Metabolites Isolated from Senecio nutans Sch. Bip and Their Synthesized Oximes Inhibit Angiotensin I-Converting Enzyme Activity in Vascular Smooth Muscle
Abstract
1. Introduction
2. Results and Discussion
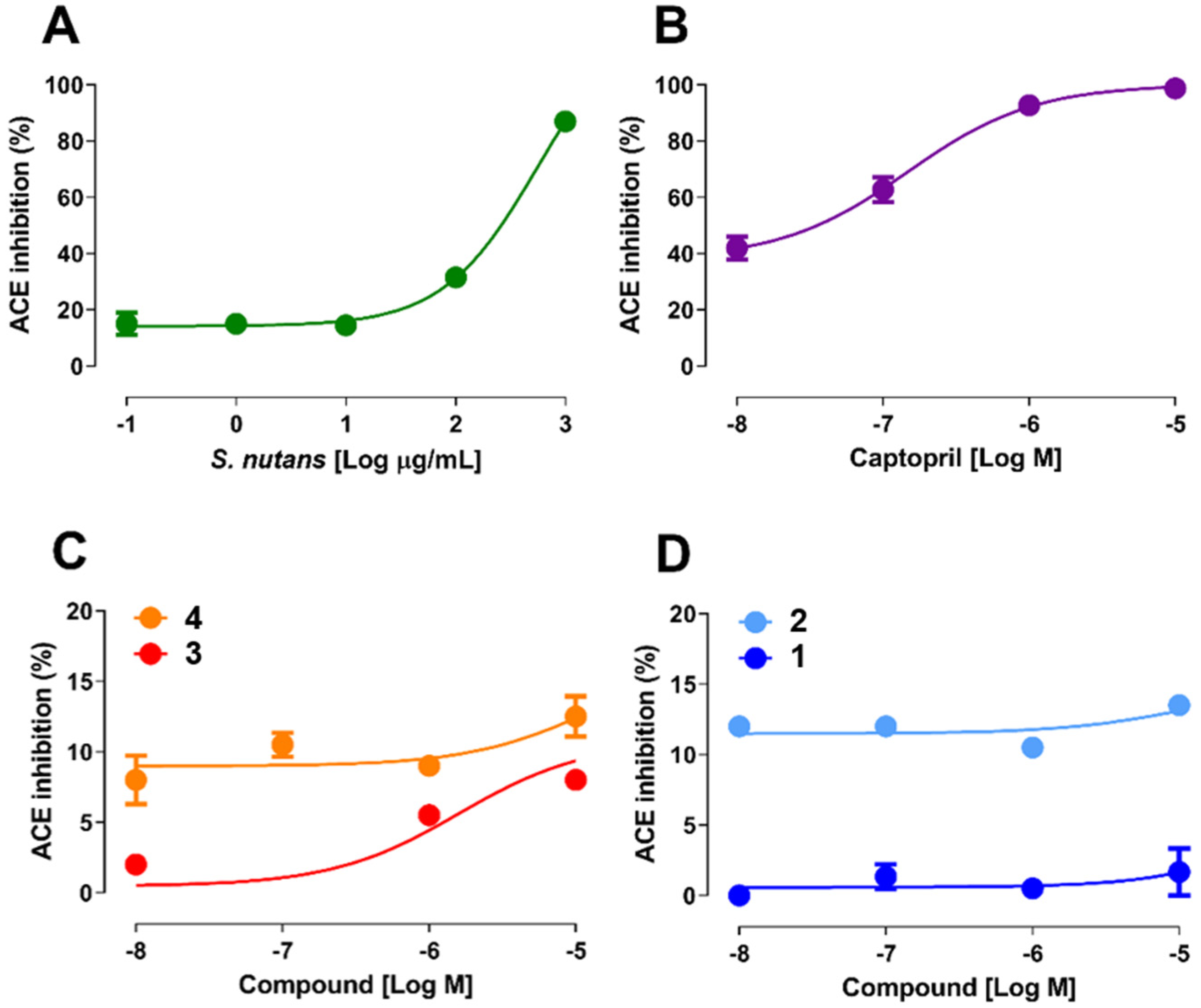
2.1. Determination of the Enzymatic Activity of ACE
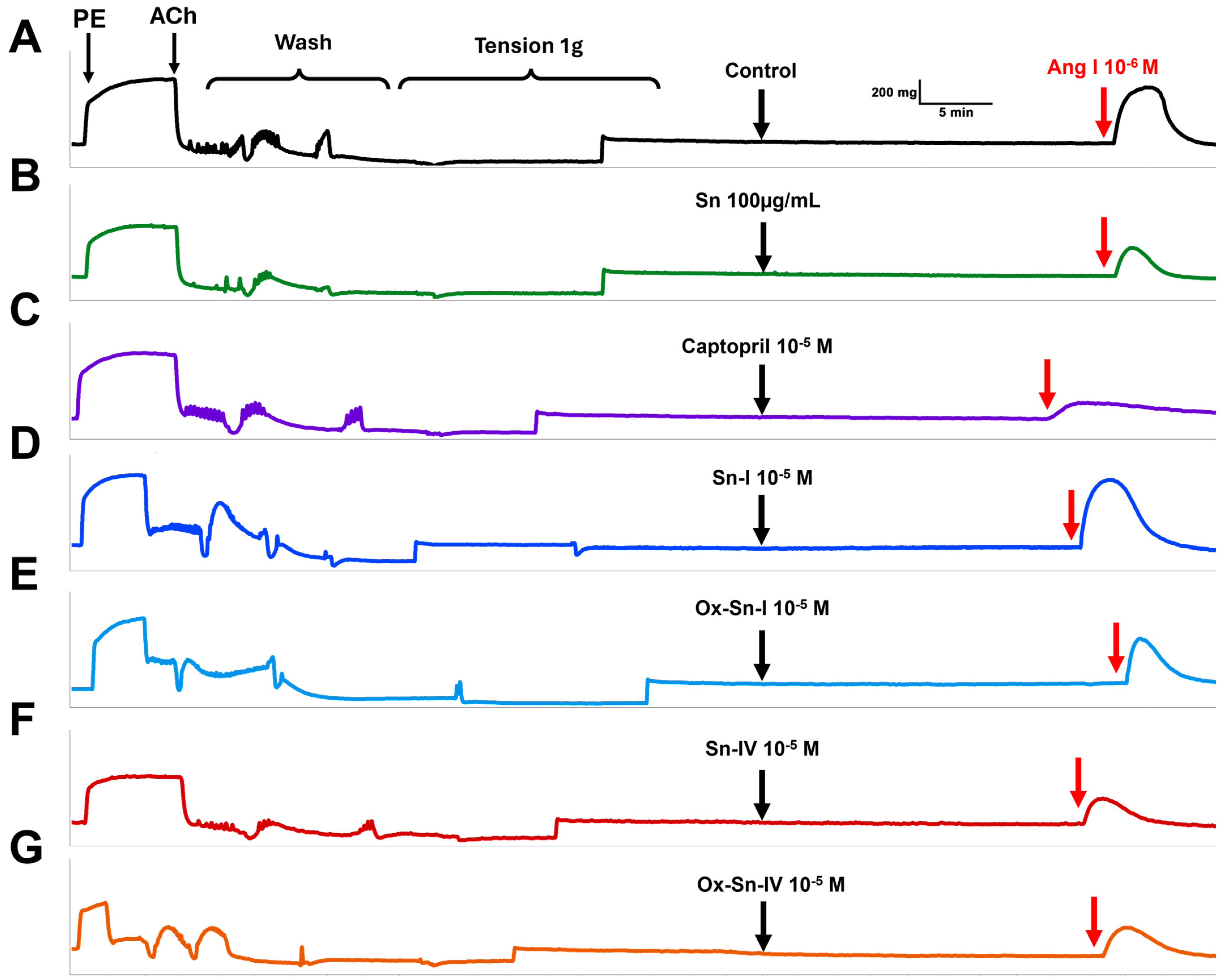
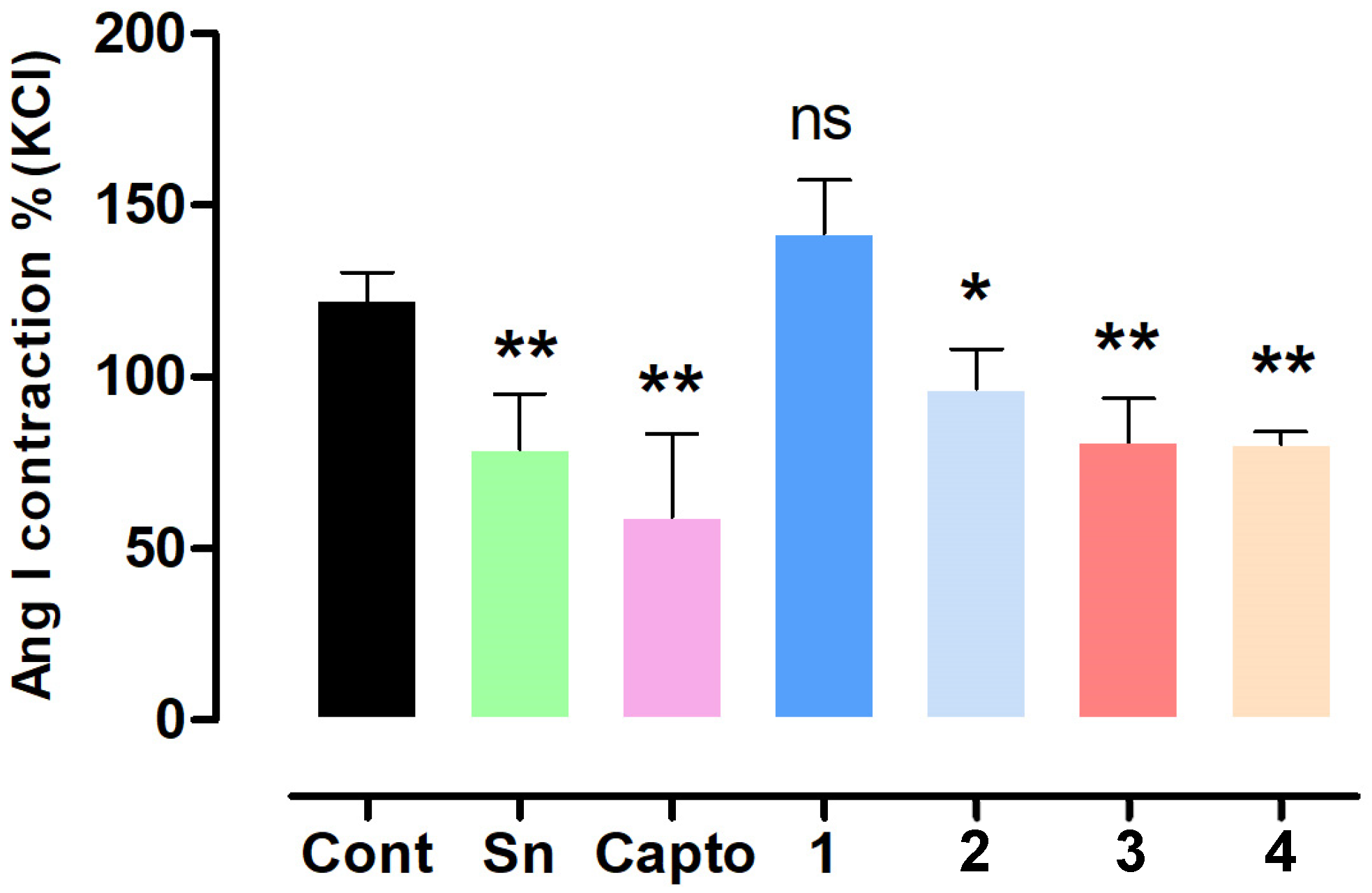
2.2. Effect of S. nutans and Pure Compounds on the Vascular Contractile Response to Angiotensin I
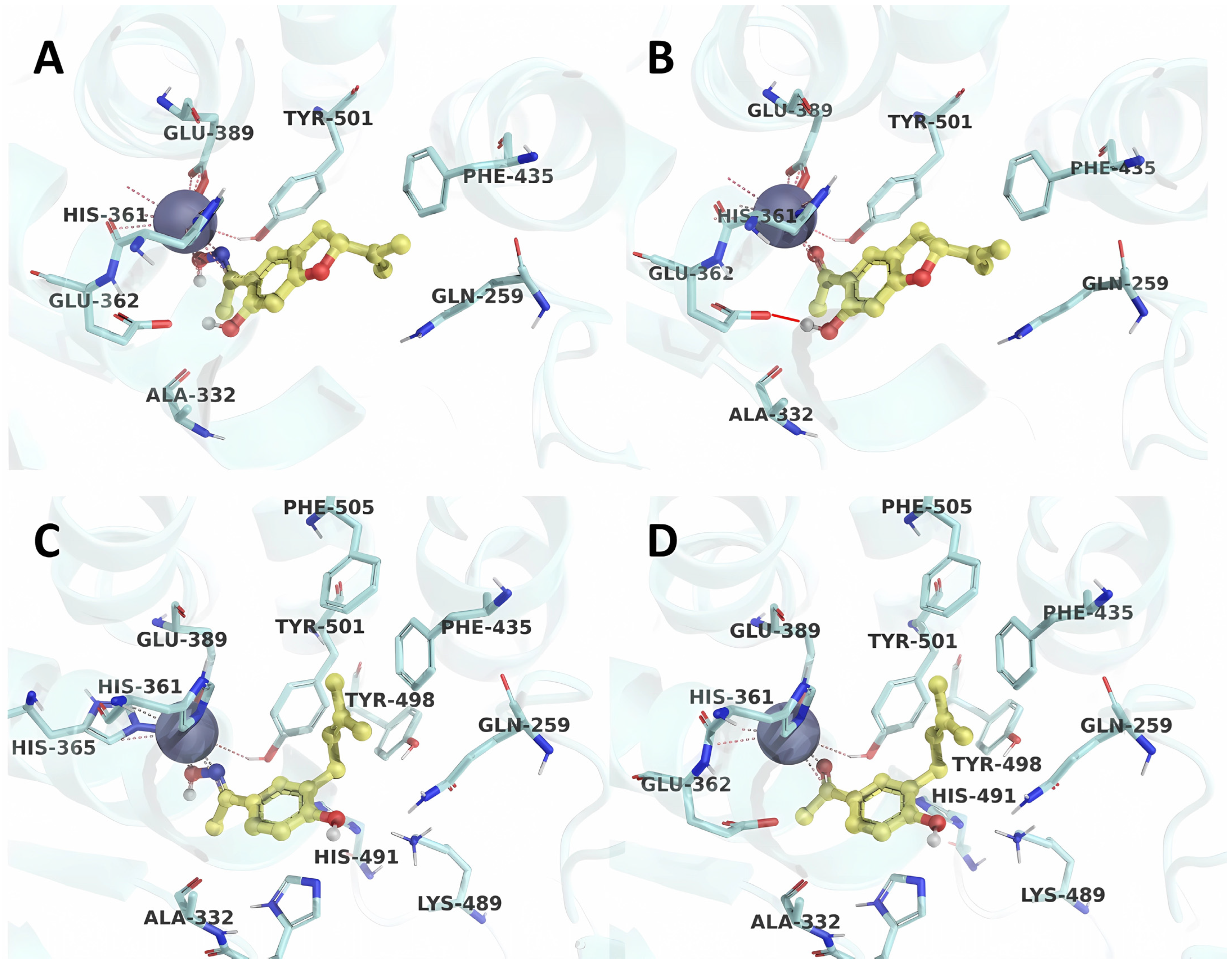
2.3. Molecular Docking Validation
2.4. Molecular Docking Analysis
3. Materials and Methods
3.1. Chemicals
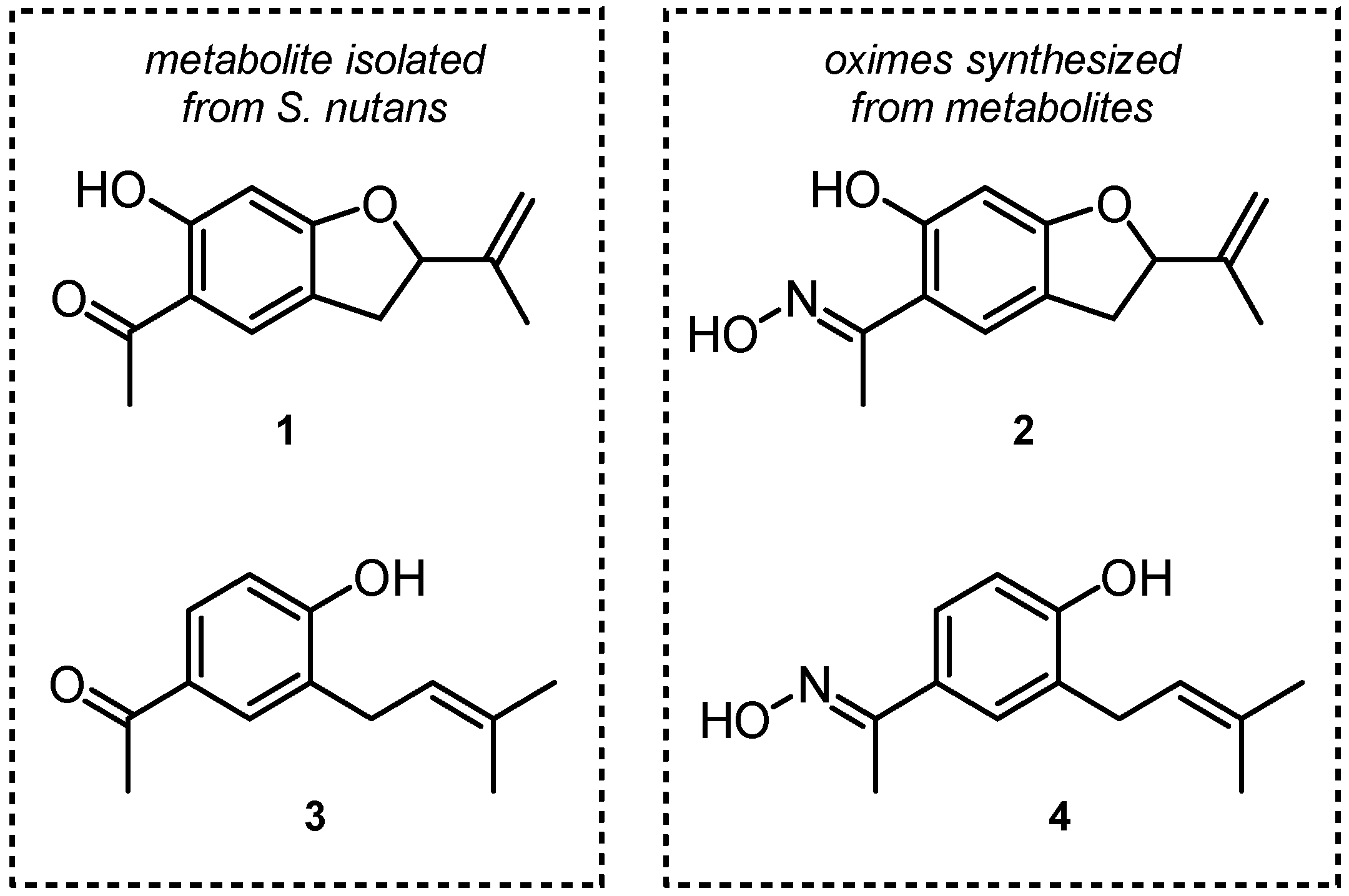
3.2. Isolation of Natural Products from S. nutans and Oxime Synthesis
3.3. Animals
3.4. Isolation of Rat Aorta and Vascular Reactivity Assays
3.5. Determination of Angiotensin I-Converting Enzyme (ACE) Activity
3.6. Preparation of Receptors and Ligands
3.7. Molecular Docking
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-Converting Enzyme |
| ACh | Acetylcholine chloride |
| Ang I | Angiotensin I |
| LTCC | L-type calcium channel |
| PE | L-phenylephrine hydrochloride |
| RAS | Renin–angiotensin system |
References
- World Health Organization. Hypertension. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 25 May 2024).
- Mills, K.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Mussa, F.; Horton, J.; Moridzadeh, R.; Nicholson, J.; Trimarchi, S.; Eagle, K. Acute Aortic Dissection and Intramural Hematoma A Systematic Review. JAMA—J. Am. Med. Assoc. 2016, 316, 754–763. [Google Scholar] [CrossRef]
- Schricker, K.; Holmer, S.; Hamann, M.; Riegger, G.; Kurtz, A. interrelation between renin mRNA levels, renin secretion, and blood-pressure in two-kidney, one-clip rats. Hypertension 1994, 24, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Mehr, A.; Kreutz, R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S. Is It Still Relevant to Discover New ACE Inhibitors from Natural Products? YES, but Only with Comprehensive Approaches to Address the Patients’ Real Problems: Chronic Dry Cough and Angioedema. Molecules 2023, 28, 4532. [Google Scholar] [CrossRef]
- Sharif, M.; Evans, B.; Pylypchuk, G. Cough induced by quinapril with resolution after changing to fosinopril. Ann. Pharmacother. 1994, 28, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Cifuentes, F.; Paredes, A.; Palacios, J.; Munoz, F.; Carvajal, L.; Nwokocha, C.R.; Morales, G. Hypotensive and antihypertensive effects of a hydroalcoholic extract from Senecio nutans Sch Bip. (Compositae) in mice: Chronotropic and negative inotropic effect, a nifedipine-like action. J. Ethnopharmacol. 2016, 179, 367–374. [Google Scholar] [CrossRef]
- Palacios, J.; Paredes, A.; Cifuentes, F.; Catalán, M.A.; García-Villalón, A.L.; Borquez, J.; Simirgiotis, M.J.; Jones, M.; Foster, A.; Greensmith, D.J. A hydroalcoholic extract of Senecio nutans SCh. Bip (Asteraceae); its effects on cardiac function and chemical characterization. J. Ethnopharmacol. 2023, 300, 115747. [Google Scholar] [CrossRef]
- Paredes, A.; Palacios, J.; Quispe, C.; Nwokocha, C.R.; Morales, G.; Kuzmicic, J.; Cifuentes, F. Hydroalcoholic extract and pure compounds from Senecio nutans Sch Bip (Compositae) induce vasodilation in rat aorta through endothelium-dependent and independent mechanisms. J. Ethnopharmacol. 2016, 192, 99–107. [Google Scholar] [CrossRef]
- Ghabbour, H.; El-Bendary, E.; El-Ashmawy, M.; El-Kerdawy, M. Synthesis, Docking Study and β-Adrenoceptor Activity of Some New Oxime Ether Derivatives. Molecules 2014, 19, 3417–3435. [Google Scholar] [CrossRef] [PubMed]
- Sahyoun, T.; Arrault, A.; Schneider, R. Amidoximes and Oximes: Synthesis, Structure, and Their Key Role as NO Donors. Molecules 2019, 24, 2470. [Google Scholar] [CrossRef] [PubMed]
- Palacios, J.; Asunción-Alvarez, D.; Aravena, D.; Chiong, M.; Catalán, M.; Parra, C.; Cifuentes, F.; Paredes, A. A new oxime synthesized from Senecio nutans SCh. Bip (chachacoma) reduces calcium influx in the vascular contractile response in rat aorta. RSC Adv. 2024, 14, 9933–9942. [Google Scholar] [CrossRef]
- Palacios, J.; Paredes, A.; Catalan, M.A.; Nwokocha, C.R.; Cifuentes, F. Novel Oxime Synthesized from a Natural Product of Senecio nutans SCh. Bip. (Asteraceae) Enhances Vascular Relaxation in Rats by an Endothelium-Independent Mechanism. Molecules 2022, 27, 3333. [Google Scholar] [CrossRef]
- de Alvarenga, E.; Fonseca, M.; Carvalho, C.; Florentino, R.; Franca, A.; Matias, E.; Guimaraes, P.; Batista, C.; Freire, V.; Carmona, A.; et al. Angiotensin Converting Enzyme Regulates Cell Proliferation and Migration. PLoS ONE 2016, 11, e0165371. [Google Scholar] [CrossRef] [PubMed]
- Korystova, A.; Emel’yanov, M.; Kublik, L.; Levitman, M.; Shaposhnikova, V.; Kim, Y.; Korystov, Y. Distribution of the activity of the angiotensin-converting enzyme in the rat aorta and changes in the activity with aging and by the action of L-NAME. Age 2012, 34, 821–830. [Google Scholar] [CrossRef]
- Chiesa, S.; Marcovecchio, M.; Benitez-Aguirre, P.; Cameron, F.; Craig, M.; Couper, J.; Davis, E.; Dalton, R.; Daneman, D.; Donaghue, K.; et al. Vascular Effects of ACE (Angiotensin-Converting Enzyme) Inhibitors and Statins in Adolescents with Type 1 Diabetes. Hypertension 2020, 76, 1734–1743. [Google Scholar] [CrossRef]
- Enseleit, F.; Hürlimann, D.; Lüscher, T. Vascular protective effects of angiotensin converting enzyme inhibitors and their relation to clinical events. J. Cardiovasc. Pharmacol. 2001, 37, S21–S30. [Google Scholar] [CrossRef]
- Virdis, A.; Neves, M.; Amiri, F.; Touyz, R.; Schiffrin, E. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J. Hypertens. 2004, 22, 535–542. [Google Scholar] [CrossRef]
- DiNicolantonio, J.; Lavie, C.; O’Keefe, J. Not All Angiotensin-Converting Enzyme Inhibitors Are Equal: Focus on Ramipril and Perindopril. Postgrad. Med. 2013, 125, 154–168. [Google Scholar] [CrossRef]
- Toda, N.; Hayashi, S.; Miyazaki, M. Contractile responses of isolated dog mesenteric-arteries to angiotensin I, II and III. Jpn. J. Pharmacol. 1978, 28, 527–534. [Google Scholar] [CrossRef]
- Natesh, R.; Schwager, S.; Sturrock, E.; Acharya, K. Crystal structure of the human angiotensin-converting enzyme-lisinopril complex. Nature 2003, 421, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Masuyer, G.; Akif, M.; Czarny, B.; Beau, F.; Schwager, S.; Sturrock, E.; Isaac, R.; Dive, V.; Acharya, K. Crystal structures of highly specific phosphinic tripeptide enantiomers in complex with the angiotensin-I converting enzyme. FEBS J. 2014, 281, 943–956. [Google Scholar] [CrossRef]
- Fienberg, S.; Cozier, G.; Acharya, K.; Chibale, K.; Sturrock, E. The Design and Development of a Potent and Selective Novel Diprolyl Derivative That Binds to the N-Domain of Angiotensin-I Converting Enzyme. J. Med. Chem. 2018, 61, 344–359. [Google Scholar] [CrossRef]
- Cozier, G.; Schwager, S.; Sharma, R.; Chibale, K.; Sturrock, E.; Acharya, K. Crystal structures of sampatrilat and sampatrilat-Asp in complex with human ACE—A molecular basis for domain selectivity. FEBS J. 2018, 285, 1477–1490. [Google Scholar] [CrossRef] [PubMed]
- Arendse, L.; Cozier, G.; Eyermann, C.; Basarab, G.; Schwager, S.; Chibale, K.; Acharya, K.; Sturrock, E. Probing the Requirements for Dual Angiotensin-Converting Enzyme C-Domain Selective/Neprilysin Inhibition. J. Med. Chem. 2022, 65, 3371–3387. [Google Scholar] [CrossRef]
- Lu, C.; Wu, C.J.; Ghoreishi, D.; Chen, W.; Wang, L.L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; et al. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef]
- Friesner, R.; Banks, J.; Murphy, R.; Halgren, T.; Klicic, J.; Mainz, D.; Repasky, M.; Knoll, E.; Shelley, M.; Perry, J.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Halgren, T.; Murphy, R.; Friesner, R.; Beard, H.; Frye, L.; Pollard, W.; Banks, J. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef] [PubMed]

| 1O86 | 4CA5 | 6EN5 | 6F9U | 7Q24 | 7Q28 | |
|---|---|---|---|---|---|---|
| Score (kcal/mol) | −12.84 | −14.21 | −9.70 | −11.62 | −9.42 | −11.02 |
| MM-GBSA (kcal/mol) | −67.58 | −89.32 | −55.32 | −78.48 | −63.32 | −69.67 |
| RMSD (Å) | 1.02 | 0.57 | 1.92 | 1.98 | 1.21 | 0.73 |
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Gln259 | - | - | 3.20 | 3.04 |
| Glu362 | 1.73 | 1.73 | - | - |
| Tyr501 | 2.30 | 2.26 | 1.88 | 2.78 |
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Gln259 | - | 3.88 | - | - |
| Phe435 | 3.28 | 3.23 | 3.5 | 3.5 |
| Phe435 | - | 3.46 | 3.4 | 3.44 |
| Tyr501 | 3.49 | 3.35 | 3.72 | 3.71 |
| Tyr501 | - | - | 3.57 | 3.74 |
| Phe505 | - | 3.84 | 3.41 | 3.38 |
| Entry | ∆GBind | ∆GCoul | ∆GHbond | ∆GLipo | ∆GPacking | ∆GSolv_GB | ∆GvdW |
|---|---|---|---|---|---|---|---|
| 1 | −39.99 | −26.80 | −1.20 | −21.74 | 0.05 | 38.62 | −32.26 |
| 2 | −42.99 | −29.05 | −1.33 | −21.50 | −0.99 | 35.67 | −30.38 |
| 3 | −31.01 | −19.07 | −0.30 | −22.23 | 1.07 | 37.60 | −29.73 |
| 4 | −34.80 | −20.60 | −0.50 | −20.36 | −0.22 | 30.35 | −28.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios, J.; Villarroel, C.; Asunción-Alvarez, D.; Cifuentes, F.; Paredes, A.; Nwokocha, C.R.; Castro-Álvarez, A.; Parra, C. Metabolites Isolated from Senecio nutans Sch. Bip and Their Synthesized Oximes Inhibit Angiotensin I-Converting Enzyme Activity in Vascular Smooth Muscle. Int. J. Mol. Sci. 2025, 26, 3786. https://doi.org/10.3390/ijms26083786
Palacios J, Villarroel C, Asunción-Alvarez D, Cifuentes F, Paredes A, Nwokocha CR, Castro-Álvarez A, Parra C. Metabolites Isolated from Senecio nutans Sch. Bip and Their Synthesized Oximes Inhibit Angiotensin I-Converting Enzyme Activity in Vascular Smooth Muscle. International Journal of Molecular Sciences. 2025; 26(8):3786. https://doi.org/10.3390/ijms26083786
Chicago/Turabian StylePalacios, Javier, Carlos Villarroel, Daniel Asunción-Alvarez, Fredi Cifuentes, Adrián Paredes, Chukwuemeka R. Nwokocha, Alejandro Castro-Álvarez, and Claudio Parra. 2025. "Metabolites Isolated from Senecio nutans Sch. Bip and Their Synthesized Oximes Inhibit Angiotensin I-Converting Enzyme Activity in Vascular Smooth Muscle" International Journal of Molecular Sciences 26, no. 8: 3786. https://doi.org/10.3390/ijms26083786
APA StylePalacios, J., Villarroel, C., Asunción-Alvarez, D., Cifuentes, F., Paredes, A., Nwokocha, C. R., Castro-Álvarez, A., & Parra, C. (2025). Metabolites Isolated from Senecio nutans Sch. Bip and Their Synthesized Oximes Inhibit Angiotensin I-Converting Enzyme Activity in Vascular Smooth Muscle. International Journal of Molecular Sciences, 26(8), 3786. https://doi.org/10.3390/ijms26083786








