A Comparative Study of Melittins from Apis florea and Apis mellifera as Cytotoxic Agents Against Non-Small Cell Lung Cancer (NSCLC) Cells and Their Combination with Gefitinib
Abstract
1. Introduction
2. Results
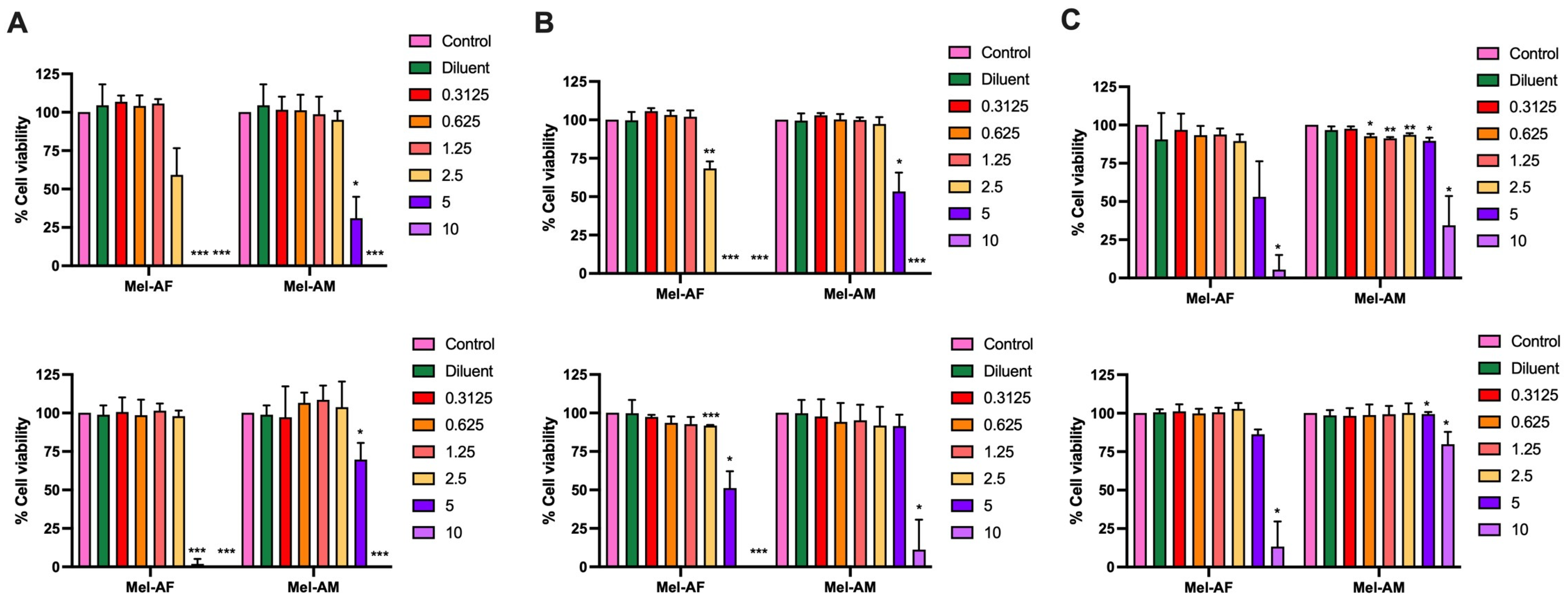
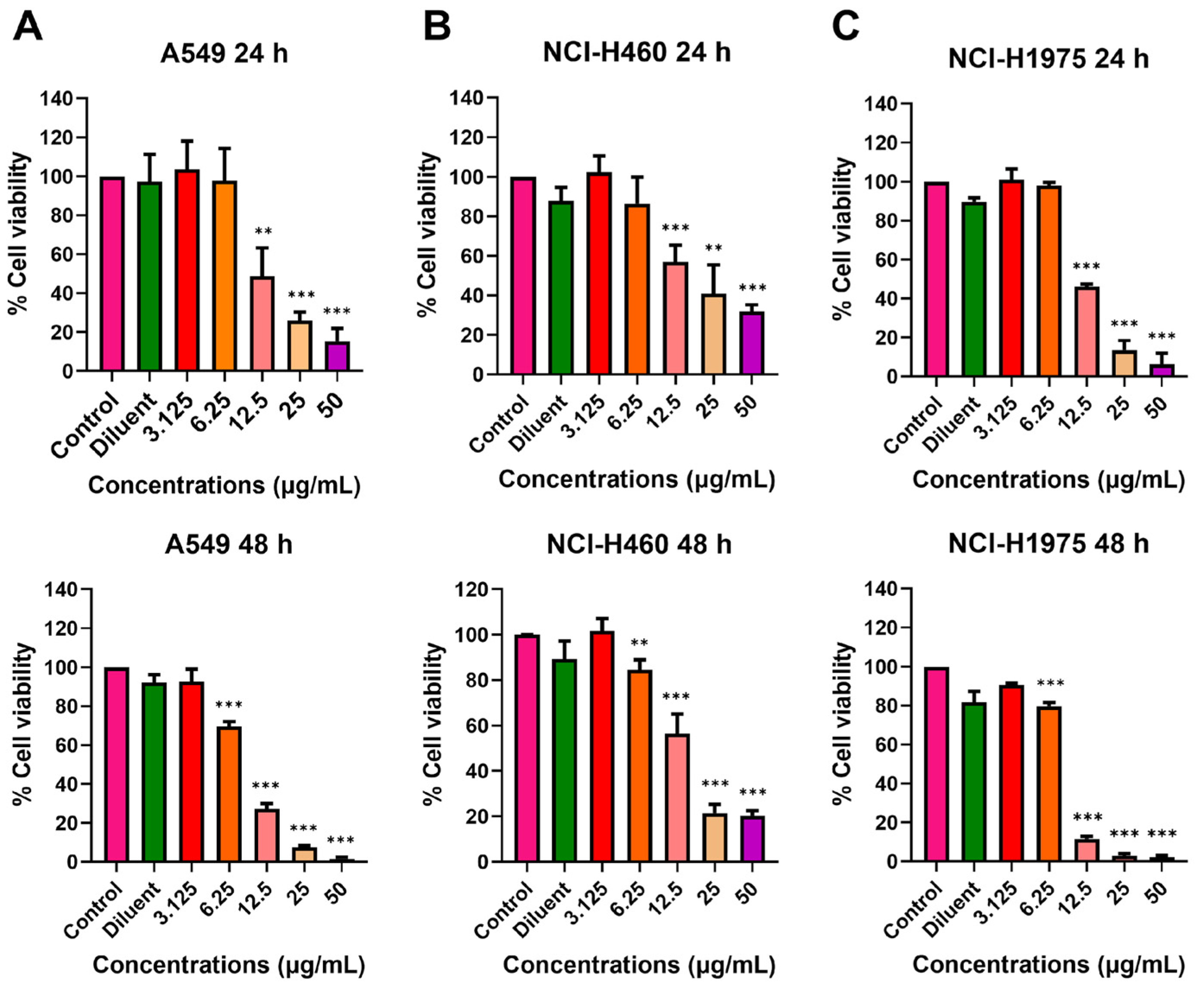
2.1. Effect of Melittin Peptides on Lung Cancer Cell Viability
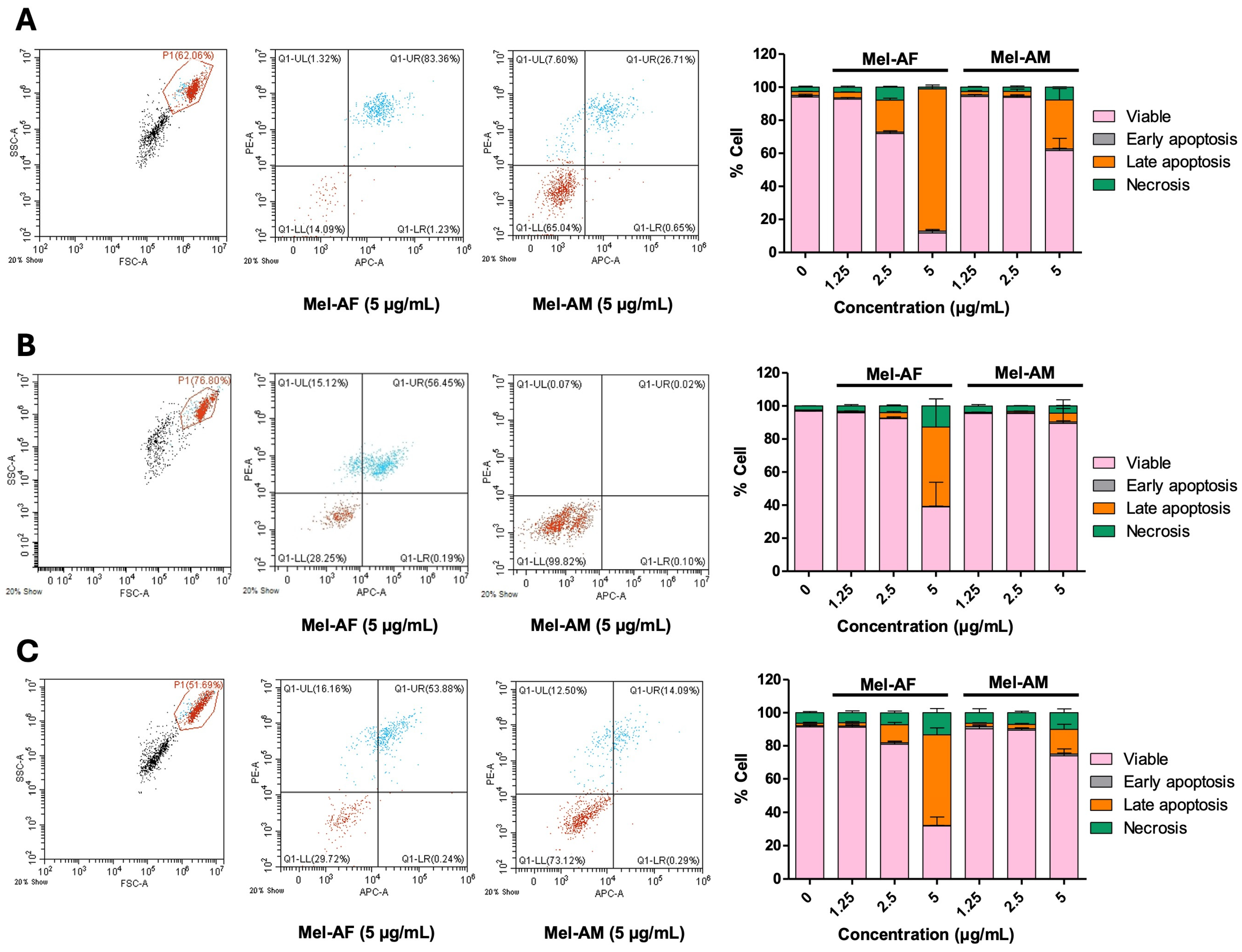
2.2. Apoptosis Induction by Melittin Peptides
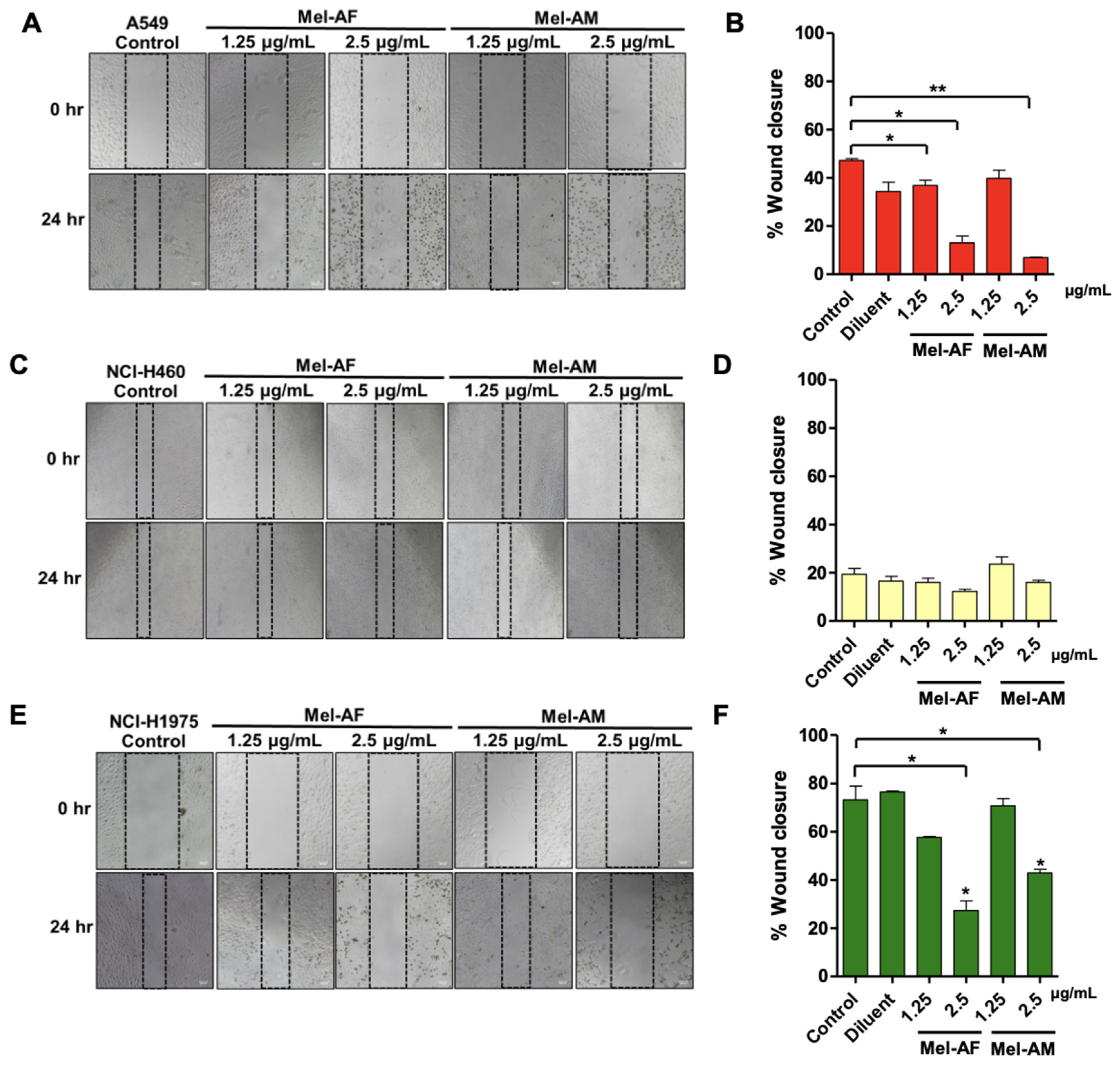
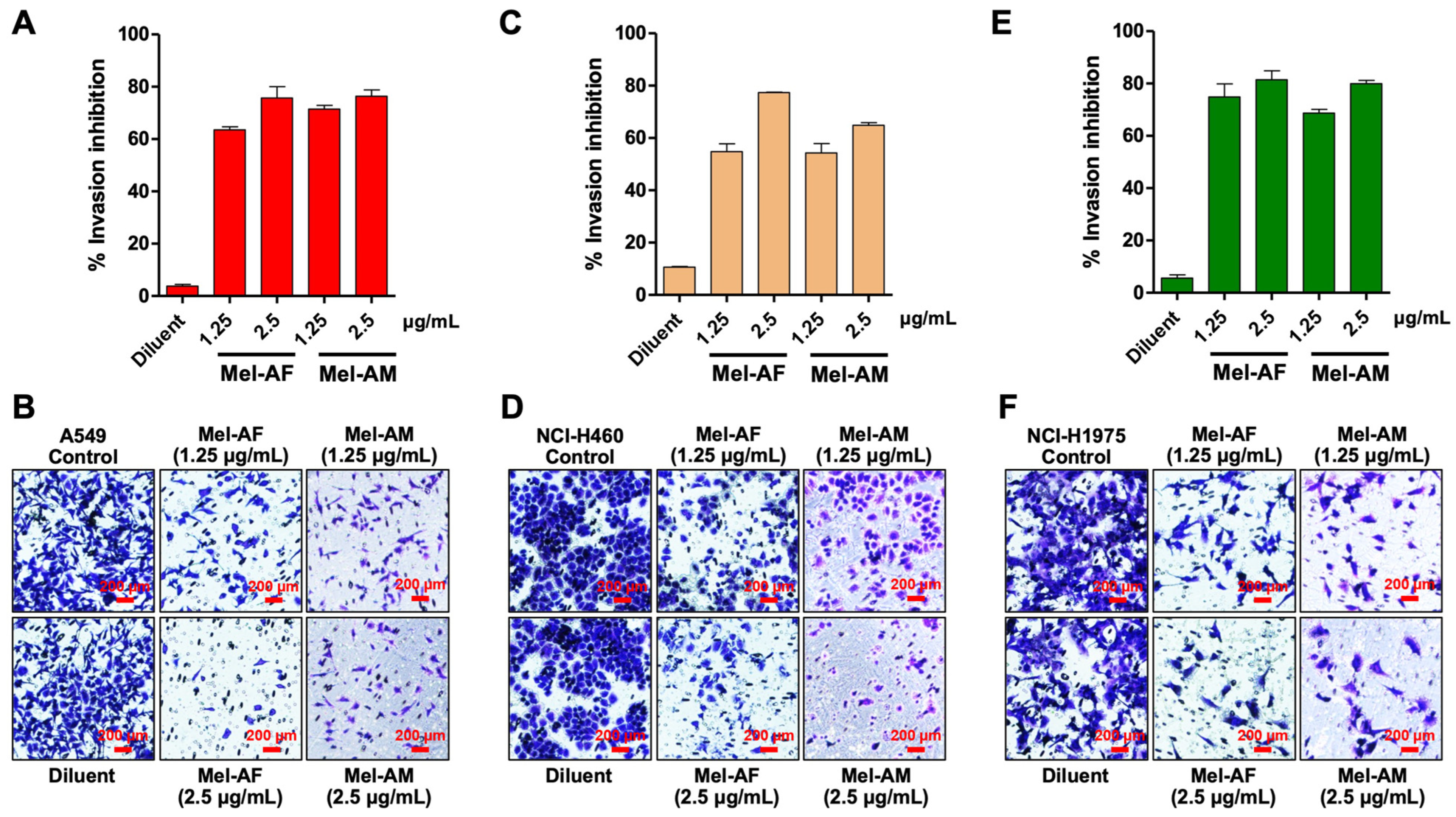
2.3. Effect of Melittin Peptides on Cell Migration and Invasion Abilities
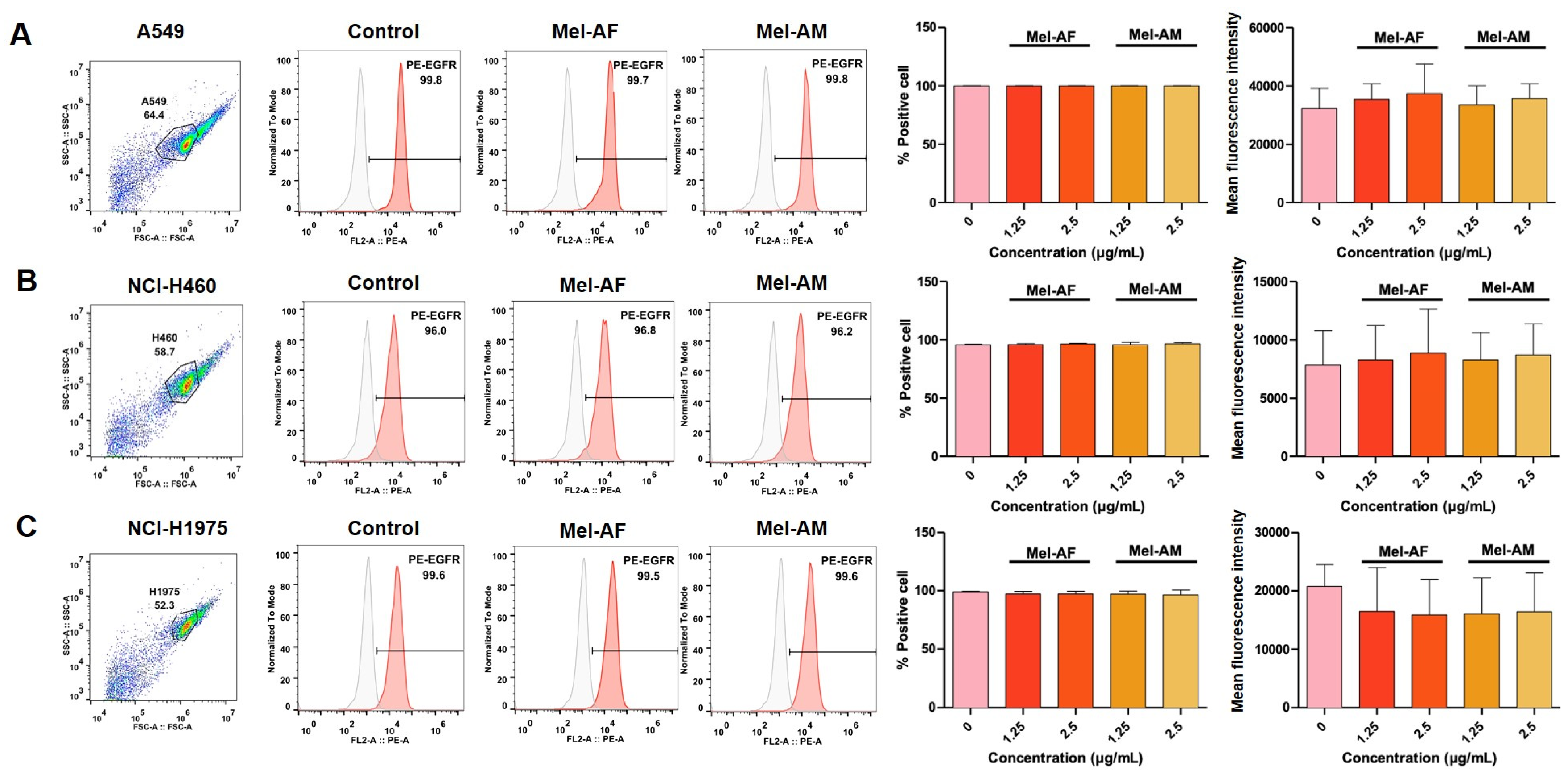
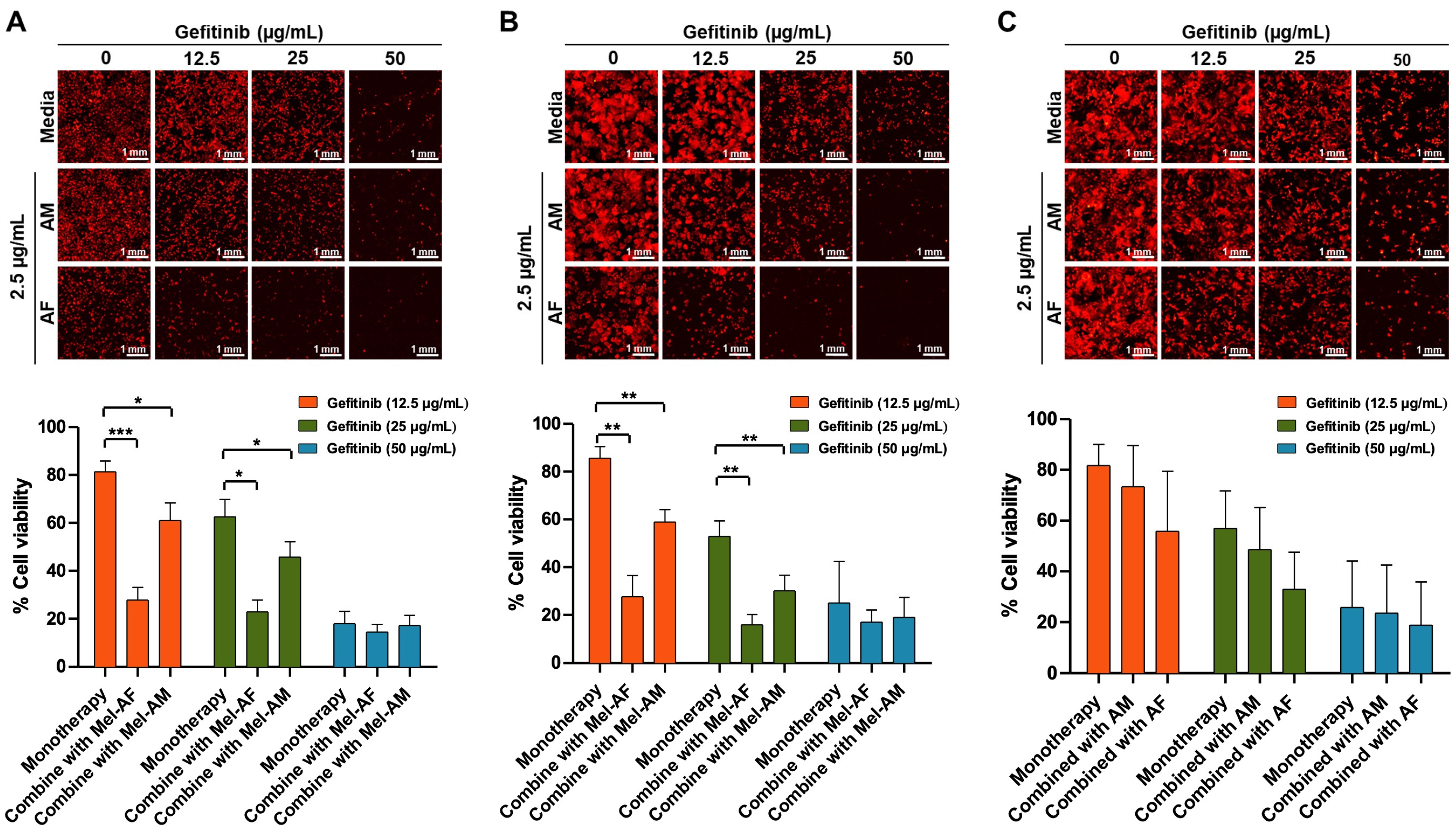
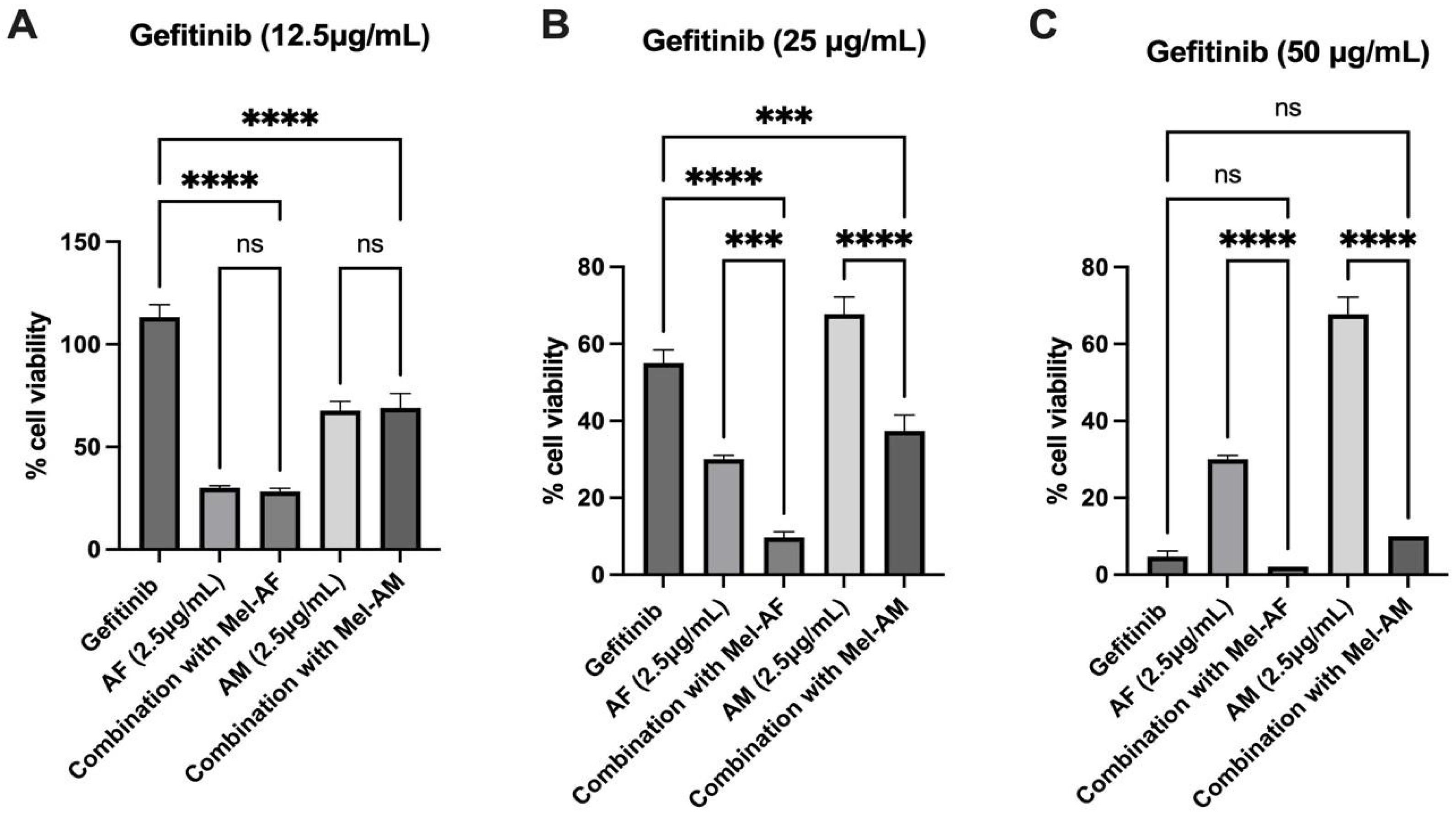
2.4. Expression of EGFR in Melittin-Treated Cells and Combination Assay of Melittin Peptides with Gefitinib
3. Discussion
4. Materials and Methods
4.1. Synthetic Melittin Peptides and Cell Cultures
4.2. Cell Viability Assay
4.3. Flow Cytometry
4.4. Western Blot Analysis
4.5. Scratch Wound and Transwell Invasion Assays
4.6. Combination Assay of Melittin Peptides with Gefitinib
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reungwetwattana, T.; Oranratnachai, S.; Puataweepong, P.; Tangsujaritvijit, V.; Cherntanomwong, P. Lung Cancer in Thailand. J. Thorac. Oncol. 2020, 15, 1714–1721. [Google Scholar] [CrossRef]
- Jarernwong, K.; Gheewala, S.H.; Sampattagul, S. Health Impact Related to Ambient Particulate Matter Exposure as a Spatial Health Risk Map Case Study in Chiang Mai, Thailand. Atmosphere 2023, 14, 261. [Google Scholar] [CrossRef]
- Amnuaylojaroen, T.; Kaewkanchanawong, P.; Panpeng, P. Distribution and Meteorological Control of PM2.5 and Its Effect on Visibility in Northern Thailand. Atmosphere 2023, 14, 538. [Google Scholar] [CrossRef]
- Basumallik, N.; Agarwal, M. Small Cell Lung Cancer. StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Hassan Lemjabbar-Alaouia, O.H.; Yanga, Y.-W.; Buchanana, P. Lung Cancer: Biology and Treatment Options. Physiol. Behav. 2016, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Praphawilai, P.; Kaewkod, T.; Suriyaprom, S.; Panya, A.; Disayathanoowat, T.; Tragoolpua, Y. Anti-Herpes Simplex Virus and Anti-Inflammatory Activities of the Melittin Peptides Derived from Apis Mellifera and Apis Florea Venom. Insects 2024, 15, 109. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Sun, C.; Xu, N.; Liu, W. The Current Landscape of the Antimicrobial Peptide Melittin and Its Therapeutic Potential. Front. Immunol. 2024, 15, 1326033. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Bae, H. Anti-Inflammatory Applications of Melittin, a Major Component of Bee Venom: Detailed Mechanism of Action and Adverse Effects. Molecules 2016, 21, 616. [Google Scholar] [CrossRef]
- Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Gu, H.; Jeon, M.; Kim, M.K.; Han, S.M.; Park, K.K. Anti-Inflammatory Effect of Melittin on Porphyromonas Gingivalis LPS-Stimulated Human Keratinocytes. Molecules 2018, 23, 332. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.F. Evaluation of the Association of Chronic Inflammation and Cancer: Insights and Implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef]
- Zhang, S.-F.; Chen, Z. Melittin Exerts an Antitumor Effect on Non-Small Celllung Cancer Cells. Mol. Med. Rep. 2017, 16, 3581–3586. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, J.; Bai, L.; Li, F.; Dong, Y.; Li, Q. Melittin Induces NSCLC Apoptosis via Inhibition of MiR-183. OncoTargets Ther. 2018, 11, 4511–4523. [Google Scholar] [CrossRef]
- Choi, K.E.; Hwang, C.J.; Gu, S.M.; Park, M.H.; Kim, J.H.; Park, J.H.; Ahn, Y.J.; Kim, J.Y.; Song, M.J.; Song, H.S.; et al. Cancer Cell Growth Inhibitory Effect of Bee Venom via Increase of Death Receptor 3 Expression and Inactivation of NF-Kappa B in NSCLC Cells. Toxins 2014, 6, 2210–2228. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Y.; He, Q.; Yang, X. Exploration of the Use of Natural Compounds in Combination with Chemotherapy Drugs for Tumor Treatment. Molecules 2023, 28, 1022. [Google Scholar] [CrossRef]
- Mansour, G.H.; El-Magd, M.A.; Mahfouz, D.H.; Abdelhamid, I.A.; Mohamed, M.F.; Ibrahim, N.S.; Wahab, A.H.A.A.; Elzayat, E.M. Bee Venom and Its Active Component Melittin Synergistically Potentiate the Anticancer Effect of Sorafenib against HepG2 Cells. Bioorg. Chem. 2021, 116, 105329. [Google Scholar] [CrossRef] [PubMed]
- Tabasum, S.; Singh, R.P. Fisetin Suppresses Migration, Invasion and Stem-Cell-like Phenotype of Human Non-Small Cell Lung Carcinoma Cells via Attenuation of Epithelial to Mesenchymal Transition. Chem. Biol. Interact. 2019, 303, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Siena, L.; Pace, E.; Ferraro, M.; Di Sano, C.; Melis, M.; Profita, M.; Spatafora, M.; Gjomarkaj, M. Gemcitabine Sensitizes Lung Cancer Cells to Fas/FasL System-Mediated Killing. Immunology 2014, 141, 242–255. [Google Scholar] [CrossRef]
- Sasaki, T.; Hiroki, K.; Yamashita, Y. The Role of Epidermal Growth Factor Receptor in Cancer Metastasis and Microenvironment. Biomed. Res. Int. 2013, 2013, 546318. [Google Scholar] [CrossRef]
- Kazandjian, D.; Blumenthal, G.M.; Yuan, W.; He, K.; Keegan, P.; Pazdur, R. FDA Approval of Gefitinib for the Treatment of Patients with Metastatic EGFR Mutation-Positive Non-Small Cell Lung Cancer. Clin. Cancer Res. 2016, 22, 1307–1312. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Yu, R.; Wang, M.; Wang, M.; Han, L. Melittin Suppresses Growth and Induces Apoptosis of Non-Small-Cell Lung Cancer Cells via down-Regulation of Tgf-β-Mediated Erk Signal Pathway. Braz. J. Med. Biol. Res. 2021, 54, e9017. [Google Scholar] [CrossRef]
- Sangboonruang, S.; Kitidee, K.; Chantawannakul, P. Melittin from Apis Florea Venom as a Promising Therapeutic Agent for Skin Cancer Treatment. Antibiotics 2020, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Liu, D.; Yang, Y. Anti-Cancer Peptides: Classification, Mechanism of Action, Reconstruction and Modification. Open Biol. 2020, 10, 200004. [Google Scholar] [CrossRef]
- Huang, Y.B.; Wang, X.F.; Wang, H.Y.; Liu, Y.; Chen, Y. Studies on Mechanism of Action of Anticancer Peptides by Modulation of Hydrophobicity within a Defined Structural Framework. Mol. Cancer Ther. 2011, 10, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.B.; He, L.Y.; Jiang, H.Y.; Chen, Y.X. Role of Helicity on the Anticancer Mechanism of Action of Cationic-Helical Peptides. Int. J. Mol. Sci. 2012, 13, 6849–6862. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Dong, J.; Wang, Y.; Zhang, J.; Zhang, M.; Wu, Z.; Lv, Y.; Wu, R. MEL-Pep, an Analog of Melittin, Disrupts Cell Membranes and Reverses 5-Fluorouracil Resistance in Human Hepatocellular Carcinoma Cells. Int. J. Biochem. Cell Biol. 2018, 101, 39–48. [Google Scholar] [CrossRef]
- Shin, M.K.; Jang, B.Y.; Bu, K.B.; Lee, S.H.; Han, D.H.; Oh, J.W.; Sung, J.S. De Novo Design of AC-P19M, a Novel Anticancer Peptide with Apoptotic Effects on Lung Cancer Cells and Anti-Angiogenic Activity. Int. J. Mol. Sci. 2022, 23, 15594. [Google Scholar] [CrossRef]
- Duffy, C.; Sorolla, A.; Wang, E.; Golden, E.; Woodward, E.; Davern, K.; Ho, D.; Johnstone, E.; Pfleger, K.; Redfern, A.; et al. Honeybee Venom and Melittin Suppress Growth Factor Receptor Activation in HER2-Enriched and Triple-Negative Breast Cancer. NPJ Precis. Oncol. 2020, 4, 24. [Google Scholar] [CrossRef]
- Shin, J.M.; Jeong, Y.J.; Cho, H.J.; Park, K.K.; Chung, I.K.; Lee, I.K.; Kwak, J.Y.; Chang, H.W.; Kim, C.H.; Moon, S.K.; et al. Melittin Suppresses HIF-1α/VEGF Expression through Inhibition of ERK and MTOR/P70S6K Pathway in Human Cervical Carcinoma Cells. PLoS ONE 2013, 8, e69380. [Google Scholar] [CrossRef]
- Xie, B.; Sun, L.; Cheng, Y.; Zhou, J.; Zheng, J.; Zhang, W. Epidermal Growth Factor Receptor Gene Mutations in Non-Small-Cell Lung Cancer Cells Are Associated with Increased Radiosensitivity in Vitro. Cancer Manag. Res. 2018, 10, 3551–3560, Erratum in Cancer Manag. Res. 2018, 10, 3983. [Google Scholar] [CrossRef]
- Talib, W.H.; Awajan, D.; Hamed, R.A.; Azzam, A.O.; Mahmod, A.I.; AL-Yasari, I.H. Combination Anticancer Therapies Using Selected Phytochemicals. Molecules 2022, 27, 5452. [Google Scholar] [CrossRef]
- Costanzo, R.; Piccirillo, M.C.; Sandomenico, C.; Carillio, G.; Montanino, A.; Daniele, G.; Giordano, P.; Bryce, J.; De Feo, G.; Di Maio, M.; et al. Gefitinib in Non Small Cell Lung Cancer. J. Biomed. Biotechnol. 2011, 2011, 815269. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Lee, V.H.F.; Yan, H. Prediction of Sensitivity to Gefitinib/Erlotinib for EGFR Mutations in NSCLC Based on Structural Interaction Fingerprints and Multilinear Principal Component Analysis. BMC Bioinform. 2018, 19, 88. [Google Scholar] [CrossRef] [PubMed]
- Ranson, M.; Hammond, L.A.; Ferry, D.; Kris, M.; Tullo, A.; Murray, P.I.; Miller, V.; Averbuch, S.; Ochs, J.; Morris, C.; et al. ZD1839, a Selective Oral Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor, Is Well Tolerated and Active in Patients with Solid, Malignant Tumors: Results of a Phase I Trial. J. Clin. Oncol. 2002, 20, 2240–2250. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Arcila, M.E.; Sima, C.S.; Riely, G.J.; Chmielecki, J.; Kris, M.G.; Pao, W.; Ladanyi, M.; Miller, V.A. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: Distinct natural history of patients with tumors harboring the T790M mutation. Clin. Cancer Res. 2011, 17, 1616–1622. [Google Scholar] [CrossRef]
- Yun, C.H.; Mengwasser, K.E.; Toms, A.V.; Woo, M.S.; Greulich, H.; Wong, K.K.; Meyerson, M.; Eck, M.J. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl. Acad. Sci. USA 2008, 105, 2070–2075. [Google Scholar] [CrossRef]
- Kobayashi, S.; Boggon, T.J.; Dayaram, T.; Jänne, P.A.; Kocher, O.; Meyerson, M.; Johnson, B.E.; Eck, M.J.; Tenen, D.G.; Halmos, B. EGFR Mutation and Resistance of Non–Small-Cell Lung Cancer to Gefitinib. N. Engl. J. Med. 2005, 352, 786–792. [Google Scholar] [CrossRef]
- Walter, A.O.; Sjin, R.T.T.; Haringsma, H.J.; Sun, J.; Ohashi, K.; Lee, K.; Dubrovskiy, A.; Labenski, M.; Wang, Z.; Zhu, Z.; et al. Discovery of a Mutant-Selective Covalent Inhibitor of EGFR That Overcomes T790M Mediated Resistance in NSCLC. Cancer Discov. 2013, 3, 1404–1415. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Mei, Q.; Chen, Y.; Yu, S.; Xia, S. Combined Inhibition of the EGFR and MTOR Pathways in EGFR Wild-Type Non-Small Cell Lung Cancer Cell Lines with Different Genetic Backgrounds. Oncol. Rep. 2013, 29, 2486–2492. [Google Scholar] [CrossRef]
- Kamal, S.F.; Ali, M.E. Melittin Enhances the Cytotoxic Effect of the Targeted Anticancer Monoclonal Antibodies on Cancer Cell Lines. 2024. Available online: https://sciety.org/articles/activity/10.21203/rs.3.rs-4837782/v1 (accessed on 5 December 2024).
- Mir Hassani, Z.; Nabiuni, M.; Parivar, K.; Abdirad, S.; Karimzadeh, L. Melittin Inhibits the Expression of Key Genes Involved in Tumor Microenvironment Formation by Suppressing HIF-1α Signaling in Breast Cancer Cells. Med. Oncol. 2021, 38, 77. [Google Scholar] [CrossRef]
- Honari, P.; Shahbazzadeh, D.; Behdani, M.; Pooshang Bagheri, K. Highly in Vitro Anti-Cancer Activity of Melittin-Loaded Niosomes on Non-Small Cell Lung Cancer Cells. Toxicon 2024, 241, 107673. [Google Scholar] [CrossRef]
- Badivi, S.; Kazemi, S.; Eskandarisani, M.; Moghaddam, N.A.; Mesbahian, G.; Karimifard, S.; Afzali, E. Targeted Delivery of Bee Venom to A549 Lung Cancer Cells by PEGylate Liposomal Formulation: An Apoptotic Investigation. Sci. Rep. 2024, 14, 17302. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-C.; Liu, L.; Yu, T.Y.; Tang, L.; Yin, M.L.; Zhu, W.H.; Jiang, X.Y.; Wang, H.Y. High-Level Expression and Purification of Melittin in Escherichia Coli Using SUMO Fusion Partner. Int. J. Pept. Res. Ther. 2021, 27, 9–15. [Google Scholar] [CrossRef]
- Buhrman, J.S.; Cook, L.C.; Rayahin, J.E.; Federle, M.J.; Gemeinhart, R.A. Active, Soluble Recombinant Melittin Purified by Extracting Insoluble Lysate of Escherichia coli without Denaturation. Biotechnol. Prog. 2013, 29, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.-M. Anti-Proliferative Activity of Recombinant Melittin Expressed in Escherichia Coli toward U937 Cells. Afr. J. Biotechnol. 2012, 11, 3026–3030. [Google Scholar] [CrossRef]
- Lee, J.; Yun, H.H.; Kim, S.; Ji, S.H.; Kuh, H.J.; Lee, J.H. Implication of BIS in the Migration and Invasion of A549 Non-Small Cell Lung Cancer Cells. Anticancer Res. 2018, 38, 4525–4533. [Google Scholar] [CrossRef]
- Oue, T.; Uehara, S.; Yamanaka, H.; Nomura, M.; Usui, N. Hedgehog Signal Inhibitors Suppress the Invasion of Human Rhabdomyosarcoma Cells. Pediatr. Surg. Int. 2013, 29, 1153–1158. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sattayawat, P.; Kaewkod, T.; Thongyim, S.; Chiawpanit, C.; Wutti-in, Y.; Thepmalee, C.; Tragoolpua, Y.; Disayathanoowat, T.; Panya, A. A Comparative Study of Melittins from Apis florea and Apis mellifera as Cytotoxic Agents Against Non-Small Cell Lung Cancer (NSCLC) Cells and Their Combination with Gefitinib. Int. J. Mol. Sci. 2025, 26, 2498. https://doi.org/10.3390/ijms26062498
Sattayawat P, Kaewkod T, Thongyim S, Chiawpanit C, Wutti-in Y, Thepmalee C, Tragoolpua Y, Disayathanoowat T, Panya A. A Comparative Study of Melittins from Apis florea and Apis mellifera as Cytotoxic Agents Against Non-Small Cell Lung Cancer (NSCLC) Cells and Their Combination with Gefitinib. International Journal of Molecular Sciences. 2025; 26(6):2498. https://doi.org/10.3390/ijms26062498
Chicago/Turabian StyleSattayawat, Pachara, Thida Kaewkod, Saruda Thongyim, Chutipa Chiawpanit, Yupanun Wutti-in, Chutamas Thepmalee, Yingmanee Tragoolpua, Terd Disayathanoowat, and Aussara Panya. 2025. "A Comparative Study of Melittins from Apis florea and Apis mellifera as Cytotoxic Agents Against Non-Small Cell Lung Cancer (NSCLC) Cells and Their Combination with Gefitinib" International Journal of Molecular Sciences 26, no. 6: 2498. https://doi.org/10.3390/ijms26062498
APA StyleSattayawat, P., Kaewkod, T., Thongyim, S., Chiawpanit, C., Wutti-in, Y., Thepmalee, C., Tragoolpua, Y., Disayathanoowat, T., & Panya, A. (2025). A Comparative Study of Melittins from Apis florea and Apis mellifera as Cytotoxic Agents Against Non-Small Cell Lung Cancer (NSCLC) Cells and Their Combination with Gefitinib. International Journal of Molecular Sciences, 26(6), 2498. https://doi.org/10.3390/ijms26062498





