Gut Microbiota and Cardiovascular Diseases: Unraveling the Role of Dysbiosis and Microbial Metabolites
Abstract
1. Introduction
2. Human Microbial Contributions
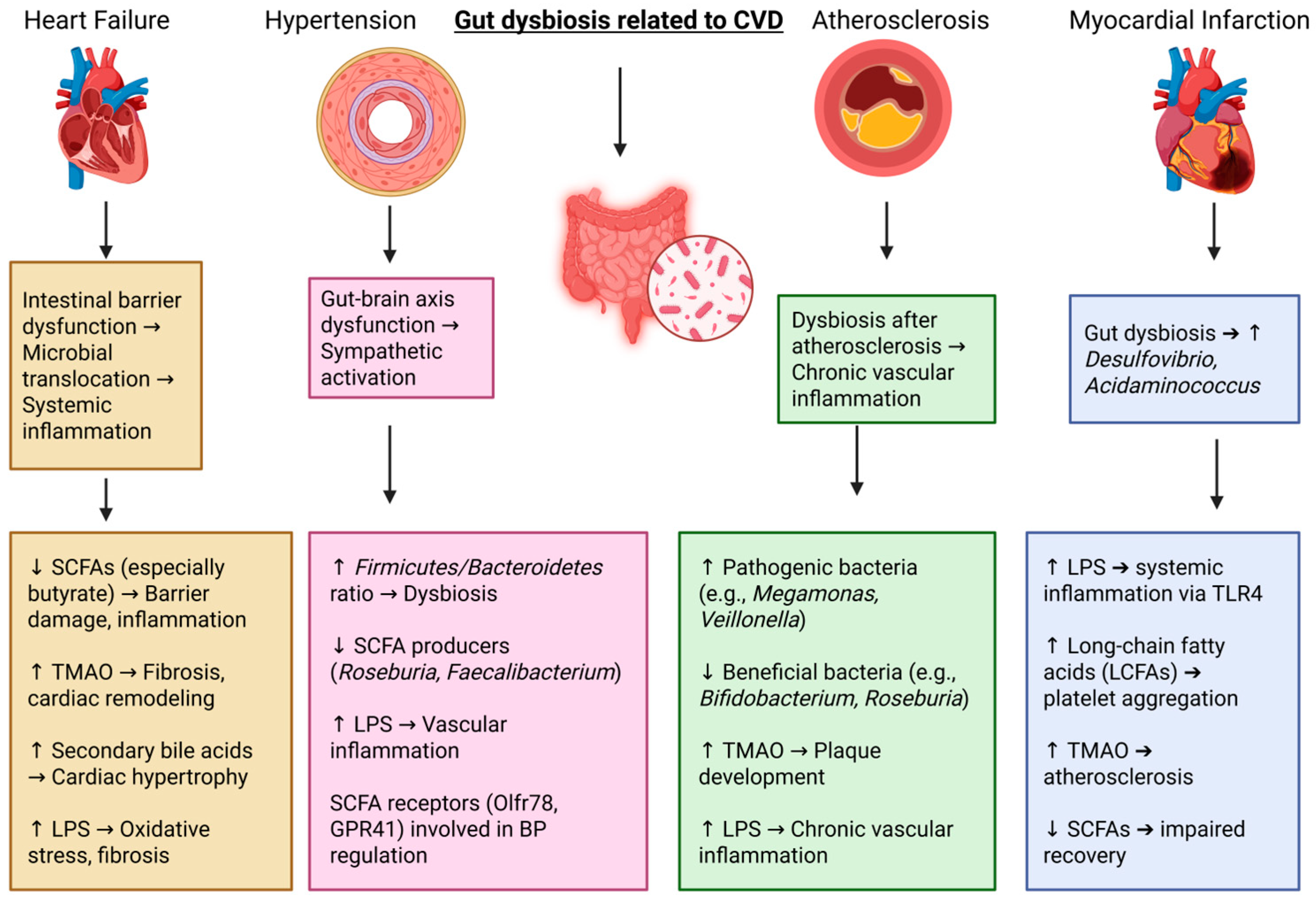
3. Cardiovascular Diseases (CVDs) and Gut Microbiota
4. HFs and Gut Microbial Changes
5. Hypertension and Gut Microbial Changes
6. Myocardial Infarction and Gut Dysbiosis
7. Atherosclerosis and Gut Microbiota Composition
8. Natural Products and Their Potential in CVD
8.1. Flavonoids
8.2. Omega-3 Fatty Acids
8.3. Resveratrol
8.4. Curcumin
8.5. Coenzyme Q10 (CoQ10)
8.6. Marine-Derived Compounds
9. Natural Products as Microbial Modulators for Cardioprotection
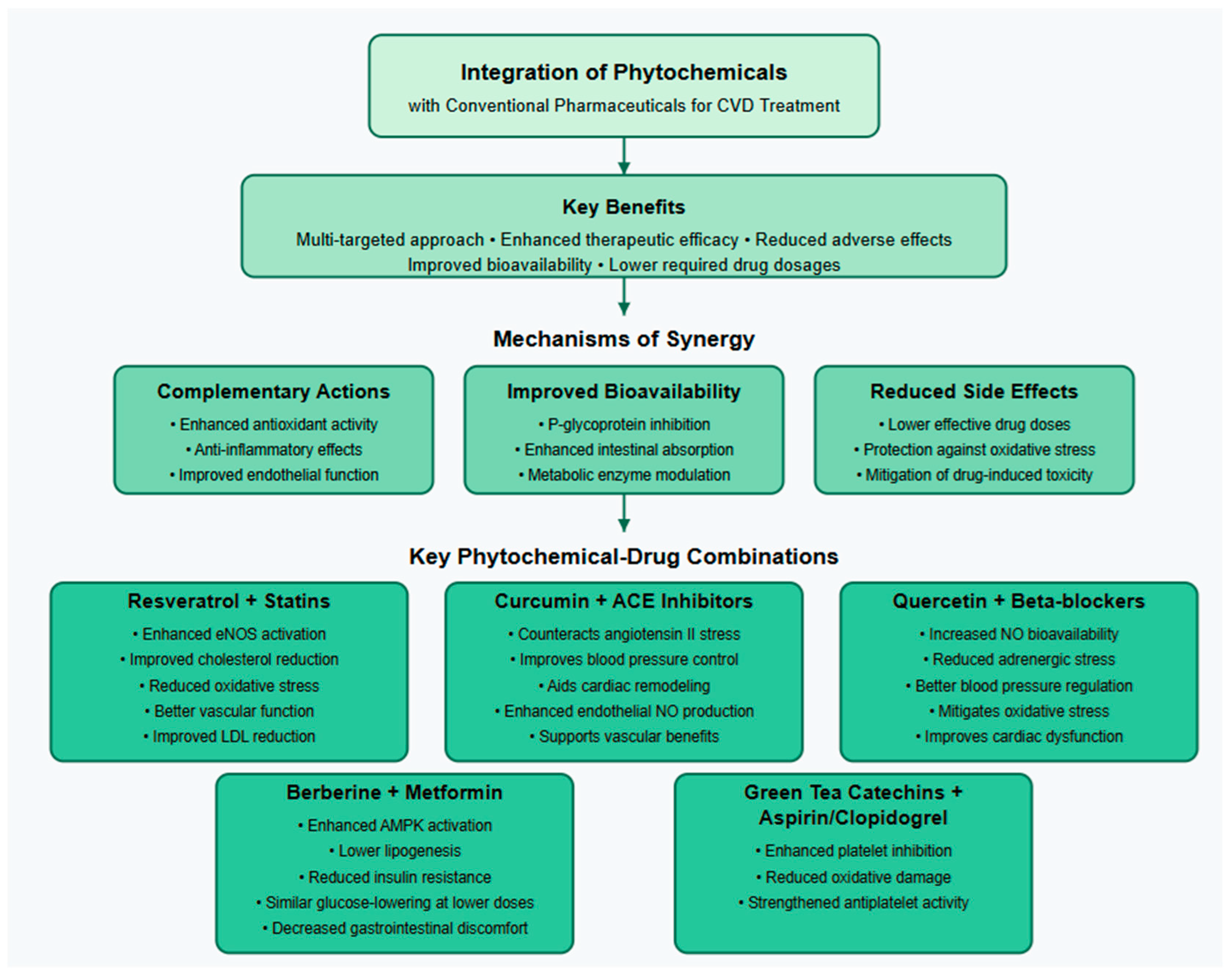
10. Synergistic Effects of Phytochemicals in CVD
11. Challenges and Future Directions
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hacker, K. The Burden of Chronic Disease. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Narula, J.; et al. The Heart of the World. Glob. Heart 2024, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Ndubuisi, N.E. Noncommunicable Diseases Prevention In Low- and Middle-Income Countries: An Overview of Health in All Policies (HiAP). Inq. A J. Med. Care Organ. Provis. Financ. 2021, 58, 46958020927885. [Google Scholar] [CrossRef]
- NCD Countdown 2030 collaborators. NCD Countdown 2030: Worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet 2018, 392, 1072–1088. [Google Scholar] [CrossRef]
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and Prevention Strategies for Non-communicable Diseases (NCDs) and Their Risk Factors. Front. Public Health 2020, 8, 574111. [Google Scholar] [CrossRef]
- Miranda, J.J.; Kinra, S.; Casas, J.P.; Davey Smith, G.; Ebrahim, S. Non-communicable diseases in low- and middle-income countries: Context, determinants and health policy. Trop. Med. Int. Health 2008, 13, 1225–1234. [Google Scholar] [CrossRef]
- Phelan, H.; Yates, V.; Lillie, E. Challenges in healthcare delivery in low- and middle-income countries. Anaesth. Intensiv. Care Med. 2022, 23, 501–504. [Google Scholar] [CrossRef]
- Piot, P.; Caldwell, A.; Lamptey, P.; Nyrirenda, M.; Mehra, S.; Cahill, K.; Aerts, A. Addressing the growing burden of non-communicable disease by leveraging lessons from infectious disease management. J. Glob. Health 2016, 6, 010304. [Google Scholar] [CrossRef]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef]
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I.; et al. Microbiota medicine: Towards clinical revolution. J. Transl. Med. 2022, 20, 111. [Google Scholar] [CrossRef]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of Gut Microbiota from the Perspective of the Gut-Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12, 1064. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Lim, K.G.; Palayan, K. A Review of Gastric Cancer Research in Malaysia. Asian Pac. J. Cancer Prev. 2019, 20, 5–11. [Google Scholar] [CrossRef]
- Hanafiah, A.; Binmaeil, H.; Raja Ali, R.A.; Mohamed Rose, I.; Lopes, B.S. Molecular characterization and prevalence of antibiotic resistance in Helicobacter pylori isolates in Kuala Lumpur, Malaysia. Infect. Drug Resist. 2019, 12, 3051–3061. [Google Scholar] [CrossRef]
- Fung, J.; Lai, C.L.; Yuen, M.F. Hepatitis B and C virus-related carcinogenesis. Clin. Microbiol. Infect. 2009, 15, 964–970. [Google Scholar] [CrossRef]
- Barathan, M.; Mohamed, R.; Yong, Y.K.; Kannan, M.; Vadivelu, J.; Saeidi, A.; Larsson, M.; Shankar, E.M. Viral Persistence and Chronicity in Hepatitis C Virus Infection: Role of T-Cell Apoptosis, Senescence and Exhaustion. Cells 2018, 7, 165. [Google Scholar] [CrossRef]
- Hatton, O.L.; Harris-Arnold, A.; Schaffert, S.; Krams, S.M.; Martinez, O.M. The interplay between Epstein-Barr virus and B lymphocytes: Implications for infection, immunity, and disease. Immunol. Res. 2014, 58, 268–276. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhu, L.; Yang, L. Gut and obesity/metabolic disease: Focus on microbiota metabolites. MedComm 2022, 3, e171. [Google Scholar] [CrossRef]
- Oka, A.; Sartor, R.B. Microbial-Based and Microbial-Targeted Therapies for Inflammatory Bowel Diseases. Dig. Dis. Sci. 2020, 65, 757–788. [Google Scholar] [CrossRef] [PubMed]
- Plamada, D.; Vodnar, D.C. Polyphenols-Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef]
- Ogunrinola, G.A.; Oyewale, J.O.; Oshamika, O.O.; Olasehinde, G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020, 2020, 8045646. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Yüksel, E.; Voragen, A.G.J.; Kort, R. The pectin metabolizing capacity of the human gut microbiota. Crit. Rev. Food Sci. Nutr. 2024, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.A.; Schmitz, R.A. Exploring the Probiotic Potential of Bacteroides spp. Within One Health Paradigm. Probiotics Antimicrob. Proteins 2025, 17, 681–704. [Google Scholar] [CrossRef]
- Petakh, P.; Oksenych, V.; Kamyshnyi, A. The F/B ratio as a biomarker for inflammation in COVID-19 and T2D: Impact of metformin. Biomed. Pharmacother. 2023, 163, 114892. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10 (Suppl. 1), S49–S66. [Google Scholar] [CrossRef]
- Moon, C.D.; Young, W.; Maclean, P.H.; Cookson, A.L.; Bermingham, E.N. Metagenomic insights into the roles of Proteobacteria in the gastrointestinal microbiomes of healthy dogs and cats. MicrobiologyOpen 2018, 7, e00677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef]
- Sejbuk, M.; Mirończuk-Chodakowska, I.; Karav, S.; Witkowska, A.M. Dietary Polyphenols, Food Processing and Gut Microbiome: Recent Findings on Bioavailability, Bioactivity, and Gut Microbiome Interplay. Antioxidants 2024, 13, 1220. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Wolters, M.; Weyh, C.; Krüger, K.; Ticinesi, A. The Effects of Lifestyle and Diet on Gut Microbiota Composition, Inflammation and Muscle Performance in Our Aging Society. Nutrients 2021, 13, 2045. [Google Scholar] [CrossRef]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr. Healthy Aging 2018, 4, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative Med. Cell Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef] [PubMed]
- Bishehsari, F.; Magno, E.; Swanson, G.; Desai, V.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. Alcohol and Gut-Derived Inflammation. Alcohol Res. Curr. Rev. 2017, 38, 163–171. [Google Scholar]
- Bruno, G.; Zaccari, P.; Rocco, G.; Scalese, G.; Panetta, C.; Porowska, B.; Pontone, S.; Severi, C. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World J. Gastroenterol. 2019, 25, 2706–2719. [Google Scholar] [CrossRef]
- Juarez, V.M.; Montalbine, A.N.; Singh, A. Microbiome as an immune regulator in health, disease, and therapeutics. Adv. Drug Deliv. Rev. 2022, 188, 114400. [Google Scholar] [CrossRef]
- Bora, S.S.; Gogoi, R.; Sharma, M.R.; Anshu Borah, M.P.; Deka, P.; Bora, J.; Naorem, R.S.; Das, J.; Teli, A.B. Microplastics and human health: Unveiling the gut microbiome disruption and chronic disease risks. Front. Cell. Infect. Microbiol. 2024, 14, 1492759. [Google Scholar] [CrossRef]
- Zhang, L.; Agrawal, M.; Ng, S.C.; Jess, T. Early-life exposures and the microbiome: Implications for IBD prevention. Gut 2024, 73, 541–549. [Google Scholar] [CrossRef]
- Ojeda-Granados, C.; Campisi, E.; Barchitta, M.; Agodi, A. Genetic, lifestyle and metabolic factors contributing to cardiovascular disease in the Italian population: A literature review. Front. Nutr. 2024, 11, 1379785. [Google Scholar] [CrossRef]
- Tang, W.H.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Feng, W.; Liu, Q.; Zhou, S.; Liu, Q.; Cai, L. The gut microbiota and its interactions with cardiovascular disease. Microb. Biotechnol. 2020, 13, 637–656. [Google Scholar] [CrossRef] [PubMed]
- Shahim, B.; Kapelios, C.J.; Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure: An Updated Review. Card. Fail. Rev. 2023, 9, e11. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Borovac, J.A.; D’Amario, D.; Bozic, J.; Glavas, D. Sympathetic nervous system activation and heart failure: Current state of evidence and the pathophysiology in the light of novel biomarkers. World J. Cardiol. 2020, 12, 373–408. [Google Scholar] [CrossRef]
- Fukui, H. Increased Intestinal Permeability and Decreased Barrier Function: Does It Really Influence the Risk of Inflammation? Inflamm. Intest. Dis. 2016, 1, 135–145. [Google Scholar] [CrossRef]
- Trehan, S.; Singh, G.; Bector, G.; Jain, P.; Mehta, T.; Goswami, K.; Chawla, A.; Jain, A.; Puri, P.; Garg, N. Gut Dysbiosis and Cardiovascular Health: A Comprehensive Review of Mechanisms and Therapeutic Potential. Cureus 2024, 16, e67010. [Google Scholar] [CrossRef]
- Méndez-Bailón, M.; Jiménez-García, R.; Hernández-Barrera, V.; Miguel-Díez, J.; Miguel-Yanes, J.M.; Muñoz-Rivas, N.; Lorenzo-Villalba, N.; Carabantes-Alarcon, D.; Zamorano-León, J.J.; Astasio-Arbiza, P.; et al. Heart Failure Is a Risk Factor for Suffering and Dying of Clostridium difficile Infection. Results of a 15-Year Nationwide Study in Spain. J. Clin. Med. 2020, 9, 614. [Google Scholar] [CrossRef]
- Duan, H.; Wang, L.; Huangfu, M.; Li, H. The impact of microbiota-derived short-chain fatty acids on macrophage activities in disease: Mechanisms and therapeutic potentials. Biomed. Pharmacother. 2023, 165, 115276. [Google Scholar] [CrossRef]
- Patil, R.S.; Maloney, M.E.; Lucas, R.; Fulton, D.J.R.; Patel, V.; Bagi, Z.; Kovacs-Kasa, A.; Kovacs, L.; Su, Y.; Verin, A.D. Zinc-Dependent Histone Deacetylases in Lung Endothelial Pathobiology. Biomolecules 2024, 14, 140. [Google Scholar] [CrossRef]
- Nagatomo, Y.; Tang, W.H. Intersections Between Microbiome and Heart Failure: Revisiting the Gut Hypothesis. J. Card. Fail. 2015, 21, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Zhang, H.; Xiang, Q.; Hu, H.; Zhai, C.; Xu, S.; Tian, H. Role of Trimethylamine N-Oxide in Heart Failure. Rev. Cardiovasc. Med. 2024, 25, 240. [Google Scholar] [CrossRef]
- Palm, C.L.; Nijholt, K.T.; Bakker, B.M.; Westenbrink, B.D. Short-Chain Fatty Acids in the Metabolism of Heart Failure—Rethinking the Fat Stigma. Front. Cardiovasc. Med. 2022, 9, 915102. [Google Scholar] [CrossRef] [PubMed]
- Riehle, C.; Abel, E.D. PGC-1 proteins and heart failure. Trends Cardiovasc. Med. 2012, 22, 98–105. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Yi, Q.; Luo, L.; Xiong, Y. Regulation of bile acids and their receptor FXR in metabolic diseases. Front. Nutr. 2024, 11, 1447878. [Google Scholar] [CrossRef] [PubMed]
- Zhenyukh, O.; González-Amor, M.; Rodrigues-Diez, R.R.; Esteban, V.; Ruiz-Ortega, M.; Salaices, M.; Mas, S.; Briones, A.M.; Egido, J. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. J. Cell Mol. Med. 2018, 22, 4948–4962. [Google Scholar] [CrossRef]
- Li, S. Modulation of immunity by tryptophan microbial metabolites. Front. Nutr. 2023, 10, 1209613. [Google Scholar] [CrossRef]
- Pereira, R.O.; Wende, A.R.; Crum, A.; Hunter, D.; Olsen, C.D.; Rawlings, T.; Riehle, C.; Ward, W.F.; Abel, E.D. Maintaining PGC-1α expression following pressure overload-induced cardiac hypertrophy preserves angiogenesis but not contractile or mitochondrial function. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2014, 28, 3691–3702. [Google Scholar] [CrossRef]
- Cianci, R.; Franza, L.; Borriello, R.; Pagliari, D.; Gasbarrini, A.; Gambassi, G. The Role of Gut Microbiota in Heart Failure: When Friends Become Enemies. Biomedicines 2022, 10, 2712. [Google Scholar] [CrossRef]
- Mamic, P.; Snyder, M.; Tang, W.H.W. Gut Microbiome-Based Management of Patients With Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023, 81, 1729–1739. [Google Scholar] [CrossRef]
- Huang, Y.J.; Ferrari, M.W.; Lin, S.; Wang, Z.H. Recent advances on the Role of Gut Microbiota in the Development of Heart Failure by Mediating Immune Metabolism. Curr. Probl. Cardiol. 2024, 49, 102128. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Shankar, R.; Singh, G.P. Prevalence and Associated Risk Factors of Hypertension: A Cross-Sectional Study in Urban Varanasi. Int. J. Hypertens 2017, 2017, 5491838. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Lu, J.; Lu, Y.; Wang, X.; Li, X.; Linderman, G.C.; Wu, C.; Cheng, X.; Mu, L.; Zhang, H.; Liu, J.; et al. Prevalence, awareness, treatment, and control of hypertension in China: Data from 1·7 million adults in a population-based screening study (China PEACE Million Persons Project). Lancet 2017, 390, 2549–2558. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, S.; Liu, Y.; Song, Z.; Ge, Z.; Fan, Y.; Chen, L.; Bi, Y.; Zhao, Z.; Wang, X.; et al. Targeting intestinal microecology: Potential intervention strategies of traditional Chinese medicine for managing hypertension. Front. Pharmacol. 2023, 14, 1171119. [Google Scholar] [CrossRef]
- Cai, M.; Lin, L.; Jiang, F.; Peng, Y.; Li, S.; Chen, L.; Lin, Y. Gut microbiota changes in patients with hypertension: A systematic review and meta-analysis. J. Clin. Hypertens 2023, 25, 1053–1068. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Microbial dysbiosis in the gut drives systemic autoimmune diseases. Front. Immunol. 2022, 13, 906258. [Google Scholar] [CrossRef]
- Drożdż, D.; Drożdż, M.; Wójcik, M. Endothelial dysfunction as a factor leading to arterial hypertension. Pediatr. Nephrol. 2023, 38, 2973–2985. [Google Scholar] [CrossRef]
- Al Khodor, S.; Reichert, B.; Shatat, I.F. The Microbiome and Blood Pressure: Can Microbes Regulate Our Blood Pressure? Front. Pediatr. 2017, 5, 138. [Google Scholar] [CrossRef]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Pawlonka, J.; Buchalska, B.; Buczma, K.; Borzuta, H.; Kamińska, K.; Cudnoch-Jędrzejewska, A. Targeting the Renin–angiotensin–aldosterone System (RAAS) for Cardiovascular Protection and Enhanced Oncological Outcomes: Review. Curr. Treat. Options Oncol. 2024, 25, 1406–1427. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.T.S.; Aguilar, E.C.; Campos, G.P.; do Couto, N.F.; Capettini, L.D.S.A.; Braga, W.F.; Andrade, L.O.; Alvarez-Leite, J. Butyrate inhibits LPC-induced endothelial dysfunction by regulating nNOS-produced NO and ROS production. Nitric Oxide Biol. Chem. 2023, 138–139, 42–50. [Google Scholar] [CrossRef]
- Beito, M.R.; Ashraf, S.; Odogwu, D.; Harmancey, R. Role of Ectopic Olfactory Receptors in the Regulation of the Cardiovascular–Kidney–Metabolic Axis. Life 2024, 14, 548. [Google Scholar] [CrossRef] [PubMed]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Hubbard, T.; Murray, I.; Bisson, W.; Lahoti, T.S.; Gowda, K.; Amin, S.G.; Patterson, A.D.; Perdew, G.H. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci. Rep. 2015, 5, 12689. [Google Scholar] [CrossRef]
- Xuan, M.; Gu, X.; Li, J.; Huang, D.; Xue, C.; He, Y. Polyamines: Their significance for maintaining health and contributing to diseases. Cell Commun. Signal. 2023, 21, 348. [Google Scholar] [CrossRef]
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A.A.; Hezarkhani, L.A.; Shohaimi, S.; Mohammadi, M. The global prevalence of myocardial infarction: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 206. [Google Scholar] [CrossRef]
- Rajati, F.; Rajati, M.; Chegeni, M.; Kazeminia, M. The prevalence of myocardial infarction in the elderly: A systematic review and meta-analysis. ARYA Atheroscler. 2024, 20, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Wereski, R.; Kimenai, D.M.; Bularga, A.; Taggart, C.; Lowe, D.J.; Mills, N.L.; Chapman, A.R. Risk factors for type 1 and type 2 myocardial infarction. Eur. Heart J. 2022, 43, 127–135. [Google Scholar] [CrossRef]
- Gaziano, T.A.; Bitton, A.; Anand, S.; Abrahams-Gessel, S.; Murphy, A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr. Probl. Cardiol. 2010, 35, 72–115. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, P.D.; Tsigalou, C.; Valsamaki, P.N.; Konstantinidis, T.G.; Voidarou, C.; Bezirtzoglou, E. The Emerging Role of the Gut Microbiome in Cardiovascular Disease: Current Knowledge and Perspectives. Biomedicines 2022, 10, 948. [Google Scholar] [CrossRef]
- Han, Y.; Gong, Z.; Sun, G.; Xu, J.; Qi, C.; Sun, W.; Jiang, H.; Cao, P.; Ju, H. Dysbiosis of Gut Microbiota in Patients With Acute Myocardial Infarction. Front. Microbiol. 2021, 12, 680101. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Bijla, M.; Saini, S.K.; Pathak, A.K.; Bharadwaj, K.P.; Sukhavasi, K.; Patil, A.; Saini, D.; Yadav, R.; Singh, S.; Leeuwenburgh, C.; et al. Microbiome interactions with different risk factors in development of myocardial infarction. Exp. Gerontol. 2024, 189, 112409. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Nieß, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2022, 26, 671–683. [Google Scholar] [CrossRef]
- Novakovic, M.; Rout, A.; Kingsley, T.; Kirchoff, R.; Singh, A.; Verma, V.; Kant, R.; Chaudhary, R. Role of gut microbiota in cardiovascular diseases. World J. Cardiol. 2020, 12, 110–122. [Google Scholar] [CrossRef]
- Zhou, X.; Li, J.; Guo, J.; Geng, B.; Ji, W.; Zhao, Q.; Li, J.; Liu, X.; Liu, J.; Guo, Z.; et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome 2018, 6, 66. [Google Scholar] [CrossRef]
- Shanmugham, M.; Bellanger, S.; Leo, C.H. Gut-Derived Metabolite, Trimethylamine-N-oxide (TMAO) in Cardio-Metabolic Diseases: Detection, Mechanism, and Potential Therapeutics. Pharmaceuticals 2023, 16, 504. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, N.K.; Kalyan, M.; Hediyal, T.A.; Anand, N.; Kendaganna, P.H.; Pendyala, G.; Yelamanchili, S.V.; Yang, J.; Chidambaram, S.B.; Sakharkar, M.K.; et al. Role of the Gut Bacteria-Derived Metabolite Phenylacetylglutamine in Health and Diseases. ACS Omega 2024, 9, 3164–3172. [Google Scholar] [CrossRef]
- Peng, J.; Xiao, X.; Hu, M.; Zhang, X. Interaction between gut microbiome and cardiovascular disease. Life Sci. 2018, 214, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Fan, N.; Ma, S.X.; Cheng, X.; Yang, X.; Wang, G. Gut Microbiota Dysbiosis: Pathogenesis, Diseases, Prevention, and Therapy. MedComm 2025, 6, e70168. [Google Scholar] [CrossRef]
- Oktaviono, Y.H.; Dyah Lamara, A.; Saputra, P.B.T.; Arnindita, J.N.; Pasahari, D.; Saputra, M.E.; Suasti, N.M.A. The roles of trimethylamine-N-oxide in atherosclerosis and its potential therapeutic aspect: A literature review. Biomol. Biomed. 2023, 23, 936–948. [Google Scholar] [CrossRef]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef]
- Soranno, D.E.; Coopersmith, C.M.; Brinkworth, J.F.; Factora, F.N.F.; Muntean, J.H.; Mythen, M.G.; Raphael, J.; Shaw, A.D.; Vachharajani, V.; Messer, J.S. A review of gut failure as a cause and consequence of critical illness. Crit. Care 2025, 29, 91. [Google Scholar] [CrossRef]
- Badimon, L.; Padró, T.; Vilahur, G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur. Heart J. Acute Cardiovasc. Care 2012, 1, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Finegold, J.A.; Asaria, P.; Francis, D.P. Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. Int. J. Cardiol. 2013, 168, 934–945. [Google Scholar] [CrossRef]
- Trimarchi, G.; Pizzino, F.; Paradossi, U.; Gueli, I.A.; Palazzini, M.; Gentile, P.; Di Spigno, F.; Ammirati, E.; Garascia, A.; Tedeschi, A.; et al. Charting the Unseen: How Non-Invasive Imaging Could Redefine Cardiovascular Prevention. J. Cardiovasc. Dev. Dis. 2024, 11, 245. [Google Scholar] [CrossRef]
- Jin, L.; Shi, X.; Yang, J.; Zhao, Y.; Xue, L.; Xu, L.; Cai, J. Gut microbes in cardiovascular diseases and their potential therapeutic applications. Protein Cell 2021, 12, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Alexandrescu, L.; Suceveanu, A.P.; Stanigut, A.M.; Tofolean, D.E.; Axelerad, A.D.; Iordache, I.E.; Herlo, A.; Nelson Twakor, A.; Nicoara, A.D.; Tocia, C.; et al. Intestinal Insights: The Gut Microbiome’s Role in Atherosclerotic Disease: A Narrative Review. Microorganisms 2024, 12, 2341. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, S.; Hu, X.; Ye, C.; Nie, Q.; Wang, K.; Yan, S.; Lin, J.; Xu, F.; Li, M.; et al. Candida albicans accelerates atherosclerosis by activating intestinal hypoxia-inducible factor2α signaling. Cell Host Microbe 2024, 32, 964–979.e7. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Laurans, L.; Mouttoulingam, N.; Chajadine, M.; Lavelle, A.; Diedisheim, M.; Bacquer, E.; Creusot, L.; Suffee, N.; Esposito, B.; Melhem, N.J.; et al. An obesogenic diet increases atherosclerosis through promoting microbiota dysbiosis-induced gut lymphocyte trafficking into the periphery. Cell Rep. 2023, 42, 113350. [Google Scholar] [CrossRef]
- Caradonna, E.; Abate, F.; Schiano, E.; Paparella, F.; Ferrara, F.; Vanoli, E.; Difruscolo, R.; Goffredo, V.M.; Amato, B.; Setacci, C.; et al. Trimethylamine-N-Oxide (TMAO) as a Rising-Star Metabolite: Implications for Human Health. Metabolites 2025, 15, 220. [Google Scholar] [CrossRef]
- Gui, Y.; Zheng, H.; Cao, R.Y. Foam Cells in Atherosclerosis: Novel Insights Into Its Origins, Consequences, and Molecular Mechanisms. Front. Cardiovasc. Med. 2022, 9, 845942. [Google Scholar] [CrossRef]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef]
- Canyelles, M.; Borràs, C.; Rotllan, N.; Tondo, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Gut Microbiota-Derived TMAO: A Causal Factor Promoting Atherosclerotic Cardiovascular Disease? Int. J. Mol. Sci. 2023, 24, 1940. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef] [PubMed]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metab. Clin. Exp. 2021, 114, 154338. [Google Scholar] [CrossRef] [PubMed]
- Lange, O.; Proczko-Stepaniak, M.; Mika, A. Short-Chain Fatty Acids-A Product of the Microbiome and Its Participation in Two-Way Communication on the Microbiome-Host Mammal Line. Curr. Obes. Rep. 2023, 12, 108–126. [Google Scholar] [CrossRef]
- Strauss, M.H.; Hall, A.S.; Narkiewicz, K. The Combination of Beta-Blockers and ACE Inhibitors Across the Spectrum of Cardiovascular Diseases. Cardiovasc. Drugs Ther. 2023, 37, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Niazi, M.; Galehdar, N.; Jamshidi, M.; Mohammadi, R.; Moayyedkazemi, A. A Review of the Role of Statins in Heart Failure Treatment. Curr. Clin. Pharmacol. 2020, 15, 30–37. [Google Scholar] [CrossRef]
- Roghani, S.H.; Khan, D.S.; Shafiq, A.; Akbar, A.; Mustafa, W.; Shah, S.Q.A.; Khan, M., Jr.; Ali, H. Efficacy of Different Beta Blockers in Reducing Mortality in Heart-Failure Patients. Cureus 2024, 16, e74171. [Google Scholar] [CrossRef]
- Alcocer, L.A.; Bryce, A.; De Padua Brasil, D.; Lara, J.; Cortes, J.M.; Quesada, D.; Rodriguez, P. The Pivotal Role of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers in Hypertension Management and Cardiovascular and Renal Protection: A Critical Appraisal and Comparison of International Guidelines. Am. J. Cardiovasc. Drugs Drugs Devices Other Interv. 2023, 23, 663–682. [Google Scholar] [CrossRef]
- Patail, H.; Sharma, T.; Bali, A.D.; Isath, A.; Aronow, W.S.; Haidry, S.A. Dual antiplatelet therapy with concomitant anticoagulation: Current perspectives on triple therapy. Arch. Med. Sci. Atheroscler. Dis. 2023, 8, e13–e18. [Google Scholar] [CrossRef]
- Velidakis, N.; Stachteas, P.; Gkougkoudi, E.; Papadopoulos, C.; Kadoglou, N.P.E. Classical and Novel Lipid-Lowering Therapies for Diabetic Patients with Established Coronary Artery Disease or High Risk of Coronary Artery Disease—A Narrative Clinical Review. Pharmaceuticals 2024, 17, 568. [Google Scholar] [CrossRef]
- Zuo, L.; Yue, X.; Bian, T.; Cai, Y.; Wang, L.; Zeng, L.; He, H.; Wang, L.; Ioannou, A.; Li, S. Coronary artery bypass surgery versus medical therapy alone for ischaemic heart disease. Cochrane Database Syst. Rev. 2021, 2021, CD013645. [Google Scholar] [CrossRef]
- Dasi, L.P.; Hatoum, H.; Kheradvar, A.; Zareian, R.; Alavi, S.H.; Sun, W.; Martin, C.; Pham, T.; Wang, Q.; Midha, P.A.; et al. On the Mechanics of Transcatheter Aortic Valve Replacement. Ann. Biomed. Eng. 2017, 45, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Ghodeshwar, G.K.; Dube, A.; Khobragade, D. Impact of Lifestyle Modifications on Cardiovascular Health: A Narrative Review. Cureus 2023, 15, e42616. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A.; Evans, M.A. Statin adverse effects: A review of the literature and evidence for a mitochondrial mechanism. Am. J. Cardiovasc. Drugs Drugs Devices Other Interv. 2008, 8, 373–418. [Google Scholar] [CrossRef]
- Sethi, Y.; Patel, N.; Kaka, N.; Kaiwan, O.; Kar, J.; Moinuddin, A.; Goel, A.; Chopra, H.; Cavalu, S. Precision Medicine and the future of Cardiovascular Diseases: A Clinically Oriented Comprehensive Review. J. Clin. Med. 2023, 12, 1799. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rodrigues, C.F.; Sharopov, F.; Docea, A.O.; Can Karaca, A.; Sharifi-Rad, M.; Kahveci Karıncaoglu, D.; Gülseren, G.; Şenol, E.; Demircan, E.; et al. Diet, Lifestyle and Cardiovascular Diseases: Linking Pathophysiology to Cardioprotective Effects of Natural Bioactive Compounds. Int. J. Environ. Res. Public Health 2020, 17, 2326. [Google Scholar] [CrossRef]
- Méndez, L.; Medina, I. Polyphenols and Fish Oils for Improving Metabolic Health: A Revision of the Recent Evidence for Their Combined Nutraceutical Effects. Molecules 2021, 26, 2438. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Drețcanu, G.; Știrbu, I.; Leoplold, N.; Cruceriu, D.; Danciu, C.; Stănilă, A.; Fărcaș, A.; Borda, I.M.; Iuhas, C.; Diaconeasa, Z. Chemical Structure, Sources and Role of Bioactive Flavonoids in Cancer Prevention: A Review. Plants 2022, 11, 1117. [Google Scholar] [CrossRef]
- Zahra, M.; Abrahamse, H.; George, B.P. Flavonoids: Antioxidant Powerhouses and Their Role in Nanomedicine. Antioxidants 2024, 13, 922. [Google Scholar] [CrossRef] [PubMed]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.G.; Donca, V.I.; Alexescu, T.G.; Para, I.; et al. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Rodríguez Guzmán, R.; Centofanti, F.; Doldo, E.; Céspedes Miranda, E.M.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P. Neuroprotective Potentials of Flavonoids: Experimental Studies and Mechanisms of Action. Antioxidants 2023, 12, 280. [Google Scholar] [CrossRef]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, Y.; Yan, F.; Dong, M.; Ren, Y. Research progress of quercetin in cardiovascular disease. Front. Cardiovasc. Med. 2023, 10, 1203713. [Google Scholar] [CrossRef]
- Cavalier, A.N.; Clayton, Z.S.; Wahl, D.; Hutton, D.A.; McEntee, C.M.; Seals, D.R.; LaRocca, T.J. Protective effects of apigenin on the brain transcriptome with aging. Mech. Ageing Dev. 2024, 217, 111889. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Targeting Cardiovascular Diseases by Flavonols: An Update. Nutrients 2022, 14, 1439. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Peng, T.; Shao, C.; Liu, Y.; Lin, H.; Liu, Y. The Antioxidant Action of Astragali radix: Its Active Components and Molecular Basis. Molecules 2024, 29, 1691. [Google Scholar] [CrossRef] [PubMed]
- Zawawi, N.A.; Ahmad, H.; Madatheri, R.; Fadilah, N.I.M.; Maarof, M.; Fauzi, M.B. Flavonoids as Natural Anti-Inflammatory Agents in the Atopic Dermatitis Treatment. Pharmaceutics 2025, 17, 261. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.K.; Bhardwaj, P.; Prajapati, V.; Bhatia, A.; Purkait, S.; Arya, D.S. Flavonoids as therapeutics for myocardial ischemia-reperfusion injury: A comprehensive review on preclinical studies. Lab. Anim. Res. 2024, 40, 32. [Google Scholar] [CrossRef]
- Kampa, R.P.; Sęk, A.; Bednarczyk, P.; Szewczyk, A.; Calderone, V.; Testai, L. Flavonoids as new regulators of mitochondrial potassium channels: Contribution to cardioprotection. J. Pharm. Pharmacol. 2023, 75, 466–481. [Google Scholar] [CrossRef]
- Kulawiak, B.; Bednarczyk, P.; Szewczyk, A. Multidimensional Regulation of Cardiac Mitochondrial Potassium Channels. Cells 2021, 10, 1554. [Google Scholar] [CrossRef]
- Si, H.; Wyeth, R.P.; Liu, D. The flavonoid luteolin induces nitric oxide production and arterial relaxation. Eur. J. Nutr. 2014, 53, 269–275. [Google Scholar] [CrossRef]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Ahmed, K.R.; Rahman, M.M.; Islam, M.N.; Fahim, M.M.H.; Rahman, M.A.; Kim, B. Antioxidants activities of phytochemicals perspective modulation of autophagy and apoptosis to treating cancer. Biomed. Pharmacother. 2024, 174, 116497. [Google Scholar] [CrossRef]
- Jubaidi, F.F.; Zainalabidin, S.; Mariappan, V.; Budin, S.B. Mitochondrial Dysfunction in Diabetic Cardiomyopathy: The Possible Therapeutic Roles of Phenolic Acids. Int. J. Mol. Sci. 2020, 21, 6043. [Google Scholar] [CrossRef]
- Jia, J.Y.; Zang, E.H.; Lv, L.J.; Li, Q.Y.; Zhang, C.H.; Xia, Y.; Zhang, L.; Dang, L.S.; Li, M.H. Flavonoids in myocardial ischemia-reperfusion injury: Therapeutic effects and mechanisms. Chin. Herb. Med. 2020, 13, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lista, J.; Perez-Martinez, P.; Lopez-Miranda, J.; Perez-Jimenez, F. Long chain omega-3 fatty acids and cardiovascular disease: A systematic review. Br. J. Nutr. 2012, 107 (Suppl. 2), S201–S213. [Google Scholar] [CrossRef]
- Rauch, B.; Senges, J. The effects of supplementation with omega-3 polyunsaturated Fatty acids on cardiac rhythm: Anti-arrhythmic, pro-arrhythmic, both or neither? It depends…. Front. Physiol. 2012, 3, 57. [Google Scholar] [CrossRef]
- Bowen, K.J.; Harris, W.S.; Kris-Etherton, P.M. Omega-3 Fatty Acids and Cardiovascular Disease: Are There Benefits? Curr. Treat. Options Cardiovasc. Med. 2016, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Rundblad, A.; Sandoval, V.; Holven, K.B.; Ordovás, J.M.; Ulven, S.M. Omega-3 fatty acids and individual variability in plasma triglyceride response: A mini-review. Redox Biol. 2023, 63, 102730. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Jannas-Vela, S.; Espinosa, A.; Candia, A.A.; Flores-Opazo, M.; Peñailillo, L.; Valenzuela, R. The Role of Omega-3 Polyunsaturated Fatty Acids and Their Lipid Mediators on Skeletal Muscle Regeneration: A Narrative Review. Nutrients 2023, 15, 871. [Google Scholar] [CrossRef]
- Adili, R.; Hawley, M.; Holinstat, M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 2018, 139, 10–18. [Google Scholar] [CrossRef]
- Sherratt, S.C.R.; Libby, P.; Budoff, M.J.; Bhatt, D.L.; Mason, R.P. Role of Omega-3 Fatty Acids in Cardiovascular Disease: The Debate Continues. Curr. Atheroscler. Rep. 2023, 25, 1–17. [Google Scholar] [CrossRef]
- Zanetti, M.; Grillo, A.; Losurdo, P.; Panizon, E.; Mearelli, F.; Cattin, L.; Barazzoni, R.; Carretta, R. Omega-3 Polyunsaturated Fatty Acids: Structural and Functional Effects on the Vascular Wall. BioMed Res. Int. 2015, 2015, 791978. [Google Scholar] [CrossRef]
- Jayedi, A.; Shab-Bidar, S.; Eimeri, S.; Djafarian, K. Fish consumption and risk of all-cause and cardiovascular mortality: A dose-response meta-analysis of prospective observational studies. Public Health Nutr. 2018, 21, 1297–1306. [Google Scholar] [CrossRef]
- Moreno, C.; Macías, A.; Prieto, A.; de la Cruz, A.; González, T.; Valenzuela, C. Effects of n-3 Polyunsaturated Fatty Acids on Cardiac Ion Channels. Front. Physiol. 2012, 3, 245. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, S.; Sun, Y.; Chen, C.; Yang, S.; Lin, M.; Long, J.; Yao, J.; Lin, Y.; Yi, F.; et al. Targeting oxidative stress as a preventive and therapeutic approach for cardiovascular disease. J. Transl. Med. 2023, 21, 519. [Google Scholar] [CrossRef]
- Xia, N.; Förstermann, U.; Li, H. Resveratrol and endothelial nitric oxide. Molecules 2014, 19, 16102–16121. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Salazar, J.; Pirela, D.; Nava, M.; Castro, A.; Angarita, L.; Parra, H.; Durán-Agüero, S.; Rojas-Gómez, D.M.; Galbán, N.; Añez, R.; et al. Specialized Proresolving Lipid Mediators: A Potential Therapeutic Target for Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3133. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, S.; Sadoshima, J. The role of sirtuins in cardiac disease. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1375–H1389. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 2015, 1852, 1155–1177. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D. Resveratrol and Cardiovascular Diseases. Nutrients 2016, 8, 250. [Google Scholar] [CrossRef]
- Cox, F.F.; Misiou, A.; Vierkant, A.; Ale-Agha, N.; Grandoch, M.; Haendeler, J.; Altschmied, J. Protective Effects of Curcumin in Cardiovascular Diseases-Impact on Oxidative Stress and Mitochondria. Cells 2022, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Młynarska, E.; Hajdys, J.; Czarnik, W.; Fularski, P.; Leszto, K.; Majchrowicz, G.; Lisińska, W.; Rysz, J.; Franczyk, B. The Role of Antioxidants in the Therapy of Cardiovascular Diseases-A Literature Review. Nutrients 2024, 16, 2587. [Google Scholar] [CrossRef]
- Singh, L.; Sharma, S.; Xu, S.; Tewari, D.; Fang, J. Curcumin as a Natural Remedy for Atherosclerosis: A Pharmacological Review. Molecules 2021, 26, 4036. [Google Scholar] [CrossRef]
- Wang, A.J.; Tang, Y.; Zhang, J.; Wang, B.J.; Xiao, M.; Lu, G.; Li, J.; Liu, Q.; Guo, Y.; Gu, J. Cardiac SIRT1 ameliorates doxorubicin-induced cardiotoxicity by targeting sestrin 2. Redox Biol. 2022, 52, 102310. [Google Scholar] [CrossRef]
- Wei, Z.; Pinfang, K.; Jing, Z.; Zhuoya, Y.; Shaohuan, Q.; Chao, S. Curcumin Improves Diabetic Cardiomyopathy by Inhibiting Pyroptosis through AKT/Nrf2/ARE Pathway. Mediat. Inflamm. 2023, 2023, 3906043. [Google Scholar] [CrossRef]
- Bahrami, A.; Montecucco, F.; Carbone, F.; Sahebkar, A. Effects of Curcumin on Aging: Molecular Mechanisms and Experimental Evidence. BioMed Res. Int. 2021, 2021, 8972074. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Funamoto, M.; Shimizu, K.; Shimizu, S.; Sari, N.; Katanasaka, Y.; Miyazaki, Y.; Kakeya, H.; Hasegawa, K.; Morimoto, T. Curcumin, an Inhibitor of p300-HAT Activity, Suppresses the Development of Hypertension-Induced Left Ventricular Hypertrophy with Preserved Ejection Fraction in Dahl Rats. Nutrients 2021, 13, 2608. [Google Scholar] [CrossRef] [PubMed]
- Santos-Parker, J.R.; Strahler, T.R.; Bassett, C.J.; Bispham, N.Z.; Chonchol, M.B.; Seals, D.R. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging 2017, 9, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Nurcahyanti, A.D.R.; Cokro, F.; Wulanjati, M.P.; Mahmoud, M.F.; Wink, M.; Sobeh, M. Curcuminoids for Metabolic Syndrome: Meta-Analysis Evidences Toward Personalized Prevention and Treatment Management. Front. Nutr. 2022, 9, 891339. [Google Scholar] [CrossRef] [PubMed]
- Roşian, Ş.H.; Boarescu, I.; Boarescu, P.-M. Antioxidant and Anti-Inflammatory Effects of Bioactive Compounds in Atherosclerosis. Int. J. Mol. Sci. 2025, 26, 1379. [Google Scholar] [CrossRef]
- Rabanal-Ruiz, Y.; Llanos-González, E.; Alcain, F.J. The Use of Coenzyme Q10 in Cardiovascular Diseases. Antioxidants 2021, 10, 755. [Google Scholar] [CrossRef]
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Ávila, M.; Fernández Vega, A.; de la Mata, M.; Delgado Pavón, A.; de Miguel, M.; Pérez Calero, C.; Villanueva Paz, M.; Cotán, D.; et al. Coenzyme q10 therapy. Mol. Syndromol. 2014, 5, 187–197. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P.; Q-SYMBIO Study Investigators. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Zozina, V.I.; Covantev, S.; Goroshko, O.A.; Krasnykh, L.M.; Kukes, V.G. Coenzyme Q10 in Cardiovascular and Metabolic Diseases: Current State of the Problem. Curr. Cardiol. Rev. 2018, 14, 164–174. [Google Scholar] [CrossRef]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; de la Mata, M.; Villanueva-Paz, M.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Suárez-Carrillo, A.; Talaverón-Rey, M.; Munuera, M.; et al. Atherosclerosis and Coenzyme Q10. Int. J. Mol. Sci. 2019, 20, 5195. [Google Scholar] [CrossRef]
- Zahedi, H.; Eghtesadi, S.; Seifirad, S.; Rezaee, N.; Shidfar, F.; Heydari, I.; Golestan, B.; Jazayeri, S. Effects of CoQ10 Supplementation on Lipid Profiles and Glycemic Control in Patients with Type 2 Diabetes: A randomized, double blind, placebo-controlled trial. J. Diabetes Metab. Disord. 2014, 13, 81. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Colletti, A.; Cicero, A.F.G. Coenzyme Q10: Clinical Applications in Cardiovascular Diseases. Antioxidants 2020, 9, 341. [Google Scholar] [CrossRef]
- Dohlmann, T.L.; Kuhlman, A.B.; Morville, T.; Dahl, M.; Asping, M.; Orlando, P.; Silvestri, S.; Tiano, L.; Helge, J.W.; Dela, F.; et al. Coenzyme Q10 Supplementation in Statin Treated Patients: A Double-Blinded Randomized Placebo-Controlled Trial. Antioxidants 2022, 11, 1698. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Dybring, A. Bioavailability of Coenzyme Q10: An Overview of the Absorption Process and Subsequent Metabolism. Antioxidants 2020, 9, 386. [Google Scholar] [CrossRef]
- Yurika, N.; Montuori, E.; Lauritano, C. Marine Microalgal Products with Activities against Age-Related Cardiovascular Diseases. Mar. Drugs 2024, 22, 229. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Berg, P.C.; Shakersain, B.; Hecht, K.; Takikawa, A.; Tao, R.; Kakuta, Y.; Uragami, C.; Hashimoto, H.; Misawa, N.; et al. Astaxanthin: Past, Present, and Future. Mar. Drugs 2023, 21, 514. [Google Scholar] [CrossRef]
- Akram, W.; Rihan, M.; Ahmed, S.; Arora, S.; Ahmad, S.; Vashishth, R. Marine-Derived Compounds Applied in Cardiovascular Diseases: Submerged Medicinal Industry. Mar. Drugs 2023, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Nagahawatta, D.P.; Liyanage, N.M.; Jayawardena, T.U.; Jeon, Y.J. Marine Polyphenols in Cardiovascular Health: Unraveling Structure-Activity Relationships, Mechanisms, and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 8419. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.; Pinto, D.C.; Silva, A.M. Seaweeds as Preventive Agents for Cardiovascular Diseases: From Nutrients to Functional Foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef]
- Lamminpää, I.; Amedei, A.; Parolini, C. Effects of Marine-Derived Components on Cardiovascular Disease Risk Factors and Gut Microbiota Diversity. Mar. Drugs 2024, 22, 523. [Google Scholar] [CrossRef]
- Han, Y.; Kim, D.H.; Pack, S.P. Marine-Derived Bioactive Ingredients in Functional Foods for Aging: Nutritional and Therapeutic Perspectives. Mar. Drugs 2024, 22, 496. [Google Scholar] [CrossRef]
- Olas, B. Probiotics, Prebiotics and Synbiotics-A Promising Strategy in Prevention and Treatment of Cardiovascular Diseases? Int. J. Mol. Sci. 2020, 21, 9737. [Google Scholar] [CrossRef]
- López-Yerena, A.; de Santisteban Villaplana, V.; Badimon, L.; Vilahur, G.; Padro, T. Probiotics: A Potential Strategy for Preventing and Managing Cardiovascular Disease. Nutrients 2025, 17, 52. [Google Scholar] [CrossRef]
- Yang, F.; Gao, R.; Luo, X.; Liu, R.; Xiong, D. Berberine influences multiple diseases by modifying gut microbiota. Front. Nutr. 2023, 10, 1187718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Feng, L.; Li, J.; Chen, L. Therapeutic Potential and Mechanisms of Berberine in Cardiovascular Disease. Curr. Pharmacol. Rep. 2016, 2, 281–292. [Google Scholar] [CrossRef]
- Deng, B.; Tao, L.; Wang, Y. Natural products against inflammation and atherosclerosis: Targeting on gut microbiota. Front. Microbiol. 2022, 13, 997056. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Hooshangi Shayesteh, M.R.; Hami, Z.; Chamanara, M.; Parvizi, M.R.; Golaghaei, A.; Nassireslami, E. Evaluation of the protective effect of coenzyme Q10 on hepatotoxicity caused by acute phosphine poisoning. Int. J. ImmunopathoL. Pharmacol. 2024, 38, 3946320241250286. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Mi, W.; Hu, Z.; Xu, L.; Bian, X.; Lian, W.; Yin, S.; Zhao, S.; Gao, W.; Guo, C.; Shi, T. Quercetin positively affects gene expression profiles and metabolic pathway of antibiotic-treated mouse gut microbiota. Front. Microbiol. 2022, 13, 983358. [Google Scholar] [CrossRef]
- Zhu, X.; Dai, X.; Zhao, L.; Li, J.; Zhu, Y.; He, W.; Guan, X.; Wu, T.; Liu, L.; Song, H.; et al. Quercetin activates energy expenditure to combat metabolic syndrome through modulating gut microbiota-bile acids crosstalk in mice. Gut Microbes 2024, 16, 2390136. [Google Scholar] [CrossRef]
- Junyuan, Z.; Hui, X.; Chunlan, H.; Junjie, F.; Qixiang, M.; Yingying, L.; Lihong, L.; Xingpeng, W.; Yue, Z. Quercetin protects against intestinal barrier disruption and inflammation in acute necrotizing pancreatitis through TLR4/MyD88/p38 MAPK and ERS inhibition. Pancreatology 2018, 18, 742–752. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef] [PubMed]
- Neto-Neves, E.M.; da Silva Maia Bezerra Filho, C.; Dejani, N.N.; de Sousa, D.P. Ferulic Acid and Cardiovascular Health: Therapeutic and Preventive Potential. Mini Rev. Med. Chem. 2021, 21, 1625–1637. [Google Scholar] [CrossRef] [PubMed]
- d’Angelo, M.; Castelli, V.; Tupone, M.G.; Catanesi, M.; Antonosante, A.; Dominguez-Benot, R.; Ippoliti, R.; Cimini, A.M.; Benedetti, E. Lifestyle and Food Habits Impact on Chronic Diseases: Roles of PPARs. Int. J. Mol. Sci. 2019, 20, 5422. [Google Scholar] [CrossRef]
- Zhou, M.; Ma, J.; Kang, M.; Tang, W.; Xia, S.; Yin, J.; Yin, Y. Flavonoids, gut microbiota, and host lipid metabolism. Eng. Life Sci. 2023, 24, 2300065. [Google Scholar] [CrossRef]
- Javadi, B.; Sobhani, Z. Role of apigenin in targeting metabolic syndrome: A systematic review. Iran. J. Basic Med. Sci. 2024, 27, 524–534. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y.; Soliman, S.M.; et al. Impacts of turmeric and its principal bioactive curcumin on human health: Pharmaceutical, medicinal, and food applications: A comprehensive review. Front. Nutr. 2023, 9, 1040259. [Google Scholar] [CrossRef]
- Ghosh, S.S.; He, H.; Wang, J.; Gehr, T.W.; Ghosh, S. Curcumin-mediated regulation of intestinal barrier function: The mechanism underlying its beneficial effects. Tissue Barriers 2018, 6, e1425085. [Google Scholar] [CrossRef]
- Zarfeshany, A.; Asgary, S.; Javanmard, S.H. Potent health effects of pomegranate. Adv. Biomed. Res. 2014, 3, 100. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M. Pomegranate Protection against Cardiovascular Diseases. Evid.-Based Complement. Altern. Med. 2012, 2012, 382763. [Google Scholar] [CrossRef]
- Festa, J.; Hussain, A.; Al-Hareth, Z.; Singh, H.; Da Boit, M. Anthocyanins and Vascular Health: A Matter of Metabolites. Foods 2023, 12, 1796. [Google Scholar] [CrossRef]
- Xiong, H.H.; Lin, S.Y.; Chen, L.L.; Ouyang, K.H.; Wang, W.J. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods 2023, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Xu, J.; Yang, M.; Hussain, M.; Liu, X.; Feng, F.; Guan, R. Protective Effect of Anthocyanins against Neurodegenerative Diseases through the Microbial-Intestinal-Brain Axis: A Critical Review. Nutrients 2023, 15, 496. [Google Scholar] [CrossRef] [PubMed]
- Mozos, I.; Flangea, C.; Vlad, D.C.; Gug, C.; Mozos, C.; Stoian, D.; Luca, C.T.; Horbańczuk, J.O.; Horbańczuk, O.K.; Atanasov, A.G. Effects of Anthocyanins on Vascular Health. Biomolecules 2021, 11, 811. [Google Scholar] [CrossRef]
- Chen, K.; Kortesniemi, M.K.; Linderborg, K.M.; Yang, B. Anthocyanins as Promising Molecules Affecting Energy Homeostasis, Inflammation, and Gut Microbiota in Type 2 Diabetes with Special Reference to Impact of Acylation. J. Agric. Food Chem. 2023, 71, 1002–1017. [Google Scholar] [CrossRef]
- Mauray, A.; Felgines, C.; Morand, C.; Mazur, A.; Scalbert, A.; Milenkovic, D. Bilberry anthocyanin-rich extract alters expression of genes related to atherosclerosis development in aorta of apo E-deficient mice. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Rotllan, N.; Price, N.; Pati, P.; Goedeke, L.; Fernández-Hernando, C. microRNAs in lipoprotein metabolism and cardiometabolic disorders. Atherosclerosis 2016, 246, 352–360. [Google Scholar] [CrossRef]
- Chihomvu, P.; Ganesan, A.; Gibbons, S.; Woollard, K.; Hayes, M.A. Phytochemicals in Drug Discovery-A Confluence of Tradition and Innovation. Int. J. Mol. Sci. 2024, 25, 8792. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Shah, S.M.A.; Akram, M.; Riaz, M.; Munir, N.; Rasool, G. Cardioprotective Potential of Plant-Derived Molecules: A Scientific and Medicinal Approach. Dose-Response A Publ. Int. Hormesis Soc. 2019, 17, 1559325819852243. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Khatiwada, N.; Hong, Z. Potential Benefits and Risks Associated with the Use of Statins. Pharmaceutics 2024, 16, 214. [Google Scholar] [CrossRef]
- Hossain, M.S.; Wazed, M.A.; Asha, S.; Amin, M.R.; Shimul, I.M. Dietary Phytochemicals in Health and Disease: Mechanisms, Clinical Evidence, and Applications-A Comprehensive Review. Food Sci. Nutr. 2025, 13, e70101. [Google Scholar] [CrossRef] [PubMed]
- Alum, E.U. Role of phytochemicals in cardiovascular disease management: Insights into mechanisms, efficacy, and clinical application. Phytomed. Plus 2025, 5, 100695. [Google Scholar] [CrossRef]
- Li, K.X.; Wang, Z.C.; Machuki, J.O.; Li, M.Z.; Wu, Y.J.; Niu, M.K.; Yu, K.Y.; Lu, Q.B.; Sun, H.J. Benefits of Curcumin in the Vasculature: A Therapeutic Candidate for Vascular Remodeling in Arterial Hypertension and Pulmonary Arterial Hypertension? Front. Physiol. 2022, 13, 848867. [Google Scholar] [CrossRef]
- Chaachouay, N. Synergy, Additive Effects, and Antagonism of Drugs with Plant Bioactive Compounds. Drugs Drug Candidates 2025, 4, 4. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.; Ying, Y.; Luo, L.; Huang, D.; Luo, Z. Metformin and berberine, two versatile drugs in treatment of common metabolic diseases. Oncotarget 2017, 9, 10135–10146. [Google Scholar] [CrossRef] [PubMed]
- Dagher, O.; Mury, P.; Thorin-Trescases, N.; Noly, P.E.; Thorin, E.; Carrier, M. Therapeutic Potential of Quercetin to Alleviate Endothelial Dysfunction in Age-Related Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 8, 658400. [Google Scholar] [CrossRef]
- Breuss, J.M.; Atanasov, A.G.; Uhrin, P. Resveratrol and Its Effects on the Vascular System. Int. J. Mol. Sci. 2019, 20, 1523. [Google Scholar] [CrossRef]
- Kong, W.J.; Wei, J.; Zuo, Z.Y.; Wang, Y.M.; Song, D.Q.; You, X.F.; Zhao, L.X.; Pan, H.N.; Jiang, J.D. Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metab. Clin. Exp. 2008, 57, 1029–1037. [Google Scholar] [CrossRef]
- Penumathsa, S.V.; Thirunavukkarasu, M.; Koneru, S.; Juhasz, B.; Zhan, L.; Pant, R.; Menon, V.P.; Otani, H.; Maulik, N. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J. Mol. Cell Cardiol. 2007, 42, 508–516. [Google Scholar] [CrossRef]
- Carrillo-Martinez, E.J.; Flores-Hernández, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernández-Ortega, L.D. Quercetin, a Flavonoid with Great Pharmacological Capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef]
- Babaei Khorzoughi, R.; Namvarjah, F.; Teimouri, M.; Hosseini, H.; Meshkani, R. In-vitro Synergistic Effect of Metformin and Berberine on High Glucose-induced Lipogenesis. Iran. J. Pharm. Res. 2019, 18, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.S.; Chung, K.H.; Chung, J.H.; Lee, J.Y.; Park, J.B.; Zhang, Y.H.; Yoo, H.S.; Yun, Y.P. Antiplatelet activity of green tea catechins is mediated by inhibition of cytoplasmic calcium increase. J. Cardiovasc. Pharmacol. 2001, 38, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cheng, T.; Xia, H.; Yang, Y.; Wang, S. Effects of Garlic on Glucose Parameters and Lipid Profile: A Systematic Review and Meta-Analysis on Randomized Controlled Trials. Nutrients 2024, 16, 1692. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wang, L.; Liu, Q.; Yang, S.; Wang, C. Advancing herbal medicine: Enhancing product quality and safety through robust quality control practices. Front. Pharmacol. 2023, 14, 1265178. [Google Scholar] [CrossRef]
- Tashkin, D.P.; Amin, A.N.; Kerwin, E.M. Comparing Randomized Controlled Trials and Real-World Studies in Chronic Obstructive Pulmonary Disease Pharmacotherapy. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1225–1243. [Google Scholar] [CrossRef]
- Gross, A.S.; Harry, A.C.; Clifton, C.S.; Della Pasqua, O. Clinical trial diversity: An opportunity for improved insight into the determinants of variability in drug response. Br. J. Clin. Pharmacol. 2022, 88, 2700–2717. [Google Scholar] [CrossRef]
- Resurreccion, E.P.; Fong, K.W. The Integration of Metabolomics with Other Omics: Insights into Understanding Prostate Cancer. Metabolites 2022, 12, 488. [Google Scholar] [CrossRef]
- Edo, G.I.; Obasohan, P.; Makia, R.S.; Abiola, O.T.; Umelo, E.C.; Jikah, A.N.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; et al. The use of quality control parameters in the evaluation of herbal drugs. A review. Discov. Med. 2024, 1, 168. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Avigan, M.I.; Mozersky, R.P.; Seeff, L.B. Scientific and Regulatory Perspectives in Herbal and Dietary Supplement Associated Hepatotoxicity in the United States. Int. J. Mol. Sci. 2016, 17, 331. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Song, X.; Wang, Y.; Cheng, M.; Lu, S.; Xu, W.; Gao, G.; Sun, L.; Tang, Z.; Wang, M.; et al. Regulatory perspectives of combination products. Bioact. Mater. 2021, 10, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Ondieki, G.; Nyagblordzro, M.; Kikete, S.; Liang, R.; Wang, L.; He, X. Cytochrome P450 and P-Glycoprotein-Mediated Interactions Involving African Herbs Indicated for Common Noncommunicable Diseases. Evid.-Based Complement. Altern. Med. 2017, 2017, 2582463. [Google Scholar] [CrossRef]
- Uno, T.; Yasui-Furukori, N. Effect of grapefruit juice in relation to human pharmacokinetic study. Curr. Clin. Pharmacol. 2006, 1, 157–161. [Google Scholar] [CrossRef]
- Borrelli, F.; Izzo, A.A. Herb-drug interactions with St John’s wort (Hypericum perforatum): An update on clinical observations. AAPS J. 2009, 11, 710–727. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Zhang, Z.; Zuo, Z. Updates on the clinical evidenced herb-warfarin interactions. Evid.-Based Complement. Altern. Med. 2014, 2014, 957362. [Google Scholar] [CrossRef]
- Duarte, J.D.; Cavallari, L.H. Pharmacogenetics to guide cardiovascular drug therapy. Nat. Rev. Cardiol. 2021, 18, 649–665. [Google Scholar] [CrossRef]
- Santhiravel, S.; Bekhit, A.E.A.; Mendis, E.; Jacobs, J.L.; Dunshea, F.R.; Rajapakse, N.; Ponnampalam, E.N. The Impact of Plant Phytochemicals on the Gut Microbiota of Humans for a Balanced Life. Int. J. Mol. Sci. 2022, 23, 8124. [Google Scholar] [CrossRef]

| Metabolite | Activated Pathway | Key Effects | Impact on Heart Failure |
|---|---|---|---|
| SCFAs (acetate, propionate, butyrate) | GPR41, GPR43, GPR109A activation; HDAC inhibition | ↑ IL-10 (anti-inflammatory cytokine), ↓ systemic inflammation, improved gut barrier integrity | Protective: maintains epithelial integrity, reduces microbial translocation, limits inflammation |
| TMAO | NF-κB activation; ROS/TXNIP/NLRP3 inflammasome; TGF-β1/Smad3 signaling | ↑ Pro-inflammatory cytokines, ↑ oxidative stress, endothelial dysfunction, fibrosis | Detrimental: promotes atherosclerosis, cardiac dysfunction, and maladaptive remodeling |
| Bile Acids (BAs) | FXR activation; AIM2 inflammasome; Type I interferon pathways | Modulation of lipid metabolism, immune responses, mitochondrial dysfunction, cardiac hypertrophy | Detrimental: worsens inflammation, fibrosis, and metabolic derangement |
| BCAAs | mTOR pathway activation | ↑ Oxidative stress, ↑ inflammation | Detrimental: exacerbates cardiac stress and injury |
| Tryptophan Derivatives (e.g., indole derivatives) | NLRP3 inflammasome modulation; PERK pathway activation | Immune regulation, cellular stress responses | Mixed: potential protective or harmful effects depending on balance |
| LPS | TLR4 signaling activation | ↑ Cytokine release, oxidative stress, fibrosis, arrhythmias | Detrimental: drives systemic inflammation and cardiac remodeling |
| Metabolite | Activated Pathway | Key Effects | Impact on Hypertension |
|---|---|---|---|
| Short-Chain Fatty Acids (SCFAs) | GPR43 activation, inhibition of RAS, modulation of ORs (Olfr78, OR10J5) | Vasodilation, reduced inflammation, regulation of sympathetic activity, enhanced NO production | Lowers blood pressure; protective |
| Trimethylamine-N-oxide (TMAO) | NF-κB activation, vascular inflammation pathways | Increases vascular inflammation, endothelial dysfunction, platelet hyperresponsiveness | Elevates blood pressure; pro-hypertensive |
| Indole Derivatives (from Tryptophan) | Aryl hydrocarbon receptor (AhR) activation | Modulates immune responses, maintains vascular integrity | Generally protective; supports vascular health |
| Kynurenine | Immune and vascular modulation | Affects vascular tone and renal function | Variable, depending on pathway balance |
| Serotonin (5-HT) | Vascular and renal regulatory pathways | Modulates vascular contraction and renal function | Complex effects; can contribute to hypertension if dysregulated |
| Polyamines (Putrescine, Spermidine) | NO synthesis modulation | Regulates vascular tone through NO activation/inhibition | Can lower or raise blood pressure depending on balance |
| Lipopolysaccharides (LPS) | TLR4-mediated inflammatory signaling | Induces systemic inflammation, endothelial dysfunction | Promotes hypertension; detrimental |
| Metabolite | Activated Pathway | Key Effects | Impact on MI |
|---|---|---|---|
| Trimethylamine N-oxide (TMAO) | NF-κB activation; calcium signaling disruption; ROS generation | Promotes inflammation, oxidative stress, endothelial dysfunction, arrhythmia, atherosclerotic plaque destabilization | Increases MI risk, worsens myocardial injury and outcomes |
| Short-Chain Fatty Acids (SCFAs) (e.g., acetate, butyrate, propionate) | GPR41/43 activation; HDAC inhibition; eNOS activation | Suppress inflammation, improve gut barrier function, enhance vasodilation | Protective role; their reduction may impair MI recovery |
| Phenylacetylglutamine (PAGln) | Adrenergic receptor activation on platelets | Enhances platelet aggregation and thrombosis | Elevates thrombotic risk, worsens MI severity |
| Lipopolysaccharide (LPS) | TLR4-NF-κB activation; ROS generation | Induces systemic inflammation, oxidative stress, endothelial dysfunction | Exacerbates myocardial injury and adverse remodeling |
| Long-Chain Fatty Acids (LCFAs) | Metabolic dysregulation (lipid metabolism) | Promote platelet aggregation and thrombus formation | Potential biomarker for early AMI; contributes to MI initiation |
| Aromatic amino acid metabolites | Oxidative stress pathways | Increase ROS generation, influence infarct size | Worsen post-MI outcomes through increased oxidative damage |
| Metabolite | Activated Pathway | Key Effects | Impact on Atherosclerosis |
|---|---|---|---|
| Trimethylamine N-oxide (TMAO) | TMAO pathway, MAPK, NF-κB signaling, inhibition of reverse cholesterol transport |
|
|
| Short-Chain Fatty Acids (SCFAs) | PPARγ activation, gut barrier integrity regulation |
|
|
| Lipopolysaccharides (LPS) | TLR4 signaling, NF-κB pathway |
|
|
| Feature | Healthy Gut Microbiota | Atherosclerosis-Associated Dysbiosis | Hypertension-Associated Dysbiosis | Heart Failure-Associated Dysbiosis | Myocardial Infarction (MI)-Associated Dysbiosis |
|---|---|---|---|---|---|
| Microbial Diversity | High microbial richness and diversity | Reduced diversity, enrichment of pro-inflammatory species | Lower diversity, increased pathobionts | Significant microbial imbalance, loss of beneficial bacteria | Decreased diversity, dominance of pro-inflammatory bacteria |
| Bacillota/Bacteroidota Ratio | Balanced ratio, supporting homeostasis | Increased Bacillota/Bacteroidota ratio (linked to inflammation) | Increased Bacillota, decreased Bacteroidota | Elevated Bacillota levels, disrupting metabolic pathways | Increased Bacillota dominance, linked to inflammation |
| SCFA-Producing Bacteria | High levels of Faecalibacterium, Roseburia, Akkermansia | Decreased Faecalibacterium, Roseburia | Reduced Akkermansia muciniphila, impairing gut barrier | Loss of Butyrivibrio and Faecalibacterium | Decreased Roseburia and Akkermansia |
| SCFA Levels (Butyrate, Acetate, Propionate) | High, maintaining gut and vascular health | Decreased SCFA levels, leading to endothelial dysfunction | Lower SCFA levels, contributing to vascular stiffness | Markedly reduced SCFAs, worsening systemic inflammation | Reduced SCFAs, promoting pro-thrombotic environment |
| Inflammatory Bacteria | Low levels of Escherichia, Enterobacter | Increased Escherichia coli, Enterobacter, Proteobacteria | Overgrowth of Desulfovibrio (H2S producer, causing damage) | Elevated Klebsiella, Enterococcus, Staphylococcus | Increased Enterobacter and Fusobacterium |
| LPS-Producing Bacteria | Low levels, preventing endotoxemia | Increased Klebsiella, Parabacteroides | Enrichment of Proteobacteria, elevating systemic LPS | High Enterobacteriaceae, promoting systemic inflammation | Increased Bacteroides and Proteobacteria |
| TMAO-Producing Bacteria | Low levels, reducing cardiovascular risk | Enrichment of Lachnoclostridium, Desulfovibrio (TMAO producers) | Increased Clostridia and Fusobacterium, promoting TMAO | Elevated Eggerthella lenta and Desulfovibrio | Increased Lachnospiraceae and Clostridium |
| Bile Acid Metabolism | Normal bile acid balance, supporting lipid metabolism | Increased secondary bile acids (pro-inflammatory effects) | Altered bile acid conversion, affecting BP regulation | Elevated toxic bile acids, worsening cardiac function | Disrupted bile acid homeostasis, impairing heart recovery |
| Inflammatory Markers | Low levels of IL-6, TNF-α, CRP | Elevated IL-6, TNF-α, CRP, promoting plaque formation | Increased pro-inflammatory cytokines (IL-17, TNF-α) | High IL-6, TNF-α, gut permeability worsens | Increased IL-1β, IL-18, contributing to clot formation |
| Endothelial Function | Intact vascular endothelium, healthy BP regulation | Endothelial dysfunction, leading to atherosclerotic plaque | Reduced nitric oxide (NO), causing vascular constriction | Endothelial damage, exacerbating heart failure risk | Vascular inflammation, increasing thrombosis risk |
| Gut Barrier Integrity | Strong tight junctions, preventing microbial translocation | Impaired barrier, microbial translocation fuels inflammation | Weakened barrier, increasing BP-related damage | Severe gut leakiness, endotoxemia worsens heart function | Increased permeability, leading to systemic inflammation |
| Compound | Sources | Cardioprotective Mechanisms | Key Molecular Targets | Clinical Implications | References |
|---|---|---|---|---|---|
| Flavonoids | Berries, citrus fruits, onions, tea | Antioxidant, anti-inflammatory, anti-atherosclerotic, anti-thrombotic, improves endothelial function, reduces blood pressure | Nrf2, NF-κB, PI3K-AKT, eNOS, COX-2, NADPH oxidase, MAPK | Potential role in preventing atherosclerosis, hypertension, and MI; needs further clinical translation | [137,138,139,140,141,142,143,144,145,146,147,148,149] |
| Omega-3 fatty acids | Fatty fish (EPA, DHA), flaxseeds, walnuts | Reduces triglycerides, lowers blood pressure, is anti-inflammatory, improves endothelial function, stabilizes cardiac electrophysiology | PPAR-α, COX-2, LOX, resolvins, prostacyclin, NO | Beneficial in reducing cardiovascular events, improving lipid profiles, and managing hypertriglyceridemia | [163,164,165,166,167,168,169,170,171,172,173,174] |
| Resveratrol | Grapes, peanuts, berries | Antioxidant, anti-inflammatory, anti-atherosclerotic, vasoprotective, inhibits LDL oxidation, enhances NO production | SIRT1, AMPK, estrogen receptor α, NF-κB, eNOS | Potential in managing hypertension, atherosclerosis, and heart failure; concerns regarding bioavailability | [175,176,177,178,179,180,181,182] |
| Curcumin | Turmeric, ginger | Reduces oxidative stress, is anti-inflammatory and anti-thrombotic, regulates lipids, inhibits platelet aggregation, prevents atherosclerosis | SIRT1, NF-κB, COX-2, NADPH oxidase | Cardioprotective in conditions like ischemia–reperfusion injury, diabetic cardiomyopathy, and drug-induced toxicity | [183,184,185,186] |
| Coenzyme Q10 (CoQ10) | Meat, fish, nuts, spinach, broccoli, whole grains | Antioxidant, mitochondrial function support, reduces oxidative stress, enhances energy production, improves endothelial function | Cytochrome c oxidase, Nrf2, ATP synthase, SIRT1 | Potential benefits in heart failure, ischemic heart disease, hypertension, and statin-induced myopathy | [187,188,189,190,191,192,193] |
| Marine-derived compounds | Fish oils, seaweed, marine algae, krill oil | Anti-inflammatory, antioxidant, reduces cholesterol and triglycerides, protects against ischemic damage, improves endothelial health | PPAR-α, SIRT1, eNOS, COX-2, TLR4 | Protective effects in cardiovascular diseases like atherosclerosis, heart failure, and inflammation | [194,195,196,197,198,199,200] |
| Natural Product | Gut Microbiota Modulation | Cardiovascular Benefits | Mechanism of Action | References |
|---|---|---|---|---|
| Berberine | Promotes SCFA-producing bacteria (Roseburia, Blautia, Alistipes) | Reduces cholesterol, triglycerides, and LDL; increases HDL-C | Increases beneficial bacteria, suppresses LPS-induced inflammation via TLR4/NF-κB, improves lipid profiles and strengthens intestinal barrier function | [212,213] |
| Polymethoxyflavones (PMFs) | Increases Akkermansia and Bifidobacterium; inhibits TMA-producing bacteria | Prevents vascular inflammation and atherosclerosis; reduces TMAO | Inhibits TMA production, reduces NF-κB/MAPK signaling, improves gut health and prevents atherosclerosis | [214] |
| Resveratrol | Promotes Bacteroides, Lactobacillus, Bifidobacterium; reduces Enterococcus faecalis | Lowers risk of atherosclerosis and hypertension; improves endothelial function | Modulates gut microbiota to enhance NO bioavailability, reduces inflammation and oxidative stress, improves bile acid metabolism, and lowers TMAO | [215,216] |
| Quercetin | Activates health-promoting bacteria; restores gut microbiota after antibiotic exposure | Anti-obesity, metabolic benefits in metabolic syndrome; reduces inflammation | Strengthens tight junctions, generates antioxidant metabolites, regulates bile acid profiles, blocks inflammasome activation and TLR4 signaling pathway | [217,218,219,220] |
| Ferulic Acid (FA) | Increases Lachnospiraceae; reduces Prevotellaceae | Lowers cholesterol, triglycerides, LDL; promotes fat metabolism | Reshapes gut microbiota, activates PPAR-α, inhibits PPAR-β/γ, decreases pro-inflammatory cytokines, and regulates lipid metabolism | [222,223,224] |
| Curcumin | Promotes beneficial bacteria; suppresses harmful bacteria | Reduces inflammation and oxidative stress; lowers atherosclerosis risk | Inhibits NF-κB pathway, strengthens intestinal barrier, modulates lipid metabolism, reduces vascular inflammation, and prevents endotoxemia | [226,227] |
| Pomegranate Juice | Enhances Bifidobacterium, Lactobacillus, Roseburia, Akkermansia | Reduces atherosclerotic lesions; increases HDL-C and lowers CVD risk | Modulates gut microbiota, reduces atherosclerotic plaque size, enhances NO production, reduces oxidative stress and inflammatory cytokines | [228,229] |
| Anthocyanins | Increases Bifidobacterium, Lactobacillus, Roseburia, Akkermansia | Enhances vascular function; reduces plaque formation and aortic inflammation | Enhances NO production, decreases oxidative stress, modulates NF-κB pathway, regulates gene expression linked to atherosclerosis, improves redox homeostasis | [230,231,232,233,234,235] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muttiah, B.; Hanafiah, A. Gut Microbiota and Cardiovascular Diseases: Unraveling the Role of Dysbiosis and Microbial Metabolites. Int. J. Mol. Sci. 2025, 26, 4264. https://doi.org/10.3390/ijms26094264
Muttiah B, Hanafiah A. Gut Microbiota and Cardiovascular Diseases: Unraveling the Role of Dysbiosis and Microbial Metabolites. International Journal of Molecular Sciences. 2025; 26(9):4264. https://doi.org/10.3390/ijms26094264
Chicago/Turabian StyleMuttiah, Barathan, and Alfizah Hanafiah. 2025. "Gut Microbiota and Cardiovascular Diseases: Unraveling the Role of Dysbiosis and Microbial Metabolites" International Journal of Molecular Sciences 26, no. 9: 4264. https://doi.org/10.3390/ijms26094264
APA StyleMuttiah, B., & Hanafiah, A. (2025). Gut Microbiota and Cardiovascular Diseases: Unraveling the Role of Dysbiosis and Microbial Metabolites. International Journal of Molecular Sciences, 26(9), 4264. https://doi.org/10.3390/ijms26094264






