Exosomal NAMPT from Engineered Mesenchymal Stem Cells Mitigates Aortic Stenosis via Metabolic and Anti-Inflammatory Pathways
Abstract
1. Introduction
1.1. Background
1.2. NAD+ Metabolism and NAMPT in Cardiovascular Disease
1.3. Therapeutic Potential of MSC-Derived Exosomes
1.4. Aortic Stenosis Model and Study Rationale
2. Results
2.1. NAMPT Expression Is Decreased in AS Mice
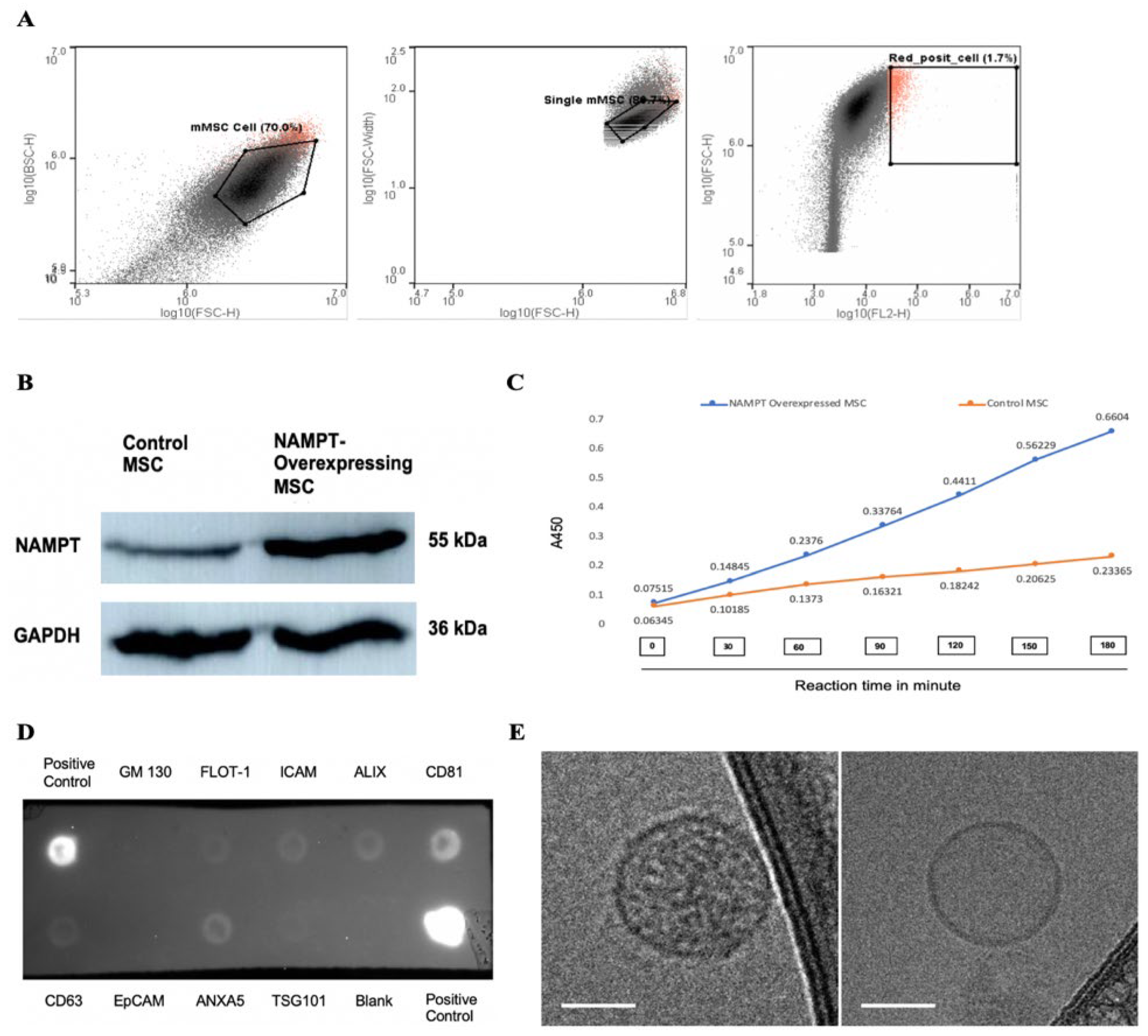
2.2. Characterization of NAMPT-Exo
2.3. NAMPT-Exo Treatment Attenuates AS Progression
2.4. In Vivo Uptake of NAMPT-Exo by Cardiac Tissue
2.5. NAMPT-Exo Suppresses EndMT in the Stenotic Aortic Valve
2.6. NAMPT-Exo Restores miR-146a-3p Levels in AS Mice
2.7. MiR-146a-3p Inhibition Suppresses EndMT in Cardiac Endothelial Cells from AS Mice
3. Discussion
3.1. Limitations
3.2. Future Perspectives
4. Materials and Methods
4.1. Animals
4.2. MSC Isolation and Culture
4.3. Lentiviral Construction
4.4. NAMPT Overexpression in MSC
4.5. NAMPT Activity Assay
4.6. Exosome Isolation and Characterization
4.7. Quantification of Exosome Particles
4.8. Cryo-Transmission Electron Microscopy
4.9. Exosome Treatment
4.10. Echocardiographic Measurements
4.11. Tissue Harvesting and Fixation
4.12. Histological Analysis
4.13. Immunohistochemistry
4.14. Endothelial Cell Isolation and Culture
4.15. EndMT Assay
4.16. Immunocytochemistry and Immunofluorescence Staining
4.17. Western Blot Analysis
4.18. MicroRNA qPCR
4.19. Data Preprocessing and Quality Control
4.20. Study Power and Sample Size
4.21. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Aortic stenosis |
| AV | Aortic Valve |
| BCA | Bicinchoninic acid |
| CD31 | cluster of differentiation 31 |
| CXCR4 | C-X-C chemokine receptor type 4 |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| EC | Endothelial cell |
| EC-CXCR4 KO | Endothelial cell-specific CXCR4 knockout |
| EndMT | Endothelial-to-mesenchymal transition |
| EV | Extracellular Vesicles |
| FBS | Fetal bovine serum |
| H&E | Hematoxylin and eosin |
| IP | Intraperitoneal |
| LVID | Left Ventricular internal diameter |
| miRNA | microRNA |
| MSC | Mesenchymal stem cell |
| NAD | Nicotinamide adenine dinucleotide |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| NAMPT-Exo | NAMPT-enriched mesenchymal stem cell-derived exosomes |
| qPCR | Quantitative polymerase chain reaction |
| RT | Room temperature |
| TEM | Transmission electron microscopy |
| TGF-β1 | Transforming growth factor-beta 1 |
| TSG101 | Tumor susceptibility gene 101 |
| WTA | Wheat Germ Agglutinin |
| α-SMA | α-smooth muscle actin |
References
- Czarny, M.J.; Resar, J.R. Diagnosis and Management of Valvular Aortic Stenosis. Clin. Med. Insights Cardiol. 2014, 8s1, CMC.S15716. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Clavel, M.-A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific Aortic Stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Wang, Z.; Sun, C.; Zhang, D.-M. Pathogenesis and Molecular Immune Mechanism of Calcified Aortic Valve Disease. Front. Cardiovasc. Med. 2021, 8, 765419. [Google Scholar] [CrossRef]
- Abdellatif, M.; Sedej, S.; Kroemer, G. NAD+ Metabolism in Cardiac Health, Aging, and Disease. Circulation 2021, 144, 1795–1817. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Guarente, L. NAD+ and Sirtuins in Aging and Disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef]
- Revollo, J.R.; Grimm, A.A.; Imai, S. The NAD Biosynthesis Pathway Mediated by Nicotinamide Phosphoribosyltransferase Regulates Sir2 Activity in Mammalian Cells. J. Biol. Chem. 2004, 279, 50754–50763. [Google Scholar] [CrossRef]
- Yoshida, M.; Satoh, A.; Lin, J.B.; Mills, K.F.; Sasaki, Y.; Rensing, N.; Wong, M.; Apte, R.S.; Imai, S. Extracellular Vesicle-Contained eNAMPT Delays Aging and Extends Lifespan in Mice. Cell Metab. 2019, 30, 329–342.e5. [Google Scholar] [CrossRef]
- Katsyuba, E.; Romani, M.; Hofer, D.; Auwerx, J. NAD+ Homeostasis in Health and Disease. Nat. Metab. 2020, 2, 9–31. [Google Scholar] [CrossRef]
- Shi, C.; Wen, Z.; Yang, Y.; Shi, L.; Liu, D. NAD+ Metabolism and Therapeutic Strategies in Cardiovascular Diseases. Atheroscler. Plus 2024, 57, 1–12. [Google Scholar] [CrossRef]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018, 27, 529–547. [Google Scholar] [CrossRef]
- Yang, M.; Wang, T.; Shao, J.; Ran, X.; Xiao, R.; Zhao, R.; Wu, C.; Ji, M.; Tian, W.; Sun, H.; et al. (+)-JQ-1 Alleviates Cardiac Injury in Myocardial Infarction by Inhibiting Ferroptosis through the NAMPT/SIRT1 Pathway. Cell Death Dis. 2025, 16, 548. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.-I.; Byun, J.; Huang, C.-Y.; Imai, N.; Ralda, G.; Zhai, P.; Xu, X.; Kashyap, S.; Warren, J.S.; Alan Maschek, J.; et al. Nampt Potentiates Antioxidant Defense in Diabetic Cardiomyopathy. Circ. Res. 2021, 129, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Manickam, R.; Santhana, S.; Xuan, W.; Bisht, K.S.; Tipparaju, S.M. Nampt: A New Therapeutic Target for Modulating NAD+ Levels in Metabolic, Cardiovascular, and Neurodegenerative Diseases. Can. J. Physiol. Pharmacol. 2025, 103, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD+ in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef]
- Hsu, C.-P.; Oka, S.; Shao, D.; Hariharan, N.; Sadoshima, J. Nicotinamide Phosphoribosyltransferase Regulates Cell Survival through NAD+ Synthesis in Cardiac Myocytes. Circ. Res. 2009, 105, 481–491. [Google Scholar] [CrossRef]
- Kosugi, S.; Yamaguchi, S.; Nishioka, K.; Nagahisa, T.; Watanabe, Y.; Kojima, D.; Kaneko, K.; Mitsuno, R.; Nakamichi, R.; Kawano, Y.; et al. Vascular Endothelial NAMPT-Mediated NAD+ Biosynthesis Regulates Angiogenesis and Cardiometabolic Functions in Male Mice. Aging Cell 2025, 24, e70222. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Y.; Hu, S.; Chen, Y.; Shen, Z. The Therapeutic Potential of Mesenchymal Stem Cells for Cardiovascular Diseases. Cell Death Dis. 2020, 11, 349. [Google Scholar] [CrossRef]
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol. Ther. 2018, 26, 1610–1623. [Google Scholar] [CrossRef]
- Kundu, D.; Shin, S.Y.; Chilian, W.M.; Dong, F. The Potential of Mesenchymal Stem Cell-Derived Exosomes in Cardiac Repair. Int. J. Mol. Sci. 2024, 25, 13494. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Immunomodulation and Regeneration: A next Generation Therapeutic Tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical Applications of Stem Cell-Derived Exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome Secreted by MSC Reduces Myocardial Ischemia/Reperfusion Injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef]
- Teng, X.; Chen, L.; Chen, W.; Yang, J.; Yang, Z.; Shen, Z. Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cell. Physiol. Biochem. 2015, 37, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, F.; Qian, H.; Xu, W.; Jiang, J. Preconditioning and Engineering Strategies for Improving the Efficacy of Mesenchymal Stem Cell-Derived Exosomes in Cell-Free Therapy. Stem Cells Int. 2022, 2022, 1779346. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, Y.; Li, H.-J. Advances in Mesenchymal Stem Cell Exosomes: A Review. Stem Cell Res. Ther. 2021, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Winnicki, A.; Gadd, J.; Ohanyan, V.; Hernandez, G.; Wang, Y.; Enrick, M.; McKillen, H.; Kiedrowski, M.; Kundu, D.; Kegecik, K.; et al. Role of Endothelial CXCR4 in the Development of Aortic Valve Stenosis. Front. Cardiovasc. Med. 2022, 9, 971321. [Google Scholar] [CrossRef]
- Sider, K.L.; Blaser, M.C.; Simmons, C.A. Animal Models of Calcific Aortic Valve Disease. Int. J. Inflamm. 2011, 2011, 364310. [Google Scholar] [CrossRef]
- Wen, D.; Hu, L.; Shan, J.; Zhang, H.; Hu, L.; Yuan, A.; Pu, J.; Xue, S. Mechanical Injury Accentuates Lipid Deposition in ApoE–/– Mice and Advance Aortic Valve Stenosis: A Novel Modified Aortic Valve Stenosis Model. Front. Cardiovasc. Med. 2023, 10, 1119746. [Google Scholar] [CrossRef]
- Gupta, D.; Zickler, A.M.; El Andaloussi, S. Dosing Extracellular Vesicles. Adv. Drug Deliv. Rev. 2021, 178, 113961. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, D.; Yuan, P.; Li, J.; Yun, Y.; Cui, Y.; Zhang, T.; Ma, J.; Sun, L.; Ma, H.; et al. Endothelial-to-Mesenchymal Transition in Calcific Aortic Valve Disease. Acta Cardiol. Sin. 2020, 36, 183–194. [Google Scholar] [CrossRef]
- Kovacic, J.C.; Mercader, N.; Torres, M.; Boehm, M.; Fuster, V. Epithelial-to-Mesenchymal and Endothelial-to-Mesenchymal Transition: From Cardiovascular Development to Disease. Circulation 2012, 125, 1795–1808. [Google Scholar] [CrossRef] [PubMed]
- Alfaidi, M.; Evans, P.C.; Pickering, J.G. Editorial: Endothelial-to-Mesenchymal Transition in Cardiovascular Disease. Front. Cardiovasc. Med. 2023, 10, 1290050. [Google Scholar] [CrossRef]
- Adewuyi, J.O.; Patel, R.; Abbasciano, R.; McCann, G.P.; Murphy, G.; Woźniak, M.J.; Singh, A. A Systematic Review of Micro-RNAs in Aortic Stenosis and Cardiac Fibrosis. Clin. Transl. Sci. 2022, 15, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Coffey, S.; Williams, M.J.A.; Phillips, L.V.; Galvin, I.F.; Bunton, R.W.; Jones, G.T. Integrated microRNA and Messenger RNA Analysis in Aortic Stenosis. Sci. Rep. 2016, 6, 36904. [Google Scholar] [CrossRef] [PubMed]
- Iacopo, F.; Lorenzo, C.; Calogero, E.; Matteo, P.; Riccardo, P.N.; Veronica, S.; Valentina, B.; Riccardo, L.; Cristian, S.; Maria, M.C.; et al. Review in Translational Cardiology: MicroRNAs and Myocardial Fibrosis in Aortic Valve Stenosis, a Deep Insight on Left Ventricular Remodeling. J. Cardiovasc. Echogr. 2016, 26, 109–114. [Google Scholar] [CrossRef]
- Suzuki, H.I.; Horie, M.; Mihira, H.; Saito, A. Molecular Analysis of Endothelial-Mesenchymal Transition Induced by Transforming Growth Factor-β Signaling. J. Vis. Exp. 2018, 57577. [Google Scholar] [CrossRef]
- Ma, J.; Sanchez-Duffhues, G.; Goumans, M.-J.; Ten Dijke, P. TGF-β-Induced Endothelial to Mesenchymal Transition in Disease and Tissue Engineering. Front. Cell Dev. Biol. 2020, 8, 260. [Google Scholar] [CrossRef]
- Chong, M.C.; Silva, A.; James, P.F.; Wu, S.S.X.; Howitt, J. Exercise Increases the Release of NAMPT in Extracellular Vesicles and Alters NAD+ Activity in Recipient Cells. Aging Cell 2022, 21, e13647. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Zhu, W.; Zhang, Y.; Hong, Y.; Liang, X.; Fan, B.; Zhao, H.; He, H.; Zhang, F. Exosomes from Mesenchymal Stem Cells Overexpressing MIF Enhance Myocardial Repair. J. Cell. Physiol. 2020, 235, 8010–8022. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, Y.; He, W.; Tian, Y.; Zhao, X. Exosomes Secreted from Bone Marrow Mesenchymal Stem Cells Suppress Cardiomyocyte Hypertrophy through Hippo-YAP Pathway in Heart Failure. Genet. Mol. Biol. 2023, 46, e20220221. [Google Scholar] [CrossRef]
- Salazar-Puerta, A.I.; Kordowski, M.; Cuellar-Gaviria, T.Z.; Rincon-Benavides, M.A.; Hussein, J.; Flemister, D.; Mayoral-Andrade, G.; Barringer, G.; Guilfoyle, E.; Blackstone, B.N.; et al. Engineered Extracellular Vesicle-Based Therapies for Valvular Heart Disease. Cell. Mol. Bioeng. 2023, 16, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.; Teo, K.; Dumesnil, J.G.; Ni, A.; Tam, J. Effect of Lipid Lowering With Rosuvastatin on Progression of Aortic Stenosis: Results of the Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin (ASTRONOMER) Trial. Circulation 2010, 121, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Rossebø, A.B.; Pedersen, T.R.; Boman, K.; Brudi, P.; Chambers, J.B.; Egstrup, K.; Gerdts, E.; Gohlke-Bärwolf, C.; Holme, I.; Kesäniemi, Y.A.; et al. Intensive Lipid Lowering with Simvastatin and Ezetimibe in Aortic Stenosis. N. Engl. J. Med. 2008, 359, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Dahal, S.; Huang, P.; Murray, B.T.; Mahler, G.J. Endothelial to Mesenchymal Transformation Is Induced by Altered Extracellular Matrix in Aortic Valve Endothelial Cells. J. Biomed. Mater. Res. 2017, 105, 2729–2741. [Google Scholar] [CrossRef]
- Wang, H.; Shi, J.; Li, B.; Zhou, Q.; Kong, X.; Bei, Y. MicroRNA Expression Signature in Human Calcific Aortic Valve Disease. BioMed Res. Int. 2017, 2017, 4820275. [Google Scholar] [CrossRef]
- Nappi, F.; Iervolino, A.; Avtaar Singh, S.S.; Chello, M. MicroRNAs in Valvular Heart Diseases: Biological Regulators, Prognostic Markers and Therapeutical Targets. Int. J. Mol. Sci. 2021, 22, 12132. [Google Scholar] [CrossRef]
- Heggermont, W.A.; Papageorgiou, A.-P.; Quaegebeur, A.; Deckx, S.; Carai, P.; Verhesen, W.; Eelen, G.; Schoors, S.; van Leeuwen, R.; Alekseev, S.; et al. Inhibition of MicroRNA-146a and Overexpression of Its Target Dihydrolipoyl Succinyltransferase Protect Against Pressure Overload-Induced Cardiac Hypertrophy and Dysfunction. Circulation 2017, 136, 747–761. [Google Scholar] [CrossRef]
- Petrkova, J.; Borucka, J.; Kalab, M.; Klevcova, P.; Michalek, J.; Taborsky, M.; Petrek, M. Increased Expression of miR-146a in Valvular Tissue From Patients With Aortic Valve Stenosis. Front. Cardiovasc. Med. 2019, 6, 86. [Google Scholar] [CrossRef]
- Demkes, C.J.; Van Rooij, E. MicroRNA-146a as a Regulator of Cardiac Energy Metabolism. Circulation 2017, 136, 762–764. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Dragoumani, K.; Chrousos, G.P.; Vlachakis, D. Exosomal Epigenetics. EMBnet J. 2024, 29, e1049. [Google Scholar] [CrossRef]
- Li, C.; Ni, Y.-Q.; Xu, H.; Xiang, Q.-Y.; Zhao, Y.; Zhan, J.-K.; He, J.-Y.; Li, S.; Liu, Y.-S. Roles and Mechanisms of Exosomal Non-Coding RNAs in Human Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 383. [Google Scholar] [CrossRef]
- Lee, Y.; El Andaloussi, S.; Wood, M.J.A. Exosomes and Microvesicles: Extracellular Vesicles for Genetic Information Transfer and Gene Therapy. Hum. Mol. Genet. 2012, 21, R125–R134. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, L.; Ocansey, D.K.W.; Wang, B.; Mao, F. The Role of Mesenchymal Stem Cell-Derived Exosome in Epigenetic Modifications in Inflammatory Diseases. Front. Immunol. 2023, 14, 1166536. [Google Scholar] [CrossRef]
- Dong, F.; Harvey, J.; Finan, A.; Weber, K.; Agarwal, U.; Penn, M.S. Myocardial CXCR4 Expression Is Required for Mesenchymal Stem Cell Mediated Repair Following Acute Myocardial Infarction. Circulation 2012, 126, 314–324. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Gao, M.; Kim, Y.; Zhang, C.; Borshch, V.; Zhou, S.; Park, H.; Jákli, A.; Lavrentovich, O.D.; Tamba, M.; Kohlmeier, A.; et al. Direct Observation of Liquid Crystals Using cryo-TEM: Specimen Preparation and Low-dose Imaging. Microsc. Res. Tech. 2014, 77, 754–772. [Google Scholar] [CrossRef]








| Primer Type | Sequence (5′-3′) | Restriction Site |
|---|---|---|
| Forward primer | CGGTGAATTCCTCGAGATGAATGCTGCGGCAGAAGC | XhoI |
| Reverse primer | GAGAGGGGCGGGATCCCTAATGAGGTGCCACGTCCTG | BamHI |
| miRNA | Sequence (5′-3′) |
|---|---|
| miR-146a-3p | CCUGUGAAAUUCAGUUCUUCAG |
| miR-NC5 negative control | ACCAUAUUGCGCGUAUAGUCGC |
| miRNA | Sequence (5′-3′) |
|---|---|
| mmu-miR-146a-5P | UGAGAACUGAAUUCCAUGGGUU |
| mmu-miR-146a-3P | CCUGUGAAAUUCAGUUCUUCAG |
| mmu-miR-142a-5P | CAUAAAGUAGAAAGCACUACU |
| mmu-miR-125b-5P | UCCCUGAGACCCUAACUUGUGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Kundu, D.K.; Kiedrowski, M.; Gadd, J.; Gao, M.; Evan, M.; Wang, Y.; Yin, L.; Ohanyan, V.; Chilian, W.M.; Dong, F. Exosomal NAMPT from Engineered Mesenchymal Stem Cells Mitigates Aortic Stenosis via Metabolic and Anti-Inflammatory Pathways. Int. J. Mol. Sci. 2026, 27, 256. https://doi.org/10.3390/ijms27010256
Kundu DK, Kiedrowski M, Gadd J, Gao M, Evan M, Wang Y, Yin L, Ohanyan V, Chilian WM, Dong F. Exosomal NAMPT from Engineered Mesenchymal Stem Cells Mitigates Aortic Stenosis via Metabolic and Anti-Inflammatory Pathways. International Journal of Molecular Sciences. 2026; 27(1):256. https://doi.org/10.3390/ijms27010256
Chicago/Turabian StyleKundu, Dipan Kumar, Matthew Kiedrowski, James Gadd, Min Gao, Madeline Evan, Yang Wang, Liya Yin, Vahagn Ohanyan, William M. Chilian, and Feng Dong. 2026. "Exosomal NAMPT from Engineered Mesenchymal Stem Cells Mitigates Aortic Stenosis via Metabolic and Anti-Inflammatory Pathways" International Journal of Molecular Sciences 27, no. 1: 256. https://doi.org/10.3390/ijms27010256
APA StyleKundu, D. K., Kiedrowski, M., Gadd, J., Gao, M., Evan, M., Wang, Y., Yin, L., Ohanyan, V., Chilian, W. M., & Dong, F. (2026). Exosomal NAMPT from Engineered Mesenchymal Stem Cells Mitigates Aortic Stenosis via Metabolic and Anti-Inflammatory Pathways. International Journal of Molecular Sciences, 27(1), 256. https://doi.org/10.3390/ijms27010256








