The Feud over Lactate and Its Role in Brain Energy Metabolism: An Unnecessary Burden on Research and the Scientists Who Practice It
Abstract
1. A Short Introduction
2. The Discoveries That Revolutionized the Study of Skeletal Muscle and Brain Energy Metabolism
3. Methodological Limitations Can Lead to Miscalculations and Wrong Conclusions
“…simultaneous measurements of glucose and oxygen consumption during rest or activation supposedly produces accurate estimate of the energy needs for the brain region under observation. However, the ratio CMRO2/CMRglucose values calculated are often significantly smaller than the expected 6/1. Such discrepancies have attributed to other glucose-consuming reactions not accompanies with oxygen consumption. Consequently, it has been a common understanding that a value of CMRO2/CMRglucose < 6 indicates that a partial non-oxidative glucose consumption (occurs). The smaller the value of CMRO2/CMRglucose, the greater is the non-oxidative consumption of glucose. This understanding makes sense when one assumes that a fully coupled glycolytic-mitochondrial respiratory apparatus should produce a CMRO2/CMRglucose value of 6 and an uncoupled apparatus (non-oxidative) should produce a CMRO2/CMRglucose value of ~0.”
“One of the most reliable techniques to measure oxygen consumption is the polarographic technique, which allows the determination of oxygen concentration via the measurement of the partial oxygen pressure (PO2) locally. Continuous measurements over a period of time when brain activity (EEG) is monitored demonstrated a correlation between increased activity and decreased tissue oxygen level. The development of oxygen microelectrodes has afforded a more accurate localization of such measurements.”
“Brain glucose uptake, oxygen metabolism, and blood flow in humans were measured with positron emission tomography, and a resting-state molar ratio of oxygen to glucose consumption of 4.1:1 was obtained. Physiological neural activity, however, increased glucose uptake and blood flow much more (51 and 50 percent, respectively) than oxygen consumption (5 percent) and produced a molar ratio for the increases of 0.4:1. Transient increases in neural activity cause a tissue uptake of glucose in excess of that consumed by oxidative metabolism, acutely consume much less energy than previously believed, and regulate local blood flow for purposes other than oxidative metabolism.”
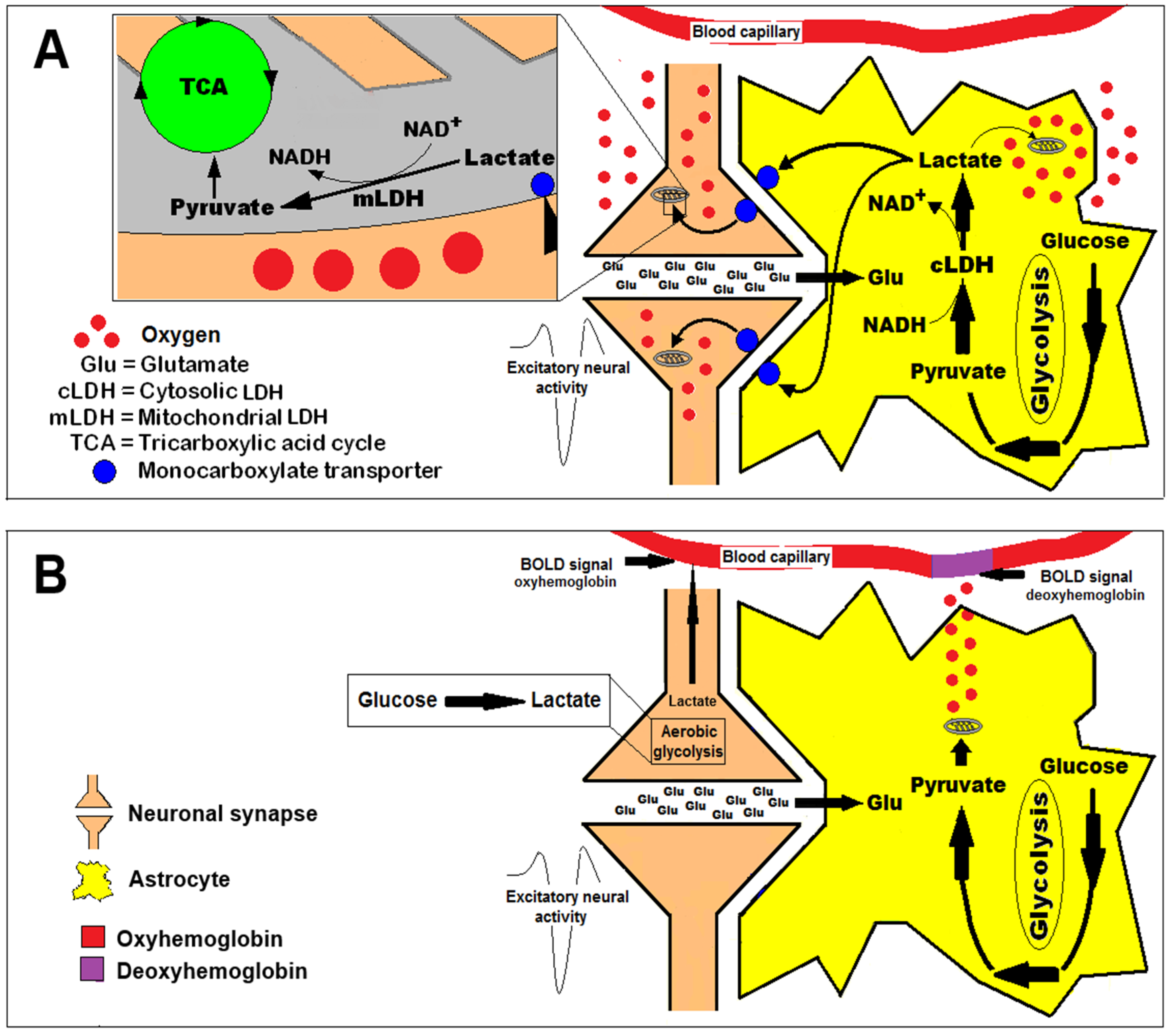
“The results of simultaneous measurements of local extracellular concentrations of lactate, glucose, and O2 first provide a comprehensive picture of complementary supply and use of lactate and glucose behind the BBB. In response to acute neuronal activation, the brain tissue shifts immediately to significant energy supply by lactate. A local temporary fuel pool behind the BBB is established by increased extracellular lactate concentration. The glutamate-stimulated astrocytic glycolysis and increase of regional blood flow may regulate the lactate concentration of the pool at different levels (i.e., molecular/cell level and anatomic level, respectively), and on different time scales, to maintain local energy homeostasis. Thus, the formation of a temporary local extracellular fuel reservoir resolves nicely the conflict between the demands of local energy homeostasis and the transport from the circulatory system.”
4. Scientific Bias May Influence the Research of Brain Activation and Its Energy Needs
“That oxygen and glucose consumption and carbon dioxide production are essentially in stochiometric balance and no other energy laten substrate is taken from the blood means, however that the net energy made available to the brain must ultimately be derived from the oxidation of glucose. It should be noted that this is the situation in the normal state; … other substrates maybe used in special circumstances or in abnormal states.” [69].
There has been a wealth of papers over the past few decades on lactate metabolism and the role(s) of lactate in the brain, with some 16,000 papers resulting from a search for lactate metabolism AND brain in PubMed. Despite all this investigation, lactate is likely the most controversial and misunderstood brain energy substrate.
Lactate is formed in all brain cells solely from pyruvate in a reversible reaction catalyzed by lactate dehydrogenase, requiring NADH as the cofactor. Lactate dehydrogenase is a ubiquitous near-equilibrium enzyme, which means it has limited capacity to influence metabolic control. While lactate itself is a “dead-end” metabolite, its immediate substrate, pyruvate, is a key molecule at a metabolic crossroad. Pyruvate can be generated by oxidative metabolism of glucose, glutamate, and other substrates via the pyruvate recycling pathway. It is converted to acetyl-CoA via the far-equilibrium pyruvate dehydrogenase complex and can also be transaminated to alanine in a pairing with glutamate and 2-oxoglutarate or carboxylated to form oxaloacetate by pyruvate carboxylase, a glial-specific enzyme. This plethora of possible pathways for pyruvate, and their respective thermodynamics, means that pyruvate clearance rates (i.e., substrate availability) tend to have more control over production of lactate than lactate dehydrogenase itself. Lactate (dehydrogenase) isoenzymes, while able to influence the rate (time course) of conversion of substrates, have no influence on the equilibrium lactate concentration.
Another important, and often forgotten factor, is that full lactate utilization in the respiratory chain requires oxygen (CMRO2), and if oxygen consumption does not match glucose utilization, then the lactate derived from glucose cannot be oxidized. During activation, glycolysis is preferentially upregulated compared with oxidation. Specific activity measurements indicate that most of the lactate produced from glucose via this upregulation is retained and oxidized in the brain. Specific activity measurements also indicate that lactate derived from glycogen in astrocytes is quickly released from the brain because, if retained, lactate-specific activity would be diluted. Lactate may escape from its “dead-end” reaction by leaving the cell, which it does through an array of transporters which mediate the uptake and release of lactate and other monocarboxylates such as acetate, pyruvate, and the ketone bodies β-hydroxybutyrate and acetoacetate. Lactate may also escape via gap junctions where it travels relatively long distances.
Lactate production in the human brain appears to be region-specific in a pattern that is conserved across individuals, with production highest in the parietal and occipital lobes (cuneus, precuneus, cingulate, and lingual gyri). Production of lactate is also not necessarily related to regional tendency to aerobic glycolysis or FDG uptake, although it may be related to the availability of NADH. Exactly when and why the brain produces lactate is still not well-understood, although many possible explanations have been advanced. It has been speculated that the need to rapidly clear glutamate from the brain is fueled by glycolysis, producing pyruvate which is excess to requirements. The excess pyruvate is then effluxed as lactate, regenerating cytosolic NAD+. Alternatively, the controversial astrocyte–lactate shuttle hypothesis posits that lactate is released by astrocytes in response to glutamate release from neurons, where the lactate is taken up and used as a fuel. Many groups have embraced the concept of an astrocyte to neuron lactate shuttle (ANLSH/ANLS) since it was first proposed by Pellerin and Magistretti based on in vitro studies. However, studies in vivo supporting this shuttle are few and not convincing. The thermodynamics of lactate metabolism, transport, and clearance go some way to explaining the attraction of the ANLS. As the reactions involving lactate are all near-equilibrium enzymes, lactate is highly correlated with many metabolites and processes, which gives lactate a seeming importance beyond the reality. This was explained succinctly by the renowned biochemist, Richard Veech, who stated that “measurements of lactate content per se are able to provide relatively little information other than the level of lactate itself. By combining the measurement of lactate with the concentration of its relevant metabolic partners, a great deal more useful information can be gained about the state of the tissue.” So is there an astrocyte–neuron lactate shuttle? Sometimes, under the right conditions but it is probably just lactate exchanging between compartments. Is it important? Well, it can happen, but in the grand scheme of things, no, it is not that important, as lactate is just as likely to efflux from neurons and is not a substrate that can maintain high level neuronal function. Giving it a name (ANLS) has given it an importance, which outstrips its role as one of many reasons that lactate enters or leaves cells and the brain.
5. A Unifying Concept of Brain Activation and the Energy Metabolic Pathways That Support It
6. Coda
Funding
Acknowledgments
Conflicts of Interest
References
- Brooks, G.A. Lactate: Glycolytic end product and oxidative substrate during sustained exercise in mammals—The “lactate shuttle”. In Circulation, Respiration, and Metabolism: Current Comparative Approaches; Springer: Berlin/Heidelberg, Germany, 1985; pp. 208–218. [Google Scholar]
- Schurr, A.; West, C.A.; Rigor, B.M. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science 1988, 240, 1326–1328. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.M.; Rajasekaran, S.; Thomsen, T.W.; Peterson, A.R. Lactate: Friend or foe. PMR 2016, 8, S8–S15. [Google Scholar] [CrossRef]
- Pellerin, L. Lactate as a pivotal element in neuron–glia metabolic cooperation. Neurochem. Int. 2003, 43, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Gladden, L.B. Lactate metabolism: A new paradigm for the third millennium. J. Physiology 2004, 558, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Gladden, L.B. A lactatic perspective on metabolism. Med. Sci. Sports Exerc. 2008, 40, 477–485. [Google Scholar] [CrossRef]
- Kravitz, L. Lactate--not guilty as charged. IDEA Fit. J. 2005, 2, 23–26. [Google Scholar]
- Handy, J. Lactate—The bad boy of metabolism, or simply misunderstood? Curr. Anaesth. Crit. Care 2006, 17, 71–76. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Sweet sixteen for ANLS. J. Cereb. Blood Flow Metab. 2012, 32, 1152–1166. [Google Scholar] [CrossRef]
- Passarella, S.; de Bari, L.; Valenti, D.; Pizzuto, R.; Paventi, G.; Atlante, A. Mitochondria and L-lactate metabolism. FEBS Lett. 2008, 582, 3569–3576. [Google Scholar] [CrossRef]
- Schurr, A. Cerebral glycolysis: A century of persistent misunderstanding and misconception. Front. Neurosci. 2014, 8, 360. [Google Scholar] [CrossRef]
- Rogatzki, M.J.; Ferguson, B.S.; Goodwin, M.L.; Gladden, L.B. Lactate is always the end product of glycolysis. Front. Neurosci. 2015, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Van Hall, G. Lactate as a fuel for mitochondrial respiration. Acta Physiol. Scand. 2000, 168, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, M.; Takeda, M. Lactate as a signaling molecule that regulates exercise-induced adaptations. Biology 2016, 5, 38. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef]
- Schurr, A. Glycolysis Paradigm Shift Dictates a Reevaluation of Glucose and Oxygen Metabolic Rates of Activated Neural Tissue. Front. Neurosci. 2018, 12, 700. [Google Scholar] [CrossRef]
- Margolis, H. Paradigms and Barriers: How Habits of Mind Govern Scientific Beliefs; University of Chicago Press: Chicago, IL, USA, 1993. [Google Scholar]
- Magistretti, P.J.; Sorg, O.; Yu, N.; Martin, J.L.; Pellerin, L. Neurotransmitters regulate energy metabolism in astrocytes: Implications for the metabolic trafficking between neural cells. Dev. Neurosci. 1993, 15, 306–312. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef]
- Sokoloff, L.; Takahashi, S.; Gotoh, J.; Driscoll, B.F.; Law, M.J. Contribution of astroglia to functionally activated energy metabolism. Dev. Neurosci. 1996, 18, 343–352. [Google Scholar] [CrossRef]
- Sokoloff, L.; Takahashi, S. Functional activation of energy metabolism in nervous tissue: Where and why. In Neurodegenerative Diseases: Molecular and Cellular Mechanisms and Therapeutic Advances; Springer: Boston, MA, USA, 1996; pp. 147–169. [Google Scholar]
- Forsyth, R.J. Astrocytes and the delivery of glucose from plasma to neurons. Neurochem. Int. 1996, 28, 231–241. [Google Scholar] [CrossRef]
- Forsyth, R.; Fray, A.; Boutelle, M.; Fillenz, M.; Middleditch, C.; Burchell, A. A role for astrocytes in glucose delivery to neurons? Dev. Neurosci. 1996, 18, 360–370. [Google Scholar] [CrossRef]
- Korf, J. Intracerebral trafficking of lactate in vivo during stress, exercise, electroconvulsive shock and ischemia as studied with microdialysis. Dev. Neurosci. 1996, 18, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Elekes, O.; Venema, K.; Postema, F.; Dringen, R.; Hamprecht, B.; Korf, J. Possible Glial Contribution of Rat Hippocampus Lactate as Assessed with Microdialysis and Stress; Springer: Vienna, Austria, 1996; pp. 1–5. [Google Scholar]
- Hertz, L.; Swanson, R.A.; Newman, G.C.; Marrif, H.; Juurlink, B.H.J.; Peng, L. Can experimental conditions explain the discrepancy over glutamate stimulation of aerobic glycolysis? Dev. Neurosci. 1998, 20, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A.; Hertz, L. Glucose and lactate metabolism during brain activation. J. Neurosci. Res. 2001, 66, 824–838. [Google Scholar] [CrossRef]
- Chih, C.P.; He, J.; Sly, T.S.; Roberts Jr, E.L. Comparison of glucose and lactate as substrates during NMDA-induced activation of hippocampal slices. Brain Res. 2001, 893, 143–154. [Google Scholar] [CrossRef]
- Chih, C.P.; Lipton, P.; Roberts, E.L. Do active cerebral neurons really use lactate rather than glucose? Trends Neurosci. 2001, 24, 573–578. [Google Scholar] [CrossRef]
- Shulman, R.G.; Hyder, F.; Rothman, D.L. Lactate efflux and the neuroenergetic basis of brain function. NMR Biomed. 2001, 14, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Bittar, P.G.; Charnay, Y.; Pellerin, L.; Bouras, C.; Magistretti, P.J. Selective distribution of lactate dehydrogenase isoenzymes in neurons and astrocytes of human brain. J. Cereb. Blood Flow Metab. 1996, 16, 1079–1089. [Google Scholar] [CrossRef]
- Pellerin, L.; Pellegri, G.; Bittar, P.G.; Charnay, Y.; Bouras, C.; Martin, J.L.; Stella, N.; Magistretti, P.J. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev. Neurosci. 1998, 20, 291–299. [Google Scholar] [CrossRef]
- Pellerin, L.; Pellegri, G.; Martin, J.L.; Magistretti, P.J. Expression of monocarboxylate transporter mRNAs in mouse brain: Support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc. Natl. Acad. Sci. USA 1998, 95, 3990–3995. [Google Scholar] [CrossRef]
- Pierre, K.; Pellerin, L.; Debernardi, R.; Riederer, B.M.; Magistretti, P.J. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience 2000, 100, 617–627. [Google Scholar] [CrossRef]
- Aubert, A.; Costalat, R.; Magistretti, P.J.; Pellerin, L. Brain lactate kinetics: Modeling evidence for neuronal lactate uptake upon activation. Proc. Natl. Acad. Sci. USA 2005, 102, 16448–16453. [Google Scholar] [CrossRef]
- Bouzier-Sore, A.K.; Voisin, P.; Canioni, P.; Magistretti, P.J.; Pellerin, L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J. Cereb. Blood Flow Metab. 2003, 23, 1298–1306. [Google Scholar] [CrossRef]
- Bouzier-Sore, A.K.; Voisin, P.; Bouchaud, V.; Bezancon, E.; Franconi, J.; Pellerin, L. Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: A comparative NMR study. Eur. J. Neurosci. 2006, 24, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Bouzier-Sore, A.K.; Aubert, A.; Serres, S.; Merle, M.; Costalat, R.; Magistretti, P.J. Activity-dependent regulation of energy metabolism by astrocytes: An update. Glia 2007, 55, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.T.; Jolivet, R.; Buck, A.; Magistretti, P.J.; Weber, B. In vivo evidence for lactate as a neuronal energy source. J. Neurosci. 2011, 31, 7477–7485. [Google Scholar] [CrossRef]
- Proia, P.; Di Liegro, C.M.; Schiera, G.; Fricano, A.; Di Liegro, I. Lactate as a m. etabolite and a regulator in the central nervous system. Int. J. Mol. Sci. 2016, 17, 1450. [Google Scholar] [CrossRef]
- Hu, Y.; Wilson, G.S. A temporary local energy pool coupled to neuronal activity: Fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J. Neurochem. 1997, 69, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A.; Payne, R.S.; Miller, J.J.; Rigor, B.M. Glia are the main source of lactate utilized by neurons for recovery of function posthypoxia. Brain Res. 1997, 774, 221–224. [Google Scholar] [CrossRef]
- Schurr, A.; Miller, J.J.; Payne, R.S.; Rigor, B.M. An increase in lactate output by brain tissue serves to meet the energy needs of glutamate-activated neurons. J. Neurosci. 1999, 19, 34–39. [Google Scholar] [CrossRef]
- Brooks, G.A. Intra-and extra-cellular lactate shuttles. Med. Sci. Sports Exerc. 2000, 32, 790–799. [Google Scholar] [CrossRef]
- Brooks, G.A. Cell–cell and intracellular lactate shuttles. J. Physiol. 2009, 587, 5591–5600. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Håberg, A.; Haraldseth, O.; Unsgård, G.; Sonnewald, U. 13C MR spectroscopy study of lactate as substrate for rat brain. Dev. Neurosci. 2000, 22, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Mangia, S.; Garreffa, G.; Bianciardi, M.; Giove, F.; Di Salle, F.; Maraviglia, B. The aerobic brain: Lactate decrease at the onset of neural activity. Neuroscience 2003, 118, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Pernet, A.; Hallett, W.A.; Bingham, E.; Marsden, P.K.; Amiel, S.A. Lactate: A preferred fuel for human brain metabolism in vivo. J. Cereb. Blood Flow Metab. 2003, 23, 658–664. [Google Scholar] [CrossRef]
- Kasischke, K.A.; Vishwasrao, H.D.; Fisher, P.J.; Zipfel, W.R.; Webb, W.W. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science 2004, 305, 99–103. [Google Scholar] [CrossRef]
- Herard, A.S.; Dubois, A.; Escartin, C.; Tanaka, K.; Delzescaux, T.; Hantraye, P.; Bonvento, G. Decreased metabolic response to visual stimulation in the superior colliculus of mice lacking the glial glutamate transporter GLT-1. Eur. J. Neurosci. 2005, 22, 1807–1811. [Google Scholar] [CrossRef]
- Schurr, A.; Payne, R.S. Lactate, not pyruvate, is neuronal aerobic glycolysis end- product: An in vitro electrophysiological study. Neuroscience 2007, 147, 613–619. [Google Scholar] [CrossRef]
- Hashimoto, T.; Hussien, R.; Cho, H.; Kaufer, D.; Brooks, G.A. Evidence for the mitochondrial lactate oxidation complex in rat neurons: Demonstration of an essential component of brain lactate shuttles. PLoS ONE 2008, 3, e2915. [Google Scholar] [CrossRef]
- Erlichman, J.S.; Hewitt, A.; Damon, T.L.; Hart, M.; Kurascz, J.; Li, A.; Leiter, J.C. Inhibition of monocarboxylate transporter 2 in the retrotrapezoid nucleus in rats: A test of the astrocyte–neuron lactate-shuttle hypothesis. J. Neurosci. 2008, 28, 4888–4896. [Google Scholar] [CrossRef]
- Gallagher, C.N.; Carpenter, K.L.; Grice, P.; Howe, D.J.; Mason, A.; Timofeev, I.; Menon, D.K.; Kirpatrick, P.J.; Pickard, J.D.; Sutherland, G.R.; et al. The human brain utilizes lactate via the tricarboxylic acid cycle: A 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain 2009, 132, 2839–2849. [Google Scholar] [CrossRef]
- Chuquet, J.; Quilichini, P.; Nimchinsky, E.A.; Buzsáki, G. Predominant enhancement of glucose uptake in astrocytes versus neurons during activation of the somatosensory cortex. J. Neurosci. 2010, 30, 15298–15303. [Google Scholar] [CrossRef] [PubMed]
- Figley, C.R. Lactate transport and metabolism in the human brain: Implications for the astrocyte-neuron lactate shuttle hypothesis. J. Neurosci. 2011, 31, 4768–4770. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.; Fernandes, E.; Barbosa, R.M.; Laranjinha, J.; Ledo, A. Astrocytic aerobic glycolysis provides lactate to support neuronal oxidative metabolism in the hippocampus. Biofactors 2023, 49, 875–886. [Google Scholar] [CrossRef]
- Rodgers, Z.B.; Detre, J.A.; Wehrli, F.W. MRI-based methods for quantification of the cerebral metabolic rate of oxygen. J. Cereb. Blood Flow Metab. 2016, 36, 1165–1185. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Torres, J.S.; Portilla, D.F.A.; Córdoba, W.J.D.; Ordoñez, B.A.R.; Cerón, L.F.Z.; Bastidas, T.A.Z. The Cerebral Metabolic Requirements Under Normal Conditions; University of Minnesota Scholarship Repository, Minnesota 002, Department of Neurology: Minneapolis, MN, USA. 2021. Available online: https://anmdecolombia.org.co/ (accessed on 1 May 2025).
- Fox, P.T.; Raichle, M.E.; Mintun, M.A.; Dence, C. Nonoxidative glucose consumption during focal physiologic neural activity. Science 1988, 24, 462–464. [Google Scholar] [CrossRef]
- Warburg, O. The metabolism of carcinoma cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Schurr, A.; Passarella, S. Aerobic glycolysis: A DeOxymoron of (neuro) biology. Metabolites 2022, 12, 72. [Google Scholar] [CrossRef]
- Schurr, A. Lactate: The ultimate cerebral oxidative energy substrate? J. Cereb. Blood Flow Metab. 2006, 26, 142–152. [Google Scholar] [CrossRef]
- Schurr, A. Lactate: A major and crucial player in normal function of both muscle and brain. J. Physiol. 2008, 586, 2665. [Google Scholar] [CrossRef]
- Schurr, A. From rags to riches: Lactate ascension as a pivotal metabolite in neuroenergetics. Front. Neurosci. 2023, 17, 1145358. [Google Scholar] [CrossRef]
- Schurr, A. How the ‘Aerobic/Anaerobic Glycolysis’ Meme Formed a ‘Habit of Mind’ Which Impedes Progress in the Field of Brain Energy Metabolism. Int. J. Mol. Sci. 2024, 25, 1433. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A.; Gozal, E. Aerobic production and utilization of lactate satisfy increased energy demands upon neuronal activation in hippocampal slices and provide neuroprotection against oxidative stress. Front. Pharmacol. 2012, 2, 96. [Google Scholar] [CrossRef] [PubMed]
- Theriault, J.E.; Shaffer, C.; Dienel, G.A.; Sander, C.Y.; Hooker, J.M.; Dickerson, B.C.; Feldman-Barrett, L.; Quigley, K.S. A functional account of stimulation-based aerobic glycolysis and its role in interpreting BOLD signal intensity increases in neuroimaging experiments. Neurosci. Biobehav. Rev. 2023, 153, 105373. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.D.; Sokoloff, L. Circulation and energy metabolism of the brain. In Basic Neurochemistry, 5th ed.; Siegle, G.J., Agranoff, B.W., Albers, R.W., Molinoff, P.B., Eds.; Raven Press: New York, NY, USA, 1999; Chapter 31; pp. 645–680. [Google Scholar]
- Ritchie, S. Science Fictions: Exposing Fraud, Bias, Negligence and Hype in Science; Random House: Bengaluru, India, 2020. [Google Scholar]
- Takahashi, S.; Driscoll, B.F.; Law, M.J.; Sokoloff, L. Role of sodium and potassium ions in regulation of glucose metabolism in cultured astroglia. Proc. Natl. Acad. Sci. USA 1995, 92, 4616–4620. [Google Scholar] [CrossRef]
- Sokoloff, L. Energetics of functional activation in neural tissues. Neurochem. Res. 1999, 24, 321–329. [Google Scholar] [CrossRef]
- Cruz, N.F.; Adachi, K.; Dienel, G.A. Rapid efflux of lactate from cerebral cortex during K+-induced spreading cortical depression. J. Cereb. Blood Flow Metab. 1999, 19, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Hertz, L.; Dienel, G.A. Energy metabolism in the brain. Int. Rev. Neurobiol. 2002, 51, 1-IN4. [Google Scholar] [CrossRef]
- Dienel, G.A.; Cruz, N.F. Nutrition during brain activation: Does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochem. Int. 2004, 45, 321–351. [Google Scholar] [CrossRef]
- Hertz, L. The astrocyte-neuron lactate shuttle: A challenge of a challenge. J. Cereb. Blood Flow Metab. 2004, 24, 1241–1248. [Google Scholar] [CrossRef]
- Dienel, G.A. Lactate muscles its way into consciousness: Fueling brain activation. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 287, R519–R521. [Google Scholar] [CrossRef]
- Sokoloff, L. Energy metabolism in neural tissues in vivo at rest and in functionally altered states. In Brain Energetics and Neuronal Activity; Wiley: Chichester, UK, 2004; pp. 11–30. [Google Scholar]
- Hertz, L.; Dienel, G.A. Lactate transport and transporters: General principles and functional roles in brain cells. J. Neurosci. Res. 2005, 79, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A.; Hertz, L. Astrocytic contributions to bioenergetics of cerebral ischemia. Glia 2005, 50, 362–388. [Google Scholar] [CrossRef]
- Abe, T.; Takahashi, S.; Suzuki, N. Metabolic properties of astrocytes differentiated from rat neurospheres. Brain Res. 2006, 1101, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A.; Cruz, N.F. Astrocyte activation in working brain: Energy supplied by minor substrates. Neurochem. Int. 2006, 48, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Hertz, L.; Peng, L.; Dienel, G.A. Energy metabolism in astrocytes: High rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J. Cereb. Blood Flow Metab. 2007, 27, 219–249. [Google Scholar] [CrossRef]
- Cruz, N.F.; Ball, K.K.; Dienel, G.A. Functional imaging of focal brain activation in conscious rats: Impact of [14C] glucose metabolite spreading and release. J. Neurosci. Res. 2007, 85, 3254–3266. [Google Scholar] [CrossRef]
- Gandhi, G.K.; Cruz, N.F.; Ball, K.K.; Dienel, G.A. Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. J. Neurochem. 2009, 111, 522–536. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain lactate metabolism: The discoveries and the controversies. J. Cereb. Blood Flow Metab. 2012, 32, 1107–1138. [Google Scholar] [CrossRef]
- Dienel, G.A. Fueling and imaging brain activation. ASN Neuro 2012, 4, AN20120021. [Google Scholar] [CrossRef]
- Dienel, G.A. Astrocytic energetics during excitatory neurotransmission: What are contributions of glutamate oxidation and glycolysis? Neurochem. Int. 2013, 63, 244–258. [Google Scholar] [CrossRef]
- Dienel, G.A. Lactate shuttling and lactate use as fuel after traumatic brain injury: Metabolic considerations. J. Cereb. Blood Flow Metab. 2014, 34, 1736–1748. [Google Scholar] [CrossRef]
- Dienel, G.A. Lack of appropriate stoichiometry: Strong evidence against an energetically important astrocyte–neuron lactate shuttle in brain. J. Neurosci. Res. 2017, 95, 2103–2125. [Google Scholar] [CrossRef]
- Dienel, G.A. The metabolic trinity, glucose–glycogen–lactate, links astrocytes and neurons in brain energetics, signaling, memory, and gene expression. Neurosci. Lett. 2017, 637, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A. Brain glucose metabolism: Integration of energetics with function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef] [PubMed]
- Rae, C.D.; Baur, J.A.; Borges, K.; Dienel, G.; Díaz-García, C.M.; Douglass, S.R.; Kelly, D.; Duarte, J.M.N.; Duran, J.; Kann, O.; et al. Brain energy metabolism: A roadmap for future research. J. Neurochem. 2024, 168, 910–954. [Google Scholar] [CrossRef]
- Dienel, G.A.; Rothman, D.L. In vivo calibration of genetically encoded metabolite biosensors must account for metabolite metabolism during calibration and cellular volume. J. Neurochem. 2024, 168, 506–532. [Google Scholar] [CrossRef]
- Brooks, G.A.; Dubouchaud, H.; Brown, M.; Sicurello, J.P.; Butz, C.E. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc. Natl. Acad. Sci. USA 1999, 96, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Mahieu, N.G.; Huang, X.; Singh, M.; Crawford, P.A.; Johnson, S.L.; Gross, R.W.; Schaefer, J.; Patti, G.J. Lactate metabolism is associated with mammalian mitochondria. Nat. Chem. Biol. 2016, 12, 937–943. [Google Scholar] [CrossRef]
- Young, A.; Oldford, C.; Mailloux, R. Lactate dehydrogenase supports lactate oxidation in mitochondria isolated from different mouse tissues. Redox Biol. 2020, 28, 101339. [Google Scholar] [CrossRef]
- Passarella, S.; Schurr, A.; Portincasa, P. Mitochondrial transport in glycolysis and gluconeogenesis: Achievements and perspectives. Int. J. Mol. Sci. 2021, 22, 12620. [Google Scholar] [CrossRef]
- Kocianova, E.; Piatrikova, V.; Golias, T. Revisiting the Warburg effect with focus on lactate. Cancers 2022, 14, 6028. [Google Scholar] [CrossRef]
- Brooks, G.A.; Curl, C.C.; Leija, R.G.; Osmond, A.D.; Duong, J.J.; Arevalo, J.A. Tracing the lactate shuttle to the mitochondrial reticulum. Exp. Mol. Med. 2022, 54, 1332–1347. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Xu, L.; Wang, A.; Zou, Y.; Li, T.; Huang, L.; Chen, W.; Liu, S.; Jiang, K.; et al. Ultrasensitive sensors reveal the spatiotemporal landscape of lactate metabolism in physiology and disease. Cell Metab. 2023, 35, 200–211. [Google Scholar] [CrossRef]
- Dalsgaard, M.K.; Quistorff, B.; Danielsen, E.R.; Selmer, C.; Vogelsang, T.; Secher, N.H. A reduced cerebral metabolic ratio in exercise reflects metabolism and not accumulation of lactate within the human brain. J. Physiol. 2004, 554, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, J.E.; Alessandri, B.; Reinert, M.; Clausen, T.; Zhou, Z.; Altememi, N.; Bullock, M.R. Lactate, not glucose, up-regulates mitochondrial oxygen consumption both in sham and lateral fluid percussed rat brains. Neurosurgery 2006, 59, 1122–1131. [Google Scholar] [CrossRef]
- Van Hall, G.; Stømstad, M.; Rasmussen, P.; Jans, Ø.; Zaar, M.; Gam, C.; Quistorff, B.; Secher, N.H.; Nielsen, H.B. Blood lactate is an important energy source for the human brain. J. Cereb. Blood Flow Metab. 2009, 29, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.; Wyss, M.T.; Lundby, C. Cerebral glucose and lactate consumption during cerebral activation by physical activity in humans. FASEB J. 2011, 25, 2865–2873. [Google Scholar] [CrossRef]
- Overgaard, M.; Rasmussen, P.; Bohm, A.M.; Seifert, T.; Brassard, P.; Zaar, M.; Homann, P.; Evans, K.A.; Nielsen, H.B.; Secher, N.H. Hypoxia and exercise provoke both lactate release and lactate oxidation by the human brain. FASEB J. 2012, 26, 3012–3020. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Tsukamoto, H.; Takenaka, S.; Olesen, N.D.; Petersen, L.G.; Sørensen, H.; Nielsen, H.B.; Secher, N.H.; Ogoh, S. Maintained exercise-enhanced brain executive function related to cerebral lactate metabolism in men. FASEB J. 1018, 32, 1417–1427. [Google Scholar] [CrossRef]
- Cai, M.; Wang, H.; Song, H.; Yang, R.; Wang, L.; Xue, X.; Sun, W.; Hu, J. Lactate Is Answerable for Brain Function and Treating Brain Diseases: Energy Substrates and Signal Molecule. Front. Nutr. 2022, 9, 800901. [Google Scholar] [CrossRef]
- Benarroch, E. What is the role of lactate in brain metabolism, plasticity, and neurodegeneration? Neurology 2024, 102, e209378. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Yoon, B.S.; Han, S.; Kim, Y.; Park, C.B. Diminished lactate utilization in LDHB-deficient neurons leads to impaired long-term memory retention. Exp. Neurol. 2025, 384, 115064. [Google Scholar] [CrossRef] [PubMed]
| 1. Takahashi et al. [71]. The first publication from Sokoloff’s laboratory, citing the ANLS hypothesis and agreeing with the observation that brain activation stimulates astrocytic glucose consumption. 2. Sokoloff et al. [20]. Another publication that agrees with the participation of astrocytes in increased glucose metabolism upon stimulation. 3. Sokoloff [72]. A paper summarizing his laboratory’s findings about astrocytic ion uptake and glucose consumption, without mentioning the other portion of the ANLS hypothesis, which is the neuronal oxidative consumption of lactate. 4. Cruz et al. [73]. The first paper from this school that relates to the lactate produced during brain activation, limiting the study to the brain cortex and only measuring lactate efflux to blood and estimates of efflux to other brain regions. A possible lactate oxidative utilization was not considered. 5. Dienel and Hertz [21]. A publication by one of Sokoloff’s disciples, who combined forces with Professor Leif Herz, an opponent of the ANLS hypothesis, in minimizing the glycolytic production of lactate to normal overflow upon brain activation, while arguing that glutamate uptake by astrocytes is supported by oxidative metabolism, not aerobic glycolysis. 6. Hertz and Dienel [74]. In a review article, the authors summarized the central role of glucose in energy metabolism, while also discounting all in vitro systems used in the study of brain energy metabolism. They focused on the use of MRI as the technique that allows the measuring of local glucose metabolism in vivo and thereby confirming many of the in vitro findings, while also ignoring any role for lactate as an oxidative energy substrate for neural activity. 7. Dienel and Cruz [75]. The first paper by Dienel and Co. to argue specifically against the ANLS hypothesis. 8. Hertz [76]. A paper that echoes Dienel and Cruz’s (2004) argument, i.e., that there is “no solid indication that lactate oxidation should primarily occur in neurons, and there is very good evidence that it is not exclusively a neuronal process”. 9. Dienel [77]. In an Editorial, the author, although accepting of the fact that lactate could be an oxidative substrate in the brain, continued to argue against the ANLS hypothesis. 10. Sokoloff [78]. In this book chapter, Sokoloff himself seemed to accept the possibility of an astrocytic–neuronal lactate shuttle and lactate oxidative utilization. 11. Hertz and Dienel [79]. Accepting the fact that lactate can be used oxidatively by brain cells, the authors argued that the neuronal lactate transporter (MCT2) is a high-affinity transporter that would not allow for a speedy uptake of lactate necessary for immediate use. 12. Dienel and Hertz [80]. The results presented in this paper are very similar to those found in the paper by Hertz and Dienel (2005). 13. Abe et al. [81]. This study found that certain astroglia, upon activation of the Na+/K+-ATPase, prefer lactate oxidation over glucose. One of the authors is S. Takahashi, who worked in Sokoloff’s laboratory. 14. Dienel and Cruze [82]. The authors tested the ability of astrocytes in vitro to utilize substrates other than glucose during activation. They tested glycogen, acetate, and glutamate, concluding that all three could be utilized by astrocytes during stimulation. Interestingly, they did not test lactate (why?). 15. Hertz et al. [83]. In this review article, the authors summarized their findings as follows: Oxidative metabolism, glycolysis, and, in some cases, glycogenolysis in astrocytes, increase when their workload rises. The energy requirement for glutamate uptake and metabolism to glutamine may be the most generally recognized energy-requiring process in astrocytes. During brain stimulation, active K+ uptake may pose the largest demand on ATP and especially on glycolytically generated ATP. Energy balance sheets and models of astrocyte–neuron interactions that do not include these functions greatly underestimate astrocytic energetics and portray a very limited context within which to understand astrocytic energetics. When lactate is produced during these processes, its fate is unknown. 16. Cruz et al. [84]. Using 14C-labled glucose and 2-deoxyglucose, the authors concluded that upon brain activation, in vivo focal CMRglucose is underestimated when using labeled glucose “because of decarboxylation reactions, spreading within tissue and via the astrocyte syncytium, and release from activated tissue”. They stated that such underestimation could “explain the fall in CMRO2/CMRglucose during brain activation and suggest that lactate and other non-oxidized metabolites of glucose are quickly shuttled away from sites of functional activation”. The possibility that the fall could be due to oxidative utilization of lactate rather than glucose was not considered. 17. Gandhi et al. [85]. Another study from Dienel’s laboratory where labeled glucose was used to measure its utilization and lactate production, which insisted that lactate is slow to be taken up by neurons compared to the ability of astrocytes, and argued that most of the lactate efflux to the blood stream and to other brain regions. Lactate oxidative utilization by neurons was not considered. 18. Dienel [86]. Another review article where the author, after a myriad of studies by various groups that consistently showed lactate to be an oxidative neuronal substrate, relented somewhat: “Brain activation in subjects with low plasma lactate causes outward, brain-to-blood lactate gradients, and lactate is quickly released in substantial amounts. Lactate utilization by the adult brain increases during lactate infusions and strenuous exercise that markedly increase blood lactate levels. Lactate can be an ‘opportunistic’, glucose-sparing substrate when present in high amounts, but most evidence supports glucose as the major fuel for normal, activated brain”. 19. Dienel [87]. A second review article with the same conclusion: “Brain activation in subjects with low blood-lactate levels causes a brain-to-blood lactate gradient, with rapid lactate release. In contrast, lactate flooding of brain during physical activity or infusion provides an opportunistic, supplemental fuel. Available evidence indicates that lactate shuttling coupled to its local oxidation during activation is a small fraction of glucose oxidation”. 20. Dienel [88]. An invited review where the author made the following conclusion: “Three lines of evidence indicate that critical cornerstones of the astrocyte-to-neuron lactate shuttle model are not established, and normal brain does not need lactate as supplemental fuel”. 21. Dienel [89]. An opinion article that touched on the potential of treating traumatic brain injury with lactate: “Results show that lactate release from human brain to blood predominates over its uptake after TBI, and strong evidence for lactate metabolism is lacking; mitochondrial dysfunction may inhibit lactate oxidation. Claims that exogenous lactate infusion is energetically beneficial for TBI patients are not based on metabolic assays and data are incorrectly interpreted”. 22. Dienel [90]. Another review that downplayed the role of lactate in astrocytic–neuronal lactate shuttle: “Glucose is the obligatory fuel for adult brain, but lactate produced from glucose by astrocytes within brain during activation has been proposed to serve as neuronal fuel. However, metabolic requirements for substantial lactate shuttling and oxidation are not fulfilled, and this notion is refuted by several independent lines of evidence. Understanding preferential upregulation of glucose compared with oxygen utilization is central to elucidating brain energetics”. 23. Dienel [91]. A research paper that highlighted the following: “Glucose and lactate enhance memory; glycogen is required for its consolidation. Hogh-dose lactate preserves memory and gene expression without glycogenolysis. High-dose lactate suppresses neuronal firing and is taken up mainly by astrocytes”. 24. Dienel [92]. Review article that concluded: “Shuttling of glucose- and glycogen-derived lactate from astrocytes to neurons during activation, neurotransmission, and memory consolidation are controversial topics for which alternative mechanisms are proposed”. 25. Rae et al. [93]. A collaboration of Dienel with 25 other authors on a review article that held to the dogmatic concept, according to which under resting conditions glycolysis ends with pyruvate, not with lactate. When lactate is produced (aerobic glycolysis), it “may escape from its “dead-end” reaction by leaving the cell, which it does through an array of transporters”. Where the ANLS hypothesis was concerned, this review stated: “Many groups have embraced the concept of an astrocyte to neuron lactate shuttle since it was first proposed … based on in vitro studies. However, studies in vivo supporting this shuttle are few and not convincing”. 26. Dienel and Rothman [94]. In this research paper, the authors used a genetically encoded lactate biosensor to measure changes in the level of the monocarboxylate both in vitro and in vivo. The impetus for this initiative was several studies that showed a gradient of lactate from astrocytes to neurons. The authors concluded that unless a calibration of the biosensor is performed both for the metabolism of lactate during the measurement and for the cellular volume of the cell-type under monitoring, any measurement is questionable. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schurr, A. The Feud over Lactate and Its Role in Brain Energy Metabolism: An Unnecessary Burden on Research and the Scientists Who Practice It. Int. J. Mol. Sci. 2025, 26, 4429. https://doi.org/10.3390/ijms26094429
Schurr A. The Feud over Lactate and Its Role in Brain Energy Metabolism: An Unnecessary Burden on Research and the Scientists Who Practice It. International Journal of Molecular Sciences. 2025; 26(9):4429. https://doi.org/10.3390/ijms26094429
Chicago/Turabian StyleSchurr, Avital. 2025. "The Feud over Lactate and Its Role in Brain Energy Metabolism: An Unnecessary Burden on Research and the Scientists Who Practice It" International Journal of Molecular Sciences 26, no. 9: 4429. https://doi.org/10.3390/ijms26094429
APA StyleSchurr, A. (2025). The Feud over Lactate and Its Role in Brain Energy Metabolism: An Unnecessary Burden on Research and the Scientists Who Practice It. International Journal of Molecular Sciences, 26(9), 4429. https://doi.org/10.3390/ijms26094429







