Mediterranean Plants and Spices as a Source of Bioactive Essential Oils for Food Applications: Chemical Characterisation and In Vitro Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of EOs Deriving from Mediterranean Plants and Spices
2.2. In Vitro Antioxidant Potential
2.3. Antimicrobial Activity of EOs Against Food-Borne Pathogens/Toxigenic Bacteria
3. Materials and Methods
3.1. Essential Oils
3.2. Characterization of EOs Through GC-MS Analysis
3.3. Antioxidant Activity
3.4. Bacterial Strains and Growth Conditions
3.5. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef] [PubMed]
- Vergis, J.; Gokulakrishnan, P.; Agarwal, R.K.; Kumar, A. Essential oils as natural food antimicrobial agents: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1320–1323. [Google Scholar] [CrossRef]
- Jackson-Davis, A.; White, S.; Kassama, L.S.; Coleman, S.; Shaw, A.; Mendonca, A.; Cooper, B.; Thomas-Popo, E.; Gordon, K.; London, L. A review of regulatory standards and advances in essential oils as antimicrobials in foods. J. Food Prot. 2023, 86, 100025. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Šimat, V.; Skroza, D.; Čagalj, M.; Smole-Možina, S.; Bassi, D.; Gardini, F.; Tabanelli, G. Effects of Rubus fruticosus and Juniperus oxycedrus derivatives on the growth of Listeria monocytogenes: Comparison between culturability and viability. Sci. Rep. 2022, 12, 13158. [Google Scholar] [CrossRef]
- Rao, J.; Chen, B.; McClements, D.J. Improving the Efficacy of Essential Oils as Antimicrobials in Foods: Mechanisms of Action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef]
- da Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Fantuzzi, E.; Domenici Roberto, C. Chemical composition, extraction sources and action mechanisms of essential oils: Natural preservative and limitations of use in meat products. Meat Sci. 2021, 176, 108463. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Li, Y.; Erhunmwunsee, F.; Liu, M.; Yang, K.; Zheng, W.; Tian, J. Antimicrobial mechanisms of spice essential oils and application in food industry. Food Chem. 2022, 382, 132312. [Google Scholar] [CrossRef]
- Dima, C.; Dima, S. Essential oils in foods: Extraction, stabilization, and toxicity. Curr. Opin. Food Sci. 2015, 5, 29–35. [Google Scholar] [CrossRef]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of essential oils: A review on their interaction with food components. Front. Microbiol. 2015, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability, and safety issues for food application—A review. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, G.; Montanari, C.; Patrignani, F.; Siroli, L.; Lanciotti, R.; Gardini, F. Modeling with the logistic regression the growth/no growth interface of Saccharomyces cerevisiae in relation to two antimicrobial terpenes (citral and linalool), pH and aw. J. Food Sci. 2014, 79, M391–M398. [Google Scholar] [CrossRef]
- Ghadermazi, R.; Keramat, J.; Goli, S.A.H. Antioxidant activity of clove (Eugenia caryophyllata Thunb), oregano (Oringanum vulgare L.) and sage (Salvia officinalis L.) essential oils in various model systems. Int. Food Res. J. 2017, 24, 1628–1635. [Google Scholar]
- Sander, A.; Bival Štefan, M.; Radetić, A.; Petracić, A.; Kucić Grgić, D.; Cvetnić, M.; Parlov Vuković, J. Advanced spectroscopic characterization, antioxidant and antibacterial activity evaluation, and trace metal analyses of essential oils from star anise, nutmeg, clove, oregano, bay leaves, and lemon peel. Appl. Sci. 2024, 14, 11094. [Google Scholar] [CrossRef]
- Sarfaraz, D.; Rahimmalek, M.; Sabzalian, M.R.; Gharibi, S.; Matkowski, A.; Szumny, A. Essential oil composition and antioxidant activity of oregano and marjoram as affected by different light-emitting diodes. Molecules 2023, 28, 3714. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant activity of spices and their impact on human health: A review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef]
- Shahat, A.A.; Ibrahim, A.Y.; Hendawy, S.F.; Omer, E.A.; Hammouda, F.M.; Abdel-Rahman, F.H.; Saleh, M.A. Chemical composition, antimicrobial and antioxidant activities of essential oils from organically cultivated fennel cultivars. Molecules 2011, 16, 1366–1377. [Google Scholar] [CrossRef]
- Naaz, S.; Ahmad, N.; Qureshi, M.I.; Hashmi, N.; Akhtar, M.S.A.; Khan, M.M. Antimicrobial and antioxidant activities of fennel oil. Bioinformation 2022, 18, 795–800. [Google Scholar] [CrossRef]
- Yang, K.; Liu, A.P.; Hu, A.X.; Li, J.X.; Zen, Z.; Liu, Y.T.; Tang, S.Y.; Li, C. Preparation and characterization of cinnamon essential oil nanocapsules and comparison of volatile components and antibacterial ability of cinnamon essential oil before and after encapsulation. Food Control 2021, 123, 107783. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Chen, X.; Li, W.; Wang, L.; Li, W.; Du, J.; Zhang, S. Effects of cinnamon essential oil on the physiological metabolism of Salmonella enteritidis. Front. Microbiol. 2022, 13, 1035894. [Google Scholar] [CrossRef] [PubMed]
- Golmakani, M.T.; Zare, M.; Razzaghi, S. Eugenol enrichment of clove bud essential oil using different microwave-assisted distillation methods. Food Sci. Technol. Res. 2017, 23, 385–394. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove essential oil (Syzygium aromaticum L. Myrtaceae): Extraction, chemical composition, food applications, and essential bioactivity for human health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef]
- Hennia, A.; Nemmiche, S.; Dandlen, S.; Miguel, M.G. Myrtus communis essential oils: Insecticidal, antioxidant and antimicrobial activities: A review. J. Essent. Oil Reserves 2019, 31, 487–545. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J.; Amensour, M.; Abrini, J. Identification of flavonoid content and chemical composition of the essential oils of Moroccan herbs: Myrtle (Myrtus communis L.), rockrose (Cistus ladanifer L.) and Montpellier cistus (Cistus monspeliensis L.). J. Essent. Oil Res. 2011, 23, 2. [Google Scholar] [CrossRef]
- Stefanova, G.; Girova, T.; Gochev, V.; Stoyanova, M.; Petkova, Z.; Stoyanova, A.; Zheljazkov, V.D. Comparative study on the chemical composition of laurel (Laurus nobilis L.) leaves from Greece and Georgia and the antibacterial activity of their essential oil. Heliyon 2020, 6, e05491. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.N.; Tang, G.Y.; Li, H.B. Antibacterial and antifungal activities of spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Garner, C.M. A review of essential oils as antimicrobials in foods with special emphasis on fresh produce. J. Food Prot. 2022, 85, 1300–1319. [Google Scholar] [CrossRef]
- Telci, I.; Demirtas, I.; Sahin, A. Variation in plant properties and essential oil composition of sweet fennel (Foeniculum vulgare Mill.) fruits during stages of maturity. Ind. Crops Prod. 2009, 30, 126–130. [Google Scholar] [CrossRef]
- Šunić, L.; Ilić, Z.S.; Stanojević, L.; Milenković, L.; Stanojević, J.; Kovac, R.; Milenković, A.; Cvetković, D. Comparison of the essential oil content, constituents and antioxidant activity from different plant parts during development stages of wild fennel (Foeniculum vulgare Mill.). Horticulturae 2023, 9, 364. [Google Scholar] [CrossRef]
- Falcão, S.; Bacém, I.; Igrejas, G.; Rodrigues, P.J.; Vilas-Boas, M.; Amaral, J.S. Chemical composition and antimicrobial activity of hydrodistilled oil from juniper berries. Ind. Crops Prod. 2018, 124, 878–884. [Google Scholar] [CrossRef]
- Abdolshahi, A.; Naybandi-Atashi, S.; Heydari-Majd, M.; Salehi, B.; Kobarfard, F.; Ayatollahi, S.A.; Ata, A.; Tabanelli, G.; Sharifi-Rad, M.; Montanari, C.; et al. Antibacterial activity of some Lamiaceae species against Staphylococcus aureus in yoghurt-based drink (Doogh). Cell. Mol. Biol. 2018, 64, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Baatour, O.; Kaddour, R.; Aidi Wannes, W.; Lachaâl, M.; Marzouk, B. Salt effects on the growth, mineral nutrition, essential oil yield and composition of marjoram (Origanum majorana). Acta Physiol. Plant 2010, 32, 45–51. [Google Scholar] [CrossRef]
- Novak, J.; Lukas, B.; Franz, C.M. The essential oil composition of wild growing sweet marjoram (Origanum majorana L., Lamiaceae) from Cyprus—Three Chemotypes. J. Essent. Oil Res. 2008, 20, 339–341. [Google Scholar] [CrossRef]
- De Mastro, G.; Tarraf, W.; Verdini, L.; Brunetti, G.; Ruta, C. Essential oil diversity of Origanum vulgare L. populations from Southern Italy. Food Chem. 2017, 235, 1–6. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daskaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Alves Borges Leal, A.L.; et al. Salvia spp. Plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Yazgan, H. Investigation of antimicrobial properties of sage essential oil and its nanoemulsion as antimicrobial agent. LWT-Food Sci. Technol. 2020, 130, 109669. [Google Scholar] [CrossRef]
- Tosun, A.; Khan, S.; Kim, Y.S.; Calín-Sánchez, A.; Hysenaj, X.; Carbonell-Barrachina, A. Essential oil composition and anti-inflammatory activity of Salvia officinalis L (Lamiaceae) in murin macrophages. Trop. J. Pharm. Res. 2014, 13, 937–942. [Google Scholar] [CrossRef]
- Mladenović, M.; Astolfi, R.; Tomašević, N.; Matić, S.; Božović, M.; Sapienza, F.; Ragno, R. In vitro antioxidant and in vivo antigenotoxic features of a series of 61 essential oils and quantitative composition–activity relationships modeled through machine learning algorithms. Antioxidants 2023, 12, 1815. [Google Scholar] [CrossRef] [PubMed]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Ramos, C.; Teixeira, B.; Batista, I.; Matos, O.; Serrano, C.; Neng, N.R.; Marques, A. Antioxidant and antibacterial activity of essential oil and extracts of bay laurel Laurus nobilis Linnaeus (Lauraceae) from Portugal. Nat. Prod. Res. 2011, 26, 518–529. [Google Scholar] [CrossRef]
- Marques, J.D.; Volcao, L.M.; Funck, G.D.; Kroning, I.S.; da Silva, W.P.; Fiorentini, A.M.; Ribeiro, G.A. Antimicrobial activity of essential oils of Origanum vulgare L. and Origanum majorana L. against Staphylococcus aureus isolated from poultry meat. Ind. Crops Prod. 2015, 77, 444–450. [Google Scholar] [CrossRef]
- Reza, Z.M.; Atefeh, J.Y.; Faezeh, F. Effect of γ-irradiation on the antibacterial activities of Cuminum cyminum L. essential oils in vitro and in vivo systems. J. Essent. Oil-Bear. Plants 2015, 18, 582–591. [Google Scholar] [CrossRef]
- Mageed, M.A.A.E.; Mansour, A.F.; El Massry, K.F.; Ramadan, M.M.; Shaheen, M.S.; Shaaban, H. Effect of microwaves on essential oils of coriander and cumin seeds and on their Antioxidant and Antimicrobial Activities. J. Essent. Oil-Bear. Plants 2012, 15, 614–627. [Google Scholar] [CrossRef]
- Xu, J.G.; Liu, T.; Hu, Q.P.; Cao, X.M. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Noshad, M.; Falah, F. Study of chemical structure, antimicrobial, cytotoxic and mechanism of action of Syzygium aromaticum essential oil on foodborne pathogens. Potravin. Slovak J. Food Sci. 2019, 13, 875–883. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A recent insight regarding the phytochemistry and bioactivity of Origanum vulgare L. essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef]
- Bautista-Hernandez, I.; Gomez-Garcia, R.; Martinez-Avila, G.C.G.; Medina-Herrera, N.; Gonzalez-Hernandez, M.D. Unlocking Essential Oils’ Potential as Sustainable Food Additives: Current State and Future Perspectives for Industrial Applications. Sustainability 2025, 17, 2053. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Maczka, W.; Twardawska, M.; Grabarczyk, M.; Winska, K. Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef] [PubMed]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef]
- Arioli, S.; Montanari, C.; Magnani, M.; Tabanelli, G.; Patrignani, F.; Lanciotti, R.; Mora, D.; Gardini, F. Modelling of Listeria monocytogenes Scott A after a mild heat treatment in the presence of thymol and carvacrol: Effects on culturability and viability. J. Food Eng. 2019, 240, 73–82. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Walasek-Janusz, M. Chemical Composition, Biological Activity, and Potential Uses of Oregano (Origanum vulgare L.) and Oregano Essential Oil. Pharmaceuticals 2025, 18, 267. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Antimicrobial Mechanisms, and Antibiotic Activities of Cinnamaldehyde against Pathogenic Bacteria in Animal Feeds and Human Foods. J. Agric. Food Chem. 2017, 65, 10406–10423. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Inhibition of membrane bound ATPases of Escherichia coli and Listeria monocytogenes by plant oil aromatics. Int. J. Food Microbiol. 2006, 111, 170–174. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Agyekumwaa Addo, K.; Yu, Y.; Xiao, X. Action mode of cuminaldehyde against Staphylococcus aureus and its application in sauced beef. LWT-Food Sci. Technol. 2022, 155, 112924. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.A. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Biomed. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.G. Plant essential oils as active antimicrobial agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. Antimicrobial activity of plant essential oils using food model media: Efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009, 26, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef]
- Angienda, P.O.; Hill, D.J. The effect of sodium chloride and pH on the antimicrobial effectiveness of essential oils against pathogenic and food spoilage bacteria: Implications in food safety. Int. J. Biol. Sci. 2011, 8, 222–227. [Google Scholar]
- Moleyar, V.; Narasimham, P. Antibacterial activity of essential oil components. Int. J. Food Microbiol. 1992, 16, 337–342. [Google Scholar] [CrossRef]
- Gālina, D.; Radenkovs, V.; Kviesis, J.; Valdovska, A. Effect of Essential Oils Supplemented with Caprylic Acid and Sodium Chloride against Faecal ESBL-Producing Escherichia coli Isolated from Pigs. Antibiotics 2022, 11, 461. [Google Scholar] [CrossRef]
- El Harsal, A.; Belmehdi, O.; Souilah, Y.; Ouzakar, S.; Farah, A.; Skali Senhaji, N.; Bouyahya, A.; Abrini, J.; Khamlichi, A. Mathematical modeling of the combined action of Origanum grosii essential oil and sodium chloride on the growth of methicillin-resistant Staphylococcus aureus. S. Afr. J. Bot. 2023, 160, 516–524. [Google Scholar] [CrossRef]
- Milat, A.M.; Boban, M.; Teissedre, P.L.; Šešelja-Perišin, A.; Jurić, D.; Skroza, D.; Generalić-Mekinić, I.; Ljubenkov, I.; Volarević, J.; Rasines-Perea, Z.; et al. Effects of oxidation and browning of macerated white wine on its antioxidant and direct vasodilatory activity. J. Funct. Foods 2019, 59, 138–147. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Burčul, F.; Generalić Mekinić, I.; Radan, M.; Rollin, P.; Blažević, I. Isothiocyanates: Cholinesterase inhibiting, antioxidant, and anti-Inflammatory Activity. J. Enzym. Inhib. Med. Chem. 2018, 33, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (Oxygen Radical Absorbance Capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
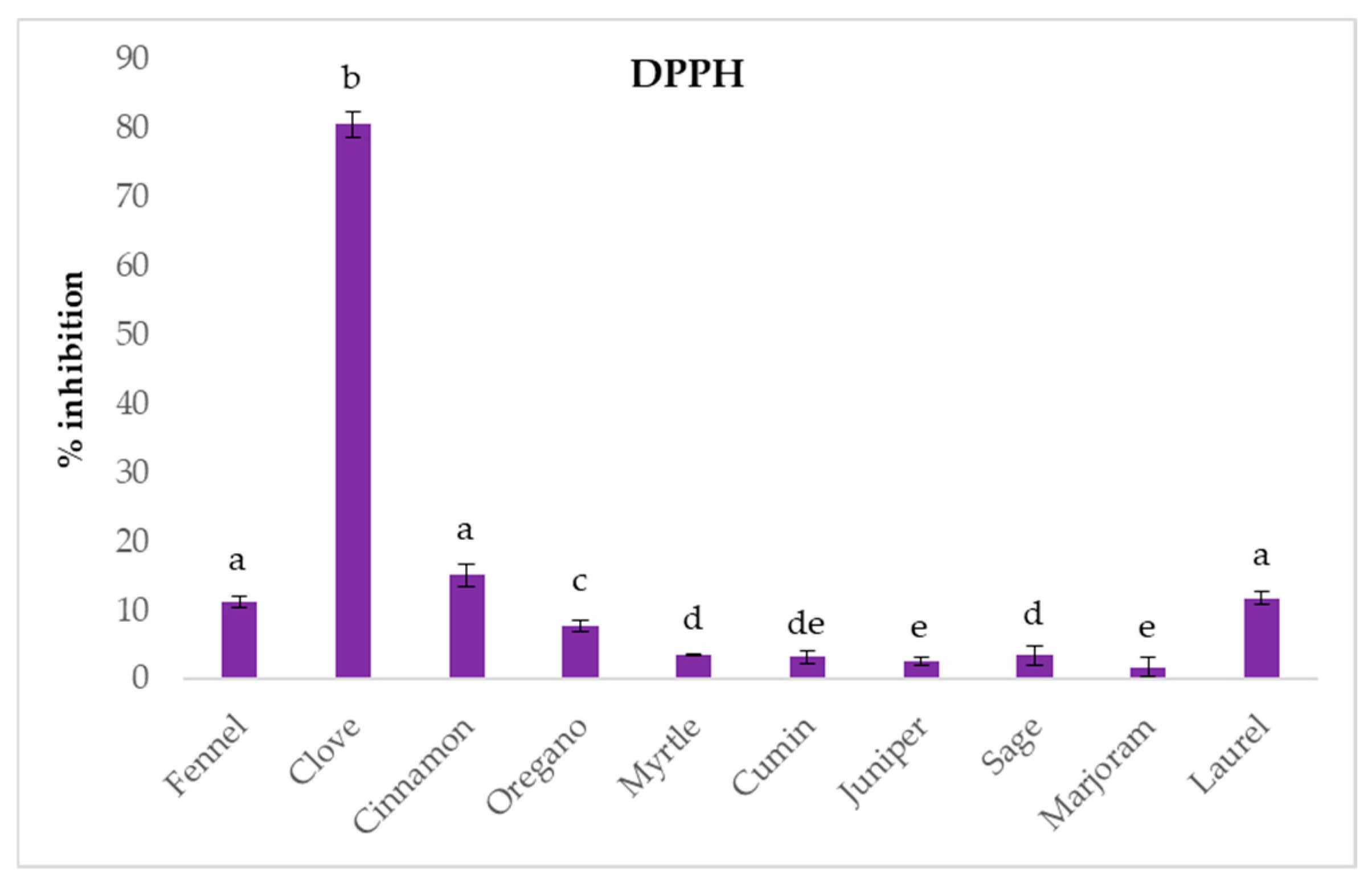
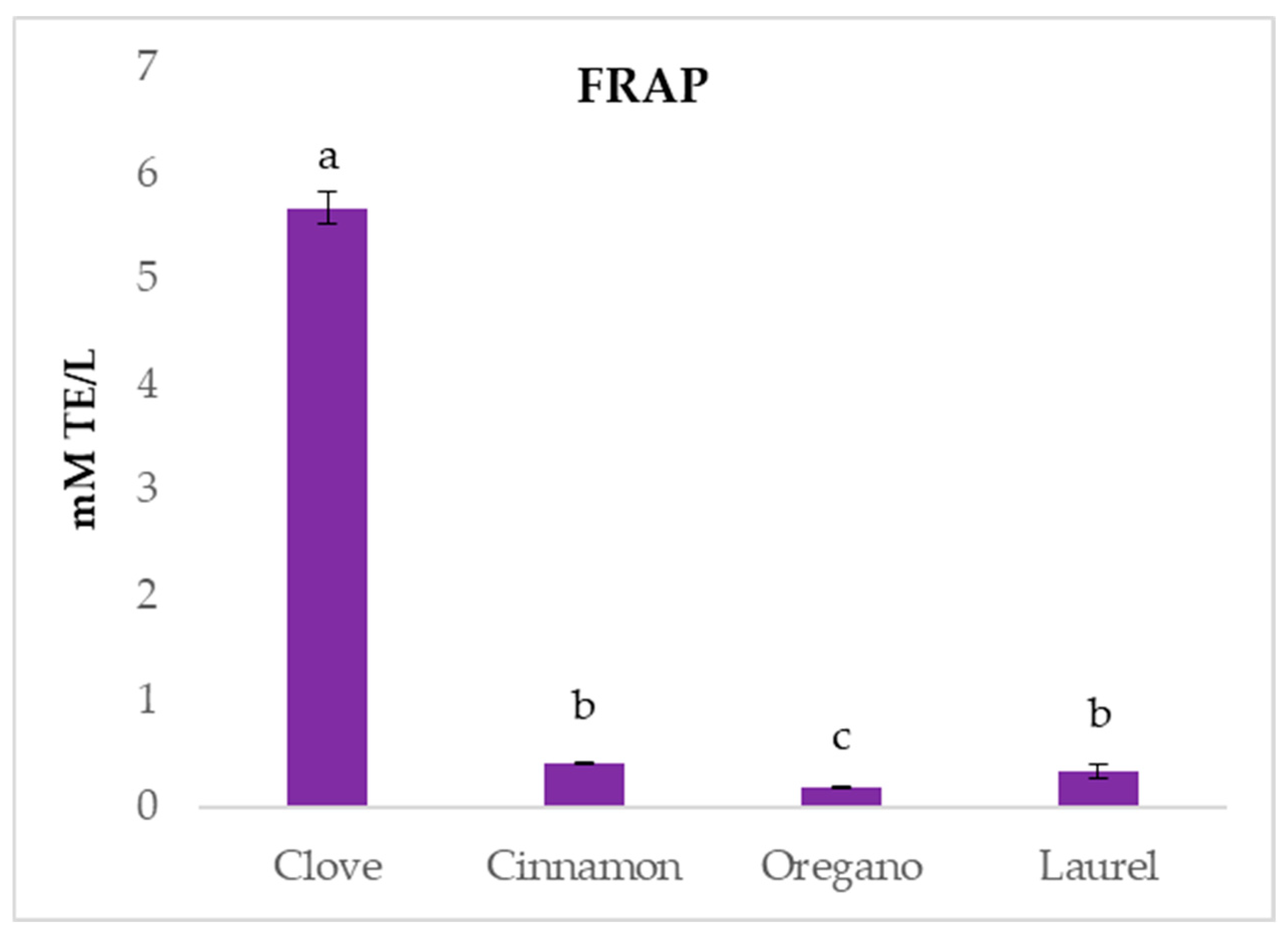

| Compound (%) | R.T. # (min) | Cinnamon | Cloves | Cumin | Fennel | Juniper | Laurel | Marjoram | Myrtle | Oregano | Sage |
|---|---|---|---|---|---|---|---|---|---|---|---|
| α-phellandrene | 5.791 | 0.65 | - * | 0.40 | 11.90 | 1.53 | 0.39 | 2.47 | 0.22 | 0.97 | 0.30 |
| α-pinene | 5.948 | 1.14 | - | 0.67 | 13.11 | 30.89 | 4.54 | 0.99 | 23.33 | 0.68 | 4.23 |
| Camphene | 6.194 | 0.57 | - | 0.02 | 0.13 | 0.20 | 0.27 | 0.03 | 0.06 | 0.08 | 4.35 |
| β-phellandrene | 6.455 | 2.03 | - | 0.27 | 1.54 | 15.16 | 9.32 | 10.61 | 0.04 | 0.13 | 0.46 |
| β-myrcene | 6.538 | 0.05 | - | 0.27 | 1.32 | 12.68 | 1.26 | 1.57 | 0.15 | 0.98 | 0.88 |
| β-pinene | 6.590 | 0.36 | - | 12.78 | 1.15 | 2.25 | 4.05 | 0.71 | 0.33 | 0.22 | 4.19 |
| 4-carene | 7.111 | 0.52 | - | 0.08 | - | 0.91 | 0.41 | 8.91 | - | 0.98 | - |
| p-cymene | 7.231 | 1.41 | - | 12.22 | 0.83 | 0.36 | 0.30 | 3.60 | 0.80 | 7.36 | 0.98 |
| trans-β-ocimene | 7.263 | - | - | - | 0.53 | - | - | - | - | - | 0.12 |
| D-limonene | 7.325 | 0.75 | - | 0.34 | 9.68 | 3.63 | 1.36 | 2.53 | 10.61 | 0.16 | 1.48 |
| Eucalyptol | 7.423 | 0.27 | - | 0.19 | 0.05 | - | 39.17 | 0.21 | 34.31 | 0.07 | 10.76 |
| γ-terpinene | 7.823 | 0.09 | - | 13.16 | 0.42 | 1.55 | 0.83 | 14.15 | 0.33 | 5.09 | 0.07 |
| β-terpineol | 8.017 | - | - | 0.10 | - | 0.05 | 0.31 | 14.39 | - | 0.22 | 0.12 |
| Terpinolene | 8.422 | - | - | 0.05 | - | 1.36 | 0.16 | 3.49 | - | - | 0.09 |
| Linalool | 8.457 | 1.42 | - | 0.03 | 0.09 | - | 5.89 | 2.20 | 4.04 | 0.95 | 0.44 |
| L-fenchone | 8.521 | - | - | - | 5.41 | - | - | - | - | - | - |
| Thujone | 8.855 | - | - | - | - | - | - | - | - | - | 35.48 |
| trans-p-Menth-2-en-1-ol | 9.127 | - | - | - | - | 0.04 | 0.05 | 0.67 | - | - | - |
| Camphor | 9.763 | 0.69 | - | - | 0.09 | - | - | - | - | 0.03 | 11.72 |
| α-terpineol | 10.108 | 0.63 | - | 0.13 | 0.03 | 0.10 | 0.89 | 4.21 | 3.70 | 0.10 | 0.13 |
| endo-borneol | 10.184 | 0.13 | - | - | - | - | 0.19 | 0.04 | - | 0.26 | 2.70 |
| Terpinen-4-ol | 10.382 | 0.35 | - | 0.26 | 0.04 | 1.22 | 1.68 | 24.33 | 0.18 | 0.58 | 0.36 |
| Estragole | 10.742 | - | - | - | 1.68 | - | - | 0.06 | 0.19 | - | - |
| Myrtenol | 10.806 | - | - | - | - | - | - | - | 0.51 | - | - |
| Fenchyl acetate | 11.314 | - | - | - | 0.72 | - | - | - | - | - | - |
| Cuminaldehyde | 11.870 | - | - | 32.61 | - | - | - | - | - | - | - |
| trans-cinnamaldehyde | 12.621 | 62.02 | - | - | - | - | - | - | - | - | - |
| Thymol | 12.766 | - | - | - | - | - | - | - | - | 2.66 | |
| Bornyl acetate | 12.924 | - | - | - | 0.05 | 0.25 | - | - | - | - | 1.47 |
| Anethole | 12.925 | - | - | - | 50.86 | - | - | - | - | - | - |
| γ-terpinen-7-al | 12.994 | - | - | 14.91 | - | - | - | - | - | - | - |
| α-terpinen-7-al | 13.082 | - | - | 9.79 | - | - | - | - | - | - | - |
| Carvacrol | 13.147 | - | - | - | - | - | - | - | - | 75.93 | - |
| ψ-Limonene | 13.589 | - | - | - | - | - | 0.58 | - | - | - | - |
| Myrtenyl acetate | 13.785 | - | - | - | - | - | - | - | 14.60 | - | - |
| Terpineol acetate | 14.215 | - | - | - | - | 0.07 | 15.20 | - | 0.36 | - | - |
| α-cubebene | 14.320 | - | - | - | - | 0.56 | - | - | 0.03 | ||
| Eugenol | 14.339 | 4.48 | 75.20 | - | - | - | 4.18 | - | - | ||
| Geranyl acetate | 14.600 | - | - | - | - | - | 0.04 | 3.35 | - | 0.02 | |
| Methyleugenol | 14.971 | - | - | - | - | - | 3.27 | - | 1.02 | - | - |
| β-elemene | 14.995 | - | - | 0.27 | - | 2.36 | 0.53 | - | - | - | |
| Caryophyllene | 15.493 | 3.01 | 4.94 | 0.06 | 0.06 | 1.74 | 1.04 | 1.51 | 0.36 | 1.38 | 4.32 |
| γ-elemene | 15.550 | - | - | 0.12 | 2.22 | - | - | - | - | - | |
| Acetic acid, cinnamyl ester | 15.579 | 14.09 | - | - | - | - | - | - | - | - | - |
| Humulene | 15.910 | 0.67 | 0.60 | 1.78 | 0.31 | 0.07 | 0.19 | 0.10 | 8.12 | ||
| β-copaene | 16.211 | - | - | 0.03 | 0.18 | 7.41 | - | 0.08 | - | - | 0.09 |
| α-muurolene | 16.362 | - | - | - | - | 1.45 | - | - | - | - | - |
| Eugenol acetate | 16.507 | - | 18.45 | - | - | - | - | - | - | - | |
| δ-cadinene | 16.545 | - | 0.07 | - | - | 4.20 | 0.13 | - | - | - | - |
| α-amorphene | 16.547 | 0.75 | - | - | - | 1.41 | - | - | - | - | |
| Ledol | 17.445 | - | - | - | - | - | - | - | - | - | 3.90 |
| o-menth-8-ene | 17.615 | - | - | - | - | - | - | - | - | - | 1.30 |
| Benzyl benzoate | 18.992 | 1.07 | - | - | - | - | - | - | - | - | - |
| Total compounds | 97.14 | 99.26 | 98.75 | 99.87 | 95.39 | 96.34 | 96.87 | 98.69 | 98.92 | 98.14 |
| Essential Oil (mg/mL) | pH | Listeria monocytogenes Scott A | Staphylococcus aureus DSM 20231t | Escherichia coli 555 | Enterococcus faecalis EF37 | ||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| Cinnamon | 7 | 0.25 | 0.50 | 0.25 | 0.50 | 0.25 | 0.50 | 0.50 | 1 |
| 6 | 0.25 | 0.50 | 0.25 | 0.50 | 0.25 | 0.50 | 0.50 | 1 | |
| 5 | 0.10 | 0.25 | 0.25 | 0.50 | 0.25 | 0.25 | 0.50 | 1 | |
| Cloves | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 5 |
| 6 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 5 | |
| 5 | 0.25 | 1 | 0.25 | 0.25 | 1 | 1 | 1 | 2 | |
| Cumin | 7 | >5 | >5 | 4 | 5 | >5 | >5 | >5 | >5 |
| 6 | 4 | >5 | 3 | 3 | >5 | >5 | >5 | >5 | |
| 5 | 2 | >5 | 1 | 1 | >5 | >5 | >5 | >5 | |
| Fennel | 7 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 |
| 6 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | |
| 5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | |
| Juniper | 7 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 |
| 6 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | |
| 5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | |
| Laurel | 7 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 |
| 6 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | |
| 5 | 4 | >5 | 2 | 2 | >5 | >5 | >5 | >5 | |
| Marjoram | 7 | 5 | >5 | >5 | >5 | 3 | 4 | >5 | >5 |
| 6 | 4 | 4 | >5 | >5 | 3 | 3 | >5 | >5 | |
| 5 | 3 | 3 | 1 | 2 | 3 | 3 | >5 | >5 | |
| Myrtle | 7 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 |
| 6 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | |
| 5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | |
| Oregano | 7 | 0.25 | 0.30 | 0.25 | 0.30 | 0.40 | 0.40 | 0.40 | 0.50 |
| 6 | 0.20 | 0.25 | 0.25 | 0.20 | 0.30 | 0.40 | 0.40 | 0.40 | |
| 5 | 0.20 | 0.20 | 0.15 | 0.15 | 0.25 | 0.25 | 0.30 | 0.40 | |
| Sage | 7 | >5 | >5 | 0.75 | 0.75 | >5 | >5 | >5 | >5 |
| 6 | >5 | >5 | 1 | 1 | >5 | >5 | >5 | >5 | |
| 5 | >5 | >5 | 1 | 1 | >5 | >5 | >5 | >5 | |
| Essential Oil (mg/mL) | NaCl | Listeria monocytogenes Scott A | Staphylococcus aureus DSM 20231t | Escherichia coli 555 | Enterococcus faecalis EF37 | ||||
|---|---|---|---|---|---|---|---|---|---|
| (%) | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Cinnamon | 0 | 0.25 | 0.50 | 0.25 | 0.50 | 0.25 | 0.50 | 0.50 | 1 |
| 3 | 0.25 | 0.25 | 0.25 | 0.25 | 0.20 | 0.20 | 0.30 | 0.75 | |
| 5 | 0.20 | 0.20 | 0.20 | 0.25 | 0.10 | 0.10 | 0.25 | 0.50 | |
| Cloves | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 5 |
| 3 | 0.75 | 1 | 0.50 | 0.50 | 0.50 | 0.50 | 5 | 5 | |
| 5 | 0.30 | 0.75 | 0.30 | 0.50 | 0.20 | 0.20 | 5 | 5 | |
| Cumin | 0 | >5 | >5 | 4 | 5 | >5 | >5 | >5 | >5 |
| 3 | >5 | >5 | 5 | 5 | 0.20 | 0.20 | >5 | >5 | |
| 5 | 1 | >5 | 5 | 5 | 0.20 | 0.20 | >5 | >5 | |
| Fennel | 0 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 |
| 3 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | |
| 5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | |
| Juniper | 0 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 |
| 3 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | |
| 5 | 5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | |
| Laurel | 0 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 |
| 3 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | |
| 5 | >5 | >5 | >5 | >5 | 0.25 | 0.25 | >5 | >5 | |
| Marjoram | 0 | 5 | >5 | >5 | >5 | 3 | 4 | >5 | >5 |
| 3 | 5 | >5 | >5 | >5 | 1 | 1 | >5 | >5 | |
| 5 | 5 | >5 | >5 | >5 | 0.50 | 0.50 | >5 | >5 | |
| Myrtle | 0 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 |
| 3 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | |
| 5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | >5 | |
| Oregano | 0 | 0.25 | 0.30 | 0.25 | 0.30 | 0.40 | 0.40 | 0.40 | 0.50 |
| 3 | 0.25 | 0.25 | 0.25 | 0.25 | 0.10 | 0.10 | 0.40 | 0.40 | |
| 5 | 0.15 | 0.20 | 0.25 | 0.25 | 0.10 | 0.10 | 0.40 | 0.40 | |
| Sage | 0 | >5 | >5 | 0.75 | 0.75 | >5 | >5 | >5 | >5 |
| 3 | >5 | >5 | 0.75 | 0.75 | >5 | >5 | >5 | >5 | |
| 5 | 5 | >5 | 0.40 | 0.40 | 0.20 | 0.20 | >5 | >5 | |
| EO | Species | Plant Source |
|---|---|---|
| Cinnamon | Cinnamomum verum formerly C. zeylanicum | Bark |
| Cloves | Eugenia caryophillata Thumb. | Leaves |
| Cumin | Cuminum cyminum | Fruits |
| Fennel | Foeniculum vulgare Mill. | Seeds |
| Juniper | Juniperus communis L. | Berries |
| Laurel | Laurus nobilis L. | Leaves |
| Marjoram | Origanum majorana | Flower parts |
| Myrtle | Myrtus communis L. | Flower parts |
| Oregano | Origanum vulgare L. | Flower parts |
| Sage | Salvia officinalis L. | Flower parts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbieri, F.; Tabanelli, G.; Braschi, G.; Bassi, D.; Morandi, S.; Šimat, V.; Čagalj, M.; Gardini, F.; Montanari, C. Mediterranean Plants and Spices as a Source of Bioactive Essential Oils for Food Applications: Chemical Characterisation and In Vitro Activity. Int. J. Mol. Sci. 2025, 26, 3875. https://doi.org/10.3390/ijms26083875
Barbieri F, Tabanelli G, Braschi G, Bassi D, Morandi S, Šimat V, Čagalj M, Gardini F, Montanari C. Mediterranean Plants and Spices as a Source of Bioactive Essential Oils for Food Applications: Chemical Characterisation and In Vitro Activity. International Journal of Molecular Sciences. 2025; 26(8):3875. https://doi.org/10.3390/ijms26083875
Chicago/Turabian StyleBarbieri, Federica, Giulia Tabanelli, Giacomo Braschi, Daniela Bassi, Sara Morandi, Vida Šimat, Martina Čagalj, Fausto Gardini, and Chiara Montanari. 2025. "Mediterranean Plants and Spices as a Source of Bioactive Essential Oils for Food Applications: Chemical Characterisation and In Vitro Activity" International Journal of Molecular Sciences 26, no. 8: 3875. https://doi.org/10.3390/ijms26083875
APA StyleBarbieri, F., Tabanelli, G., Braschi, G., Bassi, D., Morandi, S., Šimat, V., Čagalj, M., Gardini, F., & Montanari, C. (2025). Mediterranean Plants and Spices as a Source of Bioactive Essential Oils for Food Applications: Chemical Characterisation and In Vitro Activity. International Journal of Molecular Sciences, 26(8), 3875. https://doi.org/10.3390/ijms26083875












