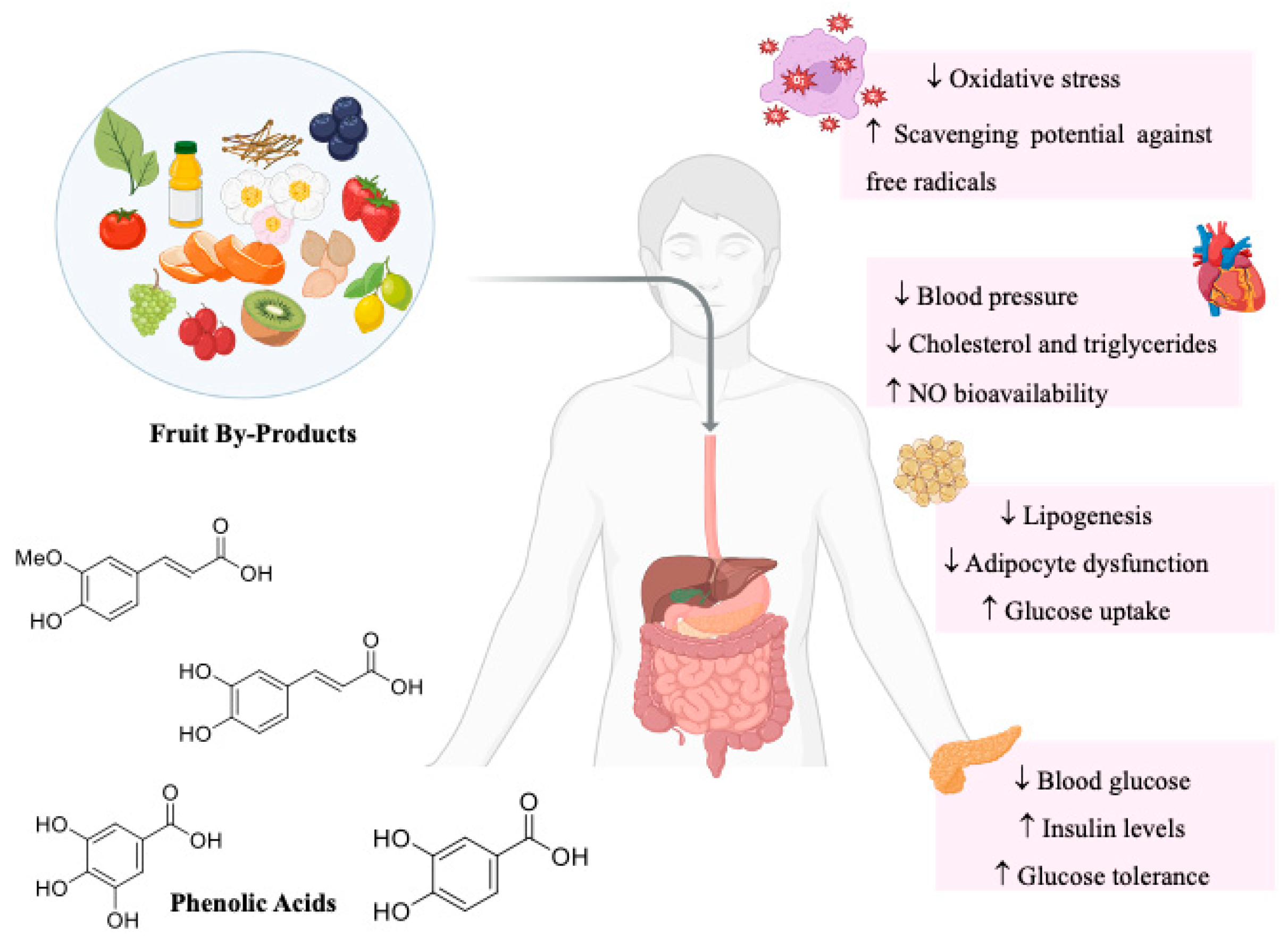
Phenolic Acids from Fruit By-Products as Therapeutic Agents for Metabolic Syndrome: A Review
Abstract
1. Introduction
2. Data Collection
3. Valorization of Fruit By-Products
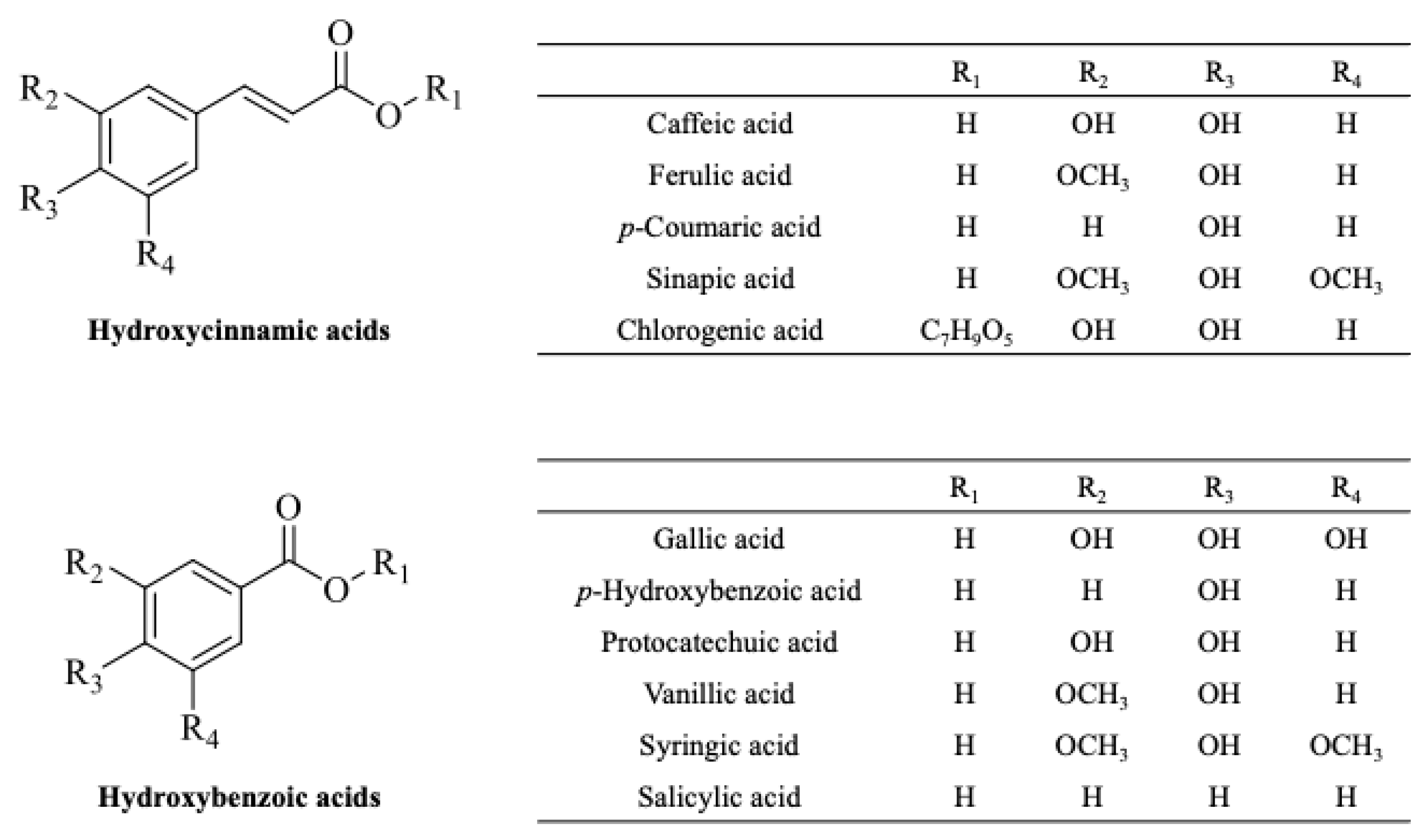
4. Phenolic Acids
4.1. Overview of Phenolic Acids Extraction Techniques
4.1.1. Solvent Extraction
4.1.2. Ultrasound-Assisted Extraction (UAE)
4.1.3. Microwave-Assisted Extraction (MAE)
4.1.4. Pressurized Liquid Extraction
4.1.5. Supercritical Fluid Extraction (SFE)
4.1.6. Enzyme-Assisted Extraction (EAE)
4.2. Main Phenolic Acids in Fruit By-Products
| Fruit | By-Product | Phenolic Acid | References |
|---|---|---|---|
| Apple | Pomace | Protocatechuic acid, chlorogenic acid, 4-hydroxybenzoic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, isoferulic acid | [57] |
| Peel | Gallic acid, vanillic acid, caffeic acid, chlorogenic acid | [58] | |
| Pulp | Gallic acid, vanillic acid, chlorogenic acid | [58] | |
| Seed | Caffeic acid, neochlorogenic acid | [27,28] | |
| Banana | Peel | 3,4-Dihydroxybenzoic acid, ferulic acid, chlorogenic acid, gallic acid | [59] |
| Inflorescence | Gallic acid, protocatechuic acid, p-hydroxybenzoic acid, syringic acid, ferulic acid | [60] | |
| Blueberry | Pomace | Gallic acid, ferulic acid, p-coumaric acid, 4-hydroxybenzoic acid | [61,62] |
| Grape | Juice | Gallic acid, vanillic acid, caffeic acid | [63] |
| Pomace | Ferulic acid, p-coumaric acid, caffeic acid, vanillic acid, gallic acid, p-hydroxybenzoic | [30] | |
| Seed | Gallic acid | [64] | |
| Lees | p-coumaric acid | [65] | |
| Peel | Hydroxycinnamic acid derivatives | [65] | |
| Kiwi | Seeds | p-coumaric acid, p-hydroxybenzoic acid | [66] |
| Leaves | Chlorogenic acid, neochlorogenic acid, caffeoylquinic acid | [66] | |
| Lemon | Peel | Caffeic acid, coumaric acid, ferulic acid, sinapic acid | [67] |
| Olive | Pomace | Gallic acid, vanillic acid, syringic acid, protocatechuic acid, caffeic acid, chlorogenic acid, ferulic acid, sinapic acid | [68] |
| Leaves | p-hydroxybenzoic acid, vanillic acid, protocatechuic acid, caffeic acid, chlorogenic acid, ferulic acid | [69] | |
| Seeds | Syringic acid, ferulic acid, caffeic acid derivative | [70] | |
| Orange | Peel | Caffeic acid, p-coumaric acid | [10] |
| Pulp | Protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, sinapic acid, chlorogenic acid | [71] | |
| Peach | Peels | p-coumaric acid, ferulic acid, caffeoylquinic acid, caffeic acid, gallic acid, protocatechuic acid, neochlorogenic acid, p-coumaroylquinic acid | [72] |
| Seeds | Chlorogenic acid, neochlorogenic acid, gallic acid, caffeic acid, cis-5-p-coumaroyloquinic acid, p-hydroxybenzoic acid | [72] | |
| Pomace | Chlorogenic acid, neochlorogenic acid | [72] | |
| Pomegranate | Peel | Gallic acid | [73,74] |
| Pomace | Gallic acid, p-coumaric acid, chlorogenic acid | [75] | |
| Rowanberry | Pomace | Chlorogenic acid | [76,77] |
| Strawberry | Pomace | Gallic acid, ferulic acid, p-coumaric acid, 4-hydroxybenzoic acid | [61] |
| Sweet cherry | Stems | Caffeoylquinic acid, protocatechuic acid, ferulic acid, hydroxybenzoic acid derivative | [8,78] |
| Leaves | Caffeoylquinic acid, p-coumaric acid, p-coumaroylquinic acid, protocatechuic acid, ferulic acid | [8,78] | |
| Flowers | Caffeoylquinic acid | [8,78] | |
| Pomace | Syringic acid, vanillic acid, chlorogenic acid, 3,5-dicaffeoylquinic acid | [79] | |
| Tomato | Peel | Caffeic acid, vanillic acid, ferulic acid, sinapic acid, chlorogenic acid, gallic acid, p-coumaric acid | [80] |
| Seeds | Caffeic acid, vanillic acid, ferulic acid, sinapic acid, chlorogenic acid, gallic acid, p-coumaric acid | [80] |
5. Phenolic Acids in the Prevention of Metabolic Syndrome
5.1. Antioxidant Activity
| By-Product/Phenolic Acid | Study Type | Main Outcomes | References |
|---|---|---|---|
| In vitro studies | |||
| Apple, lemon, and orange by-products (unspecified) | DPPH•-scavenging activity | Lemon extract presented the highest inhibition of DPPH• (51.7%), followed by apple (39.9%) | [86] |
| Tomato, grape, lemon, olive and pomegranate by-products | Olive, grape, and lemon pomaces were able to inhibit in vitro oxidation more efficiently than the rest of extracts | [87] | |
| Ferulic acid | Scavenging efficiency was in the following order: Caff > Prot > Gall > Sina >Feru > p-Coum > Vani | [88] | |
| Caffeic acid | |||
| Sinapic acid | |||
| p-Coumaric acid | |||
| Protocatechuic acid | |||
| Gallic acid | |||
| Vanillic acid | |||
| Sweet cherry petioles | All cultivars exhibited significant antioxidant potential, with DPPH-scavenging values ranging from 29.88% to 86.94%. Moreover, genotypes with the highest phenolic content showed the highest DPPH-radicals scavenging activities | [93] | |
| Sweet cherry stems | Hydromethanolic extract revealed higher antioxidant potential | [91] | |
| Sweet cherry leaves, stems, and flowers | Hydroethanolic stems extract demonstrated the strongest antioxidant activity, followed by aqueous infusions. Moreover, leaves showed better antioxidant activity than flowers | [8] | |
| Ferulic acid | ABTS+•-scavenging activity | Scavenging efficiency was in the following order: Gall > Feru > Caff > Prot > p-Coum Vanillic acid exhibited low scavenging activity Ferulic acid is more effective than p-coumaric acid due to the presence of the OCH3 group in position ortho to the hydroxyl group | [88] |
| Caffeic acid | |||
| Sinapic acid | |||
| p-Coumaric acid | |||
| Protocatechuic acid | |||
| Gallic acid | |||
| Vanillic acid | |||
| Ferulic acid | O2•−-scavenging activity | Scavenging efficiency was in the following order: Gall > Caff > Vani > Prot > Ferul > Sina Compounds that possess more than one hydroxyl group in their aromatic ring (e.g., gallic acid, caffeic acid, and protocatechuic acid) exhibited stronger inhibitory power than monohydroxyl substituents (e.g., p-coumaric acid and ferulic acid) | [88] |
| Caffeic acid | |||
| Sinapic acid | |||
| p-Coumaric acid | |||
| Protocatechuic acid | |||
| Gallic acid | |||
| Vanillic acid | |||
| Ferulic acid | Reducing power | Scavenging efficiency was in the following order: Gall > Caff > Prot > Sina > Ferul > p-Coum Gallic acid is very strong reducing agent, owing to the presence of three hydroxyl groups Vanillic acid exhibited very low reducing power | [88] |
| Caffeic acid | |||
| Sinapic acid | |||
| p-Coumaric acid | |||
| Protocatechuic acid | |||
| Gallic acid | |||
| Vanillic acid | |||
| Sweet cherry stems | Hydromethanolic extract revealed higher antioxidant potential | [91] | |
| Sweet cherry stems | β-Carotene bleaching inhibition | Hydromethanolic extract, infusion, and decoction revealed higher antioxidant potential | [91] |
Mechanisms of Action
5.2. Anti-Hyperglycemic Activity
| By-Product/Phenolic Acid | Study Type | Main Outcomes | References |
|---|---|---|---|
| In vitro studies | |||
| Sweet cherry stems, leaves, and flowers | Enzyme inhibition | Inhibition of α-glucosidase enzyme | [78] |
| Caffeic acid | [120] | ||
| Syringic acid | |||
| Chlorogenic acid | Inhibition of α-amylase and α-glucosidase enzymes | [110,111,121,121] | |
| Gallic acid | Glucose uptake | ↑ GLUT4 translocation and glucose uptake in an Akt-independent manner | [106] |
| In vivo studies | |||
| Chlorogenic acid | Glucose uptake | ↓ Body weight ↑ Glucose tolerance ↑ Insulin sensitivity | [112] |
| Ellagic acid | Stimulation of glucose-stimulated insulin secretion from isolated islets ↑ Glucose tolerance | [116,122] | |
| Gallic acid | ↑ Glucose uptake ↓ Hyperglycemia Improved oral glucose tolerance test Upregulated insulin signaling proteins Enhanced glycogenesis and glycolysis | [107,118] | |
| Caffeic, ferulic, gallic, and protocatechuic acids | Blood glucose | ↓ High-fructose-diet-induced metabolic syndrome in body mass index and blood glucose levels | [101] |
| Caffeic acid | ↓ Blood glucose ↑ Insulin levels ↑ Glucose tolerance ↑ Pancreatic β-cell function and morphology | [114,115] | |
| Ellagic acid | ↓ Fasting blood glucose ↓ Insulin resistance | [117,122] | |
| Gallic acid | ↓ Blood glucose ↑ Insulin levels | [119] | |
| Ferulic acid | ↓ Blood glucose ↑ Insulin levels | [105] | |
| Human studies | |||
| Chlorogenic acid | Glucose tolerance | ↓ Glucose and insulin concentrations 15 min after an oral glucose tolerance test | [123] |
Mechanisms of Action
5.3. Anti-Hypertensive Activity
| By-Product/Phenolic Acid | Study Type | Main Outcomes | References |
|---|---|---|---|
| In vitro studies | |||
| Gallic acid | Human umbilical vein endothelial cells (HUVECs) | ↑ NO levels Inhibit angiotensin-I converting enzyme (ACE) | [134] |
| In vivo Studies | |||
| Gallic acid | Spontaneously hypertensive rats (SHRs) | ↓ Blood pressure | [134] |
| N-nitro-L-arginine methyl ester (L-NAME)-induced hypertensive mice | ↓ Systolic blood pressure | [135] | |
| Ferulic acid | Normotensive Wistar Kyoto (WKY) rats and spontaneously hypertensive rats (SHRs) | ↑ NO bioavailability and ↓ NADPH-dependent superoxide anion levels in SHR aortas ↑Acetylcholine-induced endothelium-dependent vasodilation in SHR | [132] |
| Spontaneously hypertensive rats (SHRs) | ↓ Systolic blood pressure | [133] | |
| Male stroke-prone spontaneously hypertensive rats (SHRSP) | ↓ Angiotensin-I converting enzyme (ACE) ↓ Blood pressure ↓ Plasma total cholesterol and triglyceride levels | [136] | |
| Caffeic acid | Cyclosporine-induced hypertensive rats | ↓ Systolic blood pressure ↓ Cardiac frequency ↓ Angiotensin-I converting enzyme (ACE) ↑ NO bioavailability | [137] |
Mechanisms of Action
5.4. Anti-Obesity Activity
| By-Product/Phenolic Acid | Study Type | Main Outcomes | References |
|---|---|---|---|
| In vitro studies | |||
| p-Coumaric acid | 3T3-L1 cell model | ↓ Adipogenesis during the late phase of MDI-induced differentiation ↑ Fatty acid β-oxidation via AMPK pathway in mature adipocytes | [151] |
| Caffeic acid | ↓ Expression of transcription factors PPAR-γ and C/EBP-α with a caffeic acid treatment between 10 and 50 μM in a dose-dependent manner ↓ Intracellular ROS | [152] | |
| Vanillic acid | ↓ Expression of transcription factors PPAR-γ and C/EBP-α after 8 days of treatment with 25 μM | [153] | |
| Ellagic acid | Inhibition of lipid accumulation in 3T3-L1 cells | [165] | |
| Apple peel | [159] | ||
| Chlorogenic acid | ↓ Oxidative stress Inhibition triacylglyceride synthesis | [157] | |
| Gallic acid | ↑ Apoptosis in 3T3-L1 preadipocytes, which causes a decrease in cell size and number ↑ PPAR-γ expression, improving insulin resistance for glucose metabolism | [158] | |
| trans-Cinnamic acid | Primary subcutaneous human preadipocytes | ↓ Adipocyte lipolysis via activation of the nicotinic acid receptor GPR109A (HM74a/PUMA-G) | [156] |
| p-Coumaric acid | |||
| Benzoic acid | |||
| In vivo studies | |||
| Protocatechuic acid | Male C57BL/6J mice | ↓ Body weight and ↓ fat mass of C57BL/6J mice induced by high-fat diet | [166] |
Mechanisms of Action
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| -OH | Hydroxyl group |
| •OH | Hydroxyl radical |
| ABTS+• | 2,2′-Azinobis-3-ethylbenzothiazoline-6-sulfonic acid radical |
| ACE | Angiotensin-converting enzyme |
| AChE | Acetylcholinesterase |
| AMPK | AMP-activated protein kinase |
| BChE | Butrylcholinesterase |
| CAT | Catalase |
| C/EBP-α | CCAAT/enhancer-binding protein alpha |
| CVD | Cardiovascular disease |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DPPH• | 2,2-Diphenyl-1-picrylhydrazyl radical |
| EAE | Enzyme-assisted extraction |
| eNOS | Endothelial nitric oxide synthase |
| FAO | Food and Agriculture Organization |
| FRAP | Ferric reducing antioxidant power |
| GLUTs | Glucose transporters |
| GPx | Glutathione peroxidase |
| HSL | Hormone-sensitive lipase |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1β |
| L-NAME | L-NG-Nitro arginine methyl ester |
| MAE | Microwave-assisted extraction |
| MetS | Metabolic syndrome |
| NCBI | National Center for Biotechnology Information |
| NO | Nitric oxide |
| O2•− | Superoxide radical |
| PLE | Pressurized Liquid Extraction |
| PPAR-γ | Proliferator-activated receptor gamma |
| RAS | Renin–angiotensin system |
| ROS | Reactive oxygen species |
| SFE | Supercritical fluid Extraction |
| SHR | Spontaneously Hypertensive Rats |
| SOD | Superoxide Dismutase |
| SREBP-1c | Sterol Regulatory Element-Binding Protein 1c |
| TBARS | Thiobarbituric Acid Reactive Substance |
| TNF-α | Tumor necrosis factor |
| UAE | Ultrasound-assisted extraction |
| UCP | Uncoupling Protein-1 |
| WHO | World Health Organization |
References
- Rodríguez, L.G.R.; Gasga, V.M.Z.; Pescuma, M.; Nieuwenhove, C.V.; Mozzi, F.; Burgos, J.A.S. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 2021, 140, 109854. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef] [PubMed]
- Oleszek, M.; Kowalska, I.; Bertuzzi, T.; Oleszek, W. Phytochemicals Derived from Agricultural Residues and Their Valuable Properties and Applications. Molecules 2023, 28, 342. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, W.; Yu, J.; Zhao, L.; Wang, K.; Hu, Z.; Liu, X. By-Products of Fruit and Vegetables: Antioxidant Properties of Extractable and Non-Extractable Phenolic Compounds. Antioxidants 2023, 12, 418. [Google Scholar] [CrossRef]
- Jeria, N.; Cornejo, S.; Prado, G.; Bustamante, A.; Garcia-Diaz, D.F.; Jimenez, P.; Valenzuela, R.; Poblete-Aro, C.; Echeverria, F. Beneficial Effects of Bioactive Compounds Obtained from Agro-Industrial By-Products on Obesity and Metabolic Syndrome Components. Food Rev. Int. 2022, 39, 3753–3782. [Google Scholar] [CrossRef]
- Nunes, A.R.; Gonçalves, A.C.; Pinto, E.; Amaro, F.; Flores-Félix, J.D.; Almeida, A.; Pinho, P.G.; Falcão, A.; Alves, G.; Silva, L.R. Mineral Content and Volatile Profiling of Prunus avium L. (Sweet Cherry) By-Products from Fundão Region (Portugal). Foods 2022, 11, 751. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Nunes, A.R.; Gonçalves, A.C.; Alves, G.; Falcão, A.; García-Viguera, C.; Moreno, D.A.; Silva, L.R. Valorisation of Prunus avium L. By-Products: Phenolic Composition and Effect on Caco-2 Cells Viability. Foods 2021, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Fruit and vegetable processing wastes as natural sources of antioxidant-rich extracts: Evaluation of advanced extraction technologies by surface response methodology. J. Environ. Chem. Eng. 2021, 9, 105330. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Nattassha, R.; Handayati, Y.; Simatupang, T.M.; Siallagan, M. Understanding circular economy implementation in the agri-food supply chain: The case of an Indonesian organic fertiliser producer. Agric. Food Secur. 2020, 9, 10. [Google Scholar] [CrossRef]
- Lemieux, I.; Després, J.-P. Metabolic Syndrome: Past, Present and Future. Nutrients 2020, 12, 3501. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef] [PubMed]
- Leitner, D.R.; Frühbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and Type 2 Diabetes: Two Diseases with a Need for Combined Treatment Strategies—EASO Can Lead the Way. Obes. Facts 2017, 10, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Caleja, C.; Ribeiro, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Phenolic compounds as nutraceuticals or functional food ingredient. Curr. Pharm. Des. 2017, 23, 2787–2806. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Ditano-Vázquez, P.; Torres-Peña, J.D.; Galeano-Valle, F.; Pérez-Caballero, A.I.; Demelo-Rodríguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef]
- Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients 2021, 13, 672. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Sieniawska, E.; Ortan, A.; Fierascu, I.; Xiao, J. Fruits By-Products—A Source of Valuable Active Principles. A Short Review. Front. Bioeng. Biotechnol. 2020, 8, 319. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Esposito, B.; Sessa, M.R.; Sica, D.; Malandrino, O. Towards Circular Economy in the Agri-Food Sector. A Systematic Literature Reviewjuice. Sustainability 2020, 12, 7401. [Google Scholar] [CrossRef]
- Lau, K.Q.; Sabran, M.R.; Shafie, S.R. Utilization of Vegetable and Fruit By-products as Functional Ingredient and Food. Front. Nutr. 2021, 8, 661693. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.R.; Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R. Prunus avium L. (Sweet Cherry) By-Products: A Source of Phenolic Compounds with Antioxidant and Anti-Hyperglycemic Properties—A Review. Appl. Sci. 2021, 11, 8516. [Google Scholar] [CrossRef]
- Teshome, E.; Teka, T.A.; Nandasiri, R.; Rout, J.R.; Harouna, D.V.; Astatkie, T.; Urugo, M.M. Fruit By-Products and Their Industrial Applications for Nutritional Benefits and Health Promotion: A Comprehensive Review. Sustainability 2023, 15, 7840. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, M.; Ran, J.; Zhang, T.; Sun, H.; Dong, M.; Zhang, Z.; Zheng, H. Variation in phenolic compounds and antioxidant activity in apple seeds of seven cultivars. Saudi J. Biol. Sci. 2016, 23, 379–388. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Cyanogenic glycosides and phenolics in apple seeds and their changes during long term storage. Sci. Hortic. 2019, 255, 30–36. [Google Scholar] [CrossRef]
- Lee, G.J.; Lee, S.Y.; Kang, N.-G.; Jin, M.H. A multi-faceted comparison of phytochemicals in seven citrus peels and improvement of chemical composition and antioxidant activity by steaming. LWT 2022, 160, 113297. [Google Scholar] [CrossRef]
- Mendoza, L.; Navarro, F.; Melo, R.; Báez, F.; Cotoras, M. Characterization of polyphenol profile of extracts obtained from grap pomace and synergistic effect of these extracts and fungicides against botrytis cinerea. J. Chil. Chem. Soc. 2019, 64, 4607–4609. [Google Scholar] [CrossRef]
- Edo, G.I.; Nwachukwu, S.C.; Ali, A.B.M.; Yousif, E.; Jikah, A.N.; Zainulabdeen, K.; Ekokotu, H.A.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; et al. A review on the composition, extraction and applications of phenolic compounds. Ecol. Front. 2025, 45, 7–23. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K.; Prenzler, P.; Antolovich, M. Applications of mass spectrometry to plant phenols. TrAC Trends Anal. Chem. 1999, 18, 362–372. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Mattila, P.; Hellström, J. Phenolic acids in potatoes, vegetables, and some of their products. J. Food Compos. Anal. 2007, 20, 152–160. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, J.; Ma, Z.; Xiao, M.; Yang, L.; Tian, B.; Yu, Y.; Bi, C.; Fang, A.; Yang, Y. The Role of Hydroxycinnamic Acid Amide Pathway in Plant Immunity. Front. Plant Sci. 2022, 22, 922119. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Bento, C.; Silva, B.; Simões, M.; Silva, L.R. Nutrients, bioactive compounds and bioactivity: The health benefits of sweet cherries (Prunus avium L.). Curr. Nutr. Food Sci. 2019, 15, 208–227. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Dini, I.; Grumetto, L. Recent Advances in Natural Polyphenol Research. Molecules 2022, 27, 8777. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Kumar, J.S.P.; Verma, A.K.; Umaraw, P.; Khatkar, S.K.; Khatkar, A.B.; Pathak, D.; Kaka, U.; Sazili, A.Q. Ultrasound-Assisted Extraction and the Encapsulation of Bioactive Components for Food Applications. Foods 2022, 11, 2973. [Google Scholar] [CrossRef] [PubMed]
- Marsiglia, W.I.M.L.; Oliveira, L.S.C.; Almeida, R.L.J.; Santos, N.C.; Neto, J.M.S.; Santiago, Â.M.; Melo, B.C.A.; Silva, F.L.H. Thermal stability of total phenolic compounds and antioxidant activities of jaboticaba peel: Effect of solvents and extraction methods. J. Indian Chem. Soc. 2023, 100, 100995. [Google Scholar] [CrossRef]
- Fărcaș, A.C.; Socaci, S.A.; Nemeș, S.A.; Salanță, L.C.; Chiș, M.S.; Pop, C.R.; Borșa, A.; Diaconeasa, Z.; Vodnar, D.C. Cereal Waste Valorization through Conventional and Current Extraction Techniques—An Up-to-Date Overview. Foods 2022, 11, 2454. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, D.G.; Kitsios, A.-P.; Koutoulis, A.S.; Malisova, O.; Karabagias, I.K. Fruits, Spices and Honey Phenolic Compounds: A Comprehensive Review on Their Origin, Methods of Extraction and Beneficial Health Properties. Antioxidants 2024, 13, 1335. [Google Scholar] [CrossRef]
- Hayat, K.; Hussain, S.; Abbas, S.; Farooq, U.; Ding, B.; Xia, S.; Jia, C.; Zhang, X.; Xia, W. Optimized microwave-assisted extraction of phenolic acids from citrus mandarin peels and evaluation of antioxidant activity in vitro. Sep. Purif. Technol. 2009, 70, 63–70. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Shah, M.A.; Ahmad, N.; Vilarinho, F.; Khwaldia, K.; Silva, A.S.; Ramos, F. Citrus By-Products: Valuable Source of Bioactive Compounds for Food Applications. Antioxidants 2022, 12, 38. [Google Scholar] [CrossRef]
- Machado, T.d.O.X.; Portugal, I.; Kodel, H.d.A.C.; Fathi, A.; Fathi, F.; Oliveira, M.B.P.P.; Dariva, C.; Souto, E.B. Pressurized liquid extraction as an innovative high-yield greener technique for phenolic compounds recovery from grape pomace. Sustain. Chem. Pharm. 2025, 40, 101635. [Google Scholar] [CrossRef]
- Dobroslavić, E.; Elez Garofulić, I.; Šeparović, J.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Pressurized Liquid Extraction as a Novel Technique for the Isolation of Laurus nobilis L. Leaf Polyphenols. Molecules 2022, 27, 5099. [Google Scholar] [CrossRef]
- Yıldırım, M.; Erşatır, M.; Poyraz, S.; Amangeldinova, M.; Kudrina, N.O.; Terletskaya, N.V. Green Extraction of Plant Materials Using Supercritical CO2: Insights into Methods, Analysis, and Bioactivity. Plants 2024, 13, 2295. [Google Scholar] [CrossRef]
- Dias, A.L.B.; de Aguiar, A.C.; Rostagno, M.A. Extraction of natural products using supercritical fluids and pressurized liquids assisted by ultrasound: Current status and trends. Ultrason. Sonochem. 2021, 74, 105584. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Mancusi, A. Food Peptides for the Nutricosmetic Industry. Antioxidants 2023, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Carini, F.; Coughtrey, P.J.; Kinnersley, R.P. Radionuclide transfer to fruits: A critical review. Introduction. J. Environ. Radioact. 2001, 52, 123–129. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Paramás, A.M.; Oludemi, T.; Ayuda-Durán, B.; González-Manzano, S. Plant phenolics as functional food ingredients. Adv. Food Nutr. Res. 2019, 90, 183–257. [Google Scholar] [CrossRef]
- Chaouch, M.A.; Benvenuti, S. The Role of Fruit by-Products as Bioactive Compounds for Intestinal Health. Foods 2020, 9, 1716. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.P.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products—Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 1388–1416. [Google Scholar] [CrossRef]
- Li, W.; Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M.A. Analysis of polyphenols in apple pomace: A comparative study of different extraction and hydrolysis procedures. Ind. Crops Prod. 2020, 147, 112250. [Google Scholar] [CrossRef]
- Du, G.; Zhu, Y.; Wang, X.; Zhang, J.; Tian, C.; Liu, L.; Meng, Y.; Guo, Y. Phenolic composition of apple products and by-products based on cold pressing technology. J. Food Sci. Technol. 2019, 56, 1389–1397. [Google Scholar] [CrossRef]
- Zhang, J.W.; Wang, J.H.; Wang, G.H.; Wang, C.C.; Huang, R.Q. Extraction and characterization of phenolic compounds and dietary fibres from banana peel. Acta Aliment. 2019, 48, 525–537. [Google Scholar] [CrossRef]
- Choudhury, N.; Nickhil, C.; Deka, S.C. Comprehensive review on the nutritional and therapeutic value of banana by-products and their applications in food and non-food sectors. Food Biosci. 2023, 56, 103416. [Google Scholar] [CrossRef]
- Hurtado-Romero, A.; Garcia-Amezquita, L.E.; Carrillo-Nieves, D.; Montilla, A.; Villamiel, M.; Requena, T.; García-Cayuela, T. Characterization of berry by-products as fermentable substrates: Proximate and phenolic composition, antimicrobial activity, and probiotic growth dynamics. LWT 2024, 204, 116468. [Google Scholar] [CrossRef]
- Zhang, M.-Q.; Zhang, J.; Zhang, Y.-T.; Sun, J.-Y.; Prieto, M.A.; Simal-Gandara, J.; Putnik, P.; Li, N.-Y.; Liu, C. The link between the phenolic composition and the antioxidant activity in different small berries: A metabolomic approach. LWT 2023, 182, 114853. [Google Scholar] [CrossRef]
- Pezzini, V.; Agostini, F.; Smiderle, F.; Touguinha, L.; Salvador, M.; Moura, S. Grape juice by-products extracted by ultrasound and microwave-assisted with different solvents: A rich chemical composition. Food Sci. Biotechnol. 2019, 28, 691–699. [Google Scholar] [CrossRef]
- Kulichova, J.; Buaong, M.; Balik, J.; Hic, P.; Triska, J. Juices enriched with phenolic extracts from grapes. Czech J. Food Sci. 2018, 36, 261–267. [Google Scholar] [CrossRef]
- Barcia, M.T.; Pertuzatti, P.B.; Gómez-Alonso, S.; Godoy, H.T.; Hermosín-Gutiérrez, I. Phenolic composition of grape and winemaking by-products of Brazilian hybrid cultivars BRS Violeta and BRS Lorena. Food Chem. 2014, 159, 95–105. [Google Scholar] [CrossRef]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Riaz Rajoka, M.S.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: A circular economy model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef]
- Elkhatim, K.A.S.; Elagib, R.A.A.; Hassan, A.B. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef]
- Khwaldia, K.; Attour, N.; Matthes, J.; Beck, L.; Schmid, M. Olive byproducts and their bioactive compounds as a valuable source for food packaging applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1218–1253. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Maestri, D.; Barrionuevo, D.; Bodoira, R.; Zafra, A.; Jiménez-López, J.; Alché, J.D. Nutritional profile and nutraceutical components of olive (Olea europaea L.) seeds. J. Food Sci. Technol. 2019, 56, 4359–4370. [Google Scholar] [CrossRef]
- Xi, W.; Zhang, Y.; Sun, Y.; Shen, Y.; Ye, X.; Zhou, Z. Phenolic composition of Chinese wild mandarin (Citrus reticulata Balnco.) pulps and their antioxidant properties. Ind. Crops Prod. 2014, 52, 466–474. [Google Scholar] [CrossRef]
- Solomakou, N.; Drosaki, A.M.; Kaderides, K.; Mourtzinos, I.; Goula, A.M. Valorization of Peach By-Products: Utilizing Them as Valuable Resources in a Circular Economy Model. Sustainability 2024, 16, 1289. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, H.P.; Verma, A.; Chahal, A.S.; Jajoria, K.; Rasane, P.; Kaur, S.; Kaur, J.; Gunjal, M.; Ercisli, S.; et al. Pomegranate Peel Phytochemistry, Pharmacological Properties, Methods of Extraction, and Its Application: A Comprehensive Review. ACS Omega 2023, 8, 35452–35469. [Google Scholar] [CrossRef]
- Basiri, S.; Shekarforoush, S.S.; Aminlari, M.; Akbari, S. The effect of pomegranate peel extract (PPE) on the polyphenol oxidase (PPO) and quality of Pacific white shrimp (Litopenaeus vannamei) during refrigerated storage. LWT—Food Sci. Technol. 2015, 60, 1025–1033. [Google Scholar] [CrossRef]
- Dimitrijevic, J.; Tomovic, M.; Bradic, J.; Petrovic, A.; Jakovljevic, V.; Andjic, M.; Živković, J.; Milošević, S.Đ.; Simanic, I.; Dragicevic, N. Punica granatum L. (Pomegranate) Extracts and Their Effects on Healthy and Diseased Skin. Pharmaceutics 2024, 16, 458. [Google Scholar] [CrossRef]
- Tańska, M.; Roszkowska, B.; Czaplicki, S.; Borowska, E.J.; Bojarska, J.; Dąbrowska, A. Effect of Fruit Pomace Addition on Shortbread Cookies to Improve Their Physical and Nutritional Values. Plant Foods Hum. Nutr. 2016, 71, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Reißner, A.-M.; Al-Hamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turner, C.; Rohm, H. Composition and physicochemical properties of dried berry pomace. J. Sci. Food Agric. 2019, 99, 1284–1293. [Google Scholar] [CrossRef]
- Jesus, F.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Exploring the phenolic profile, antioxidant, antidiabetic and anti-hemolytic potential of Prunus avium vegetal parts. Food Res. Int. 2019, 116, 600–610. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Görgüç, A.; Karaaslan, M.; Vardin, H.; Bilek, S.; Uygun, Ö.; Bircan, C. Sour Cherry By-products: Compositions, Functional Properties and Recovery Potentials—A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3549–3563. [Google Scholar] [CrossRef]
- Valdez-Morales, M.; Espinosa-Alonso, L.G.; Espinoza-Torres, L.C.; Delgado-Vargas, F.; Medina-Godoy, S. Phenolic Content and Antioxidant and Antimutagenic Activities in Tomato Peel, Seeds, and Byproducts. J. Agric. Food Chem. 2014, 62, 5281–5289. [Google Scholar] [CrossRef]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hyperten. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Niewiadomska, J.; Gajek-Marecka, A.; Gajek, J.; Noszczyk-Nowak, A. Biological Potential of Polyphenols in the Context of Metabolic Syndrome: An Analysis of Studies on Animal Models. Biology 2022, 11, 559. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Bin Dukhyil, A.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.H.; Andrade, M.A.; Séndon, R.; Silva, A.S.; Ramos, F.; Vilarinho, F.; Khwaldia, K.; Barbosa-Pereira, L. Industrial Fruits By-Products and Their Antioxidant Profile: Can They Be Exploited for Industrial Food Applications? Foods 2021, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Timón, M.L.; Andrés, A.I.; Petrón, M.J. Antioxidant Activity of Aqueous Extracts Obtained from By-Products of Grape, Olive, Tomato, Lemon, Red Pepper and Pomegranate. Foods 2024, 13, 1802. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Godon, B.; Aires, A.; Saavedra, M.J.; Gonçalves, B. Phenolic Profile and Bioactive Potential of Stems and Seed Kernels of Sweet Cherry Fruit. Antioxidants 2020, 9, 1295. [Google Scholar] [CrossRef]
- Willig, g.; Brunissen, F.; brunois, F.; Godon, B.; Magro, C.; Monteux, C.; Peyrot, C.; Ioannou, I. Phenolic Compounds Extracted from Cherry Tree (Prunus avium) Branches: Impact of the Process on Cosmetic Properties. Antioxidants 2022, 11, 813. [Google Scholar] [CrossRef]
- Bastos, C.; Barros, L.; Duenas, M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterisation and bioactive properties of Prunus avium L.: The widely studied fruits and the unexplored stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R.; Flores-Félix, J.D. Antioxidant activity of the main phenolics found in red fruits: An in vitro and in silico study. Food Chem. 2024, 452, 139459. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Hao, L.; Yi, W.; Ding, S.; Xu, F. Polyphenols, Oxidative Stress, and Metabolic Syndrome. Oxid. Med. Cell. Longev. 2020, 2020, 7398453. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Hu, P.; Feng, L.-P.; Huang, L.-L.; Wang, Y.; Yan, X.; Xiong, J.; Xia, H.-L. Protective Effects of Ferulic Acid on Metabolic Syndrome: A Comprehensive Review. Molecules 2023, 28, 281. [Google Scholar] [CrossRef]
- Singh, S.; Arthur, R.; Upadhayay, S.; Kumar, P. Ferulic acid ameliorates neurodegeneration via the Nrf2/ARE signalling pathway: A Review. Pharmacol. Res.—Mod. Chin. Med. 2022, 5, 100190. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, W.; Sair, A.T.; Li, T.; Liu, R.H. Ferulic acid restores mitochondrial dynamics and autophagy via AMPK signaling pathway in a palmitate-induced hepatocyte model of metabolic syndrome. Sci. Rep. 2024, 14, 18970. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef]
- Ibitoye, O.B.; Ajiboye, T.O. Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch. Physiol. Biochem. 2018, 124, 410–417. [Google Scholar] [CrossRef]
- Martín, M.Á.; Ramos, S. Impact of Dietary Flavanols on Microbiota, Immunity and Inflammation in Metabolic Diseases. Nutrients 2021, 13, 850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, Y.; Zhao, Y.; Zhou, X.; Ji, L. Is visceral abdominal fat area a better indicator for hyperglycemic risk? Results from the Pinggu Metabolic Disease Study. J. Diabetes Investig. 2020, 11, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Jung, E.H.; Kim, S.R.; Hwang, I.K.; Ha, T.Y. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J. Agric. Food Chem. 2007, 55, 9800–9804. [Google Scholar] [CrossRef]
- Prasad, C.N.V.; Anjana, T.; Banerji, A.; Gopalakrishnapillai, A. Gallic acid induces GLUT4 translocation and glucose uptake activity in 3T3-L1 cells. FEBS Lett. 2010, 584, 531–536. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Jothi, G.; Antony, P.J.; Balakrishna, K.; Paulraj, M.G.; Ignacimuthu, S.; Stalin, A.; Al-Dhabi, N.A. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARγ in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur. J. Pharmacol. 2014, 745, 201–216. [Google Scholar] [CrossRef]
- Ćorković, I.; Gašo-Sokač, D.; Pichler, A.; Šimunović, J.; Kopjar, M. Dietary Polyphenols as Natural Inhibitors of α-Amylase and α-Glucosidase. Life 2022, 12, 1692. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J. Funct. Foods 2020, 64, 103587. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Huang, D.; Chen, S.; Xia, Y.; Zhu, S. The inhibitory mechanism of chlorogenic acid and its acylated derivatives on α-amylase and α-glucosidase. Food Chem. 2022, 372, 131334. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Q.; Shen, L.; Guo, K.; Zhou, X. Chlorogenic acid improves glucose tolerance, lipid metabolism, inflammation and microbiota composition in diabetic db/db mice. Front. Endocrinol. 2022, 13, 1042044. [Google Scholar] [CrossRef] [PubMed]
- Zuñiga, L.Y.; Mora, M.C.A.A.; González-Ortiz, M.; Ramos-Núñez, J.L.; Martínez-Abundis, E. Effect of Chlorogenic Acid Administration on Glycemic Control, Insulin Secretion, and Insulin Sensitivity in Patients with Impaired Glucose Tolerance. J. Med. Food. 2018, 21, 469–473. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Ijomone, O.M.; Islam, M.S. Caffeic acid regulates glucose homeostasis and inhibits purinergic and cholinergic activities while abating oxidative stress and dyslipidaemia in fructose-streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2022, 74, 973–984. [Google Scholar] [CrossRef]
- Oršolić, N.; Sirovina, D.; Odeh, D.; Gajski, G.; Balta, V.; Šver, L.; Jembrek, M.J. Efficacy of Caffeic Acid on Diabetes and Its Complications in the Mouse. Molecules 2021, 26, 3262. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Hafizur, R.M.; Hameed, A.; Ahmed, S.; Nisar, M.; Kabir, N. Ellagic acid in Emblica officinalis exerts anti-diabetic activity through the action on β-cells of pancreas. Eur. J. Nutr. 2017, 56, 591–601. [Google Scholar] [CrossRef]
- Polce, S.A.; Burke, C.; França, L.M.; Kramer, B.; Paes, A.M.A.; Carrillo-Sepulveda, M.A. Ellagic Acid Alleviates Hepatic Oxidative Stress and Insulin Resistance in Diabetic Female Rats. Nutrients 2018, 10, 531. [Google Scholar] [CrossRef]
- Huang, D.-W.; Chang, W.-C.; Wu, J.S.-B.; Shih, R.-W.; Shen, S.-C. Gallic acid ameliorates hyperglycemia and improves hepatic carbohydrate metabolism in rats fed a high-fructose diet. Nutr. Res. 2016, 36, 150–160. [Google Scholar] [CrossRef]
- Oboh, G.; Ogunbadejo, M.D.; Ogunsuyi, O.B.; Oyeleye, S.I. Can gallic acid potentiate the antihyperglycemic effect of acarbose and metformin? Evidence from streptozotocin-induced diabetic rat model. Arch. Physiol. Biochem. 2022, 128, 619–627. [Google Scholar] [CrossRef]
- Maradesha, T.; Patil, S.M.; Al-Mutairi, K.A.; Ramu, R.; Madhunapantula, S.V.; Alqadi, T. Inhibitory Effect of Polyphenols from the Whole Green Jackfruit Flour against α-Glucosidase, α-Amylase, Aldose Reductase and Glycation at Multiple Stages and Their Interaction: Inhibition Kinetics and Molecular Simulations. Molecules 2022, 27, 1888. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.M.; Arbid, M.S. Estimation of ellagic acid and/or repaglinide effects on insulin signaling, oxidative stress, and inflammatory mediators of liver, pancreas, adipose tissue, and brain in insulin resistant/type 2 diabetic rats. Appl. Physiol. Nutr. Metab. 2017, 42, 181–192. [Google Scholar] [CrossRef]
- Dijk, A.E.v.; Olthof, M.R.; Meeuse, J.C.; Seebus, E.; Heine, R.J.; Dam, R.M.v. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care 2009, 32, 1023–1025. [Google Scholar] [CrossRef]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic Effects of Simple Phenolic Acids: A Comprehensive Review. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, W.; Kang, S.; Liu, P.; Yao, Y.; Liu, W.; Aikemu, A.; Pang, K.; Yang, X. Phenolic Constituents with Glucose Uptake and GLUT4 Translocation Bioactivities from the Fruits of Cordia dichotoma. J. Agric. Food Chem. 2024, 72, 16298–16311. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.B.; Chiang, B.H. A novel phenolic formulation for treating hepatic and peripheral insulin resistance by regulating GLUT4-mediated glucose uptake. J. Tradit. Complement. Med. 2022, 12, 195–205. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Z.; Xie, J.; Yu, J.; Xiong, S.; Xiang, F.; Ma, X.; Yang, C.; Lin, L. Research Progress on the Mechanism for Improving Glucose and Lipid Metabolism Disorders Using Phenolic Acid Components from Medicinal and Edible Homologous Plants. Molecules 2024, 29, 4790. [Google Scholar] [CrossRef]
- Duvnjak, L. Hypertension and the Metabolic Syndrome. EJIFCC 2007, 18, 55–60. [Google Scholar]
- Maruhashi, T.; Higashi, Y. Pathophysiological Association between Diabetes Mellitus and Endothelial Dysfunction. Antioxidants 2021, 10, 1306. [Google Scholar] [CrossRef]
- Yu, M.; Kim, H.J.; Heo, H.; Kim, M.; Jeon, Y.; Lee, H.; Lee, J. Comparison of the Antihypertensive Activity of Phenolic Acids. Molecules 2022, 27, 6185. [Google Scholar] [CrossRef]
- Alam, M.A. Anti-hypertensive Effect of Cereal Antioxidant Ferulic Acid and Its Mechanism of Action. Front. Nutr. 2019, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yamamoto, M.; Jokura, H.; Fujii, A.; Tokimitsu, I.; Hase, T.; Saito, I. Ferulic acid restores endothelium-dependent vasodilation in aortas of spontaneously hypertensive rats. Am. J. Hypertens. 2007, 20, 508–513. [Google Scholar] [CrossRef]
- Suzuki, A.; Kagawa, D.; Fujii, A.; Ochiai, R.; Tokimitsu, I.; Saito, I. Short- and long-term effects of ferulic acid on blood pressure in spontaneously hypertensive rats. Am. J. Hypertens. 2002, 15, 351–357. [Google Scholar] [CrossRef]
- Kang, N.; Lee, J.-H.; Lee, W.; Ko, J.-Y.; Kim, E.-A.; Kim, J.-S.; Heu, M.-S.; Kim, G.H.; Jeon, Y.-J. Gallic acid isolated from Spirogyra sp. improves cardiovascular disease through a vasorelaxant and antihypertensive effect. Environ. Toxicol. Pharmacol. 2015, 39, 764–772. [Google Scholar] [CrossRef]
- Jin, L.; Lin, M.Q.; Piao, Z.H.; Cho, J.Y.; Kim, G.R.; Choi, S.Y.; Ryu, Y.; Sun, S.; Kee, H.J.; Jeong, M.H. Gallic acid attenuates hypertension, cardiac remodeling, and fibrosis in mice with NG-nitro-L-arginine methyl ester-induced hypertension via regulation of histone deacetylase 1 or histone deacetylase 2. J. Hypertens. 2017, 35, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Ardiansyah; Ohsaki, Y.; Shirakawa, H.; Koseki, T.; Komai, M. Novel effects of a single administration of ferulic acid on the regulation of blood pressure and the hepatic lipid metabolic profile in stroke-prone spontaneously hypertensive rats. J. Agric. Food Chem. 2008, 56, 2825–2830. [Google Scholar] [CrossRef] [PubMed]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Role of Dietary Polyphenols in the Activity and Expression of Nitric Oxide Synthases: A Review. Antioxidants 2023, 12, 147. [Google Scholar] [CrossRef]
- Ozay, C.; Aksoyalp, Z.S.; Erdogan, B.R. Chapter 9—Plant phenolic acids modulating the renin-angiotensin system in the management of cardiovascular diseases. In Studies in Natural Products Chemistry; Atta-ur-Rahman, E.F., Ed.; Elsevier: Karachi, Pakistan, 2024; pp. 285–314. [Google Scholar] [CrossRef]
- Ontawong, A.; Duangjai, A.; Vaddhanaphuti, C.S.; Amornlerdpison, D.; Pengnet, S.; Kamkaew, N. Chlorogenic acid rich in coffee pulp extract suppresses inflammatory status by inhibiting the p38, MAPK, and NF-κB pathways. Heliyon 2023, 9, e13917. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Yin, Y.; Li, D.-N.; Zhao, D.-Y.; Huang, J.-Q. Biological Activities of p-Hydroxycinnamic Acids in Maintaining Gut Barrier Integrity and Function. Foods 2023, 12, 2636. [Google Scholar] [CrossRef]
- Silva, A.A.; Pereira-de-Morais, L.; Silva, R.E.R.; Dantas, M.M.; Milfont, C.G.B.; Gomes, M.F.; Araújo, I.M.; Kerntopf, M.R.; Menezes, I.R.A.; Barbosa, R. Pharmacological screening of the phenolic compound caffeic acid using rat aorta, uterus and ileum smooth muscle. Chem. Biol. Interact. 2020, 332, 109269. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Lister, N.B.; Baur, L.A.; Felix, J.F.; Hill, A.J.; Marcus, C.; Reinehr, T.; Summerbell, C.; Wabitsch, M. Child and adolescent obesity. Nat. Rev. Dis. Prim. 2023, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Aloo, S.-O.; Ofosu, F.K.; Kim, N.-H.; Kilonzi, S.M.; Oh, D.-H. Insights on Dietary Polyphenols as Agents against Metabolic Disorders: Obesity as a Target Disease. Antioxidants 2023, 12, 416. [Google Scholar] [CrossRef]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef]
- Buchholz, T.; Melzig, M.F. Polyphenolic Compounds as Pancreatic Lipase Inhibitors. Planta Med. 2015, 81, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, P.; Navarro-Herrera, D.; Zabala, M.; Miguéliz, I.; Romo-Hualde, A.; López-Yoldi, M.; Martínez, J.A.; Vizmanos, J.L.; Milagro, F.I.; González-Navarro, C.J. Phenolic Compounds Inhibit 3T3-L1 Adipogenesis Depending on the Stage of Differentiation and Their Binding Affinity to PPARγ. Molecules 2019, 24, 61045. [Google Scholar] [CrossRef]
- Li, K.K.; Liu, C.L.; Shiu, H.T.; Wong, H.L.; Siu, W.S.; Zhang, C.; Han, X.Q.; Ye, C.X.; Leung, P.C.; Ko, C.H. Cocoa tea (Camellia ptilophylla) water extract inhibits adipocyte differentiation in mouse 3T3-L1 preadipocyte. Sci. Rep. 2016, 6, 20172. [Google Scholar] [CrossRef]
- Kang, S.-W.; Kang, S.-I.; Shin, H.-S.; Yoon, S.-A.; Kim, J.-H.; Ko, H.-C.; Kim, S.-J. Sasa quelpaertensis Nakai extract and its constituent p-coumaric acid inhibit adipogenesis in 3T3-L1 cells through activation of the AMPK pathway. Food Chem. Toxicol. 2013, 59, 380–385. [Google Scholar] [CrossRef]
- Lee, D. P-13—Inhibitory effect of caffeic acid phenethyl on adipocyte differentiation through regulation of reactive oxygen species in the 3T3-L1 cell model. Free Radic. Biol. Med. 2018, 120, S48–S49. [Google Scholar] [CrossRef]
- Mosqueda-Solís, A.; Lasa, A.; Gómez-Zorita, S.; Eseberri, I.; Picó, C.; Portillo, M.P. Screening of potential anti-adipogenic effects of phenolic compounds showing different chemical structure in 3T3-L1 preadipocytes. Food Funct. 2017, 8, 3576–3586. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-R.; Lazarenko, O.P.; Zhang, J.; Blackburn, M.L.; Ronis, M.J.J.; Badger, T.M. Diet-derived phenolic acids regulate osteoblast and adipocyte lineage commitment and differentiation in young mice. J. Bone Miner. Res. 2014, 29, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef]
- Ren, N.; Kaplan, R.; Hernandez, M.; Cheng, K.; Jin, L.; Taggart, A.K.P.; Zhu, A.Y.; Gan, X.; Wright, S.D.; Cai, T.-Q. Phenolic acids suppress adipocyte lipolysis via activation of the nicotinic acid receptor GPR109A (HM74a/PUMA-G). J. Lipid Res. 2009, 50, 908–914. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C.; Zhai, M.; Yu, T.; Pei, M.; Du, P.; Li, A.; Yan, J.; Li, C.; Zhang, G. Effect of chlorogenic acid on lipid metabolism in 3T3-L1 cells induced by oxidative stress. Food Biosci. 2023, 51, 102330. [Google Scholar] [CrossRef]
- Behera, P.K.; Devi, S.; Mittal, N. Therapeutic potential of gallic acid in obesity: Considerable shift! Obes. Med. 2023, 37, 100473. [Google Scholar] [CrossRef]
- Ko, D.-Y.; Ku, K.-M. Effect of Anti-Obesity and Antioxidant Activity through the Additional Consumption of Peel from “Fuji” Pre-Washed Apple. Foods 2022, 11, 40497. [Google Scholar] [CrossRef]
- Geng, J.; Ni, Q.; Sun, W.; Li, L.; Feng, X. The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother. 2022, 147, 112678. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 73715. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Karamać, M.; Amarowicz, R. Inhibition of pancreatic lipase by phenolic acids—Examination in vitro. Z. Naturforsch. C 1996, 51, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Colantuono, A.; Ferracane, R.; Vitaglione, P. In vitro bioaccessibility and functional properties of polyphenols from pomegranate peels and pomegranate peels-enriched cookies. Food Funct. 2016, 7, 4247–4258. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.-S.; Choi, H.-S.; Seo, M.-J.; Jeon, H.-J.; Lee, B.-Y. Ellagic acid suppresses lipid accumulation by suppressing early adipogenic events and cell cycle arrest. Phytother. Res. 2015, 29, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Peng, S.; Ye, H.; Li, D.; Granato, D.; Guo, H.; Xie, Z. Metabolite differentiation and antiobesity effects between different grades of Yuexi Cuilan green tea. J. Funct. Foods 2021, 87, 104794. [Google Scholar] [CrossRef]
- Xu, M.; Yang, L.; Zhu, Y.; Liao, M.; Chu, L.; Li, X.; Lin, L.; Zheng, G. Collaborative effects of chlorogenic acid and caffeine on lipid metabolism via the AMPKα-LXRα/SREBP-1c pathway in high-fat diet-induced obese mice. Food Funct. 2019, 10, 7489–7497. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Jiang, K.; Shi, B.; Liu, L.; Hou, R.; Chen, G.; Farag, M.A.; Yan, N.; Liu, L. Dietary polyphenols regulate appetite mechanism via gut-brain axis and gut homeostasis. Food Chem. 2024, 446, 138739. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Y.; Guo, J.; You, Y.; Zhan, J.; Huang, W. Chlorogenic Acid Stimulates the Thermogenesis of Brown Adipocytes by Promoting the Uptake of Glucose and the Function of Mitochondria. J. Food Sci. 2019, 84, 3815–3824. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, A.R.; Alves, G.; Falcão, A.; Lopes, J.A.; Silva, L.R. Phenolic Acids from Fruit By-Products as Therapeutic Agents for Metabolic Syndrome: A Review. Int. J. Mol. Sci. 2025, 26, 3834. https://doi.org/10.3390/ijms26083834
Nunes AR, Alves G, Falcão A, Lopes JA, Silva LR. Phenolic Acids from Fruit By-Products as Therapeutic Agents for Metabolic Syndrome: A Review. International Journal of Molecular Sciences. 2025; 26(8):3834. https://doi.org/10.3390/ijms26083834
Chicago/Turabian StyleNunes, Ana R., Gilberto Alves, Amílcar Falcão, João A. Lopes, and Luís R. Silva. 2025. "Phenolic Acids from Fruit By-Products as Therapeutic Agents for Metabolic Syndrome: A Review" International Journal of Molecular Sciences 26, no. 8: 3834. https://doi.org/10.3390/ijms26083834
APA StyleNunes, A. R., Alves, G., Falcão, A., Lopes, J. A., & Silva, L. R. (2025). Phenolic Acids from Fruit By-Products as Therapeutic Agents for Metabolic Syndrome: A Review. International Journal of Molecular Sciences, 26(8), 3834. https://doi.org/10.3390/ijms26083834








