Abstract
(1) Background: Second-generation integrase strand transfer inhibitors (INSTIs) are now the preferred first-line therapies for human immunodeficiency virus (HIV). However, concerns regarding their side effects, such as weight gain and metabolic disturbances, have emerged. This scoping review aims to assess the effects of INSTIs on the gut microbiota, with a focus on differences between agents and their clinical implications. (2) Methods: A scoping review was conducted using PubMed, Web of Science, and Embase, with reports collected following PRISMA for Scoping Reviews (PRISMA-ScR). (3) Results: The majority of available evidence focused on dolutegravir, which demonstrated beneficial effects on microbiota diversity and composition. However, factors such as younger age, lower CD4+ counts, and extreme BMI were associated with proinflammatory changes. Limited data on bictegravir also suggested favorable alterations in the gut microbiota. Raltegravir, a first-generation INSTI, was associated with improvements in alpha diversity and microbial composition, although these changes were not consistently beneficial. Moreover, associated changes in inflammatory and microbial translocation markers suggested unfavorable alterations. (4) Conclusions: Based on the evidence mapped, second-generation INSTIs may generally induce favorable changes in the gut microbiota. However, further research is needed to explore the clinical implications of these microbiota alterations, particularly in specific patient groups.
1. Introduction
1.1. Development and Characteristics of Antiretroviral Therapy
Since the introduction of antiretroviral therapy (ART) in 1987, the management of HIV has undergone a paradigm shift, transforming it from a fatal disease into a chronic but manageable condition. This advancement has significantly reduced HIV-related morbidity and mortality, improving the overall quality of life for people living with HIV (PLWH) [1,2]. Over the years, ART has evolved to include seven major drug classes. Each class targets distinct stages of the HIV replication cycle, thereby enhancing viral suppression and treatment outcomes [3]. The standard treatment for newly diagnosed ART-naïve patients usually consists of a combination of three active drugs, most commonly two nucleoside reverse transcriptase inhibitors (NRTIs) combined with either a second-generation INSTI, a boosted protease inhibitor (PI), or a non-nucleoside reverse transcriptase inhibitor (NNRTI) [4,5].
PIs were introduced in the 1990s as potent antiretroviral agents with a high genetic barrier to resistance [6,7]. They function by inhibiting HIV protease, preventing the cleavage of viral polyproteins, and thereby blocking the maturation of viral particles [8,9,10]. However, their use has been associated with serious metabolic and cardiovascular side effects and significant drug–drug interactions [6,7]. Nonetheless, PIs continue to play a crucial role in first-line therapy for select patients and remain an option for those who experience treatment failure with other ART drug classes [5,11,12].
Since the approval of the NNRTIs in 1996, this drug class has been a cornerstone of ART regimens [4,5,13]. First-generation NNRTIs, such as efavirenz, function by directly binding to and inhibiting reverse transcriptase, thereby preventing the enzyme from functioning [8,9,10]. Despite their effectiveness, these agents have notable limitations, including a low genetic barrier to resistance, which increases the risk of developing both drug-specific and cross-class resistance. In contrast, second-generation NNRTIs exhibit improved resistance profiles, lower toxicity, fewer drug–drug interactions, and enhanced synergistic effects when used in combination with other antiretroviral agents [14,15]. Although efavirenz was widely used for an extended period as a first-line treatment, its use has become more limited due to the emergence of neuropsychiatric side effects, the potential for the transmission of resistance mutations, and the emergence of novel INSTIs [16].
1.2. Integrase Strand Transfer Inhibitors
The first-generation INSTIs raltegravir and elvitegravir were introduced in 2007 and 2012, respectively, and provided effective viral suppression with minimal drug–drug interactions for raltegravir and good tolerability for both [2,17,18]. These agents work by preventing the integration of viral DNA into the host genome through direct binding to the integrase enzyme [8,9,10]. Compared to first-line NNRTI- and PI-based regimens, raltegravir- and elvitegravir-containing ART demonstrated good efficacy and safety, leading to their incorporation into treatment regimens for ART-naïve patients [19,20]. However, both agents exhibited a low genetic barrier to resistance, increasing the risk of cross-resistance and limiting subsequent treatment options within the INSTI class [16,21].
The second-generation INSTIs, including dolutegravir and bictegravir, were developed to address these limitations. Dolutegravir, introduced in 2013, has demonstrated superior efficacy when compared to darunavir/ritonavir and efavirenz/emtricitabine/tenofovir disoproxil fumarate, as well as non-inferiority to raltegravir [22,23,24]. Furthermore, its improved tolerability, fewer drug–drug interactions, and a significantly higher resistance barrier compared to first-generation INSTIs led the World Health Organization (WHO) in 2019 to recommend the transition from efavirenz-based regimens to dolutegravir-based therapy as the preferred first-line treatment for PLWH [16,25,26]. Bictegravir, a novel INSTI introduced in 2018, has also been introduced as part of a fixed-dose combination regimen [27]. It offered additional advantages such as suitability for rapid treatment initiation, no requirement for prior HLA-B*5701 testing, compatibility with hepatitis B virus coinfection, and possibility of use in ART-experienced patients undergoing hemodialysis [28,29]. Additionally, cabotegravir, a long-acting INSTI, has been approved in combination with rilpivirine as a complete parenteral regimen for virally suppressed patients [30].
As the use of INSTIs continues to expand, their tolerability and side-effect profiles are becoming more clearly defined. Increasing evidence has raised concerns that second-generation INSTIs may be linked to observed side effects, such as adverse metabolic effects, including significant weight gain, as well as cardiovascular complications and other metabolic disturbances [26,29,31].
Currently, second-generation INSTIs are considered the preferred first-line therapies, being the most commonly administered ART and taken by millions of PLWH, whereas first-generation INSTIs, PIs, and NNRTIs receive varying levels of support from current treatment guidelines [4,5,16]. With accumulating clinical data, extensive research is ongoing to better characterize the differences between individual INSTIs, with a focus on their respective advantages and disadvantages in terms of efficacy, resistance, safety, and long-term outcomes [32,33,34,35].
1.3. The Role of Gut Microbiota in Chronic Inflammation Among PLWH
A crucial aspect of HIV infection is its impact on the human microbiome, particularly the composition of the gut microbiota. The gut microbiota comprises a diverse array of bacteria, viruses, and eukaryotic cells that maintain homeostasis with the human host [36,37]. The predominant bacterial phyla include Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia [38,39,40]. The composition of the microbiota can be described using alpha diversity (defined by the evenness and richness of individual samples, measured by Shannon diversity and Chao1 index), beta diversity (defined by the variability of samples within a specific habitat), microbiota composition changes, and alterations in systemic inflammation or translocation markers [41]. The review that follows adheres to this framework.
During HIV infection, CD4+ T-cell depletion begins, significantly affecting the gut-associated lymphoid tissue (GALT). The compromised lymphoid system weakens the integrity of the intestinal barrier, allowing microbiota and associated metabolites to translocate into the systemic circulation, thereby triggering systemic inflammation. The translocation of diverse microbial antigens across the gut barrier activates the immune system, resulting in chronic immune activation and systemic inflammation [42,43]. Although findings on HIV-associated alterations in gut microbiota composition vary, a general trend toward reduced alpha diversity and shifts in beta diversity has been consistently reported [44]. An increase in Proteobacteria genera and a decrease in Bacteroidetes and several Firmicutes-associated genera is frequently reported. Additionally, Ruminococcaceae and Prevotellaceae have been linked to inflammatory properties and CD4+ T-cell counts [45,46,47]. Beyond HIV infection itself, other factors such as age, gender, geographical context, and comorbidities also contribute to the specific composition of the microbiota [48,49,50,51]. The previously observed changes in the gut microbiota results in dysbiosis and persistent systemic inflammation, which contributes to the development of noncommunicable diseases (NCDs), such as diabetes, metabolic syndrome, and cardiovascular diseases, and to an increased risk of mortality among PLWH [52,53].
Dysbiosis in the gut also contributes to significant alterations in the gut’s metabolomic profile, with one of the key markers being changes in short-chain fatty acid (SCFA) levels [54,55]. A commonly observed change is the alteration of propionate, which primarily exhibits anti-inflammatory properties. HIV infection disrupts the abundance of propionate-producing bacteria, particularly Ruminococcaceae and Lachnospiraceae from the Firmicutes phylum, resulting in decreased concentrations [54].
Beyond metabolic alterations, chronic inflammation in HIV infection is characterized by elevated levels of systemic inflammatory markers, including soluble cluster of differentiation 14 (sCD14), various interleukins, C-reactive protein (CRP), and intercellular adhesion molecule 1 (ICAM-1) [52,56,57,58]. Concurrently, microbial translocation markers such as lipopolysaccharides (LPS) and intestinal fatty acid-binding protein (I-FABP) are also altered, indicating increased permeability of the gut barrier and translocation of microbial components into the bloodstream [52,59].
Following HIV diagnosis, ART is initiated. While ART effectively suppresses viral replication and restores immune function, its impact on gut microbiota composition and the associated inflammatory and translocation markers remains an area of active investigation [60]. INSTIs have emerged as preferred first-line agents due to their high efficacy and tolerability [16,26]. However, understanding the differential effects of individual INSTIs on the gut microbiota is crucial, particularly in determining which agent offers the most favorable balance between efficacy and overall tolerability. The first-generation INSTIs remain a key focus, as their effects on the gut microbiota are often grouped with those of newer agents despite potential differences in their impact.
1.4. Aims of the Scoping Review
The objective of this scoping review was to systematically examine and update the current body of evidence regarding the effects of individual INSTIs on the gut microbiota, with an emphasis on identifying differences between agents and their potential clinical implications. This review builds upon our previous report, which explored changes in human gut microbiota following NNRTI- and INSTI-based therapies [61].
This review addresses two central research aims:
- To map alterations in the gut microbiota of PLWH receiving specific INSTI-based therapy in comparison to those undergoing NNRTI or PI-based regimens.
- To assess differences in gut microbiota composition between PLWH treated with dolutegravir-based regimens and those receiving bictegravir-based therapy.
2. Materials and Methods
This scoping review was conducted following the guidelines outlined in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) extension for scoping reviews [62]. Comprehensive literature searches were conducted in PubMed (21 April 2025), Web of Science (21 April 2025), and Embase (21 April 2025) from their respective inception dates, without applying restrictions on language or publication type. To ensure exhaustive search, a combination of Medical Subject Headings (MeSH) terms and free-text keywords was employed. The search approach was evaluated using seven preselected studies known to be relevant and was refined accordingly. Detailed information on the search methodology and the PRISMA extension checklist for scoping reviews are available in Supplementary Materials S1 and S2, respectively. Additionally, reference lists from relevant systematic reviews were manually examined to identify any further eligible studies.
The study selection process comprised three stages: (1) removal of duplicates, (2) screening of titles and abstracts, and (3) full-text review. Selection criteria were based on predefined inclusion and exclusion parameters (Table 1). The first reviewer completed all stages, with the final stage reviewed for accuracy by a second reviewer. Duplicate detection and data management was performed using Rayyan and Excel (version 2013). Any disagreements during the selection process were resolved through discussion.

Table 1.
Inclusion and exclusion criteria.
Relevant data were extracted by one reviewer and independently verified by another to ensure accuracy. The extracted information included the following: study title, authors, year of publication, country of origin, study design, number of participants, participant characteristics, details of the intervention, methods of stool sample data collection, duration of follow-up, and key outcomes (including alpha and beta diversity indices, microbiota composition, and changes in markers of bacterial translocation or systemic inflammation).
The study selection process is depicted in Figure 1, while the findings from the included studies are summarized in Table 2 and Figure 2. These summaries provide a comprehensive update of the current research landscape and highlight potential gaps in knowledge. Publications that detail the effects of specific INSTI therapies but were excluded due to unsuitable publication types were incorporated into the Discussion section to provide a broader perspective. As this is a scoping review, a formal assessment of the risk of bias was not conducted.
3. Results
3.1. Study Selection Process
A total of 13,140 articles were retrieved from the PubMed, Web of Science, and Embase databases. After removing 3852 duplicates, 9081 records were excluded through title and abstract screening based on relevance, language, publication type, and text availability. Of the 224 full-text articles assessed for eligibility, 201 were excluded for the following reasons: (1) absence of detailed information regarding the effects of ART on the gut microbiota or on markers of inflammation; (2) inclusion of ART-naïve participants; (3) focus on non-INSTI-based ART regimens; and (4) failure to specify the particular INSTI administered or the individual effects of specific INSTIs. The screening and selection process is illustrated in Figure 1. Ultimately, six studies met the inclusion criteria and were included in the final analysis.
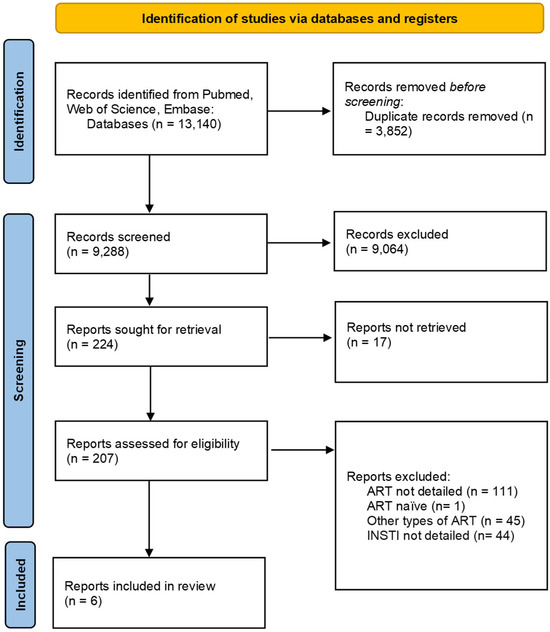

Figure 1.
Prisma flowchart on the study selection process.
3.2. Specific INSTI Related Changes in the Gut Microbiota
A total of six studies were included in the final analysis. These consisted of three randomized controlled trials and one cross-sectional study comparing raltegravir-based regimens with NNRTI- and/or PI-based therapies; one non-randomized trial assessing the effects of dolutegravir and bictegravir in comparison with NNRTI-based regimens; and one comparative study investigating the effects of dolutegravir versus an NNRTI-based regimen.
Hanttu et al. examined gut microbiota composition in patients receiving efavirenz or a PI compared to those who switched from efavirenz- or PI-based therapy to raltegravir as well as to HIV-seronegative controls. Serum levels of I-FABP, LPS, calprotectin, and vitamin D were also measured, with a follow-up period of 24 weeks [63]. At baseline, the efavirenz-treated group exhibited lower alpha diversity (Chao-1 index) compared to HIV-seronegative individuals. Beta diversity was significantly altered in both PI- and efavirenz-treated groups relative to HIV-seronegative individuals, with distinct microbial composition differences among the three subgroups [63]. Following the switch to raltegravir, alpha diversity (Chao-1 and Shannon indices) significantly increased in the efavirenz-to-raltegravir group, approaching levels seen in HIV-seronegative individuals [63]. Microbial composition showed a trend towards increased Prevotella-9 and decreased Phascolarctobacterium and Bacteroides after switching from PI or efavirenz to raltegravir, though these changes could not be directly attributed to either prior regimen. Biomarker analysis indicated higher baseline I-FABP levels in the efavirenz-treated group, but switching to raltegravir did not significantly affect either biomarker levels [63].
Villanueva-Millán et al. conducted a cross-sectional study involving PLWH receiving raltegravir-based (n = 8), NNRTI-based (n = 22), or PI-based (n = 15) ART, alongside ART-naïve PLWH (n = 5) and seronegative individuals (n = 21) [64]. Alpha diversity in the raltegravir-treated group was comparable to that of healthy controls and significantly higher than in ART-naïve individuals. In terms of microbiota composition, Bacteroidetes and Firmicutes were the dominant phyla across the entire study population. Raltegravir treatment was associated with an increased abundance of the δ-Proteobacteria class; the Desulfovibrionales and Selenomonadales orders; the Desulfovibrionaceae and Lachnospiraceae families; the Desulfovibrio genus; as well as Blautia sp. 3 and Parabacteroides merdae species and decreased levels of the Clostridiales order [64]. Furthermore, compared to PI-based regimens, raltegravir was linked to a lesser reduction in bacterial species richness. Regarding translocation markers, sCD14 levels in the raltegravir group were similar to those observed in healthy controls, unlike in other ART-treated groups. However, levels of ICAM-1 were elevated [64].
El Kamari et al. conducted a randomized controlled trial in which ART-naïve PLWH were assigned to receive either raltegravir- (n = 82), darunavir/ritonavir- (n = 82), or atazanavir/ritonavir-based (n = 67) ART regimens. The study assessed markers of gut integrity, including lipopolysaccharide-binding protein (LBP), zonulin, I-FABP, and ileal bile acid-binding protein (I-BABP), over a 96-week period [65]. Participants in the raltegravir arm exhibited significantly higher zonulin levels compared to those in the other treatment groups, while increases in I-BABP and LBP levels were observed but did not reach statistical significance. I-FABP levels—associated with increases in BMI, as well as visceral, subcutaneous, and total adipose tissue—showed a consistent elevation across all treatment arms and remained comparable among the different regimens [65].
Kelesidis et al. conducted a study similar to that of El Kamari et al., enrolling ART-naïve PLWH and assigning them to receive raltegravir- (n = 106), darunavir/ritonavir- (n = 113), or atazanavir/ritonavir-based (n = 109) ART regimens. The study evaluated a panel of inflammatory and coagulation markers, including CRP, IL-6, glycoprotein acetylation (GlycA), D-dimer, sCD14, soluble CD163 (sCD163), and soluble interleukin-2 receptor (sIL-2r), measured at 24 or 48 weeks and at 96 weeks [66]. In the raltegravir treatment arm, reductions in hs-CRP, IL-6, GlycA, sCD14, sCD163, and sIL-2r levels were observed. However, changes in inflammatory markers did not differ significantly between the raltegravir and PI-based treatment arms [66].
Narayanan et al. conducted a study involving 80 HIV-seronegative individuals and 69 PLWH receiving long-term ART with PI-, NNRTI-, or INSTI-based regimens. Among individuals on long-term treatment, the bictegravir-based ART subgroup exhibited a higher abundance of Bifidobacterium, Anaerostipes, Butyricimonas, and Butyricicoccus, while Faecalibacterium and Ruminococcus gauvreauii were enriched in those receiving dolutegravir. In contrast, Megasphaera was more abundant in NNRTI-treated individuals [67]. Further analysis focused on patients receiving dolutegravir. Those with a high BMI exhibited increased abundances of Bifidobacterium, Dorea, and Streptococcus, whereas those with a low BMI showed higher levels of Bacteroides and Escherichia-Shigella. Age-related differences were also observed: younger PLWH on dolutegravir had lower alpha diversity, with an enrichment of Lachnospira and Eggerthella, while older PLWH exhibited higher alpha diversity with increased Coprococcus and Dorea levels. Beta diversity also differed significantly between these age groups [67]. Additionally, long-term dolutegravir-treated patients had higher alpha diversity and an increased abundance of Succinivibrio compared to those on short-term treatment. CD4 cell count was also associated with microbial composition: patients with high CD4 counts had greater species richness, with elevated levels of Dialister, Ruminococcus, and Agathobacter, whereas those with lower CD4 counts exhibited enrichment of Fusobacterium and Ruminococcus gnavus [67].
Roux et al. analyzed inflammatory markers in PLWH who switched from efavirenz to a dolutegravir-based regimen (n = 9) and compared them to HIV-seronegative controls (n = 20). Inflammatory markers were assessed at baseline and after six months of dolutegravir treatment. Based on CRP levels, with a threshold of 5 mg/L, participants were stratified into two subgroups for further analysis [68]. At six months, all dolutegravir-treated patients exhibited significantly elevated levels of leucine, MIP-1α, sCD40L, and RANTES compared to healthy controls. However, after excluding patients with persistently elevated CRP (n = 4/9), only MIP-1α remained significantly increased [68]. Regarding metabolic profiles, at baseline, the efavirenz-treated group had higher levels of AMP, 1,7-dimethylxanthine, formic acid, glucose, and glycolic acid, while acetoacetic acid, creatinine, lactic acid, myo-inositol, and urea were reduced compared to seronegative individuals. Following the switch to dolutegravir, only 3-hydroxybutyric acid showed a significant increase, whereas other metabolites exhibited less pronounced changes [68]. In terms of inflammatory markers, the efavirenz-treated group demonstrated elevated G-CSF, GM-CSF, and PDGF-BB levels compared to healthy controls. After six months on dolutegravir, G-CSF and MIP-1α remained elevated, while IL-6, GM-CSF, and PDGF-BB decreased to levels comparable to seronegative individuals. Additionally, platelet activation markers, including sCD40L and RANTES, were elevated during efavirenz treatment but normalized following the switch to dolutegravir [68].
The study results are summarized in Table 2, while the specific changes induced by dolutegravir, bictegravir, and raltegravir are presented in Figure 2, along with the taxonomy of the bacteria discussed in this article.
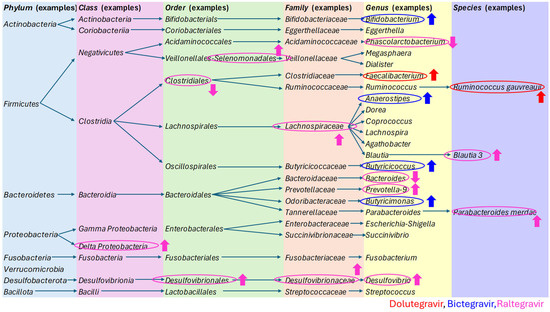


Figure 2.
The taxonomy of the bacteria discussed in this article, with a particular focus on the specific changes induced by dolutegravir, bictegravir, and raltegravir [40,63,64,65,66,67,68,69,70,71,72,73].

Table 2.
Raltegravir-, dolutegravir-, and bictegravir-specific changes on the gut microbiota in PLWH.
Table 2.
Raltegravir-, dolutegravir-, and bictegravir-specific changes on the gut microbiota in PLWH.
| Study Title | Author | Publication Year | Participants | INSTI-Mediated Alpha Diversity Changes | INSTI-Mediated Beta Diversity Changes | INSTI-Mediated Changes in Microbiome Composition | Stool Sample Analysis | INSTI-Mediated Change on Translocation or Inflammation Markers |
|---|---|---|---|---|---|---|---|---|
| Gut microbiota alterations after switching from a protease inhibitor or efavirenz to raltegravir in a randomized, controlled study [63] | Hanttu et al. | 2023 | PLWH on either efavirenz or PI (n = 41) vs. 24 weeks after switch to raltegravir (n = 19) vs. negative controls (n = 10) | Raltegravir treatment approximated the alpha diversity of HIV-seronegative individuals | - | Raltegravir:
| 16S rRNA sequencing | No changes |
| Differential effects of antiretrovirals on microbial translocation and gut microbiota composition of HIV-infected patients [64] | Villanueva-Millán et al. | 2017 | PLWH on either NNRI (n = 22) or PI (n = 15) or raltegravir (n = 8) vs. ART-naïve (n = 5) and negative controls (n = 21) | Raltegravir treatment approximated the alpha diversity of HIV-seronegative individuals | - | Raltegravir:
| 16S rDNA pyrosequencing | Raltegravir
|
| Lower Pretreatment Gut Integrity Is Independently Associated With Fat Gain on Antiretroviral Therapy [65] | El Kamari et al. | 2018 | PLWH randomized to raltegravir (n = 82), darunavir/ritonavir (n = 82), and atazanavir/ritonavir (n = 67) therapies | - | - | - | - | Raltegravir
|
| Changes in Inflammation and Immune Activation With Atazanavir-, Raltegravir-, Darunavir-Based Initial Antiviral Therapy: ACTG 5260s [66] | Kelesidis et al. | 2015 | PLWH randomized to raltegravir (n = 106), darunavir/ritonavir (n = 113), and atazanavir/ritonavir (n = 109) therapies | - | - | - | - | Raltegravir
|
| Exploring the interplay between antiretroviral therapy and the gut-oral microbiome axis in people living with HIV [67] | Narayanan et al. | 2023 | [INSTI (bictegravir vs. dolutegravir) vs. NNRTI vs. PI] (n = 69) vs. [negative controls] (n = 80) | Dolutegravir:
| Dolutegravir:
| Bictegravir:
| 16S rRNA sequencing | - |
| Comparative Effects of Efavirenz and Dolutegravir on Metabolomic and Inflammatory Profiles, and Platelet Activation of People Living with HIV: A Pilot Study [68] | Roux et al. | 2024 | Efavirenz (n = 9) vs. 6 months after dolutegravir switch (n = 9) vs. negative controls (n = 20) | - | - | - | 16S rRNA sequencing | Dolutegravir:
|
4. Discussion
In the present scoping review, our objective was to synthesize the existing knowledge on gut microbiota changes associated with specific INSTI-based therapies. This review builds upon our previous scoping review, which detailed microbiota alterations related to INSTI- and NNRTI-based therapies. The previous review summarized that INSTI-based regimens may improve alpha diversity, approaching levels observed in healthy controls, while beta diversity remains relatively unchanged. The findings also suggested that INSTI therapies enhance microbiome composition and may contribute to a reduction in inflammatory markers [61]. The present findings reinforce these observations, with dolutegravir demonstrating beneficial effects on both alpha and beta diversity as well as on overall gut microbiota composition. However, factors such as younger age, persistently low CD4+ T-cell counts, and extreme BMI were associated with unfavorable microbiota shifts. Available evidence also suggested that bictegravir promotes favorable gut microbiota alterations. Furthermore, raltegravir may be linked to improvements in alpha diversity and composition, though these changes were not universally beneficial. Notably, changes in gut integrity and inflammatory markers associated with raltegravir did not differ significantly from those observed with PI-based regimens [63,64,65,66,67,68]. While the current review builds on prior systematic findings, it provides a more detailed, agent-specific analysis of individual INSTIs.
In more detail, among the current studies, the most comprehensive data from second-line INSTIs was available for dolutegravir. Dolutegravir treatment was linked to improved alpha diversity in most subgroups except for younger patients (<40 years). Significant differences in beta diversity were observed exclusively in relation to age. Moreover, dolutegravir use was linked to the enrichment of Faecalibacterium and Ruminococcus gauvreauii [67,68]. Faecalibacterium has strong anti-inflammatory properties, and its reduced levels are associated with inflammatory bowel disease, diabetes mellitus, and obesity [74]. Ruminococcus gauvreauii is indicative of adequate thiamin levels, essential for immune regulation, and its depletion may suggest a shift toward a proinflammatory immune profile [75]. The study further emphasized the role of age, BMI, CD4+ T-cell count, and treatment duration in shaping microbiota composition, alongside the effects of dolutegravir treatment [67].
Narayana et al. observed that age significantly influenced both alpha and beta diversity. In the literature, De la Cuesta-Zuluaga et al. reported that alpha diversity increases until approximately 40 years before plateauing [76], whereas Badal et al. observed a subsequent rise in the oldest individuals [77]. The literature data also suggest that aging induces shifts in beta diversity, marked by an increase in proinflammatory facultative anaerobes and a decline in obligate anaerobes, which are essential for gut homeostasis and immune regulation [78]. Additionally, the enrichment of Lachnospira and Eggerthella among younger PLWH on dolutegravir suggests metabolic disturbances linked to obesity, diabetes, chronic kidney disease, and liver disorders [79]. In contrast, among older PLWH on dolutegravir, an increase in the beneficial butyrate-producing genus Coprococcus was observed alongside elevated Dorea levels. Dorea is associated with insulin production and fasting blood glucose levels, potentially contributing to type 2 diabetes mellitus and weight gain [80,81,82].
Treatment duration and CD4 cell count were also significant determinants of gut microbiota composition in PLWH receiving dolutegravir-based treatment. Consistent with previous findings, lower CD4 cell counts were associated with an increased abundance of Fusobacterium, while long-term treatment was linked to Succinivibrio enrichment, both of which are implicated in microbial alterations seen in immunological nonresponders and responders, respectively [67,83,84]. Additionally, the study reported elevated Ruminococcus gnavus levels in PLWH with low CD4 cell counts on dolutegravir treatment. This bacterium plays a complex role by producing SCFAs while simultaneously disrupting the gut mucus barrier. It has also been associated with metabolic syndrome, diabetes, and obesity [85].
This study further analyzed microbiota composition in PLWH receiving dolutegravir therapy in relation to BMI. High BMI was associated with Bifidobacterium enrichment [67]. Bifidobacterium is commonly associated with markers of good health. It functions as a primary degrader in the gut microbiome, contributes to the maintenance of gut homeostasis and has been linked to protective effects against weight gain [86,87,88,89]. However, elevated Dorea levels were also observed, suggesting a microbiome alteration that could facilitate weight gain and metabolic disorders [67,86,87,89]. Conversely, low BMI was linked to increased levels of opportunistic pathogens such as Escherichia-Shigella [90]. Surprisingly, elevated levels of Bacteroides were also observed, with Bacteroides promoting immune modulation through invariant natural killer T cells and IL-4 production [91]. Interestingly, a preprint study by Blazquez-Bondia et al. examined the human gut microbiome composition of PLWH initiating either DTG (n = 46) or darunavir/ritonavir (n = 42) therapy and reported an increased abundance of multiple Bifidobacterium species in the DTG treatment arm. However, these microbial changes were not associated with alterations of body weight [92].
Regarding inflammatory and microbial translocation biomarkers, Roux et al. demonstrated a favorable immunological shift following the transition from efavirenz to dolutegravir. Specifically, platelet activation markers such as RANTES and sCD40, along with proinflammatory markers including IL-6, GM-CSF, and PDGF-BB, significantly decreased after dolutegravir initiation. This reduction suggests a decline in chronic immune activation, which is associated with the development of NCD such as atherosclerosis and cardiovascular diseases [68,93]. Additionally, an elevation in 3-hydroxybutyrate was observed, a key energy source essential for gut homeostasis [68,94]. However, an increase in MIP-1α, which plays a role in myeloid cell recruitment, was also noted, while the inflammatory marker G-CSF remained unchanged [68,95].
With respect to bictegravir therapy, compositional changes were observed in a current study by Narayanan et al., including the enrichment of Bifidobacterium, Anaerostipes, Butyricimonas, and Butyricicoccus [67]. Evidence suggests that Bifidobacterium, Anaerostipes, Butyricimonas, and Butyricicoccus are beneficial due to their ability to produce the SCFA butyrate, which plays a crucial role in gut health [96,97,98,99,100]. In vitro models further suggested that Butyricimonas may play a protective role against diabetes mellitus and metabolic disorders [101]. Although an in vitro study by Rubio-García et al. also demonstrated antimicrobial effects of bictegravir against Enterococcus spp., pharmacokinetic studies suggest that bictegravir does not reach sufficient concentrations in the gut microbiota to significantly impact their abundance [102,103].
Although raltegravir is now primarily considered a second-line treatment, it is frequently assessed alongside second-generation INSTI therapies in studies examining gut microbiota changes following INSTI initiation. However, potential differences in their effects should be considered. The studies included in the present review suggested that raltegravir therapy enhances alpha diversity, approaching levels observed in healthy individuals. This effect was observed almost uniformly alongside INSTI therapies [64,104,105].
Regarding bacterial composition, the study by Hanttu et al. observed increased abundances of Prevotella-9 and a decrease in Phascolarctobacterium and Bacteroides levels [63]. In general, Prevotella, a proinflammatory taxon, is associated with the microbiota composition of MSM and has been linked to both improved glucose metabolism and insulin resistance [48,106,107]. Additionally, Hishiya et al. found that Prevotella-9 was specifically more abundant in patients with higher CD4 counts, while elevated fecal succinic acid in low CD4 patients negatively impacted its levels [108]. Furthermore, Phascolarctobacterium is linked to inflammatory cytokines, with its abundance positively correlating with weight loss and its reduction associated with inflammatory bowel disease [109,110,111]. Villanueva-Millán et al. reported increases in microbial taxa traditionally considered markers of dysbiosis, including the genus Desulfovibrio, the class δ-Proteobacteria, and members of the family Lachnospiraceae, all of which are implicated in metabolic disorders and atherosclerosis [64,112,113]. The observed reduction in the order Clostridiales, which plays a key role in maintaining gut homeostasis and overall gastrointestinal function, is also conflicting [64,114]. Conversely, increases in bacteria known for their production of SCFAs—such as those from the order Selenomonadales, family Lachnospiraceae, and species Blautia and Parabacteroides merdae—may indicate protective microbial shifts associated with RAL-based therapy [64,79,115,116]. Pharmacokinetic studies by Patterson et al. and Thompson et al. offer a potential mechanistic explanation, demonstrating that raltegravir (RAL) accumulates at high concentrations across multiple sites within the human gastrointestinal tract. This accumulation may underlie its distinct and diverse effects on gut microbiota composition [117,118].
Regarding alterations in gut integrity and systemic inflammatory markers, both the study by Hanttu et al. and Kelesidis et al. reported a favorable reduction in sCD14 levels—a biomarker of monocyte activation and a well-established predictor of HIV-associated morbidity and mortality [63,66,119]. In contrast, the study by Kelesidis et al. demonstrated non-consistent reductions of inflammatory markers after RAL and the changes did not differ significantly from the PI treatment arm. These alterations are indicative of a sustained proinflammatory state that may contribute to ongoing immune activation despite virologic suppression [66,120,121]. On the other hand, elevated zonulin levels—an established regulator of intestinal permeability—were particularly noted in the RAL treatment group in the study conducted by El Kamari et al. Previous research has suggested that zonulin production is suppressed in untreated HIV infection due to epithelial damage in the intestinal barrier. This suppression may be partially reversed upon initiation of ART, resulting in increased zonulin levels and consequently enhanced gut permeability [65,122].
Weight gain and the associated risk of NCDs among PLWH represent a significant challenge in modern HIV therapy. The factors contributing to weight gain have been extensively analyzed, with a particular focus on the potential role of INSTI-based therapies [29,123,124,125]. The present scoping review did not aim to synthesize evidence related to weight gain, and the existing data on microbiome alterations in association with weight changes remain inconclusive. Nevertheless, existing studies suggest that dolutegravir therapy may offer some protection against weight gain except for individuals with a high BMI, with a low CD4 count, or at certain age thresholds. In the case of bictegravir, the available evidence remains limited, and a protective effect against metabolic dysregulation has been hypothesized. However, a conference presentation by Pinto-Cardoso et al. reported significant weight gain following a switch from efavirenz to bictegravir-based therapy among treatment-experienced PLWH [67,126].
Regarding the effects of RAL therapies El Kamari et al. demonstrated that RAL-based regimens apply effects on gut integrity markers that are comparable to those observed with PI-based therapies. Both treatment modalities were associated with elevated and sustained levels of I-FABP. Increased I-FABP levels have been linked to weight gain, and it has been hypothesized that this may be mediated through the stimulation of hepatic triglyceride synthesis and subsequent adipose tissue accumulation [65,127]. Importantly, the study also emphasized the critical influence of baseline gut function on BMI and fat distribution patterns [65]. Overall, this complex phenomenon appears to be multifactorial and may be partly explained by the “return to health” effect, implying a limited contribution from direct ART-associated metabolic adverse effects [128,129,130,131,132].
To our knowledge, this is the first scoping review to assess specific changes in gut microbiota and associated inflammatory markers in response to dolutegravir-, bictegravir-, and raltegravir-based ART treatment. However, our study has several limitations. First, the included studies examined a relatively small patient population, which may limit the generalizability of the findings. Second, the microbiota analysis was mostly based on the 16S rRNA sequencing method. Evidence suggests that 16S rRNA sequencing primarily predicts specific genes based on amplified regions rather than directly sequencing the entire rRNA gene. Additionally, this method may not detect subtle alterations in microbial composition [133]. Moreover, Roux et al. applied a 10% significance level, which may have further influenced the results and their interpretation. In spite of these limitations, this scoping review is first of its kind aligning the available current scientific literature on the effects of the most commonly used INSTIs on the gut microbiota.
5. Conclusions
To summarize, among the studies reviewed, the most substantial body of evidence of second-line INSTIs was found for dolutegravir, which demonstrated beneficial effects on both alpha and beta diversity, as well as on the overall composition of the gut microbiota. However, factors such as younger age, persistently lower CD4+ T-cell counts, and extreme BMI were associated with unfavorable shifts in microbiota composition. Although data on bictegravir was more limited, the available evidence suggested that bictegravir also induces favorable alterations in the gut microbiota. Furthermore, raltegravir was linked to improvements in alpha diversity and composition, although these changes were not universally beneficial. Moreover, associated changes in inflammatory and microbial translocation markers suggested unfavorable alterations.
Additional studies are required to better define the impact of first-line ART therapies on the gut microbiota and to investigate its role in disease progression and inflammation. Gaining deeper insight into these relationships could aid clinicians in choosing treatments that reduce gut dysbiosis-related chronic inflammation while identifying the key factors driving microbiota alterations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26136366/s1.
Author Contributions
Conceptualization, Z.G. and B.L.; methodology, Z.G.; formal analysis, Z.G.; writing—original draft preparation, Z.G.; writing—review and editing, B.L.; visualization, Z.G.; supervision, B.L. All authors have read and agreed to the published version of the manuscript.
Funding
The manuscript receipt no external funding.
Acknowledgments
The 4.0 version of ChatGPT, developed by OpenAI, was used as a language tool to refine our writing and enhancing the clarity of our work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ART | Antiretroviral therapy |
| BMI | Body mass index |
| CCR5 | C-C chemokine receptor type 5 |
| CRP | C-reactive protein |
| DNA | Deoxyribonucleic acid |
| GALT | Gut-associated lymphoid tissue |
| G-CSF | Granulocyte colony-stimulating factor |
| GlycA | Glycoprotein acetylation |
| GM-CSF | Granulocyte macrophage colony-stimulating factor |
| HIV | Human immunodeficiency virus |
| I-BABP | Ileal bile acid-binding protein |
| ICAM-1 | Intercellular adhesion molecule 1 |
| I-FABP | Intestinal fatty acid-binding protein |
| IL-6 | Iinterleukin-6 |
| INSTI | Integrase strand transfer inhibitor |
| LPS | Lipopolysaccharide |
| MeSH | Medical Subject Headings |
| NCD | Noncommunicable disease |
| NRTI | Nucleoside reverse transcriptase inhibitor |
| NNRTI | Non-nucleoside reverse transcriptase inhibitor |
| PDGF-BB | Platelet derived growth factor subunit B |
| PI | Protease inhibitor |
| PLWH | People living with HIV |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRISMA-ScR | PRISMA for Scoping Reviews |
| RANTES | Regulated on activation, normal T-cell expressed and secreted |
| RNA | Ribonucleic acid |
| rRNA | Ribosomal ribonucleic acid |
| sCD14 | Soluble cluster of differentiation 14 |
| sCD40 | Soluble cluster of differentiation 40 |
| sCD163 | Soluble cluster of differentiation 163 |
| SCFA | Short-chain fatty acid |
| sIL-2r | Soluble interleukin-2 receptor |
| WHO | World Health Organization |
References
- Pandhi, D.; Ailawadi, P. Initiation of antiretroviral therapy. Indian J. Sex. Transm. Dis. AIDS 2014, 35, 1–11. [Google Scholar] [CrossRef]
- Tseng, A.; Seet, J.; Phillips, E.J. The evolution of three decades of antiretroviral therapy: Challenges, triumphs and the promise of the future. Br. J. Clin. Pharmacol. 2015, 79, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Eggleton, J.S.; Nagalli, S. Highly Active Antiretroviral Therapy (HAART). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- EACS. EACS Guidelines v12.0. Available online: https://www.eacsociety.org/media/guidelines-12.0.pdf (accessed on 15 February 2025).
- Clinicalinfo.HIV.gov. What to Start: Initial Combination Antiretroviral Regimens for People with HIV. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/what-start-initial-combination (accessed on 15 February 2025).
- LiverTox. Protease Inhibitors (HIV). In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Weber, I.T.; Wang, Y.F.; Harrison, R.W. HIV Protease: Historical Perspective and Current Research. Viruses 2021, 13, 839. [Google Scholar] [CrossRef]
- Colloty, J.; Teixeira, M.; Hunt, R. Advances in the treatment and prevention of HIV: What you need to know. Br. J. Hosp. Med. 2023, 84, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kemnic, T.R.; Gulick, P.G. HIV Antiretroviral Therapy. In StatPearls; National Library of Medicine: Treasure Island, FL, USA, 2025. [Google Scholar]
- NIH. The HIV Life Cycle. Available online: https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-life-cycle (accessed on 27 June 2025).
- Lee, Y.L.; Lin, K.Y.; Cheng, S.H.; Lu, P.L.; Wang, N.C.; Ho, M.W.; Yang, C.J.; Liou, B.H.; Tang, H.J.; Huang, S.S.; et al. Dual therapy with dolutegravir plus boosted protease inhibitor as maintenance or salvage therapy in highly experienced people living with HIV. Int. J. Antimicrob. Agents 2021, 58, 106403. [Google Scholar] [CrossRef]
- Figueroa, M.I.; Camiro-Zuniga, A.; Belaunzaran-Zamudio, P.F.; Sierra Madero, J.; Andrade Villanueva, J.; Arribas, J.R.; Lama, J.R.; Cecchini, D.M.; Lopardo, G.; Crabtree-Ramirez, B.; et al. The effect of protease inhibitor-based dual antiretroviral regimens on CD4/CD8 ratio during the first year of therapy in ART-naive patients with HIV-infection. HIV Med. 2021, 22, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Vanangamudi, M.; Palaniappan, S.; Kathiravan, M.K.; Namasivayam, V. Strategies in the Design and Development of Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs). Viruses 2023, 15, 1992. [Google Scholar] [CrossRef]
- Wang, Y.; De Clercq, E.; Li, G. Current and emerging non-nucleoside reverse transcriptase inhibitors (NNRTIs) for HIV-1 treatment. Expert. Opin. Drug Metab. Toxicol. 2019, 15, 813–829. [Google Scholar] [CrossRef]
- Vanangamudi, M.; Kurup, S.; Namasivayam, V. Non-nucleoside reverse transcriptase inhibitors (NNRTIs): A brief overview of clinically approved drugs and combination regimens. Curr. Opin. Pharmacol. 2020, 54, 179–187. [Google Scholar] [CrossRef]
- Kelly, S.G.; Masters, M.C.; Taiwo, B.O. Initial Antiretroviral Therapy in an Integrase Inhibitor Era: Can We Do Better? Infect. Dis. Clin. N. Am. 2019, 33, 681–692. [Google Scholar] [CrossRef]
- Zhao, A.V.; Crutchley, R.D.; Guduru, R.C.; Ton, K.; Lam, T.; Min, A.C. A clinical review of HIV integrase strand transfer inhibitors (INSTIs) for the prevention and treatment of HIV-1 infection. Retrovirology 2022, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.M.; Sherman, E.M.; Egelund, E.F.; Brotherton, A.; Durham, S.; Badowski, M.E.; Cluck, D.B. Integrase Inhibitors: After 10 Years of Experience, Is the Best Yet to Come? Pharmacotherapy 2019, 39, 576–598. [Google Scholar] [CrossRef] [PubMed]
- Rockstroh, J.K.; DeJesus, E.; Lennox, J.L.; Yazdanpanah, Y.; Saag, M.S.; Wan, H.; Rodgers, A.J.; Walker, M.L.; Miller, M.; DiNubile, M.J.; et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: Final 5-year results from STARTMRK. J. Acquir. Immune Defic. Syndr. 2013, 63, 77–85. [Google Scholar] [CrossRef]
- Lennox, J.L.; Landovitz, R.J.; Ribaudo, H.J.; Ofotokun, I.; Na, L.H.; Godfrey, C.; Kuritzkes, D.R.; Sagar, M.; Brown, T.T.; Cohn, S.E.; et al. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: A randomized, controlled equivalence trial. Ann. Intern. Med. 2014, 161, 461–471. [Google Scholar] [CrossRef]
- Smith, S.J.; Zhao, X.Z.; Passos, D.O.; Lyumkis, D.; Burke, T.R., Jr.; Hughes, S.H. Integrase Strand Transfer Inhibitors Are Effective Anti-HIV Drugs. Viruses 2021, 13, 205. [Google Scholar] [CrossRef]
- Raffi, F.; Rachlis, A.; Stellbrink, H.J.; Hardy, W.D.; Torti, C.; Orkin, C.; Bloch, M.; Podzamczer, D.; Pokrovsky, V.; Pulido, F.; et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 2013, 381, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, S.L.; Antela, A.; Clumeck, N.; Duiculescu, D.; Eberhard, A.; Gutierrez, F.; Hocqueloux, L.; Maggiolo, F.; Sandkovsky, U.; Granier, C.; et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N. Engl. J. Med. 2013, 369, 1807–1818. [Google Scholar] [CrossRef]
- Clotet, B.; Feinberg, J.; van Lunzen, J.; Khuong-Josses, M.A.; Antinori, A.; Dumitru, I.; Pokrovskiy, V.; Fehr, J.; Ortiz, R.; Saag, M.; et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014, 383, 2222–2231. [Google Scholar] [CrossRef]
- WHO. WHO Recommends Dolutegravir as Preferred HIV Treatment Option in All Populations. Available online: https://www.who.int/news/item/22-07-2019-who-recommends-dolutegravir-as-preferred-hiv-treatment-option-in-all-populations (accessed on 17 February 2025).
- Scarsi, K.K.; Havens, J.P.; Podany, A.T.; Avedissian, S.N.; Fletcher, C.V. HIV-1 Integrase Inhibitors: A Comparative Review of Efficacy and Safety. Drugs 2020, 80, 1649–1676. [Google Scholar] [CrossRef]
- LiverTox. Bictegravir. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Deeks, E.D. Bictegravir/Emtricitabine/Tenofovir Alafenamide: A Review in HIV-1 Infection. Drugs 2018, 78, 1817–1828. [Google Scholar] [CrossRef]
- Shah, S.; Hindley, L.; Hill, A. Are New Antiretroviral Treatments Increasing the Risk of Weight Gain? Drugs 2021, 81, 299–315. [Google Scholar] [CrossRef]
- Taki, E.; Soleimani, F.; Asadi, A.; Ghahramanpour, H.; Namvar, A.; Heidary, M. Cabotegravir/Rilpivirine: The last FDA-approved drug to treat HIV. Expert. Rev. Anti-Infect. Ther. 2022, 20, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Hill, A. Risks of metabolic syndrome and diabetes with integrase inhibitor-based therapy. Curr. Opin. Infect. Dis. 2021, 34, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Sax, P.E.; DeJesus, E.; Crofoot, G.; Ward, D.; Benson, P.; Dretler, R.; Mills, A.; Brinson, C.; Peloquin, J.; Wei, X.; et al. Bictegravir versus dolutegravir, each with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection: A randomised, double-blind, phase 2 trial. Lancet HIV 2017, 4, e154–e160. [Google Scholar] [CrossRef] [PubMed]
- Orkin, C.; Antinori, A.; Rockstroh, J.K.; Moreno-Guillen, S.; Martorell, C.T.; Molina, J.M.; Lazzarin, A.; Maggiolo, F.; Yazdanpanah, Y.; Andreatta, K.; et al. Switch to bictegravir/emtricitabine/tenofovir alafenamide from dolutegravir-based therapy. AIDS 2024, 38, 983–991. [Google Scholar] [CrossRef]
- Nunez, I.; Caro-Vega, Y.; MacDonald, C.; Mosqueda, J.L.; Pineirua-Menendez, A.; Matthews, A.A. Comparative effectiveness of bictegravir versus dolutegravir, raltegravir, and efavirenz-based antiretroviral therapy among treatment-naive individuals with HIV. Eur. J. Intern. Med. 2025, 133, 86–92. [Google Scholar] [CrossRef]
- Gan, L.; Xie, X.; Fu, Y.; Yang, X.; Ma, S.; Kong, L.; Song, C.; Song, Y.; Ren, T.; Long, H. Bictegravir/Emtricitabine/Tenofovir Alafenamide Versus Dolutegravir Plus Lamivudine for Switch Therapy in Patients with HIV-1 Infection: A Real-World Cohort Study. Infect. Dis. Ther. 2023, 12, 2581–2593. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Bandera, A.; De Benedetto, I.; Bozzi, G.; Gori, A. Altered gut microbiome composition in HIV infection: Causes, effects and potential intervention. Curr. Opin. HIV AIDS 2018, 13, 73–80. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Seekatz, A.M.; Safdar, N.; Khanna, S. The role of the gut microbiome in colonization resistance and recurrent Clostridioides difficile infection. Ther. Adv. Gastroenterol. 2022, 15, 17562848221134396. [Google Scholar] [CrossRef]
- Kers, J.G.; Saccenti, E. The Power of Microbiome Studies: Some Considerations on Which Alpha and Beta Metrics to Use and How to Report Results. Front. Microbiol. 2021, 12, 796025. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, A.; Innocenti, G.P.; Santinelli, L.; Pinacchio, C.; De Girolamo, G.; Vassalini, P.; Fanello, G.; Mastroianni, C.M.; Ceccarelli, G.; d’Ettorre, G. Antiretroviral Therapy Dampens Mucosal CD4(+) T Lamina Propria Lymphocytes Immune Activation in Long-Term Treated People Living with HIV-1. Microorganisms 2021, 9, 1624. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Wu, N.; Jin, C. Intestinal Microbiota Dysbiosis Promotes Mucosal Barrier Damage and Immune Injury in HIV-Infected Patients. Can. J. Infect. Dis. Med. Microbiol. 2023, 2023, 3080969. [Google Scholar] [CrossRef] [PubMed]
- Ashuro, A.A.; Lobie, T.A.; Ye, D.Q.; Leng, R.X.; Li, B.Z.; Pan, H.F.; Fan, Y.G. Review on the Alteration of Gut Microbiota: The Role of HIV Infection and Old Age. AIDS Res. Hum. Retroviruses 2020, 36, 556–565. [Google Scholar] [CrossRef]
- Salvador, P.B.U.; Altavas, P.; Del Rosario, M.A.S.; Ornos, E.D.B.; Dalmacio, L.M.M. Alterations in the Gut Microbiome Composition of People Living with HIV in the Asia-Pacific Region: A Systematic Review. Clin. Pract. 2024, 14, 846–861. [Google Scholar] [CrossRef]
- Vujkovic-Cvijin, I.; Somsouk, M. HIV and the Gut Microbiota: Composition, Consequences, and Avenues for Amelioration. Curr. HIV/AIDS Rep. 2019, 16, 204–213. [Google Scholar] [CrossRef]
- Satish, S.; Abu, Y.; Gomez, D.; Kumar Dutta, R.; Roy, S. HIV, opioid use, and alterations to the gut microbiome: Elucidating independent and synergistic effects. Front. Immunol. 2023, 14, 1156862. [Google Scholar] [CrossRef]
- Rocafort, M.; Gootenberg, D.B.; Luévano, J.M., Jr.; Paer, J.M.; Hayward, M.R.; Bramante, J.T.; Ghebremichael, M.S.; Xu, J.; Rogers, Z.H.; Munoz, A.R.; et al. HIV-associated gut microbial alterations are dependent on host and geographic context. Nat. Commun. 2024, 15, 1055. [Google Scholar] [CrossRef]
- Salazar, N.; Arboleya, S.; Fernandez-Navarro, T.; de Los Reyes-Gavilan, C.G.; Gonzalez, S.; Gueimonde, M. Age-Associated Changes in Gut Microbiota and Dietary Components Related with the Immune System in Adulthood and Old Age: A Cross-Sectional Study. Nutrients 2019, 11, 1765. [Google Scholar] [CrossRef] [PubMed]
- Trøseid, M.; Nielsen, S.D.; Vujkovic-Cvijin, I. Gut microbiome and cardiometabolic comorbidities in people living with HIV. Microbiome 2024, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Valeri, F.; Endres, K. How biological sex of the host shapes its gut microbiota. Front. Neuroendocrinol. 2021, 61, 100912. [Google Scholar] [CrossRef] [PubMed]
- Mac Cann, R.; Newman, E.; Devane, D.; Sabin, C.; Cotter, A.G.; Landay, A.; O’Toole, P.W.; Mallon, P.W. HIV, the gut microbiome and clinical outcomes, a systematic review. PLoS ONE 2024, 19, e0308859. [Google Scholar] [CrossRef]
- Ishizaka, A.; Koga, M.; Mizutani, T.; Parbie, P.K.; Prawisuda, D.; Yusa, N.; Sedohara, A.; Kikuchi, T.; Ikeuchi, K.; Adachi, E.; et al. Unique Gut Microbiome in HIV Patients on Antiretroviral Therapy (ART) Suggests Association with Chronic Inflammation. Microbiol. Spectr. 2021, 9, e0070821. [Google Scholar] [CrossRef]
- Sereti, I.; Verburgh, M.L.; Gifford, J.; Lo, A.; Boyd, A.; Verheij, E.; Verhoeven, A.; Wit, F.; Schim van der Loeff, M.F.; Giera, M.; et al. Impaired gut microbiota-mediated short-chain fatty acid production precedes morbidity and mortality in people with HIV. Cell Rep. 2023, 42, 113336. [Google Scholar] [CrossRef]
- González-Hernández, L.A.; Ruiz-Briseño, M.D.R.; Sánchez-Reyes, K.; Alvarez-Zavala, M.; Vega-Magaña, N.; López-Iñiguez, A.; Díaz-Ramos, J.A.; Martínez-Ayala, P.; Soria-Rodriguez, R.A.; Ramos-Solano, M.; et al. Alterations in bacterial communities, SCFA and biomarkers in an elderly HIV-positive and HIV-negative population in western Mexico. BMC Infect. Dis. 2019, 19, 234. [Google Scholar] [CrossRef]
- Fulcher, J.A.; Li, F.; Tobin, N.H.; Zabih, S.; Elliott, J.; Clark, J.L.; D’Aquila, R.; Mustanski, B.; Kipke, M.D.; Shoptaw, S.; et al. Gut dysbiosis and inflammatory blood markers precede HIV with limited changes after early seroconversion. EBioMedicine 2022, 84, 104286. [Google Scholar] [CrossRef]
- Ryu, A.; Clagett, B.M.; Freeman, M.L. Inflammation and Microbial Translocation Correlate with Reduced MAIT Cells in People with HIV. Pathog. Immun. 2024, 10, 19–46. [Google Scholar] [CrossRef]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Ouyang, J.; Yan, J.; Zhou, X.; Isnard, S.; Harypursat, V.; Cui, H.; Routy, J.P.; Chen, Y. Relevance of biomarkers indicating gut damage and microbial translocation in people living with HIV. Front. Immunol. 2023, 14, 1173956. [Google Scholar] [CrossRef]
- Wilson, E.M.; Sereti, I. Immune restoration after antiretroviral therapy: The pitfalls of hasty or incomplete repairs. Immunol. Rev. 2013, 254, 343–354. [Google Scholar] [CrossRef]
- Gaspar, Z.; Nagavci, B.; Szabo, B.G.; Lakatos, B. Gut Microbiome Alteration in HIV/AIDS and the Role of Antiretroviral Therapy-A Scoping Review. Microorganisms 2024, 12, 2221. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Hanttu, A.M.; Pekkala, S.; Satokari, R.; Hartikainen, A.K.; Arkkila, P.; Pietiläinen, K.H.; Sutinen, J.P. Gut microbiota alterations after switching from a protease inhibitor or efavirenz to raltegravir in a randomized, controlled study. AIDS 2023, 37, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Millán, M.J.; Pérez-Matute, P.; Recio-Fernández, E.; Lezana Rosales, J.M.; Oteo, J.A. Differential effects of antiretrovirals on microbial translocation and gut microbiota composition of HIV-infected patients. J. Int. AIDS Soc. 2017, 20, 21526. [Google Scholar] [CrossRef] [PubMed]
- El Kamari, V.; Moser, C.; Hileman, C.O.; Currier, J.S.; Brown, T.T.; Johnston, L.; Hunt, P.W.; McComsey, G.A. Lower Pretreatment Gut Integrity Is Independently Associated With Fat Gain on Antiretroviral Therapy. Clin. Infect. Dis. 2019, 68, 1394–1401. [Google Scholar] [CrossRef]
- Kelesidis, T.; Tran, T.T.; Stein, J.H.; Brown, T.T.; Moser, C.; Ribaudo, H.J.; Dube, M.P.; Murphy, R.; Yang, O.O.; Currier, J.S.; et al. Changes in Inflammation and Immune Activation With Atazanavir-, Raltegravir-, Darunavir-Based Initial Antiviral Therapy: ACTG 5260s. Clin. Infect. Dis. 2015, 61, 651–660. [Google Scholar] [CrossRef]
- Narayanan, A.; Kieri, O.; Vesterbacka, J.; Manoharan, L.; Chen, P.; Ghorbani, M.; Ljunggren, H.G.; Sällberg Chen, M.; Aleman, S.; Sönnerborg, A.; et al. Exploring the interplay between antiretroviral therapy and the gut-oral microbiome axis in people living with HIV. Sci. Rep. 2024, 14, 17820. [Google Scholar] [CrossRef]
- Roux, C.G.; Mason, S.; du Toit, L.D.V.; Nel, J.G.; Rossouw, T.M.; Steel, H.C. Comparative Effects of Efavirenz and Dolutegravir on Metabolomic and Inflammatory Profiles, and Platelet Activation of People Living with HIV: A Pilot Study. Viruses 2024, 16, 1462. [Google Scholar] [CrossRef]
- Otsuka, K.; Isobe, J.; Asai, Y.; Nakano, T.; Hattori, K.; Ariyoshi, T.; Yamashita, T.; Motegi, K.; Saito, A.; Kohmoto, M.; et al. Butyricimonas is a key gut microbiome component for predicting postoperative recurrence of esophageal cancer. Cancer Immunol. Immunother. 2024, 73, 23. [Google Scholar] [CrossRef] [PubMed]
- Domingo, M.C.; Huletsky, A.; Boissinot, M.; Bernard, K.A.; Picard, F.J.; Bergeron, M.G. Ruminococcus gauvreauii sp. nov., a glycopeptide-resistant species isolated from a human faecal specimen. Int. J. Syst. Evol. Microbiol. 2008, 58, 1393–1397. [Google Scholar] [CrossRef]
- Maheshwari, P.; Murali Sankar, P. Microbial Symbionts. 2023. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780323993340000189 (accessed on 27 June 2025).
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; McVeigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Dueholm, M.K.D.; Andersen, K.S.; Korntved, A.C.; Rudkjobing, V.; Alves, M.; Bajon-Fernandez, Y.; Batstone, D.; Butler, C.; Cruz, M.C.; Davidsson, A.; et al. MiDAS 5: Global diversity of bacteria and archaea in anaerobic digesters. Nat. Commun. 2024, 15, 5361. [Google Scholar] [CrossRef]
- Martin, R.; Rios-Covian, D.; Huillet, E.; Auger, S.; Khazaal, S.; Bermudez-Humaran, L.G.; Sokol, H.; Chatel, J.M.; Langella, P. Faecalibacterium: A bacterial genus with promising human health applications. FEMS Microbiol. Rev. 2023, 47, fuad039. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, A.; Mizutani, T.; Suzuki, Y.; Matano, T.; Yotsuyanagi, H. Thiamine deficiency underlies persistent gut dysbiosis and inflammation in people living with HIV on antiretroviral therapy. Transl. Med. Commun. 2024, 9, 25. [Google Scholar] [CrossRef]
- de la Cuesta-Zuluaga, J.; Kelley, S.T.; Chen, Y.; Escobar, J.S.; Mueller, N.T.; Ley, R.E.; McDonald, D.; Huang, S.; Swafford, A.D.; Knight, R.; et al. Age- and Sex-Dependent Patterns of Gut Microbial Diversity in Human Adults. mSystems 2019, 4, e00261-19. [Google Scholar] [CrossRef] [PubMed]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.M.; Wilson, C.C. What is the collective effect of aging and HIV on the gut microbiome? Curr. Opin. HIV AIDS 2020, 15, 94–100. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Baltazar-Díaz, T.A.; Amador-Lara, F.; Andrade-Villanueva, J.F.; González-Hernández, L.A.; Cabrera-Silva, R.I.; Sánchez-Reyes, K.; Álvarez-Zavala, M.; Valenzuela-Ramírez, A.; Del Toro-Arreola, S.; Bueno-Topete, M.R. Gut Bacterial Communities in HIV-Infected Individuals with Metabolic Syndrome: Effects of the Therapy with Integrase Strand Transfer Inhibitor-Based and Protease Inhibitor-Based Regimens. Microorganisms 2023, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Notting, F.; Pirovano, W.; Sybesma, W.; Kort, R. The butyrate-producing and spore-forming bacterial genus Coprococcus as a potential biomarker for neurological disorders. Gut Microbiome 2023, 4, e16. [Google Scholar] [CrossRef] [PubMed]
- Companys, J.; Gosalbes, M.J.; Pla-Paga, L.; Calderon-Perez, L.; Llaurado, E.; Pedret, A.; Valls, R.M.; Jimenez-Hernandez, N.; Sandoval-Ramirez, B.A.; Del Bas, J.M.; et al. Gut Microbiota Profile and Its Association with Clinical Variables and Dietary Intake in Overweight/Obese and Lean Subjects: A Cross-Sectional Study. Nutrients 2021, 13, 2032. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Sun, J.; Wei, L.; Jiang, H.; Hu, C.; Yang, J.; Huang, Y.; Ruan, B.; Zhu, B. Altered gut microbiota correlate with different immune responses to HAART in HIV-infected individuals. BMC Microbiol. 2021, 21, 11. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Rojo, D.; Martínez-Martínez, M.; Deusch, S.; Vázquez-Castellanos, J.F.; Bargiela, R.; Sainz, T.; Vera, M.; Moreno, S.; Estrada, V.; et al. Gut Bacteria Metabolism Impacts Immune Recovery in HIV-infected Individuals. EBioMedicine 2016, 8, 203–216. [Google Scholar] [CrossRef]
- Crost, E.H.; Coletto, E.; Bell, A.; Juge, N. Ruminococcus gnavus: Friend or foe for human health. FEMS Microbiol. Rev. 2023, 47, fuad014. [Google Scholar] [CrossRef]
- Ku, S.; Haque, M.A.; Jang, M.J.; Ahn, J.; Choe, D.; Jeon, J.I.; Park, M.S. The role of Bifidobacterium in longevity and the future of probiotics. Food Sci. Biotechnol. 2024, 33, 2097–2110. [Google Scholar] [CrossRef] [PubMed]
- Saa, P.; Urrutia, A.; Silva-Andrade, C.; Martin, A.J.; Garrido, D. Modeling approaches for probing cross-feeding interactions in the human gut microbiome. Comput. Struct. Biotechnol. J. 2022, 20, 79–89. [Google Scholar] [CrossRef]
- Martin, A.J.M.; Serebrinsky-Duek, K.; Riquelme, E.; Saa, P.A.; Garrido, D. Microbial interactions and the homeostasis of the gut microbiome: The role of Bifidobacterium. Microbiome Res. Rep. 2023, 2, 17. [Google Scholar] [CrossRef]
- Mulders, R.J.; de Git, K.C.G.; Schele, E.; Dickson, S.L.; Sanz, Y.; Adan, R.A.H. Microbiota in obesity: Interactions with enteroendocrine, immune and central nervous systems. Obes. Rev. 2018, 19, 435–451. [Google Scholar] [CrossRef]
- Li, S.; Guo, J.; Liu, R.; Zhang, F.; Wen, S.; Liu, Y.; Ren, W.; Zhang, X.; Shang, Y.; Gao, M.; et al. Predominance of Escherichia-Shigella in Gut Microbiome and Its Potential Correlation with Elevated Level of Plasma Tumor Necrosis Factor Alpha in Patients with Tuberculous Meningitis. Microbiol. Spectr. 2022, 10, e0192622. [Google Scholar] [CrossRef] [PubMed]
- Paquin-Proulx, D.; Ching, C.; Vujkovic-Cvijin, I.; Fadrosh, D.; Loh, L.; Huang, Y.; Somsouk, M.; Lynch, S.V.; Hunt, P.W.; Nixon, D.F.; et al. Bacteroides are associated with GALT iNKT cell function and reduction of microbial translocation in HIV-1 infection. Mucosal Immunol. 2017, 10, 69–78. [Google Scholar] [CrossRef]
- Blázquez-Bondia, C.; Català-Moll, F.; Torres, F.; Manzardo, C.; Bonfill, E.; Falcó, V.; Domingo, P.; Podzamczer, D.; Force, L.; Curran, A.; et al. Gut Microbiota Recovery in Late HIV-1 Presenters Initiating First-Line Dolutegravir-Based Antiretroviral Therapy: Results from a 2-Year, Open-Label, Randomized Clinical Trial. 2024. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4934503 (accessed on 27 June 2025).
- Madzime, M.; Rossouw, T.M.; Theron, A.J.; Anderson, R.; Steel, H.C. Interactions of HIV and Antiretroviral Therapy With Neutrophils and Platelets. Front. Immunol. 2021, 12, 634386. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Sasaki, K. 3-Hydroxybutyrate could serve as a principal energy substrate for human microbiota. Med. Hypotheses 2024, 182, 111217. [Google Scholar] [CrossRef]
- Jones, E.; Price, D.A.; Dahm-Vicker, M.; Cerundolo, V.; Klenerman, P.; Gallimore, A. The influence of macrophage inflammatory protein-1alpha on protective immunity mediated by antiviral cytotoxic T cells. Immunology 2003, 109, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xie, L.S.; Lian, S.; Li, K.; Yang, Y.; Wang, W.Z.; Hu, S.; Liu, S.J.; Liu, C.; He, Z. Anaerostipes hadrus, a butyrate-producing bacterium capable of metabolizing 5-fluorouracil. mSphere 2024, 9, e0081623. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, W.; Shin, N.R.; Hyun, D.W.; Kim, P.S.; Kim, H.S.; Lee, J.Y.; Tak, E.J.; Sung, H.; Bae, J.W. Anaerostipes hominis sp. nov., a novel butyrate-producing bacteria isolated from faeces of a patient with Crohn’s disease. Int. J. Syst. Evol. Microbiol. 2021, 71, 005129. [Google Scholar] [CrossRef]
- Sakamoto, M.; Takagaki, A.; Matsumoto, K.; Kato, Y.; Goto, K.; Benno, Y. Butyricimonas synergistica gen. nov., sp. nov. and Butyricimonas virosa sp. nov., butyric acid-producing bacteria in the family ‘Porphyromonadaceae’ isolated from rat faeces. Int. J. Syst. Evol. Microbiol. 2009, 59, 1748–1753. [Google Scholar] [CrossRef]
- Boesmans, L.; Valles-Colomer, M.; Wang, J.; Eeckhaut, V.; Falony, G.; Ducatelle, R.; Van Immerseel, F.; Raes, J.; Verbeke, K. Butyrate Producers as Potential Next-Generation Probiotics: Safety Assessment of the Administration of Butyricicoccus pullicaecorum to Healthy Volunteers. mSystems 2018, 3, e00094-18. [Google Scholar] [CrossRef]
- Chang, S.C.; Shen, M.H.; Liu, C.Y.; Pu, C.M.; Hu, J.M.; Huang, C.J. A gut butyrate-producing bacterium Butyricicoccus pullicaecorum regulates short-chain fatty acid transporter and receptor to reduce the progression of 1,2-dimethylhydrazine-associated colorectal cancer. Oncol. Lett. 2020, 20, 327. [Google Scholar] [CrossRef]
- Lee, H.; An, J.; Kim, J.; Choi, D.; Song, Y.; Lee, C.K.; Kong, H.; Kim, S.B.; Kim, K. A Novel Bacterium, Butyricimonas virosa, Preventing HFD-Induced Diabetes and Metabolic Disorders in Mice via GLP-1 Receptor. Front. Microbiol. 2022, 13, 858192. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Garcia, E.; Ferrando, N.; Martin, N.; Ballesté-Delpierre, C.; Miró, J.M.; Paredes, R.; Casals-Pascual, C.; Vila, J. In vitro antibacterial activity of antiretroviral drugs on key commensal bacteria from the human microbiota. Front. Cell Infect. Microbiol. 2023, 13, 1306430. [Google Scholar] [CrossRef] [PubMed]
- Wallace, V.J.; Sakowski, E.G.; Preheim, S.P.; Prasse, C. Bacteria exposed to antiviral drugs develop antibiotic cross-resistance and unique resistance profiles. Commun. Biol. 2023, 6, 837. [Google Scholar] [CrossRef] [PubMed]
- Villoslada-Blanco, P.; Pérez-Matute, P.; Íñiguez, M.; Recio-Fernández, E.; Blanco-Navarrete, P.; Metola, L.; Ibarra, V.; Alba, J.; de Toro, M.; Oteo, J.A. Integrase Inhibitors Partially Restore Bacterial Translocation, Inflammation and Gut Permeability Induced by HIV Infection: Impact on Gut Microbiota. Infect. Dis. Ther. 2022, 11, 1541–1557. [Google Scholar] [CrossRef]
- Villoslada-Blanco, P.; Pérez-Matute, P.; Íñiguez, M.; Recio-Fernández, E.; Jansen, D.; De Coninck, L.; Close, L.; Blanco-Navarrete, P.; Metola, L.; Ibarra, V.; et al. Impact of HIV infection and integrase strand transfer inhibitors-based treatment on the gut virome. Sci. Rep. 2022, 12, 21658. [Google Scholar] [CrossRef]
- Armstrong, A.J.S.; Shaffer, M.; Nusbacher, N.M.; Griesmer, C.; Fiorillo, S.; Schneider, J.M.; Preston Neff, C.; Li, S.X.; Fontenot, A.P.; Campbell, T.; et al. An exploration of Prevotella-rich microbiomes in HIV and men who have sex with men. Microbiome 2018, 6, 198. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Hishiya, N.; Uno, K.; Nakano, A.; Konishi, M.; Higashi, S.; Eguchi, S.; Ariyoshi, T.; Matsumoto, A.; Oka, K.; Takahashi, M.; et al. Association between the gut microbiome and organic acid profiles in a Japanese population with HIV infection. J. Infect. Chemother. 2024, 30, 58–66. [Google Scholar] [CrossRef]
- Ling, Z.; Jin, C.; Xie, T.; Cheng, Y.; Li, L.; Wu, N. Alterations in the Fecal Microbiota of Patients with HIV-1 Infection: An Observational Study in A Chinese Population. Sci. Rep. 2016, 6, 30673. [Google Scholar] [CrossRef]
- Bajer, L.; Kverka, M.; Kostovcik, M.; Macinga, P.; Dvorak, J.; Stehlikova, Z.; Brezina, J.; Wohl, P.; Spicak, J.; Drastich, P. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J. Gastroenterol. 2017, 23, 4548–4558. [Google Scholar] [CrossRef]
- Muniz Pedrogo, D.A.; Jensen, M.D.; Van Dyke, C.T.; Murray, J.A.; Woods, J.A.; Chen, J.; Kashyap, P.C.; Nehra, V. Gut Microbial Carbohydrate Metabolism Hinders Weight Loss in Overweight Adults Undergoing Lifestyle Intervention With a Volumetric Diet. Mayo Clin. Proc. 2018, 93, 1104–1110. [Google Scholar] [CrossRef]
- Singh, S.B.; Carroll-Portillo, A.; Lin, H.C. Desulfovibrio in the Gut: The Enemy within? Microorganisms 2023, 11, 1772. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. Biomed. Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal Clostridia: Leading players in the maintenance of gut homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Chiu, F.C.; Tsai, C.F.; Huang, P.S.; Shih, C.Y.; Tsai, M.H.; Hwang, J.J.; Wang, Y.C.; Chuang, E.Y.; Tsai, C.T.; Chang, S.N. The Gut Microbiome, Seleno-Compounds, and Acute Myocardial Infarction. J. Clin. Med. 2022, 11, 1462. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lu, X. Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.B.; Prince, H.A.; Stevens, T.; Shaheen, N.J.; Dellon, E.S.; Madanick, R.D.; Jennings, S.; Cohen, M.S.; Kashuba, A.D. Differential penetration of raltegravir throughout gastrointestinal tissue: Implications for eradication and cure. AIDS 2013, 27, 1413–1419. [Google Scholar] [CrossRef]
- Thompson, C.G.; Rosen, E.P.; Prince, H.M.A.; White, N.; Sykes, C.; de la Cruz, G.; Mathews, M.; Deleage, C.; Estes, J.D.; Charlins, P.; et al. Heterogeneous antiretroviral drug distribution and HIV/SHIV detection in the gut of three species. Sci. Transl. Med. 2019, 11, eaap8758. [Google Scholar] [CrossRef]
- Sandler, N.G.; Wand, H.; Roque, A.; Law, M.; Nason, M.C.; Nixon, D.E.; Pedersen, C.; Ruxrungtham, K.; Lewin, S.R.; Emery, S.; et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J. Infect. Dis. 2011, 203, 780–790. [Google Scholar] [CrossRef]
- Tenorio, A.R.; Zheng, Y.; Bosch, R.J.; Krishnan, S.; Rodriguez, B.; Hunt, P.W.; Plants, J.; Seth, A.; Wilson, C.C.; Deeks, S.G.; et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J. Infect. Dis. 2014, 210, 1248–1259. [Google Scholar] [CrossRef]
- Akinkuolie, A.O.; Buring, J.E.; Ridker, P.M.; Mora, S. A novel protein glycan biomarker and future cardiovascular disease events. J. Am. Heart Assoc. 2014, 3, e001221. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.W.; Sinclair, E.; Rodriguez, B.; Shive, C.; Clagett, B.; Funderburg, N.; Robinson, J.; Huang, Y.; Epling, L.; Martin, J.N.; et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J. Infect. Dis. 2014, 210, 1228–1238. [Google Scholar] [CrossRef]
- Hester, E.K.; Greenlee, S.; Durham, S.H. Weight Changes With Integrase Strand Transfer Inhibitor Therapy in the Management of HIV Infection: A Systematic Review. Ann. Pharmacother. 2022, 56, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Savinelli, S.; Newman, E.; Mallon, P.W.G. Metabolic Complications Associated with Use of Integrase Strand Transfer Inhibitors (InSTI) for the Treatment of HIV-1 Infection: Focus on Weight Changes, Lipids, Glucose and Bone Metabolism. Curr. HIV/AIDS Rep. 2024, 21, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, C.; Bremer, A.; Alba, D.; Apovian, C.; Koethe, J.R.; Koliwad, S.; Lewis, D.; Lo, J.; McComsey, G.A.; Eckard, A.; et al. Obesity and Fat Metabolism in Human Immunodeficiency Virus-Infected Individuals: Immunopathogenic Mechanisms and Clinical Implications. J. Infect. Dis. 2019, 220, 420–431. [Google Scholar] [CrossRef]
- Pinto-Cardoso, S.M.; Chávez-Torres, M.; Aguilar, A.; Avila-Rios, S. CHANGES IN GUT MICROBIOTA PROFILE IN PWHIV WHO SWITCH FROM EFV/FTC/TDF TO BIC/FTC/TAF. In Proceedings of the 30th CROI 2023, Seattle, WD, USA, 19–22 February 2023. [Google Scholar]
- van Wijk, J.P.; Cabezas, M.C. Hypertriglyceridemia, Metabolic Syndrome, and Cardiovascular Disease in HIV-Infected Patients: Effects of Antiretroviral Therapy and Adipose Tissue Distribution. Int. J. Vasc. Med. 2012, 2012, 201027. [Google Scholar] [CrossRef]
- Gilberti, G.; Tiecco, G.; Marconi, S.; Marullo, M.; Zanini, B.; Quiros-Roldan, E. Weight gain, obesity, and the impact of lifestyle factors among people living with HIV: A systematic review. Obes. Rev. 2025, 26, e13908. [Google Scholar] [CrossRef]
- Guaraldi, G.; Milic, J.; Bacchi, E.; Carli, F.; Menozzi, M.; Franconi, I.; Raimondi, A.; Ciusa, G.; Masi, V.; Belli, M.; et al. Contribution of integrase inhibitor use, body mass index, physical activity and caloric intake to weight gain in people living with HIV. HIV Res. Clin. Pract. 2022, 24, 2150815. [Google Scholar] [CrossRef]
- Byonanebye, D.M.; Polizzotto, M.N.; Maltez, F.; Rauch, A.; Grabmeier-Pfistershammer, K.; Wit, F.; De Wit, S.; Castagna, A.; d’Arminio Monforte, A.; Mussini, C.; et al. Associations between change in BMI and the risk of hypertension and dyslipidaemia in people receiving integrase strand-transfer inhibitors, tenofovir alafenamide, or both compared with other contemporary antiretroviral regimens: A multicentre, prospective observational study from the RESPOND consortium cohorts. Lancet HIV 2024, 11, e321–e332. [Google Scholar] [CrossRef]
- Rupasinghe, D.; Bansi-Matharu, L.; Law, M.; Zangerle, R.; Rauch, A.; Tarr, P.E.; Greenberg, L.; Neesgaard, B.; Jaschinski, N.; De Wit, S.; et al. Integrase Strand Transfer Inhibitor-Related Changes in Body Mass Index and Risk of Diabetes: A Prospective Study From the RESPOND Cohort Consortium. Clin. Infect. Dis. 2025, 80, 404–416. [Google Scholar] [CrossRef]
- Lam, J.O.; Leyden, W.A.; Alexeeff, S.; Lea, A.N.; Hechter, R.C.; Hu, H.; Marcus, J.L.; Pitts, L.; Yuan, Q.; Towner, W.J.; et al. Changes in Body Mass Index Over Time in People With and Without HIV Infection. Open Forum Infect. Dis. 2024, 11, ofad611. [Google Scholar] [CrossRef] [PubMed]
- Muhamad Rizal, N.S.; Neoh, H.M.; Ramli, R.; PR, A.L.K.P.; Hanafiah, A.; Abdul Samat, M.N.; Tan, T.L.; Wong, K.K.; Nathan, S.; Chieng, S.; et al. Advantages and Limitations of 16S rRNA Next-Generation Sequencing for Pathogen Identification in the Diagnostic Microbiology Laboratory: Perspectives from a Middle-Income Country. Diagnostics 2020, 10, 816. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).