Enhancing the Therapeutic Potential of Human Umbilical Cord Mesenchymal Stem Cells for Osteoarthritis: The Role of Platelet-Rich Plasma and Extracellular Vesicles
Abstract
1. Introduction
2. Results
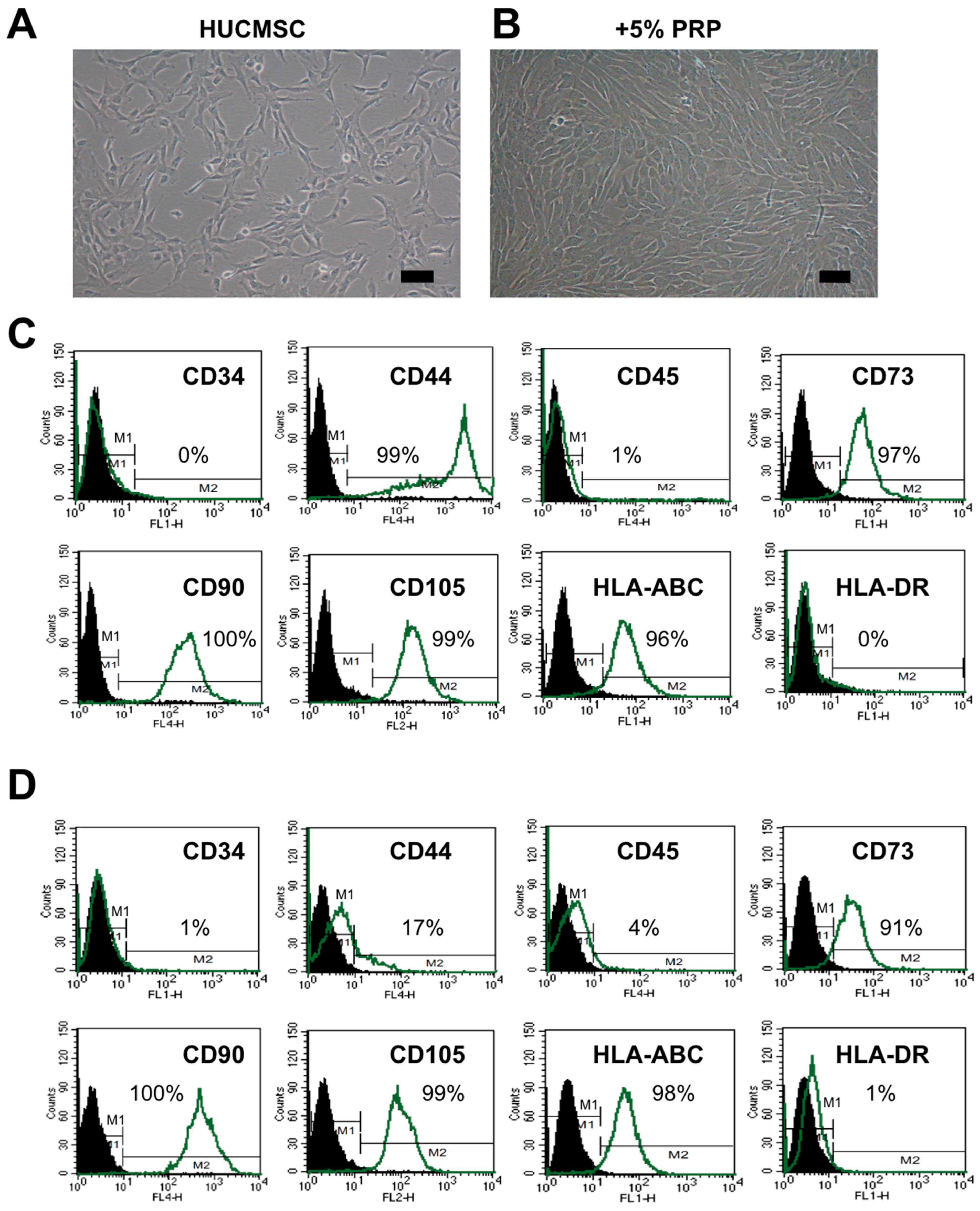
2.1. HUCMSCs Present Typical MSC Characteristics After PRP Treatment
2.2. PRP-Treated HUCMSCs Could Differentiate into Adipocytes, Osteoblasts, and Chondrocytes
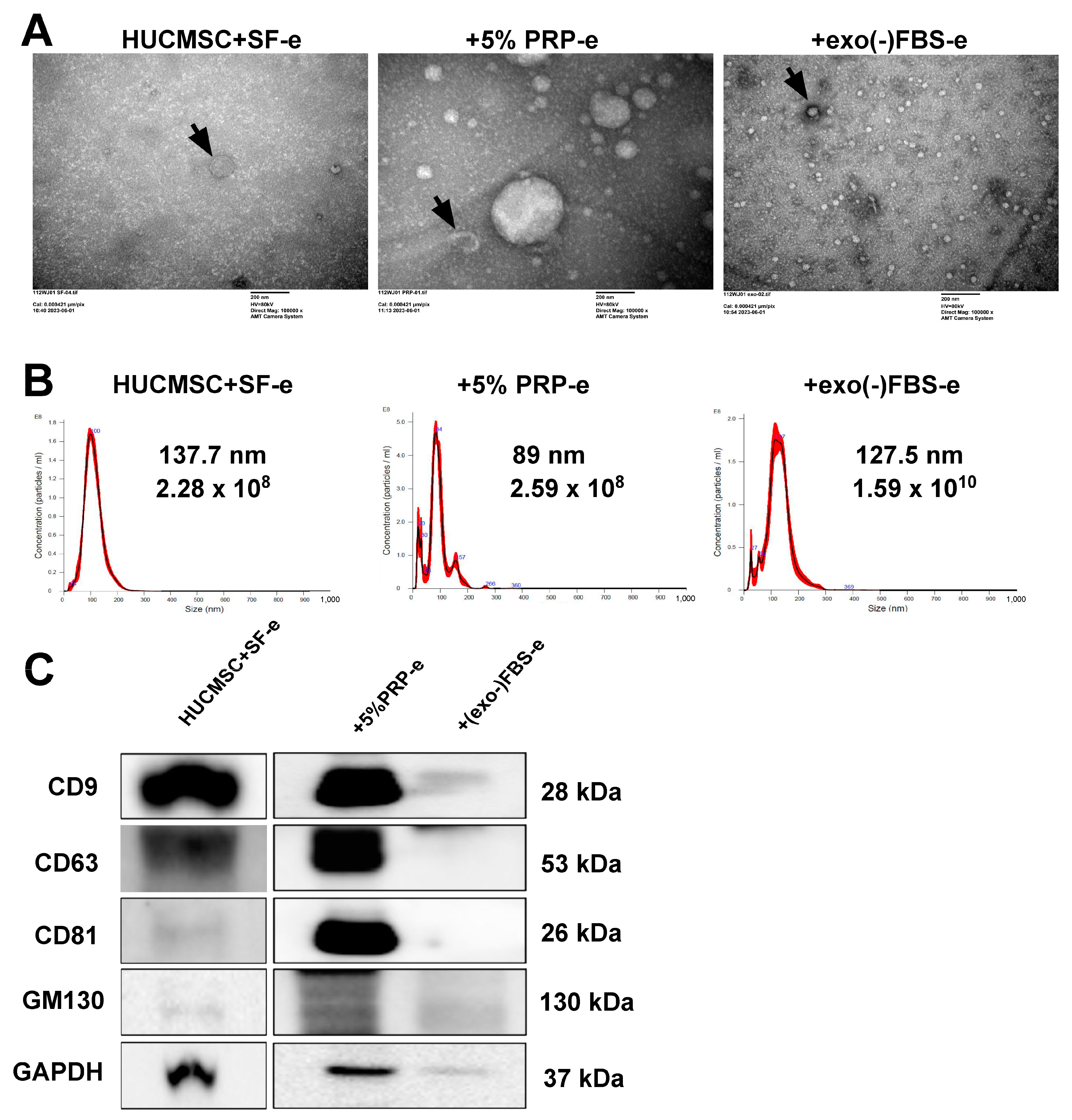
2.3. Characterization of EVs Secreted by HUCMSCs
2.4. EVs Secreted from PRP-Treated HUCMSCs Could Attenuate the OA
2.5. Improvement in the Histology of Cartilage Recovery After EV Transplant
2.6. Improvement in International Cartilage Repair Society Histological Score After EV Transplant
2.7. Transplantation of EVs Increased Type II Collagen and Aggrecan Expressions
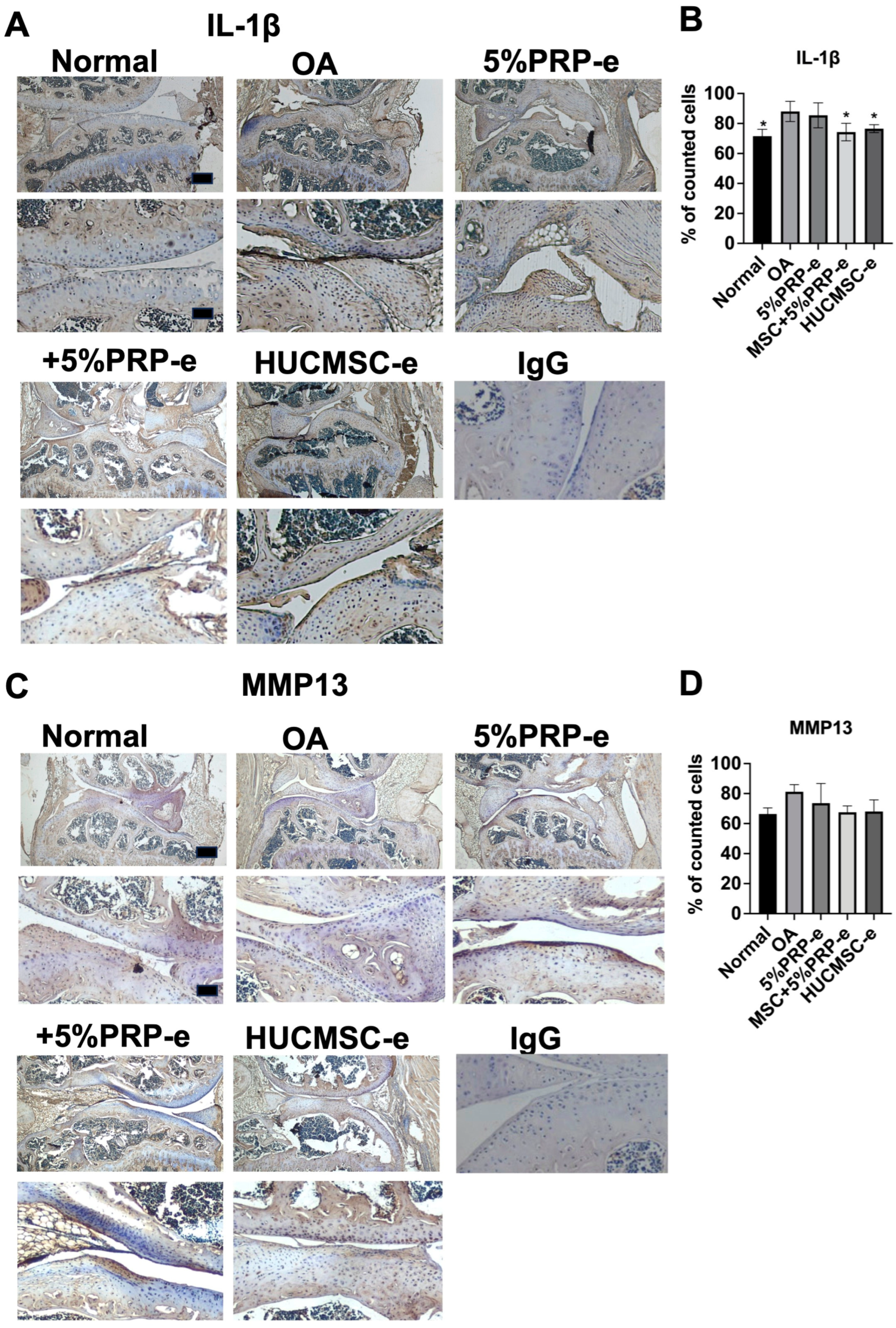
2.8. EV Transplant Decreased IL-1beta Expressions
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Ethics
4.2. HUCMSC Culture and Identification of the MSC Characteristics
4.3. Platelet-Rich Plasma Preparation
4.4. Flow Cytometry
4.5. HUCMSC Trilineage Differentiation
4.6. Adipocyte Differentiation
4.7. Osteocyte Differentiation
4.8. Chondrocyte Differentiation
4.9. qRT-PCR
4.10. EV Isolation and Identification
4.11. Characterization of EVs Using Nanoparticle Tracking Analysis
4.12. Western Blot Analysis
4.13. Transmission Electron Microscopy
4.14. Collagenase-Induced Osteoarthritis Model
4.15. Extracellular Vesicle Injection
4.16. Function Assessments
4.17. Macroscopic Examination
4.18. Histological Evaluation
4.19. Immunohistochemical Staining
4.20. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Nicola, V. Degenerative Osteoarthritis a Reversible Chronic Disease. Regen. Ther. 2020, 15, 149–160. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of Osteoarthritis: Risk Factors, Regulatory Pathways in Chondrocytes, and Experimental Models. Biology 2020, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.J.; Driban, J.B.; McAlindon, T.E. Pharmaceutical Treatment of Osteoarthritis. Osteoarthr. Cartil. 2023, 31, 458–466. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Xu, X.; Liu, J. Intra-Articular Injections of Platelet-Rich Plasma, Hyaluronic Acid or Corticosteroids for Knee Osteoarthritis: A Prospective Randomized Controlled Study. Orthopade 2019, 48, 239–247. [Google Scholar] [CrossRef]
- Altman, R.; Lim, S.; Steen, R.G.; Dasa, V. Hyaluronic Acid Injections Are Associated with Delay of Total Knee Replacement Surgery in Patients with Knee Osteoarthritis: Evidence from a Large US Health Claims Database. PLoS ONE 2015, 10, e0145776. [Google Scholar] [CrossRef]
- Berkani, S.; Courties, A.; Eymard, F.; Latourte, A.; Richette, P.; Berenbaum, F.; Sellam, J.; Louati, K. Time to Total Knee Arthroplasty after Intra-Articular Hyaluronic Acid or Platelet-Rich Plasma Injections: A Systematic Literature Review and Meta-Analysis. J. Clin. Med. 2022, 11, 3985. [Google Scholar] [CrossRef]
- Ding, D.-C.; Shyu, W.-C.; Lin, S.-Z. Mesenchymal Stem Cells. Cell Transplant 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Novel Frontiers in Regenerative Medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Fong, C.Y.; Richards, M.; Manasi, N.; Biswas, A.; Bongso, A. Comparative Growth Behaviour and Characterization of Stem Cells from Human Wharton’s Jelly. Reprod. Biomed. Online 2007, 15, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-C.; Chang, Y.-H.; Shyu, W.-C.; Lin, S.-Z. Human Umbilical Cord Mesenchymal Stem Cells: A New Era for Stem Cell Therapy. Cell Transplant. 2015, 24, 339–347. [Google Scholar] [CrossRef]
- Fong, C.-Y.; Chak, L.-L.; Biswas, A.; Tan, J.-H.; Gauthaman, K.; Chan, W.-K.; Bongso, A. Human Wharton’s Jelly Stem Cells Have Unique Transcriptome Profiles Compared to Human Embryonic Stem Cells and Other Mesenchymal Stem Cells. Stem Cell Rev. 2011, 7, 1–16. [Google Scholar] [CrossRef]
- Ip, H.L.; Nath, D.K.; Sawleh, S.H.; Kabir, M.H.; Jahan, N. Regenerative Medicine for Knee Osteoarthritis—The Efficacy and Safety of Intra-Articular Platelet-Rich Plasma and Mesenchymal Stem Cells Injections: A Literature Review. Cureus 2020, 12, e10575. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Y.; Zheng, F.; Ma, N.; Qin, R.; Qin, W.; Liu, B.; Qin, A. Activated Human Umbilical Cord Blood Platelet-Rich Plasma Enhances the Beneficial Effects of Human Umbilical Cord Mesenchymal Stem Cells in Chemotherapy-Induced POF Rats. Stem Cells Int. 2021, 2021, 8293699. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Karpiński, R.; Maciejewski, R.; Jonak, J.; Jurkiewicz, A. Short-Term Effects of Arthroscopic Microfracturation of Knee Chondral Defects in Osteoarthritis. Appl. Sci. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Boffa, A.; Previtali, D.; Altamura, S.A.; Zaffagnini, S.; Candrian, C.; Filardo, G. Platelet-Rich Plasma Augmentation to Microfracture Provides a Limited Benefit for the Treatment of Cartilage Lesions: A Meta-Analysis. Orthop. J. Sports Med. 2020, 8, 2325967120910504. [Google Scholar] [CrossRef]
- Dellar, E.R.; Hill, C.; Melling, G.E.; Carter, D.R.F.; Baena-Lopez, L.A. Unpacking Extracellular Vesicles: RNA Cargo Loading and Function. J. Extracell. Biol. 2022, 1, e40. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Wu, K.-C.; Harn, H.-J.; Lin, S.-Z.; Ding, D.-C. Exosomes and Stem Cells in Degenerative Disease Diagnosis and Therapy. Cell Transplant. 2018, 27, 349–363. [Google Scholar] [CrossRef]
- Amsar, R.M.; Wijaya, C.H.; Ana, I.D.; Hidajah, A.C.; Notobroto, H.B.; Kencana Wungu, T.D.; Barlian, A. Extracellular Vesicles: A Promising Cell-Free Therapy for Cartilage Repair. Future Sci. OA 2022, 8, FSO774. [Google Scholar] [CrossRef]
- Huang, L.; Dong, G.; Peng, J.; Li, T.; Zou, M.; Hu, K.; Shu, Y.; Cheng, T.; Hao, L. The Role of Exosomes and Their Enhancement Strategies in the Treatment of Osteoarthritis. Hum. Cell 2023, 36, 1887–1900. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liang, G.; Han, Y.; Yang, W.; Xu, N.; Luo, M.; Pan, J.; Liu, J.; Zeng, L.-F. Combination of Mesenchymal Stem Cells (MSCs) and Platelet-Rich Plasma (PRP) in the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomised Controlled Trials. BMJ Open 2022, 12, e061008. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Fraser, A.; Fearon, U.; Billinghurst, R.C.; Ionescu, M.; Reece, R.; Barwick, T.; Emery, P.; Poole, A.R.; Veale, D.J. Turnover of Type II Collagen and Aggrecan in Cartilage Matrix at the Onset of Inflammatory Arthritis in Humans: Relationship to Mediators of Systemic and Local Inflammation. Arthritis Rheum. 2003, 48, 3085–3095. [Google Scholar] [CrossRef]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Watson, N.; Divers, R.; Kedar, R.; Mehindru, A.; Mehindru, A.; Borlongan, M.C.; Borlongan, C.V. Discarded Wharton Jelly of the Human Umbilical Cord: A Viable Source for Mesenchymal Stromal Cells. Cytotherapy 2015, 17, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-C.; Chang, Y.-H.; Ding, D.-C.; Lin, S.-Z. Mesenchymal Stromal Cells for Aging Cartilage Regeneration: A Review. Int. J. Mol. Sci. 2024, 25, 12911. [Google Scholar] [CrossRef]
- Ding, D.-C.; Shyu, W.-C.; Lin, S.-Z.; Liu, H.-W.; Chiou, S.-H.; Chu, T.-Y. Human Umbilical Cord Mesenchymal Stem Cells Support Nontumorigenic Expansion of Human Embryonic Stem Cells. Cell Transplant. 2012, 21, 1515–1527. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Wu, K.-C.; Ding, D.-C. Chondrogenic Potential of Human Umbilical Cord Mesenchymal Stem Cells Cultured with Exosome-Depleted Fetal Bovine Serum in an Osteoarthritis Mouse Model. Biomedicines 2022, 10, 2773. [Google Scholar] [CrossRef]
- Yu, Y.; Valderrama, A.V.; Han, Z.; Uzan, G.; Naserian, S.; Oberlin, E. Human Fetal Liver MSCs Are More Effective than Adult Bone Marrow MSCs for Their Immunosuppressive, Immunomodulatory, and Foxp3+ T Reg Induction Capacity. Stem Cell Res. Ther. 2021, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhu, H.; Li, S.; Wang, Z.; Xiao, J. Mesenchymal Stem Cell-Derived Exosomes as a Promising Cell-Free Therapy for Knee Osteoarthritis. Front. Bioeng. Biotechnol. 2024, 12, 1309946. [Google Scholar] [CrossRef]
- Li, K.; Yan, G.; Huang, H.; Zheng, M.; Ma, K.; Cui, X.; Lu, D.; Zheng, L.; Zhu, B.; Cheng, J.; et al. Anti-Inflammatory and Immunomodulatory Effects of the Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells on Osteoarthritis via M2 Macrophages. J. Nanobiotechnology 2022, 20, 38. [Google Scholar] [CrossRef]
- Liu, W.; Liu, A.; Li, X.; Sun, Z.; Sun, Z.; Liu, Y.; Wang, G.; Huang, D.; Xiong, H.; Yu, S.; et al. Dual-Engineered Cartilage-Targeting Extracellular Vesicles Derived from Mesenchymal Stem Cells Enhance Osteoarthritis Treatment via miR-223/NLRP3/pyroptosis Axis: Toward a Precision Therapy. Bioact. Mater. 2023, 30, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-C.; Yang, H.-I.; Chang, Y.-H.; Chiang, R.Y.-S.; Ding, D.-C. Extracellular Vesicles Derived from Human Umbilical Mesenchymal Stem Cells Transfected with miR-7704 Improved Damaged Cartilage and Reduced Matrix Metallopeptidase 13. Cells 2025, 14, 82. [Google Scholar] [CrossRef]
- Abbaszadeh, H.; Ghorbani, F.; Derakhshani, M.; Movassaghpour, A.; Yousefi, M. Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Novel Therapeutic Paradigm. J. Cell. Physiol. 2020, 235, 706–717. [Google Scholar] [CrossRef]
- Forteza-Genestra, M.A.; Antich-Rosselló, M.; Ráez-Meseguer, C.; Ramis-Munar, G.; Sangenís, A.T.; Calvo, J.; Gayà, A.; Monjo, M.; Ramis, J.M. Exploring the Potential of Extracellular Vesicles as Cell-Free Therapeutics for Osteoarthritis: In Vivo and Ex Vivo Comparative Studies. Bone Joint J. 2024, 106-B, 92. [Google Scholar] [CrossRef]
- Otahal, A.; Kramer, K.; Kuten-Pella, O.; Weiss, R.; Stotter, C.; Lacza, Z.; Weber, V.; Nehrer, S.; De Luna, A. Characterization and Chondroprotective Effects of Extracellular Vesicles from Plasma- and Serum-Based Autologous Blood-Derived Products for Osteoarthritis Therapy. Front. Bioeng. Biotechnol. 2020, 8, 584050. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Ni, J.; Witherel, C.E.; Yang, M.; Burdick, J.A.; Wen, C.; Wong, S.H.D. Harnessing Tissue-Derived Extracellular Vesicles for Osteoarthritis Theranostics. Theranostics 2022, 12, 207–231. [Google Scholar] [CrossRef]
- Lin, J.; Wang, L.; Lin, J.; Liu, Q. The Role of Extracellular Vesicles in the Pathogenesis, Diagnosis, and Treatment of Osteoarthritis. Molecules 2021, 26, 4987. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, S.; Shojaei, F.; Shojaeian, A.; Rezakhani, L.; Dehkordi, M.B. An Overview of Current Knowledge in Biological Functions and Potential Theragnostic Applications of Exosomes. Chem. Phys. Lipids 2020, 226, 104836. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Caponnetto, F.; Manini, I.; Skrap, M.; Palmai-Pallag, T.; Di Loreto, C.; Beltrami, A.P.; Cesselli, D.; Ferrari, E. Size-Dependent Cellular Uptake of Exosomes. Nanomedicine 2017, 13, 1011–1020. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Zhang, S.; Deng, Z.-B.; Grizzle, W.; Miller, D.; Zhang, H.-G. Exosomes Are Endogenous Nanoparticles That Can Deliver Biological Information between Cells. Adv. Drug Deliv. Rev. 2013, 65, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Tabak, S.; Schreiber-Avissar, S.; Beit-Yannai, E. Extracellular Vesicles Have Variable Dose-Dependent Effects on Cultured Draining Cells in the Eye. J. Cell. Mol. Med. 2018, 22, 1992–2000. [Google Scholar] [CrossRef]
- Yahata, S.; Hirose, M.; Ueno, T.; Nagumo, H.; Sakai-Kato, K. Effect of Sample Concentration on Nanoparticle Tracking Analysis of Small Extracellular Vesicles and Liposomes Mimicking the Physicochemical Properties of Exosomes. Chem. Pharm. Bull. 2021, 69, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Spasovski, D.; Spasovski, V.; Bascarevic, Z.; Stojiljkovic, M.; Andjelkovic, M.; Pavlovic, S. Seven-Year Longitudinal Study: Clinical Evaluation of Knee Osteoarthritic Patients Treated with Mesenchymal Stem Cells. J. Clin. Med. 2024, 13, 3861. [Google Scholar] [CrossRef]
- Delling, U.; Brehm, W.; Ludewig, E.; Winter, K.; Jülke, H. Longitudinal Evaluation of Effects of Intra-Articular Mesenchymal Stromal Cell Administration for the Treatment of Osteoarthritis in an Ovine Model. Cell Transplant. 2015, 24, 2391–2407. [Google Scholar] [CrossRef]
- Malemud, C.J. Prospects for Drug Discovery in Osteoarthritis. Future Drug Discov. 2019, 1, FDD13. [Google Scholar] [CrossRef]
- Pak, J.; Chang, J.-J.; Lee, J.H.; Lee, S.H. Safety Reporting on Implantation of Autologous Adipose Tissue-Derived Stem Cells with Platelet-Rich Plasma into Human Articular Joints. BMC Musculoskelet. Disord. 2013, 14, 337. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, S.; Fercher, D.; Barreto, G.; Frondelius, T.; Morgese, G.; Benetti, E.; Saarakkala, S.; Zenobi-Wong, M.; Finnilä, M.A. Structural Cartilage Changes and Pain in a Collagenase-Induced Osteoarthritis Rat Model. Osteoarthr. Cartil. 2022, 30, S281–S282. [Google Scholar] [CrossRef]
- Weber, P.; Bevc, K.; Fercher, D.; Kauppinen, S.; Zhang, S.; Asadikorayem, M.; Marin, L.B.; Zhang, T.; Frondelius, T.; Salzmann, G.; et al. The Collagenase-Induced Osteoarthritis (CIOA) Model: Where Mechanical Damage Meets Inflammation. Osteoarthr. Cartil. Open 2024, 6, 100539. [Google Scholar] [CrossRef]
- Yoshioka, N.K.; Young, G.M.; Khajuria, D.K.; Karuppagounder, V.; Pinamont, W.J.; Fanburg-Smith, J.C.; Abraham, T.; Elbarbary, R.A.; Kamal, F. Structural Changes in the Collagen Network of Joint Tissues in Late Stages of Murine OA. Sci. Rep. 2022, 12, 9159. [Google Scholar] [CrossRef]
- Bodic, B.; Boutet, M.A.; Boyer, C.; Metayer, B.; Vignes, C.; Lesoeur, J.; Veziers, J.; Daguin, V.; Haspot, F.; Maugars, Y.; et al. Development and Characterization of a Humanized Mouse Model of Osteoarthritis. Osteoarthritis Cartilage 2022, 30, 875–885. [Google Scholar] [CrossRef]
- Dou, H.; Wang, S.; Hu, J.; Song, J.; Zhang, C.; Wang, J.; Xiao, L. Osteoarthritis Models: From Animals to Tissue Engineering. J. Tissue Eng. 2023, 14, 20417314231172584. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-C.; Shyu, W.-C.; Chiang, M.-F.; Lin, S.-Z.; Chang, Y.-C.; Wang, H.-J.; Su, C.-Y.; Li, H. Enhancement of Neuroplasticity through Upregulation of beta1-Integrin in Human Umbilical Cord-Derived Stromal Cell Implanted Stroke Model. Neurobiol. Dis. 2007, 27, 339–353. [Google Scholar] [CrossRef]
- Araki, J.; Jona, M.; Eto, H.; Aoi, N.; Kato, H.; Suga, H.; Doi, K.; Yatomi, Y.; Yoshimura, K. Optimized Preparation Method of Platelet-Concentrated Plasma and Noncoagulating Platelet-Derived Factor Concentrates: Maximization of Platelet Concentration and Removal of Fibrinogen. Tissue Eng. Part C Methods 2012, 18, 176–185. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of Exosomes Secreted by Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells and Synovial Membrane-Derived Mesenchymal Stem Cells for the Treatment of Osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef]
- Pritzker, K.P.H.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.-P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis Cartilage Histopathology: Grading and Staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef]





| Gene | Forward | Reverse | Bp |
|---|---|---|---|
| FABP4 | ATGGGATGGAAAATCAACCA | GTGGAAGTGACGCCTTTCAT | 87 |
| PPARγ | CCAGAAAGCGATTCCTTCAC | TGCAACCACTGGATCTGTTC | 240 |
| RUNX2 | CGGAATGCCTCTGCTGTTAT | TTCCCGAGGTCCATCTACTG | 174 |
| ALPL | CCACGTCTTCACATTTGGTG | GCAGTGAAGGGCTTCTTGTC | 96 |
| COL2A1 | GAGAGGTCTTCCTGGCAAAG | AAGTCCCTGGAAGCCAGAT | 118 |
| ACAN | GAGATGGAGGGTGAGGTC | ACGCTGCCTCGGGCTTC | 443 |
| GAPDH | GGTCTCCTCTGACTTGAACA | GTGAGGGTCTCTCTCTTCCT | 221 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-H.; Wu, K.-C.; Ding, D.-C. Enhancing the Therapeutic Potential of Human Umbilical Cord Mesenchymal Stem Cells for Osteoarthritis: The Role of Platelet-Rich Plasma and Extracellular Vesicles. Int. J. Mol. Sci. 2025, 26, 3785. https://doi.org/10.3390/ijms26083785
Chang Y-H, Wu K-C, Ding D-C. Enhancing the Therapeutic Potential of Human Umbilical Cord Mesenchymal Stem Cells for Osteoarthritis: The Role of Platelet-Rich Plasma and Extracellular Vesicles. International Journal of Molecular Sciences. 2025; 26(8):3785. https://doi.org/10.3390/ijms26083785
Chicago/Turabian StyleChang, Yu-Hsun, Kun-Chi Wu, and Dah-Ching Ding. 2025. "Enhancing the Therapeutic Potential of Human Umbilical Cord Mesenchymal Stem Cells for Osteoarthritis: The Role of Platelet-Rich Plasma and Extracellular Vesicles" International Journal of Molecular Sciences 26, no. 8: 3785. https://doi.org/10.3390/ijms26083785
APA StyleChang, Y.-H., Wu, K.-C., & Ding, D.-C. (2025). Enhancing the Therapeutic Potential of Human Umbilical Cord Mesenchymal Stem Cells for Osteoarthritis: The Role of Platelet-Rich Plasma and Extracellular Vesicles. International Journal of Molecular Sciences, 26(8), 3785. https://doi.org/10.3390/ijms26083785





