Soybean Trypsin Inhibitor Possesses Potency Against SARS-CoV-2 Infection by Blocking the Host Cell Surface Receptors ACE2, TMPRSS2, and CD147
Abstract
1. Introduction
2. Results
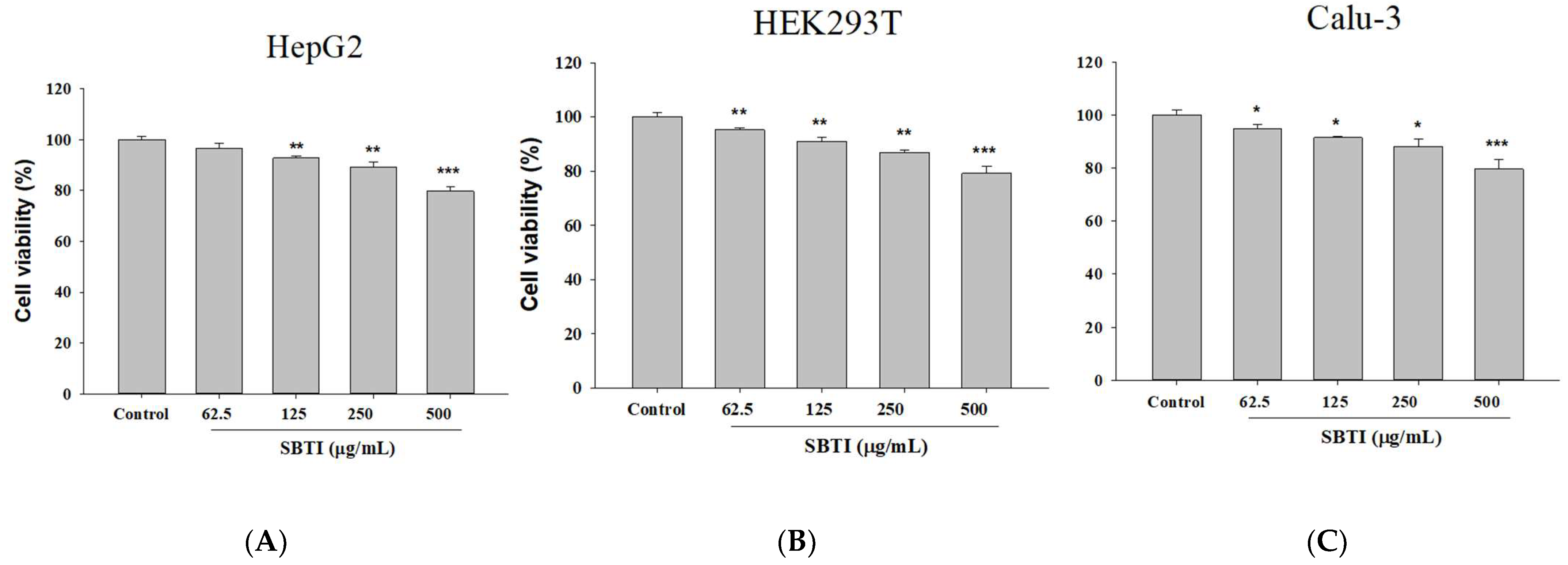
2.1. Confirmation of the Effect on Cell Growth Using Different Concentrations of SBTI
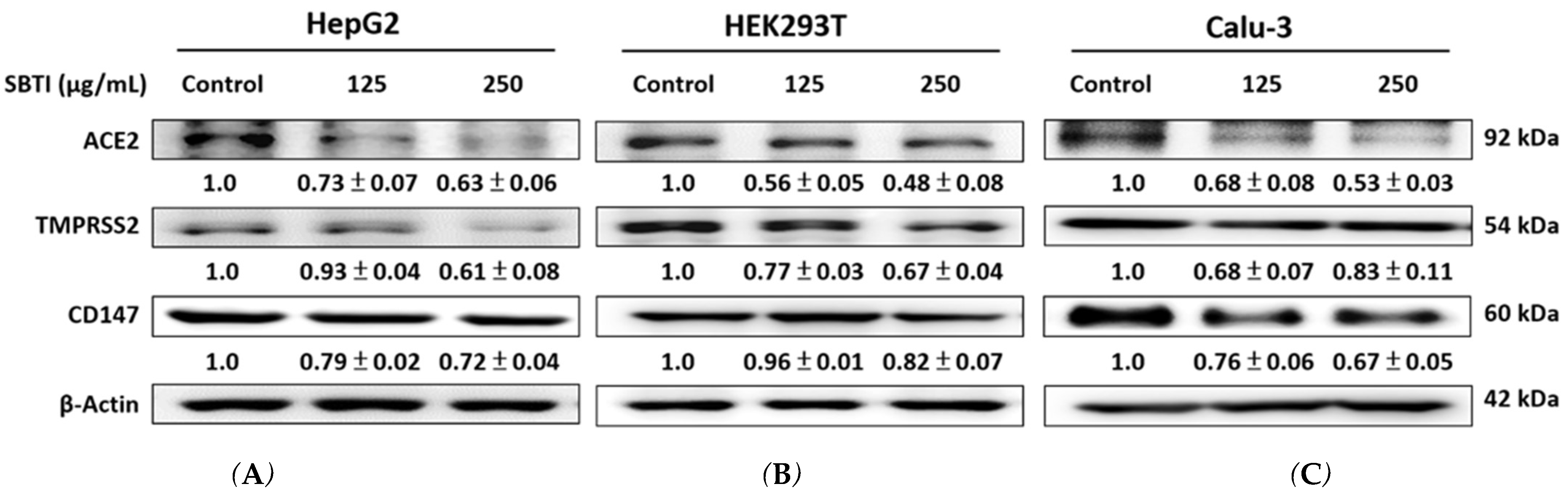
2.2. Expression of ACE2, TMPRSS2, and CD147 Induced by SBTI in the In Vitro Experiment
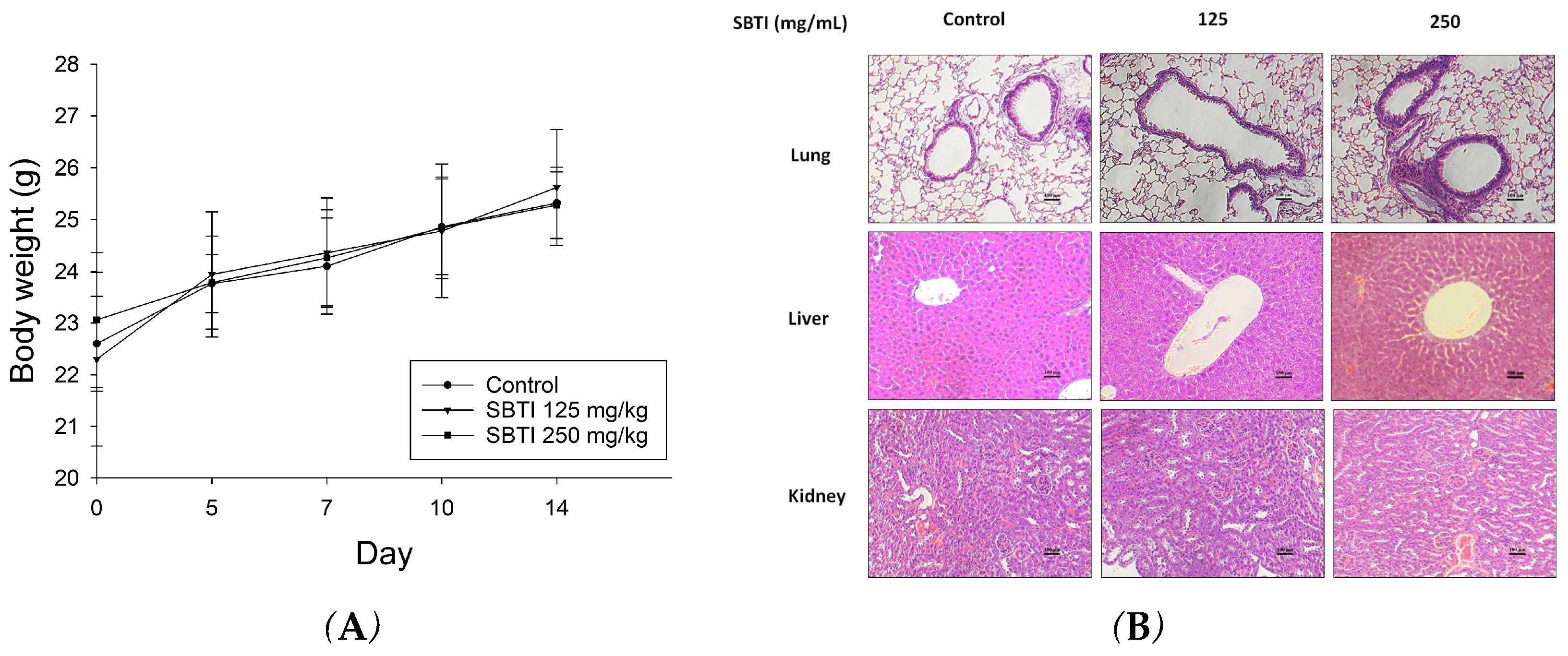
2.3. Evaluation of the Effect of SBTI on Animal Models
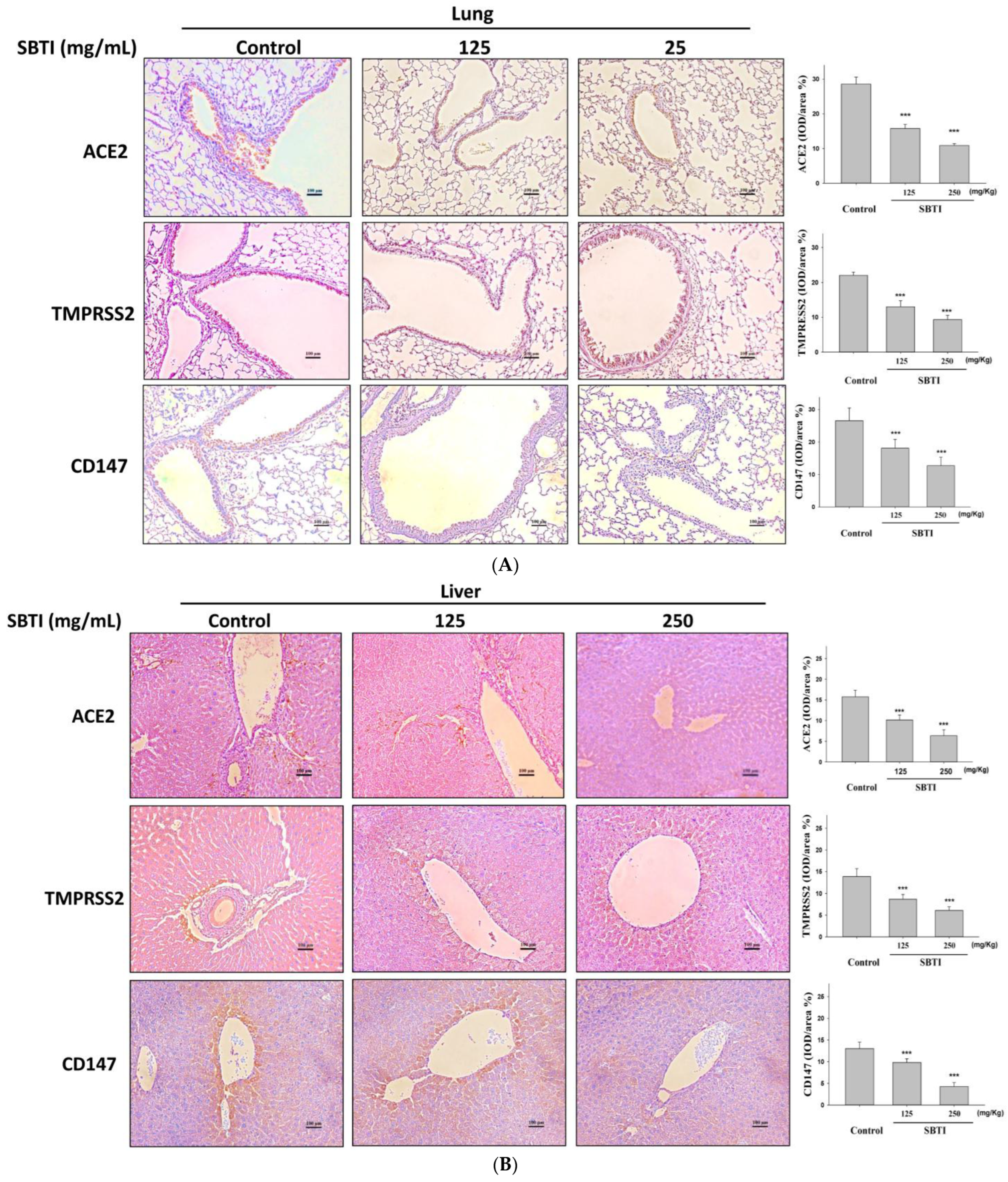
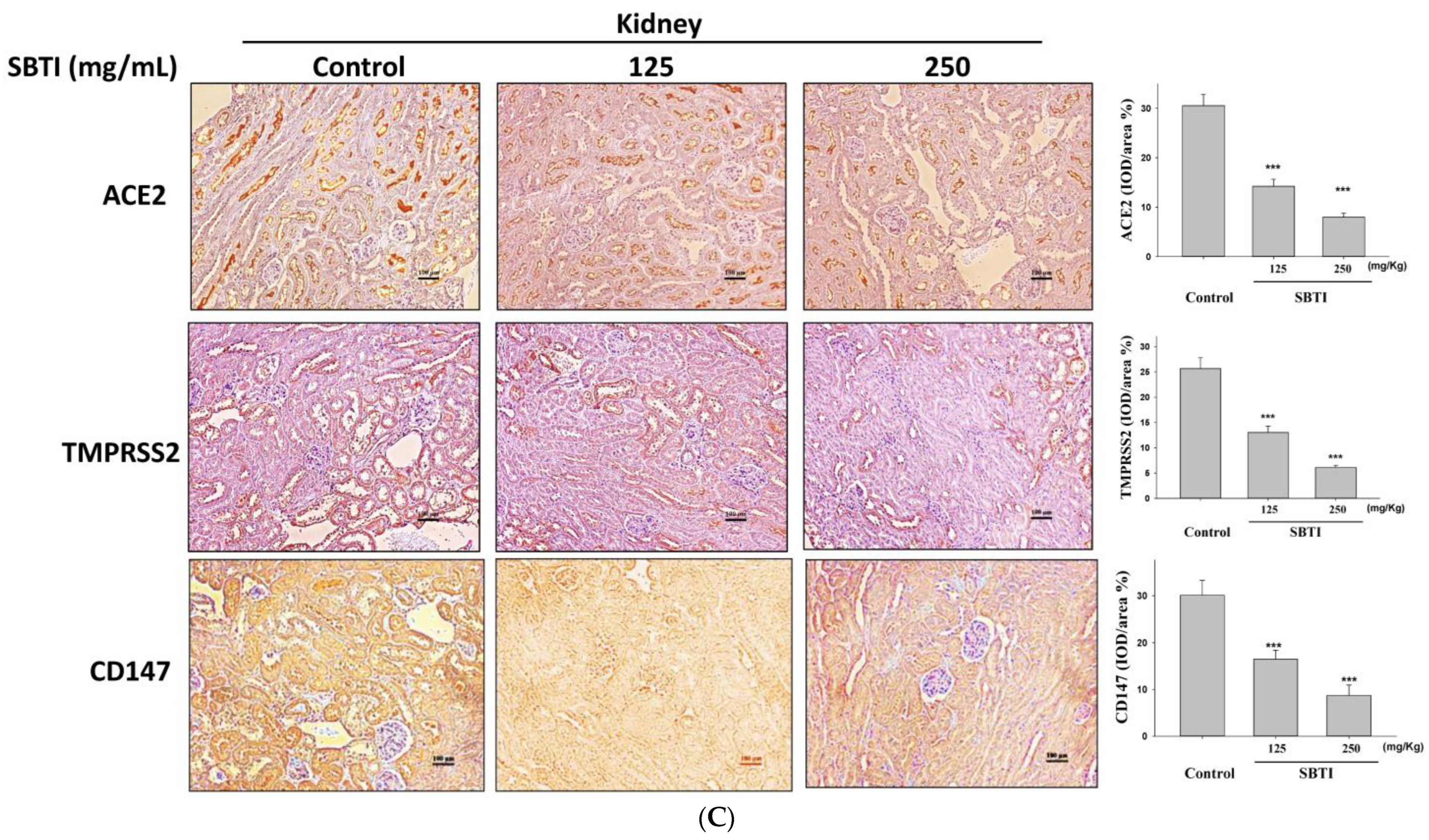
2.4. Observing the Inhibitory Effects of SBTI on the Expression of ACE2, TMPRSS2, and CD147 Proteins by the IHC Method
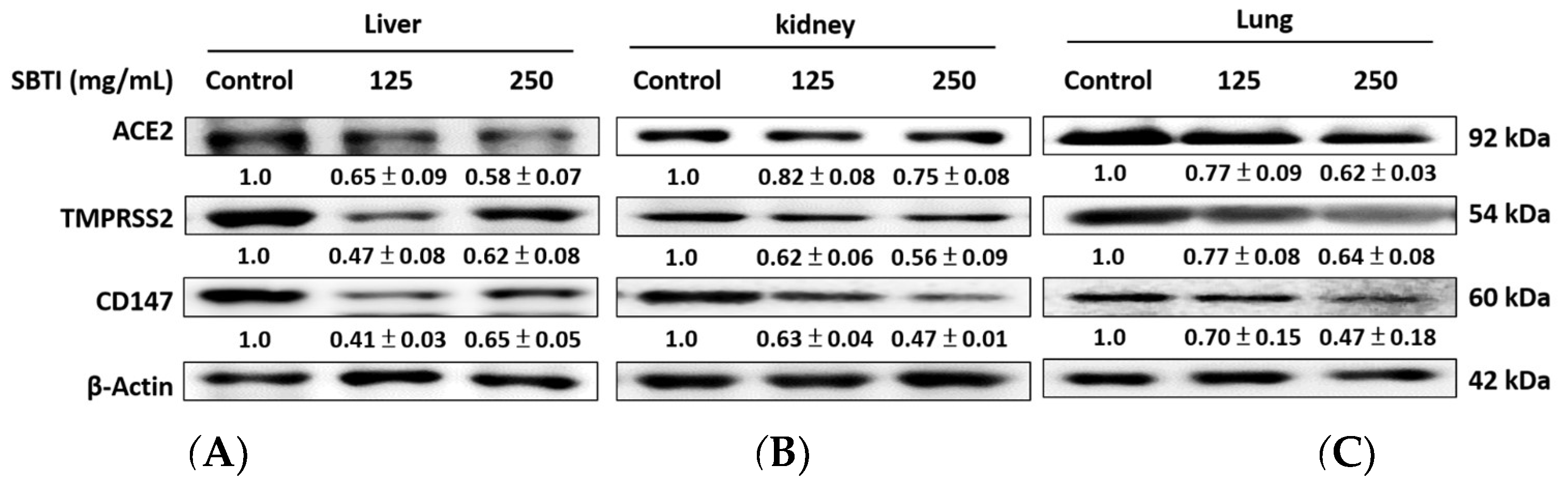
2.5. Evaluation of the Effect of SBTI on ACE2, TMPRSS2, and CD147 Expression in Diverse Tissues
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Viability Assay
4.2. Western Blot Analysis
4.3. Mouse Experiment
4.4. Hematoxylin and Eosin (H&E) Staining
4.5. Immunohistochemistry (IHC) Staining
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halawa, S.; Pullamsetti, S.S.; Bangham, C.R.M.; Stenmark, K.R.; Dorfmuller, P.; Frid, M.G.; Butrous, G.; Morrell, N.W.; de Jesus Perez, V.A.; Stuart, D.I.; et al. Potential long-term effects of SARS-CoV-2 infection on the pulmonary vasculature: A global perspective. Nat. Rev. Cardiol. 2022, 19, 314–331. [Google Scholar] [PubMed]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [PubMed]
- Wong, S.C.; Chan, V.W.; Yuen, L.L.; AuYeung, C.H.; Leung, J.O.; Li, C.K.; Kwok, M.O.; So, S.Y.; Chen, J.H.; Chiu, K.H.; et al. Infection of healthcare workers despite a high vaccination rate during the fifth wave of COVID-19 due to Omicron variant in Hong Kong. Infect. Prev. Pract. 2023, 5, 100261. [Google Scholar] [PubMed]
- Choudhary, S.; Sreenivasulu, K.; Mitra, P.; Misra, S.; Sharma, P. Role of Genetic Variants and Gene Expression in the Susceptibility and Severity of COVID-19. Ann. Lab. Med. 2021, 41, 129–138. [Google Scholar]
- Lanjanian, H.; Moazzam-Jazi, M.; Hedayati, M.; Akbarzadeh, M.; Guity, K.; Sedaghati-Khayat, B.; Azizi, F.; Daneshpour, M.S. SARS-CoV-2 infection susceptibility influenced by ACE2 genetic polymorphisms: Insights from Tehran Cardio-Metabolic Genetic Study. Sci. Rep. 2021, 11, 1529. [Google Scholar]
- Su, Y.C.; Huang, G.J.; Lin, J.G. Chinese herbal prescriptions for COVID-19 management: Special reference to Taiwan Chingguan Yihau (NRICM101). Front. Pharmacol. 2022, 13, 928106. [Google Scholar]
- Devaux, C.A.; Rolain, J.M.; Raoult, D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. Infect. 2020, 53, 425–435. [Google Scholar]
- Abbasi, A.Z.; Kiyani, D.A.; Hamid, S.M.; Saalim, M.; Fahim, A.; Jalal, N. Spiking dependence of SARS-CoV-2 pathogenicity on TMPRSS2. J. Med. Virol. 2021, 93, 4205–4218. [Google Scholar]
- Huang, J.; Song, W.; Huang, H.; Sun, Q. Pharmacological Therapeutics Targeting RNA-Dependent RNA Polymerase, Proteinase and Spike Protein: From Mechanistic Studies to Clinical Trials for COVID-19. J. Clin. Med. 2020, 9, 1131. [Google Scholar]
- Luan, B.; Huynh, T.; Cheng, X.; Lan, G.; Wang, H.R. Targeting Proteases for Treating COVID-19. J. Proteome Res. 2020, 19, 4316–4326. [Google Scholar]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Author Correction: Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2021, 12, 2144. [Google Scholar] [PubMed]
- Bojkova, D.; McGreig, J.E.; McLaughlin, K.-M.; Masterson, S.G.; Widera, M.; Krähling, V.; Ciesek, S.; Wass, M.N.; Michaelis, M.; Jindrich Cinatl, J. SARS-CoV-2 and SARS-CoV differ in their cell tropism and drug sensitivity profiles. bioRxiv 2020. [Google Scholar] [CrossRef]
- Shouman, S.; El-Kholy, N.; Hussien, A.E.; El-Derby, A.M.; Magdy, S.; Abou-Shanab, A.M.; Elmehrath, A.O.; Abdelwaly, A.; Helal, M.; El-Badri, N. SARS-CoV-2-associated lymphopenia: Possible mechanisms and the role of CD147. Cell Commun. Signal. 2024, 22, 349. [Google Scholar] [PubMed]
- Cavezzi, A.; Menicagli, R.; Troiani, E.; Corrao, S. COVID-19, Cation dysmetabolism, sialic acid, CD147, ACE2, viroporins, hepcidin and ferroptosis: A possible unifying hypothesis. F1000Research 2022, 11, 102. [Google Scholar]
- Fang, E.F.; Wong, J.H.; Ng, T.B. Thermostable Kunitz trypsin inhibitor with cytokine inducing, antitumor and HIV-1 reverse transcriptase inhibitory activities from Korean large black soybeans. J. Biosci. Bioeng. 2010, 109, 211–217. [Google Scholar]
- Fang, E.F.; Wong, J.H.; Bah, C.S.; Lin, P.; Tsao, S.W.; Ng, T.B. Bauhinia variegata var. variegata trypsin inhibitor: From isolation to potential medicinal applications. Biochem. Biophys. Res. Commun. 2010, 396, 806–811. [Google Scholar]
- Luz, A.B.S.; de Medeiros, A.F.; Bezerra, L.L.; Lima, M.S.R.; Pereira, A.S.; EGO, E.S.; Passos, T.S.; Monteiro, N.K.V.; Morais, A.H.A. Prospecting native and analogous peptides with anti-SARS-CoV-2 potential derived from the trypsin inhibitor purified from tamarind seeds. Arab. J. Chem. 2023, 16, 104886. [Google Scholar]
- Gupta, S.; Kanwar, S.S. Plant protease inhibitors and their antiviral activities-Potent therapeutics for SARS-CoV-2. J. Med. Discov. 2021, 6, 1–14. [Google Scholar]
- Kobayashi, H. Prevention of cancer and inflammation by soybean protease inhibitors. Front Biosci 2013, 5, 966–973. [Google Scholar]
- Pires De Souza, G.A.; Le Bideau, M.; Boschi, C.; Wurtz, N.; Colson, P.; Aherfi, S.; Devaux, C.; La Scola, B. Choosing a cellular model to study SARS-CoV-2. Front. Cell Infect. Microbiol. 2022, 12, 1003608. [Google Scholar]
- Smirnova, O.A.; Ivanova, O.N.; Fedyakina, I.T.; Yusubalieva, G.M.; Baklaushev, V.P.; Yanvarev, D.V.; Kechko, O.I.; Mitkevich, V.A.; Vorobyev, P.O.; Fedorov, V.S.; et al. SARS-CoV-2 Establishes a Productive Infection in Hepatoma and Glioblastoma Multiforme Cell Lines. Cancers 2023, 15, 632. [Google Scholar] [PubMed]
- WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 4 July 2025).
- Mitja, O.; Clotet, B. Use of antiviral drugs to reduce COVID-19 transmission. Lancet Glob. Health 2020, 8, e639–e640. [Google Scholar] [PubMed]
- Lin, J.G.; Huang, G.J.; Su, Y.C. Efficacy analysis and research progress of complementary and alternative medicines in the adjuvant treatment of COVID-19. J. Biomed. Sci. 2023, 30, 30. [Google Scholar]
- Vasquez-Bonilla, W.O.; Orozco, R.; Argueta, V.; Sierra, M.; Zambrano, L.I.; Muñoz-Lara, F.; López-Molina, D.S.; Arteaga-Livias, K.; Grimes, Z.; Bryce, C. A review of the main histopathological findings in coronavirus disease 2019. Hum. Pathol. 2020, 105, 74–83. [Google Scholar]
- Munro, A.P.; Jones, C.E. Immunity debt and unseasonal childhood respiratory viruses. Br. J. Hosp. Med. 2022, 83, 1–3. [Google Scholar]
- Billard, M.N.; Bont, L.J. Quantifying the RSV immunity debt following COVID-19: A public health matter. Lancet Infect. Dis. 2023, 23, 3–5. [Google Scholar]
- Principi, N.; Autore, G.; Ramundo, G.; Esposito, S. Epidemiology of Respiratory Infections during the COVID-19 Pandemic. Viruses 2023, 15, 1160. [Google Scholar] [CrossRef]
- Yang, M.C.; Su, Y.T.; Chen, P.H.; Tsai, C.C.; Lin, T.I.; Wu, J.R. Changing patterns of infectious diseases in children during the COVID-19 pandemic. Front. Cell Infect. Microbiol. 2023, 13, 1200617. [Google Scholar]
- Rubin, R. From “Immunity Debt” to “Immunity Theft”-How COVID-19 Might Be Tied to Recent Respiratory Disease Surges. JAMA 2024, 331, 378–381. [Google Scholar]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar]
- Li, Y.; Zhou, W.; Yang, L.; You, R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol. Res. 2020, 157, 104833. [Google Scholar] [PubMed]
- Raghav, P.K.; Kalyanaraman, K.; Kumar, D. Human cell receptors: Potential drug targets to combat COVID-19. Amino Acids 2021, 53, 813–842. [Google Scholar] [PubMed]
- Chien, L.H.; Deng, J.S.; Jiang, W.P.; Chen, C.C.; Chou, Y.N.; Lin, J.G.; Huang, G.J. Study on the potential of Sanghuangporus sanghuang and its components as COVID-19 spike protein receptor binding domain inhibitors. Biomed. Pharmacother. 2022, 153, 113434. [Google Scholar]
- Zhang, Q.; Xiang, R.; Huo, S.; Zhou, Y.; Jiang, S.; Wang, Q.; Yu, F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct. Tar. 2021, 6, 233. [Google Scholar]
- Hoffmann, M.; Hofmann-Winkler, H.; Smith, J.C.; Kruger, N.; Arora, P.; Sorensen, L.K.; Sogaard, O.S.; Hasselstrom, J.B.; Winkler, M.; Hempel, T.; et al. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine 2021, 65, 103255. [Google Scholar]
- Ren, H.L.; Wen, G.M.; Zhao, Z.Y.; Liu, D.H.; Xia, P. Can CD147 work as a therapeutic target for tumors through COVID-19 infection? Int. J. Med. Sci. 2022, 19, 2087–2092. [Google Scholar]
- Behl, T.; Kaur, I.; Aleya, L.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target. Sci. Total Environ. 2022, 808, 152072. [Google Scholar]
- Wu, J.; Chen, L.; Qin, C.; Huo, F.; Liang, X.; Yang, X.; Zhang, K.; Lin, P.; Liu, J.; Feng, Z.; et al. CD147 contributes to SARS-CoV-2-induced pulmonary fibrosis. Signal Transduct. Target. Ther. 2022, 7, 382. [Google Scholar]
- Sakr, Y.; Bensasi, H.; Taha, A.; Bauer, M.; Ismail, K.; UAE-Jena Research Group; Belhaj, G.; Afet, K.M.; Munde, D.; Monk, D.; et al. Camostat mesylate therapy in critically ill patients with COVID-19 pneumonia. Intensive Care Med. 2021, 47, 707–709. [Google Scholar]
- Zhu, H.; Du, W.; Song, M.; Liu, Q.; Herrmann, A.; Huang, Q. Spontaneous binding of potential COVID-19 drugs (Camostat and Nafamostat) to human serine protease TMPRSS2. Comput. Struct. Biotechnol. J. 2020, 19, 467–476. [Google Scholar]
- Oliva, M.L.; Ferreira Rda, S.; Ferreira, J.G.; de Paula, C.A.; Salas, C.E.; Sampaio, M.U. Structural and functional properties of kunitz proteinase inhibitors from leguminosae: A mini review. Curr. Protein Pept. Sci. 2011, 12, 348–357. [Google Scholar] [PubMed]
- Roosta, H.R.; Javadi, T.; Nazari, F. Isolation and characterization of trypsin inhibitors (Kunitz soybean trypsin inhibitor, Bowman-Birk inhibitor) in soybean. Adv. Environ. Biol. 2011, 5, 145–154. [Google Scholar]
- Bendre, A.D.; Ramasamy, S.; Suresh, C.G. Analysis of Kunitz inhibitors from plants for comprehensive structural and functional insights. Int. J. Biol. Macromol. 2018, 113, 933–943. [Google Scholar] [PubMed]
- Bonturi, C.R.; Silva Teixeira, A.B.; Rocha, V.M.; Valente, P.F.; Oliveira, J.R.; Filho, C.M.B.; Fátima Correia Batista, I.; Oliva, M.L.V. Plant Kunitz inhibitors and their interaction with proteases: Current and potential pharmacological targets. Int. J. Mol. Sci. 2022, 23, 4742. [Google Scholar]
- Flavin, D.F. The effects of soybean trypsin inhibitors on the pancreas of animals and man: A review. Vet. Hum. Toxicol. 1982, 24, 25–28. [Google Scholar]
- Cid-Gallegos, M.S.; Corzo-Ríos, L.J.; Jiménez-Martínez, C.; Sánchez-Chino, X.M. Protease inhibitors from plants as therapeutic agents- a review. Plant Foods Hum. Nutr. 2022, 77, 20–29. [Google Scholar]
- Kennedy, A.R. Proteases, protease inhibitors and radiation carcinogenesis. Int. J. Radiat. Biol. 2023, 99, 882–890. [Google Scholar]
- Nath, A.; Ahmad, A.S.; Amankwaa, A.; Csehi, B.; Mednyánszky, Z.; Szerdahelyi, E.; Tóth, A.; Tormási, J.; Truong, D.H.; Abrankó, L.; et al. Hydrolysis of soybean milk protein by papain: Antioxidant, anti-Angiotensin, antigenic and digestibility perspectives. Bioengineering 2022, 9, 418. [Google Scholar] [CrossRef]
- De La Barca, A.M.; Wall, A.; López-Díaz, J.A. Allergenicity, trypsin inhibitor activity and nutritive quality of enzymatically modified soy proteins. Int. J. Food Sci. Nutr. 2005, 56, 203–211. [Google Scholar]
- Visser, N.; Herreman, L.C.M.; Vandooren, J.; Pereira, R.V.S.; Opdenakker, G.; Spelbrink, R.E.J.; Wilbrink, M.H.; Bremer, E.; Gosens, R.; Nawijn, M.C.; et al. Novel high-yield potato protease inhibitor panels block a wide array of proteases involved in viral infection and crucial tissue damage. J. Mol. Med. 2024, 102, 521–536. [Google Scholar]
- Kirar, M.; Singh, H.; Sehrawat, N. Virtual screening and molecular dynamics simulation study of plant protease inhibitors against SARS-CoV-2 envelope protein. Informatics Med. Unlocked 2022, 30, 100909. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Saldívar, S.O.S. Inactivation methods of trypsin inhibitor in legumes: A review. J. Food Sci. 2018, 83, 17–29. [Google Scholar] [PubMed]
- Jain, A.; Kasliwal, R.; Jain, S.S.; Jain, R.; Gupta, D.; Gupta, P.; Jain, A.; Tambi, R.; Panwar, P.; Meena, M.; et al. Effect of urinary trypsin inhibitor (ulinastatin) therapy in COVID-19. Indian J. Crit. Care Med. 2022, 26, 696–703. [Google Scholar] [PubMed]
- Mehta, Y.; Zirpe, K.; Dixit, S.; Ansari, A.; Mehta, C.; Deshmukh, A.; Ambapkar, S.; Ambapkar, S.; Joshi, M.; Joshi, A.; et al. Ulinastatin add-on to standard of care in critically Ill COVID-19 patients: A multicenter, retrospective study. J. Assoc. Physicians India. 2023, 71, 11–12. [Google Scholar]
- Wu, C.Y.; Lin, Y.S.; Yang, Y.H.; Shu, L.H.; Cheng, Y.C.; Liu, H.T. GB-2 inhibits ACE2 and TMPRSS2 expression: In vivo and in vitro studies. Biomed. Pharmacother. 2020, 132, 110816. [Google Scholar]
- Rasmi, Y.; Hatamkhani, S.; Naderi, R.; Shokati, A.; Zadeh, V.N.; Hosseinzadeh, F.; Farnamian, Y.; Jalali, L. Molecular signaling pathways, pathophysiological features in various organs, and treatment strategies in SARS-CoV2 infection. Acta Histochem. 2022, 124, 151908. [Google Scholar]
- Qiao, Y.; Wang, X.-M.; Mannan, R.; Pitchiaya, S.; Zhang, Y.; Wotring, J.W.; Xiao, L.; Robinson, D.R.; Wu, Y.-M.; Tien, J.C.-Y.; et al. Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2. Proc. Natl. Acad. Sci. USA 2021, 118, e2021450118. [Google Scholar]
- Schwartz, J.; Capistrano, K.J.; Gluck, J.; Hezarkhani, A.; Naqvi, A.R. SARS-CoV-2, periodontal pathogens, and host factors: The trinity of oral post-acute sequelae of COVID-19. Rev. Med. Virol. 2024, 34, e2543. [Google Scholar]
- Jiang, W.P.; Deng, J.S.; Yu, C.C.; Lin, J.G.; Huang, G.J. Anti-SARS-CoV-2 viral activity of sweet potato trypsin inhibitor via downregulation of TMPRSS2 Activity and ACE2 expression in vitro and in vivo. Int. J. Mol. Sci. 2024, 25, 6067. [Google Scholar]
- Sun, T.K.; Huang, W.C.; Sun, Y.W.; Deng, J.S.; Chien, L.H.; Chou, Y.N.; Jiang, W.P.; Lin, J.G.; Huang, G.J. Schizophyllum commune Reduces Expression of the SARS-CoV-2 Receptors ACE2 and TMPRSS2. Int. J. Mol. Sci. 2022, 23, 14766. [Google Scholar]
- Chen, Y.R.; Jiang, W.P.; Deng, J.S.; Chou, Y.N.; Wu, Y.B.; Liang, H.J.; Lin, J.G.; Huang, G.J. Anisomeles indica extracts and their constituents suppress the protein expression of ACE2 and TMPRSS2 in vivo and in vitro. Int. J. Mol. Sci. 2023, 24, 15062. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.-L.; Lin, J.-G.; Jiang, W.-P.; Hung, H.-P.; Inose, A.; Huang, G.-J. Soybean Trypsin Inhibitor Possesses Potency Against SARS-CoV-2 Infection by Blocking the Host Cell Surface Receptors ACE2, TMPRSS2, and CD147. Int. J. Mol. Sci. 2025, 26, 6583. https://doi.org/10.3390/ijms26146583
Wu W-L, Lin J-G, Jiang W-P, Hung H-P, Inose A, Huang G-J. Soybean Trypsin Inhibitor Possesses Potency Against SARS-CoV-2 Infection by Blocking the Host Cell Surface Receptors ACE2, TMPRSS2, and CD147. International Journal of Molecular Sciences. 2025; 26(14):6583. https://doi.org/10.3390/ijms26146583
Chicago/Turabian StyleWu, Wen-Liang, Jaung-Geng Lin, Wen-Ping Jiang, Hsi-Pin Hung, Atsushi Inose, and Guan-Jhong Huang. 2025. "Soybean Trypsin Inhibitor Possesses Potency Against SARS-CoV-2 Infection by Blocking the Host Cell Surface Receptors ACE2, TMPRSS2, and CD147" International Journal of Molecular Sciences 26, no. 14: 6583. https://doi.org/10.3390/ijms26146583
APA StyleWu, W.-L., Lin, J.-G., Jiang, W.-P., Hung, H.-P., Inose, A., & Huang, G.-J. (2025). Soybean Trypsin Inhibitor Possesses Potency Against SARS-CoV-2 Infection by Blocking the Host Cell Surface Receptors ACE2, TMPRSS2, and CD147. International Journal of Molecular Sciences, 26(14), 6583. https://doi.org/10.3390/ijms26146583






