5 | 26.0 ± 6.1 | 102.5 ± 4.1 | 59.2 ± 9.4 | 2876.6± 841.3 | 364.0 ± 54.2 | 325.6 ± 42.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Strzelec, M.; Detka, J.; Kot, M.; Wang, Q.; Sobocińska, M.K.; Mikkelsen, J.D.; Majka, M. Cytoprotective and Immunomodulatory Properties of Mesenchymal Stem Cell Secretome and Its Effect on Organotypic Hippocampal Cultures in Mouse Model of Temporal Lobe Epilepsy. Int. J. Mol. Sci. 2026, 27, 265. https://doi.org/10.3390/ijms27010265
Strzelec M, Detka J, Kot M, Wang Q, Sobocińska MK, Mikkelsen JD, Majka M. Cytoprotective and Immunomodulatory Properties of Mesenchymal Stem Cell Secretome and Its Effect on Organotypic Hippocampal Cultures in Mouse Model of Temporal Lobe Epilepsy. International Journal of Molecular Sciences. 2026; 27(1):265. https://doi.org/10.3390/ijms27010265
Chicago/Turabian StyleStrzelec, Martyna, Jan Detka, Marta Kot, Qi Wang, Małgorzata K. Sobocińska, Jens D. Mikkelsen, and Marcin Majka. 2026. "Cytoprotective and Immunomodulatory Properties of Mesenchymal Stem Cell Secretome and Its Effect on Organotypic Hippocampal Cultures in Mouse Model of Temporal Lobe Epilepsy" International Journal of Molecular Sciences 27, no. 1: 265. https://doi.org/10.3390/ijms27010265
APA StyleStrzelec, M., Detka, J., Kot, M., Wang, Q., Sobocińska, M. K., Mikkelsen, J. D., & Majka, M. (2026). Cytoprotective and Immunomodulatory Properties of Mesenchymal Stem Cell Secretome and Its Effect on Organotypic Hippocampal Cultures in Mouse Model of Temporal Lobe Epilepsy. International Journal of Molecular Sciences, 27(1), 265. https://doi.org/10.3390/ijms27010265







