Flavonoids from Scutellaria baicalensis: Promising Alternatives for Enhancing Swine Production and Health
Abstract
1. Introduction
2. Methods
2.1. Predicted Potential Targets of SFs
2.2. Predicted Potential Targets of SFs in Inflammation and Oxidative Stress
2.3. Construction of Drug–Ingredients–Disease–Targets–KEGG Pathway Network
3. Results and Discussion
3.1. Active Ingredients and Target Genes of SFs
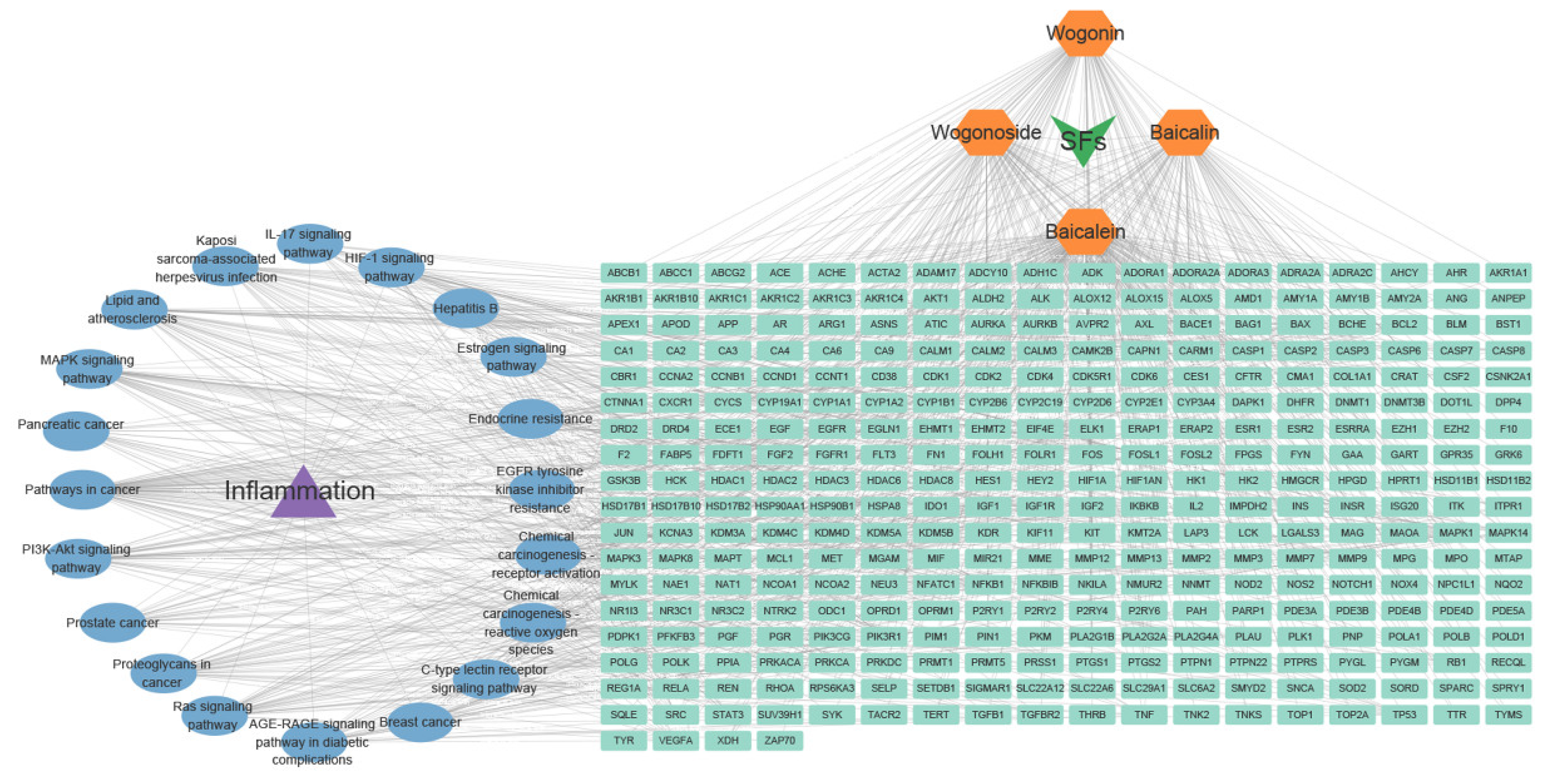
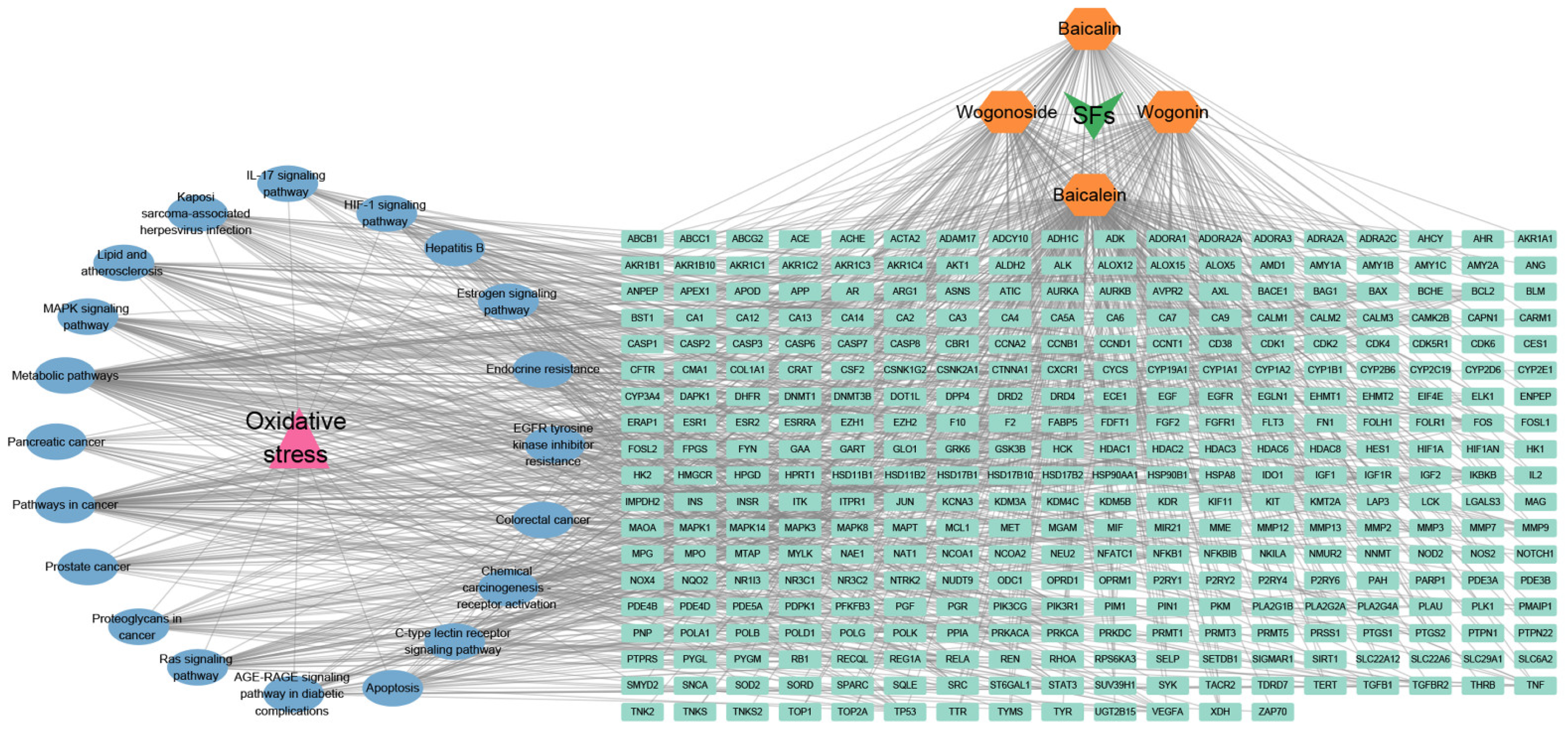
3.2. Pharmacological Effects and Mechanisms of SFs
3.2.1. Anti-Inflammatory Effects
3.2.2. Antioxidant Effects
3.2.3. Antibacterial Effects
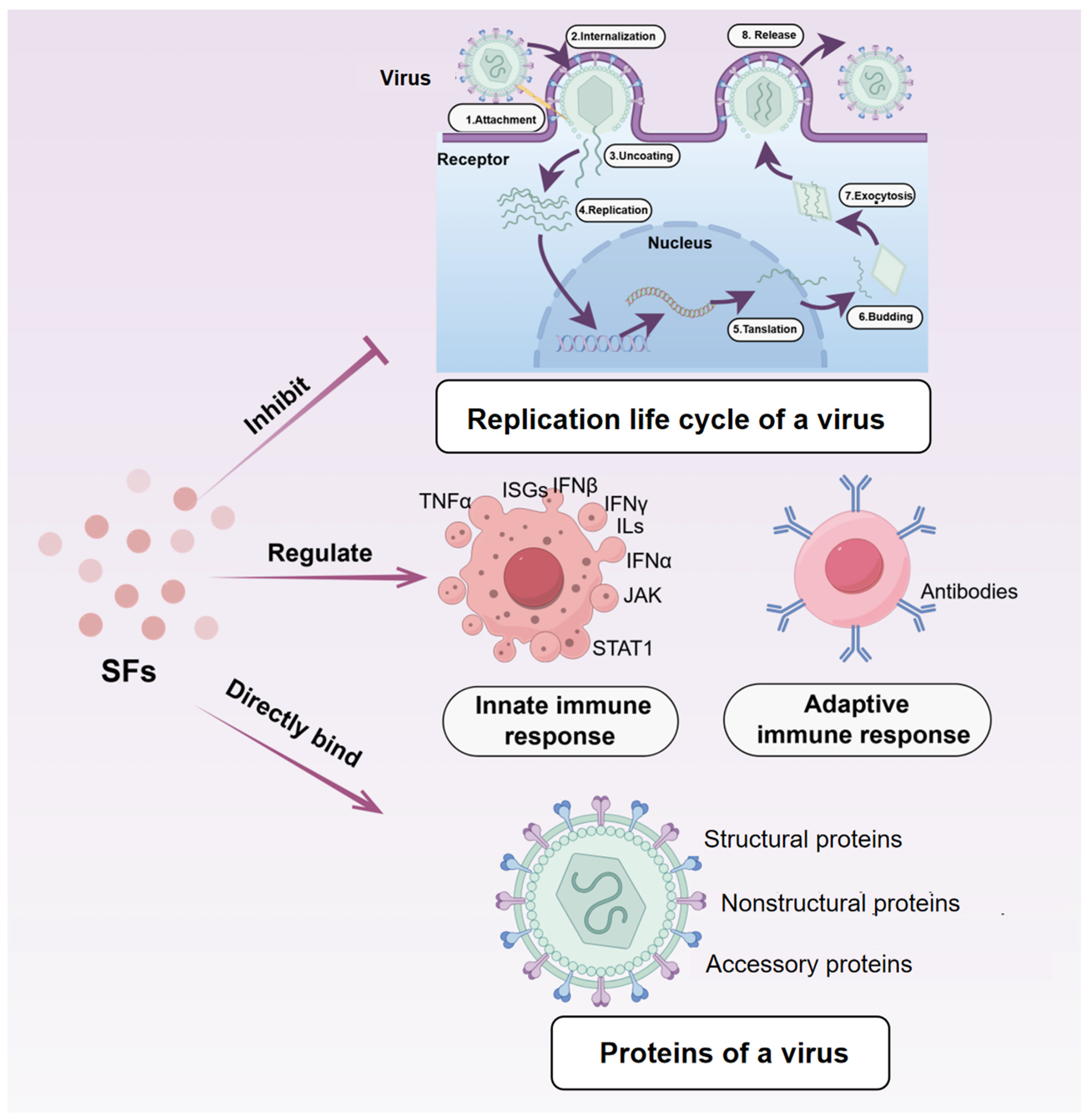
3.2.4. Antiviral Effects
3.3. Application of SFs in Swine Health Management
3.3.1. Improvement in Growth Performance
3.3.2. Enhancement of Immunity
3.3.3. Regulation of the Intestinal Microbiome
3.3.4. Disease Prevention and Treatment
4. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SFs | Scutellaria baicalensis’s Flavonoids |
| ADG | Average Daily Gain |
| ADFI | Average Daily Feed Intake |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MAPK | Mitogen-Activated Protein Kinase |
| AKT | Protein Kinase B |
| PI3K | Phosphoinositide 3-Kinase |
| EGFR | Epidermal Growth Factor Receptor |
| NF-kB | Nuclear Factor kappa-light-chain-enhancer of Activated B cells |
| NLRP3 | NOD-like Receptor Family Pyrin Domain Containing 3 |
| ROS | Reactive Oxygen Species |
| HIV | Human Immunodeficiency Virus |
| TCM | Traditional Chinese Medicine |
References
- Cabello, F.C. Heavy Use of Prophylactic Antibiotics in Aquaculture: A Growing Problem for Human and Animal Health and for the Environment. Environ. Microbiol. 2010, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology and Toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, P.; Liu, S.; Liu, Q.; Li, Y.; Sun, Y.; He, C.; Xiao, P. Traditional Uses, Ten-Years Research Progress on Phytochemistry and Pharmacology, and Clinical Studies of the Genus Scutellaria. J. Ethnopharmacol. 2021, 265, 113198. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, C.; Xiao, X.; Liu, H.; Wang, M.; Zhou, X.; He, J. Effects of Extracts of Scutellaria baicalensis and Lonicerae flos on Reproductive Performance, Milk Quality and Serum Indexes in Sows and Growth Performance in Suckling Piglets. Livest. Sci. 2023, 275, 105295. [Google Scholar] [CrossRef]
- Zhang, F.; Jin, E.; Liu, X.; Ji, X.; Hu, H. Effect of Dietary Fructus Mume and Scutellaria baicalensis Georgi on the Fecal Microbiota and Its Correlation with Apparent Nutrient Digestibility in Weaned Piglets. Animals 2022, 12, 2418. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Bochořáková, H.; Paulová, H.; Slanina, J.; Musil, P.; Táborská, E. Main Flavonoids in the Root of Scutellaria baicalensis Cultivated in Europe and Their Comparative Antiradical Properties. Phytotherapy Res. 2003, 17, 640–644. [Google Scholar] [CrossRef]
- Li, C.; Lin, G.; Zuo, Z. Pharmacological Effects and Pharmacokinetics Properties of Radix scutellariae and Its Bioactive Flavones. Biopharm. Drug Disp. 2011, 32, 427–445. [Google Scholar] [CrossRef]
- Li, H.; Jiang, Y.; Chen, F. Separation Methods Used for Scutellaria baicalensis Active Components. J. Chromatogr. B 2004, 812, 277–290. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.; Yang, Z.; Tang, Q.; Tan, H.; Wang, X.; Cai, S. Differences in Anti-Inflammatory Effects between Two Specifications of Scutellariae Radix in LPS-Induced Macrophages in Vitro. Chin. J. Nat. Med. 2017, 15, 515–524. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. The In Vitro Antibacterial Activity of Dietary Spice and Medicinal Herb Extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Eiden, M.; Leidel, F.; Strohmeier, B.; Fast, C.; Groschup, M.H. A Medicinal Herb Scutellaria Lateriflora Inhibits PrP Replication in Vitro and Delays the Onset of Prion Disease in Mice. Front. Psychiatry 2012, 3, 20692. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cao, J.; Zhu, B.; Wang, Z.; Wang, T.; Tang, J. Baicalin Attenuates Joint Pain and Muscle Dysfunction by Inhibiting Muscular Oxidative Stress in an Experimental Osteoarthritis Rat Model. Arch. Immunol. Ther. Exp. 2018, 66, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Chen, J.; Tan, H.; Wang, N.; Chen, Z.; Feng, Y. Scutellaria baicalensis and Cancer Treatment: Recent Progress and Perspectives in Biomedical and Clinical Studies. Am. J. Chin. Med. 2018, 46, 25–54. [Google Scholar] [CrossRef]
- Dong, Q.; Chu, F.; Wu, C.; Huo, Q.; Gan, H.; Li, X.; Liu, H. Scutellaria baicalensis Georgi Extract Protects against Alcohol-Induced Acute Liver Injury in Mice and Affects the Mechanism of ER Stress. Mol. Med. Rep. 2016, 13, 3052–3062. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Smith, J.F.; Starr, E.G.; Goodman, M.A.; Hanson, R.B.; Palmer, T.A.; Woolstenhulme, J.B.; Weyand, J.A.; Marchant, A.D.; Bueckers, S.L.; Nelson, T.K.; et al. Topical Application of Wogonin Provides a Novel Treatment of Knee Osteoarthritis. Front. Physiol. 2020, 11, 80. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic Potentials of Baicalin and Its Aglycone, Baicalein against Inflammatory Disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.; Zhang, D.; Zhong, X.; Du, K.; Qian, P.; Yao, W.; Gao, H.; Wei, M. Baicalin Mitigates Cognitive Impairment and Protects Neurons from Microglia-mediated Neuroinflammation via Suppressing NLRP 3 Inflammasomes and TLR4/NF-κB Signaling Pathway. CNS Neurosci. Ther. 2019, 25, 575–590. [Google Scholar] [CrossRef]
- Rui, W.; Li, S.; Xiao, H.; Xiao, M.; Shi, J. Baicalein Attenuates Neuroinflammation by Inhibiting NLRP3/Caspase-1/GSDMD Pathway in MPTP-Induced Mice Model of Parkinson’s Disease. Int. J. Neuropsychopharmacol. 2020, 23, 762–773. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, Y.; Zhao, C.; Zhao, B.; Wang, J. The Pharmacological Efficacy of Baicalin in Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 9317. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.K.; Kim, H.; Shin, H.; Song, J.; Lee, M.K.; Park, B.; Lee, K.Y. Characterization of Anti-Inflammatory and Antioxidant Constituents from Scutellaria baicalensis Using LC-MS Coupled with a Bioassay Method. Molecules 2020, 25, 3617. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, A.; Liu, R. Separation and Purification of Baicalin and Wogonoside from the Chinese Medicinal Plant Scutellaria baicalensis Georgi by High-Speed Counter-Current Chromatography. J. Chromatogr. A 2005, 1066, 243–247. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, N.; Li, D.; Liang, D.; Liu, Z.; Li, F.; Fu, Y.; Cao, Y.; Deng, X.; Yang, Z. Baicalin Plays an Anti-Inflammatory Role through Reducing Nuclear Factor-κB and P38 Phosphorylation in S. aureus-Induced Mastitis. Int. Immunopharmacol. 2013, 16, 125–130. [Google Scholar] [CrossRef]
- Yang, W.; Li, H.; Cong, X.; Wang, X.; Jiang, Z.; Zhang, Q.; Qi, X.; Gao, S.; Cao, R.; Tian, W. Baicalin Attenuates Lipopolysaccharide Induced Inflammation and Apoptosis of Cow Mammary Epithelial Cells by Regulating NF-κB and HSP72. Int. Immunopharmacol. 2016, 40, 139–145. [Google Scholar] [CrossRef]
- Chen, Z.; Su, Y.; Bi, Y.; Tsang, S.; Huang, Y. Effect of Baicalein and Acetone Extract of Scutellaria baicalensis on Canola Oil Oxidation. J. Am. Oil Chem. Soc. 2000, 77, 73–78. [Google Scholar] [CrossRef]
- Cheng, F.; Lu, Y.; Zhong, X.; Song, W.; Wang, X.; Sun, X.; Qin, J.; Guo, S.; Wang, Q. Baicalin’s Therapeutic Time Window of Neuroprotection during Transient Focal Cerebral Ischemia and Its Antioxidative Effects in Vitro and in Vivo. Evidence-Based Complement. Altern. Med. 2013, 2013, 120261. [Google Scholar] [CrossRef]
- Tong, R.; Mehendale, S.R.; Wang, C.-Z.; Shao, Z.; Yuan, C.-S. Letter to the Editor—Comparison of Antioxidant Effects of Various Scutellaria baicalensis Fractions and the Potential Role of Catalase Upregulation. Am. J. Chin. Med. 2009, 37, 621–623. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, Y.; An, L.; Dou, Y.; Liu, Y. Density Functional Theory Study of the Structure–Antioxidant Activity of Polyphenolic Deoxybenzoins. Food Chem. 2014, 151, 198–206. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, X.; Yang, R.; Liu, L.; Wang, L.; Li, M.; Du, W. Baicalein Is Neuroprotective in Rat MCAO Model: Role of 12/15-Lipoxygenase, Mitogen-Activated Protein Kinase and Cytosolic Phospholipase A2. Pharmacol. Biochem. Behav. 2010, 96, 469–475. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.; Gao, M.; Li, X.; Zhang, T.; Du, G. Baicalein Protects Rat Brain Mitochondria against Chronic Cerebral Hypoperfusion-Induced Oxidative Damage. Brain Res. 2009, 1249, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, Pathophysiology and Development of Therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Chen, X.; Gu, Y.; Peng, T.; Yang, D.; Chang, R.C.-C.; So, K.-F.; Liu, K.; Shen, J. Baicalin Can Scavenge Peroxynitrite and Ameliorate Endogenous Peroxynitrite-Mediated Neurotoxicity in Cerebral Ischemia-Reperfusion Injury. J. Ethnopharmacol. 2013, 150, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Huang, K.; Xu, H. Protective Effects of Flavonoids in the Roots of Scutellaria baicalensis Georgi against Hydrogen Peroxide-Induced Oxidative Stress in Hs-Sy5y Cells. Pharmacol. Res. 2001, 43, 173–178. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Xu, K.; Cai, F.; Gu, J.; Ma, L.; Chen, J. Neuroprotection by Baicalein in Ischemic Brain Injury Involves PTEN/AKT Pathway. J. Neurochem. 2010, 112, 1500–1512. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Wang, J.; Chen, T.; Jiang, J.; Shi, H.; Gui, C.; Luo, R.; Xie, X.; Xiang, M.; Zhang, X. Hypoxic Preconditioning Attenuates Hypoxia/Reoxygenation-Induced Apoptosis in Mesenchymal Stem Cells. Acta Pharmacol. Sin. 2008, 29, 74–82. [Google Scholar] [CrossRef]
- Gabrielska, J.; Oszmiańsk, J.; Żyłka, R.; Komorowska, M. Antioxidant Activity of Flavones from Scutellaria baicalensis in Lecithin Liposomes. Z. Naturforsch. C J. Biosci. 1997, 52, 817–823. [Google Scholar] [CrossRef]
- Gao, B.; Zhu, H.; Liu, Z.; He, X.; Sun, J.; Li, Y.; Wu, X.; Pehrsson, P.; Zhang, Y.; Yu, L. Chemical Compositions of Scutellaria baicalensis Georgi. (Huangqin) Extracts and Their Effects on ACE2 Binding of SARS-CoV-2 Spike Protein, ACE2 Activity, and Free Radicals. Int. J. Mol. Sci. 2024, 25, 2045. [Google Scholar] [CrossRef]
- Chan, E.; Wong, C.Y.-K.; Wan, C.-W.; Kwok, C.-Y.; Wu, J.-H.; Ng, K.-M.; So, C.-H.; Au, A.L.-S.; Poon, C.C.-W.; Seto, S.-W.; et al. Evaluation of Anti-Oxidant Capacity of Root of Scutellaria baicalensis Georgi, in Comparison with Roots of Polygonum Multiflorum Thunb and Panax Ginseng CA Meyer. Am. J. Chin. Med. 2010, 38, 815–827. [Google Scholar] [CrossRef]
- Xue, Y.; Wei, Y.; Cao, L.; Shi, M.; Sheng, J.; Xiao, Q.; Cheng, Z.; Luo, T.; Jiao, Q.; Wu, A.; et al. Protective Effects of Scutellaria-Coptis Herb Couple against Non-Alcoholic Steatohepatitis via Activating NRF2 and FXR Pathways in Vivo and in Vitro. J. Ethnopharmacol. 2024, 318, 116933. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Li, W.; Qin, H.; Yun, J.; Sun, X. The Use of Chinese Skullcap (Scutellaria baicalensis) and Its Extracts for Sustainable Animal Production. Animals 2021, 11, 1039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 Signaling: An Important Molecular Mechanism of Herbal Medicine in the Treatment of Atherosclerosis via the Protection of Vascular Endothelial Cells from Oxidative Stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Matsumura, S.; Kariya, N.; Nishimura, M.; Shimono, T. In Vitro Antibacterial Activities of Scutellaria baicalensis Georgi against Cariogenic Bacterial. Pediatr. Dent. J. 2007, 17, 58–64. [Google Scholar] [CrossRef]
- Jiang, H.; Bai, Z.; Xu, Z.; Sun, J.; Françoise, H.; Luan, Z.; Wang, H. Antimicrobial Mechanism of Semi-Bionic Extracts of Three Traditional Medicinal Plants—Rheum Palmatum L., Scutellaria baicalensis Georgi, and Houttuynia cordata Thunb—That Can Be Used as Antibiotic Alternatives. Front. Vet. Sci. 2023, 9, 1083223. [Google Scholar] [CrossRef]
- Pant, C.C.; Melkani, A.B.; Mohan, L.; Dev, V. Composition and Antibacterial Activity of Essential Oil from Scutellaria Grossa Wall Ex Benth. Nat. Prod. Res. 2012, 26, 190–192. [Google Scholar] [CrossRef]
- Xia, Y.; Chan, G.K.; Wang, H.; Dong, T.T.; Duan, R.; Hu, W.; Qin, Q.; Wang, W.; Tsim, K.W. The Anti-Bacterial Effects of Aerial Parts of Scutellaria baicalensis: Potential Application as an Additive in Aquaculture Feedings. Aquaculture 2020, 526, 735418. [Google Scholar] [CrossRef]
- Blaszczyk, T.; Krzyzanowska, J.; Lamer-Zarawska, E. Screening for Antimycotic Properties of 56 Traditional Chinese Drugs. Phytother. Res. 2000, 14, 210–212. [Google Scholar] [CrossRef]
- Qiu, F.; Meng, L.; Chen, J.; Jin, H.; Jiang, L. In Vitro Activity of Five Flavones from Scutellaria baicalensis in Combination with Cefazolin against Methicillin Resistant Staphylococcus Aureus (MRSA). Med. Chem. Res. 2016, 25, 2214–2219. [Google Scholar] [CrossRef]
- Wu, J.; Hu, D.; Wang, K.-X. Study of Scutellaria baicalensis and Baicalin against antimicrobial susceptibility of Helicobacter pylori strains in vitro. Zhong Yao Cai 2008, 31, 707–710. [Google Scholar]
- Chan, B.C.; Ip, M.; Lau, C.B.; Lui, S.; Jolivalt, C.; Ganem-Elbaz, C.; Litaudon, M.; Reiner, N.E.; Gong, H.; See, R.H.; et al. Synergistic Effects of Baicalein with Ciprofloxacin against NorA Over-Expressed Methicillin-Resistant Staphylococcus aureus (MRSA) and Inhibition of MRSA Pyruvate Kinase. J. Ethnopharmacol. 2011, 137, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Tang, S.; Wu, C.; Wang, Y.; He, T.; Chen, T.; Xiao, X. Synergy between Baicalein and Penicillins against Penicillinase-Producing Staphylococcus aureus. Int. J. Med. Microbiol. 2015, 305, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Liu, P.; Chen, H.; Wang, Y.; Li, C.; Wang, Q. Baicalein Inhibits the Staphylococcus aureus Biofilm and the LuxS/AI-2 System in Vitro. Infect. Drug Res. 2023, 16, 2861–2882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luan, Y.; Jing, S.; Wang, Y.; Gao, Z.; Yang, P.; Ding, Y.; Wang, L.; Wang, D.; Wang, T. Baicalein Mediates Protection against Staphylococcus aureus-Induced Pneumonia by Inhibiting the Coagulase Activity of vWbp. Biochem. Pharmacol. 2020, 178, 114024. [Google Scholar] [CrossRef]
- Chen, W.; Li, B.; Li, S.; Ou, Y.; Ou, Q. Effects of Scutellaria baicalensis on Activity and Biofilm Formation of Klebsiella pneumoniae. Chin. Med. Sci. J. 2016, 31, 180–184. [Google Scholar] [CrossRef]
- Dharani, R.S.; Ranjitha, R.; Sripathi, R.; Ali Muhammad, K.S.; Ravi, S. Docking Studies in Target Proteins Involved in Antibacterial Action Mechanisms: Alkaoids Isolated from Scutellaria Genus. Asian J. Pharm. Clin. Res. 2016, 9, 121. [Google Scholar] [CrossRef]
- Fu, S.; Liu, H.; Xu, L.; Qiu, Y.; Liu, Y.; Wu, Z.; Ye, C.; Hou, Y.; Hu, C.-A.A. Baicalin Modulates NF-κB and NLRP3 Inflammasome Signaling in Porcine Aortic Vascular Endothelial Cells Infected by Haemophilus parasuis Causing Glässer’s Disease. Sci. Rep. 2018, 8, 807. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; He, X.; Jiao, N.; Zhang, X.; Qiu, K.; Piao, X.; Yin, J. The Involvement of NF-κB/P38 Pathways in Scutellaria baicalensis Extracts Attenuating of Escherichia coli K88-Induced Acute Intestinal Injury in Weaned Piglets. Br. J. Nutr. 2019, 122, 152–161. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F.; Ma, Y.; Li, H.; Ju, X.; Xu, J. Effect of Puerarin, Baicalin and Berberine Hydrochloride on the Regulation of IPEC-J2 Cells Infected with Enterotoxigenic Escherichia coli. Evid. Based Complement. Alternat. Med. 2019, 2019, 7438593. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, C.; Hu, Y.; Xia, T.; Zhang, B.; Chen, F.; Tan, X.; Zheng, Z. Gegen Qinlian Decoction Restores the Intestinal Barrier in Bacterial Diarrhea Piglets by Promoting Lactobacillus Growth and Inhibiting the TLR2/MyD88/NF-κB Pathway. Biomed. Pharmacother. 2022, 155, 113719. [Google Scholar] [CrossRef]
- Zong, B.; Xiao, Y.; Wang, P.; Liu, W.; Ren, M.; Li, C.; Fu, S.; Zhang, Y.; Qiu, Y. Baicalin Weakens the Virulence of Porcine Extraintestinal Pathogenic Escherichia coli by Inhibiting the LuxS/AI-2 Quorum-Sensing System. Biomolecules 2024, 14, 452. [Google Scholar] [CrossRef] [PubMed]
- Zong, B.; Xiao, Y.; Ren, M.; Wang, P.; Fu, S.; Qiu, Y. Baicalin Weakens the Porcine ExPEC-Induced Inflammatory Response in 3D4/21 Cells by Inhibiting the Expression of NF-κB/MAPK Signaling Pathways and Reducing NLRP3 Inflammasome Activation. Microorganisms 2023, 11, 2126. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Tian, X.; Peng, C.; Zhang, D.; Zhou, L.; Yuan, Y.; He, J.; Guo, L.; Qiu, Y.; Ye, C.; et al. Baicalin Inhibited PANX-1/P2Y6 Signaling Pathway Activation in Porcine Aortic Vascular Endothelial Cells Infected by Glaesserella parasuis. Heliyon 2024, 10, e23632. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhuang, F.; Guo, L.; Qiu, Y.; Xiong, J.; Ye, C.; Liu, Y.; Wu, Z.; Hou, Y.; Hu, C.-A.A. Effect of Baicalin-Aluminum Complexes on Fecal Microbiome in Piglets. Int. J. Mol. Sci. 2019, 20, 2390. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Zhang, Z.; Zhang, J.; Xu, J.; Qiu, Y.; Ye, C.; Fu, S.; Wu, Z.; Hu, C.-A.A. Baicalin Protects Vascular Tight Junctions in Piglets during Glaesserella parasuis Infection. Front. Vet. Sci. 2021, 8, 671936. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, B.; Xu, J.; Ren, Q.; Wang, Z.; Wang, S.; Dong, Y.; Yang, G. Baicalin Suppress Growth and Virulence-Related Factors of Methicillin-Resistant Staphylococcus aureus in Vitro and Vivo. Microb. Pathog. 2020, 139, 103899. [Google Scholar] [CrossRef]
- Fu, S.; Meng, Q.; Zhang, D.; Zuo, S.; He, J.; Guo, L.; Qiu, Y.; Ye, C.; Liu, Y.; Hu, C.-A.A. Effect of Baicalin on Transcriptome Changes in Piglet Vascular Endothelial Cells Induced by a Combination of Glaesserella parasuis and Lipopolysaccharide. DNA Cell Biol. 2021, 40, 776–790. [Google Scholar] [CrossRef]
- Fu, S.; Liu, J.; Xu, J.; Zuo, S.; Zhang, Y.; Guo, L.; Qiu, Y.; Ye, C.; Liu, Y.; Wu, Z.; et al. The Effect of Baicalin on microRNA Expression Profiles in Porcine Aortic Vascular Endothelial Cells Infected by Haemophilus parasuis. Mol. Cell. Biochem. 2020, 472, 45–56. [Google Scholar] [CrossRef]
- Guo, L.; Liu, J.; Zhang, Y.; Fu, S.; Qiu, Y.; Ye, C.; Liu, Y.; Wu, Z.; Hou, Y.; Hu, C.-A.A. The Effect of Baicalin on the Expression Profiles of Long Non-Coding RNAs and mRNAs in Porcine Aortic Vascular Endothelial Cells Infected with Haemophilus parasuis. DNA Cell Biol. 2020, 39, 801–815. [Google Scholar] [CrossRef]
- Ye, C.; Li, R.; Xu, L.; Qiu, Y.; Fu, S.; Liu, Y.; Wu, Z.; Hou, Y.; Hu, C.-A.A. Effects of Baicalin on Piglet Monocytes Involving PKC-MAPK Signaling Pathways Induced by Haemophilus parasuis. BMC Vet. Res. 2019, 15, 98. [Google Scholar] [CrossRef]
- Fu, S.; Liu, H.; Chen, X.; Qiu, Y.; Ye, C.; Liu, Y.; Wu, Z.; Guo, L.; Hou, Y.; Hu, C.-A.A. Baicalin Inhibits Haemophilus parasuis-Induced High-Mobility Group Box 1 Release during Inflammation. Int. J. Mol. Sci. 2018, 19, 1307. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Chen, Y.; Wang, D.; Ma, L.; Guo, M.; Zhou, C.; Dou, J. The Inhibitory Effect of Sodium Baicalin on Oseltamivir-Resistant Influenza A Virus via Reduction of Neuraminidase Activity. Arch. Pharm. Res. 2018, 41, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Huang, L.; Wang, H.; Wang, C.; Chen, J.; Yang, P.; Ni, M.; Chen, C.; Li, C.; Xie, Y.; et al. An Ultrasensitive High-Performance Baicalin Sensor Based on C3N4-SWCNTs/Reduced Graphene Oxide/Cyclodextrin Metal-Organic Framework Nanocomposite. Sens. Actuators B Chem. 2022, 350, 130853. [Google Scholar] [CrossRef]
- Sawadpongpan, S.; Jaratsittisin, J.; Hitakarun, A.; Roytrakul, S.; Wikan, N.; Smith, D.R. Investigation of the Activity of Baicalein towards Zika Virus. BMC Complement. Med. Ther. 2023, 23, 143. [Google Scholar] [CrossRef]
- Low, Z.X.; OuYong, B.M.; Hassandarvish, P.; Poh, C.L.; Ramanathan, B. Antiviral Activity of Silymarin and Baicalein against Dengue Virus. Sci. Rep. 2021, 11, 21221. [Google Scholar] [CrossRef]
- Qian, X.; Zhou, H.; Liu, Y.; Dong, J.; Tang, W.; Zhao, P.; Tang, H.; Jin, Y. Synthesis of Baicalein Derivatives and Evaluation of Their Antiviral Activity against Arboviruses. Bioorg. Med. Chem. Lett. 2022, 72, 128863. [Google Scholar] [CrossRef]
- Chang, W.; Wang, J.; Wu, F.; Zhang, H.; Yang, M. Antiviral Activity and Underlying Mechanisms of Baicalin against Porcine Reproductive and Respiratory Syndrome Virus in Vitro. Microb. Pathog. 2024, 193, 106712. [Google Scholar] [CrossRef]
- Shu, X.; Zhang, Y.; Zhang, X.; Zhang, Y.; Shu, Y.; Wang, Y.; Zhang, Z.; Song, C. Therapeutic and Immune-Regulation Effects of Scutellaria baicalensis Georgi Polysaccharide on Pseudorabies in Piglets. Front. Vet. Sci. 2024, 11, 1356819. [Google Scholar] [CrossRef]
- Leonova, G.; Shutikova, A.; Lubova, V.; Maistrovskaya, O. Inhibitory Activity of Scutellaria baicalensis Flavonoids against Tick-Borne Encephalitis Virus. Bull. Exp. Biol. Med. 2020, 168, 665–668. [Google Scholar] [CrossRef]
- Błach-Olszewska, Z.; Jatczak, B.; Rak, A.; Lorenc, M.; Gulanowski, B.; Drobna, A.; Lamer-Zarawska, E. Production of Cytokines and Stimulation Ofresistance to Viral Infection in Human Leukocytes by Scutellaria baicalensis Flavones. J. Interf. Cytokine Res. 2008, 28, 571–582. [Google Scholar] [CrossRef]
- Kitamura, K.; Honda, M.; Yoshizaki, H.; Yamamoto, S.; Nakane, H.; Fukushima, M.; Ono, K.; Tokunaga, T. Baicalin, an Inhibitor of HIV-1 Production in Vitro. Antivir. Res. 1998, 37, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Fu, T.; Dongyan, Y.; Mikovits, J.A.; Ruscetti, F.W.; Wang, J.M. Flavonoid Baicalin Inhibits HIV-1 Infection at the Level of Viral Entry. Biochem. Biophys. Res. Commun. 2000, 276, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Z.; Chen, H.; Zhang, X.; Chen, X. Synthesis of baicalin derivatives and evaluation of their anti-human immunodeficiency virus (HIV-1) activity. Yao Xue Xue Bao 1998, 33, 22–27. [Google Scholar]
- Jiang, Y.; Ng, T.B.; Wang, C.R.; Zhang, D.; Cheng, Z.H.; Liu, Z.K.; Qiao, W.T.; Geng, Y.Q.; Li, N.; Liu, F. Inhibitors from Natural Products to HIV-1 Reverse Transcriptase, Protease and Integrase. Mini-Rev. Med. Chem. 2010, 10, 1331–1344. [Google Scholar] [CrossRef]
- Li, G.; Diao, X. Effect of Adding Fermented Scutellaria in Feed on Production Performance of Weaned Piglets. Chin. Feed 2012, 12, 27–28. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, I.H. Effect of Probiotic Bacteria-fermented Medicinal Plants Ynura procumbens, Ehmannia glutinosa, Cutellaria baicalensis as Performance Enhancers in Growing Pigs. Anim. Sci. J. 2015, 86, 603–609. [Google Scholar] [CrossRef]
- Liu, W.; Pi, S.; Kim, I. Effects of Scutellaria baicalensis and Lonicera Japonicaextract Mixture Supplementation on Growth Performance, Nutrient Digestibility, Blood Profiles and Meat Quality in Finishing Pigs. Itali. J. Anim. Sci. 2016, 15, 446–452. [Google Scholar] [CrossRef]
- Gheisar, M.M.; Cheong, J.Y.; Zhao, P.; Kim, I.H. Evaluating the Influence of Dietary Phytogenic Blends on Gestating and Lactating Sows and Suckling Piglets. Anim. Prod. Sci. 2018, 58, 2071. [Google Scholar] [CrossRef]
- Chang, C.; Chen, Y.; Chiou, M.; Su, C.; Chen, D.S.; Tsai, C.-E.; Yu, B.; Hsu, Y.-M. Application of Scutellariae Radix, Gardeniae Fructus, and Probiotics to Prevent Salmonella enterica Serovar Choleraesuis Infection in Swine. Evidence-Based Complement. Altern. Med. 2013, 2013, 568528. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, S.; Zhong, R.; Chen, L.; Zhang, H. PSVIII-26 Baicalin Alleviates Lincomycin-Induced Intestinal Injury by Regulating Gut Microbiota in Weaned Pigs. J. Anim. Sci. 2023, 101, 662–663. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, N.; Wen, D.; Guo, P.; Liu, Y.; Fu, S.; Ye, C.; Wu, Z.; Qiu, Y. Baicalin Attenuates Lipopolysaccharide-Induced Intestinal Inflammatory Injury via Suppressing PARP1-Mediated NF-κB and NLRP3 Signalling Pathway. Toxicon 2024, 239, 107612. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yun, H.; Pi, S.; Kim, I. Supplementing Lactation Diets with Herbal Extract Mixture during Summer Improves the Performance of Sows and Nursing Piglets. Ann. Anim. Sci. 2017, 17, 835–847. [Google Scholar] [CrossRef]
- Zhao, P.; Li, H.; Lei, Y.; Li, T.; Kim, S.; Kim, I. Effect of Fermented Medicinal Plants on Growth Performance, Nutrient Digestibility, Fecal Noxious Gas Emissions, and Diarrhea Score in Weanling Pigs. J. Sci. Food Agric. 2016, 96, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Cui, Y.; Hu, X.; Zhen, Y.; Lv, H.; Huang, D.; Deng, Y.; Deng, X. Treatment Test of Skullcap Injection on the Artificial Infection of Swine Edema Disease. Chin. J. Vet. Sci. 2016, 5, 4. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, S.; Liu, Z.; Lv, H.; Cai, X.; Zhong, R.; Chen, L.; Zhang, H. Baicalin Restore Intestinal Damage after Early-Life Antibiotic Therapy: The Role of the MAPK Signaling Pathway. Pharmacol. Res. 2024, 204, 107194. [Google Scholar] [CrossRef]
- Wang, L.; Huo, B.; Huang, L.; Che, L.; Feng, B.; Lin, Y.; Xu, S.; Wu, D.; Fang, Z. Dietary Supplementation with a Mixture of Herbal Extracts during Late Gestation and Lactation Improves Performance of Sows and Nursing Piglets through Regulation of Maternal Metabolism and Transmission of Antibodies. Front. Vet. Sci. 2022, 9, 1026088. [Google Scholar] [CrossRef]
- Guo, Q.; Xuan, M.; Luo, Z.; Wang, J.; Han, S.; Ri, M.; Choe, Y.; Hwang, K.; Yin, X.; Kang, J. Baicalin Improves the in Vitro Developmental Capacity of Pig Embryos by Inhibiting Apoptosis, Regulating Mitochondrial Activity and Activating Sonic Hedgehog Signaling. Mol. Hum. Reprod. 2019, 25, 538–549. [Google Scholar] [CrossRef]
- Ling, X.; Xiang, Y.; Chen, F.; Tang, Q.; Zhang, W.; Tan, X. Intestinal Absorption Differences of Major Bioactive Compounds of Gegenqinlian Decoction between Normal and Bacterial Diarrheal Mini-Pigs in Vitro and in Situ. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1083, 93–101. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Askari, V.R.; Hosseinzadeh, H. Promising Influences of Scutellaria baicalensis and Its Two Active Constituents, Baicalin, and Baicalein, against Metabolic Syndrome: A Review. Phytother. Res. 2021, 35, 3558–3574. [Google Scholar] [CrossRef]
- Ge, P.; Qi, Y.; Qu, S.; Zhao, X.; Ni, S.; Yao, Z.-Y.; Guo, R.; Yang, N.-Y.; Zhang, Q.-C.; Zhu, H.-X. Potential Mechanism of S. baicalensis on Lipid Metabolism Explored via Network Pharmacology and Untargeted Lipidomics. Drug Des. Dev. Ther. 2021, 15, 1915–1930. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, M.; Yu, H.; Li, J.; Wang, S.; Zhang, Y.; Qiu, F.; Wang, T. Scutellaria baicalensis Regulates FFA Metabolism to Ameliorate NAFLD through the AMPK-Mediated SREBP Signaling Pathway. J. Nat. Med. 2018, 72, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Na, H.-Y.; Lee, B.-C. Scutellaria baicalensis Alleviates Insulin Resistance in Diet-Induced Obese Mice by Modulating Inflammation. Int. J. Mol. Sci. 2019, 20, 727. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Bose, S.; Lim, S.-K.; Wang, J.-H.; Choi, Y.-H.; Kim, H. Combination of Scutellaria baicalensis and Metformin Ameliorates Diet-Induced Metabolic Dysregulation in Mice via the Gut-Liver-Brain Axis. Am. J. Chin. Med. 2020, 48, 1409–1433. [Google Scholar] [CrossRef] [PubMed]
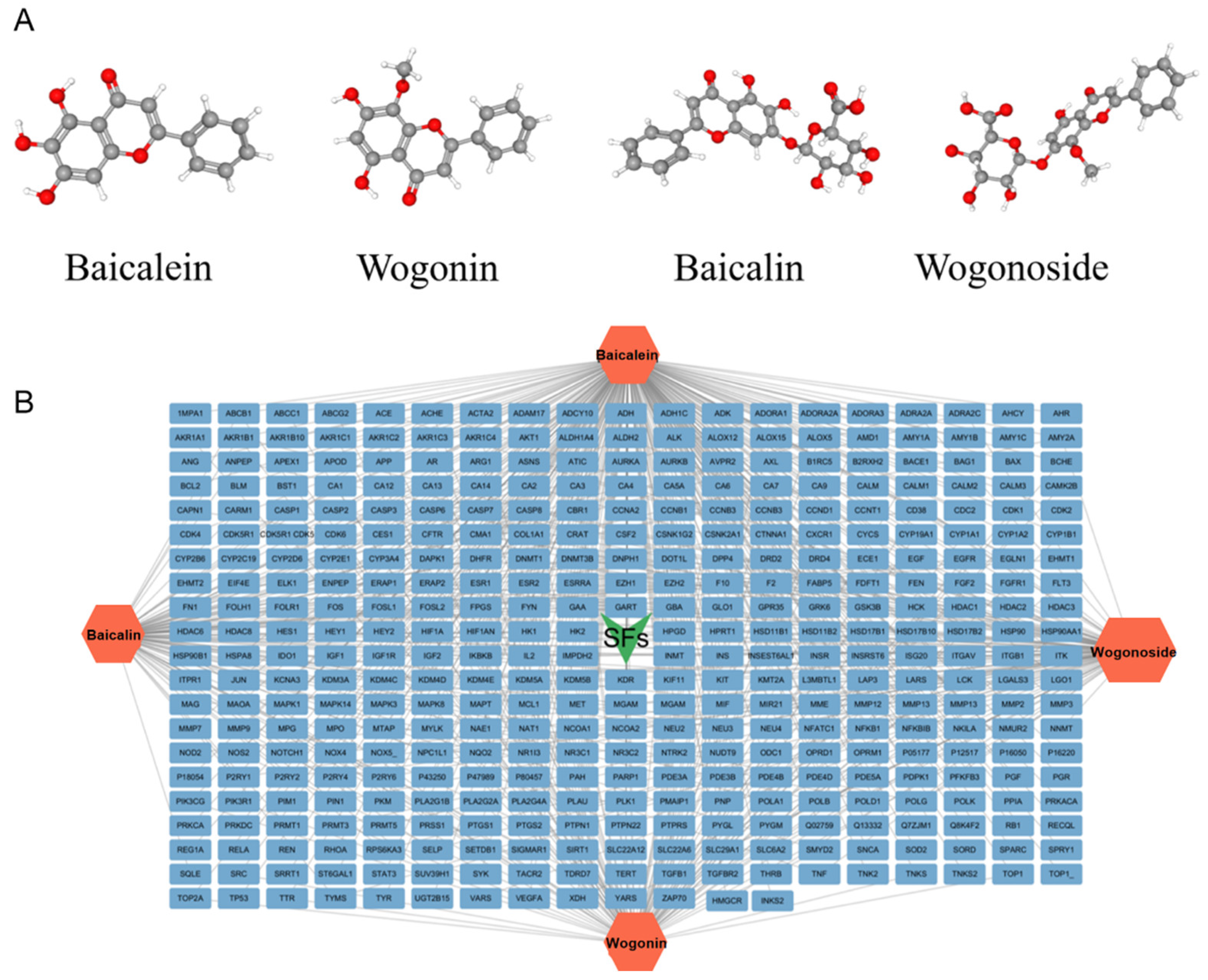
| Mol ID | Molecule Name | Structure | MW | Alogp | Hdon | Hacc | OB (%) | DL |
|---|---|---|---|---|---|---|---|---|
| MOL001689 | Acacetin | 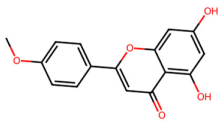 | 284.28 | 2.59 | 2 | 5 | 34.97 | 0.24 |
| MOL000173 | Wogonin | 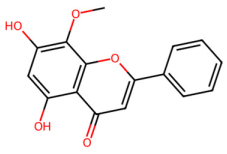 | 284.28 | 2.59 | 2 | 5 | 30.68 | 0.23 |
| MOL000228 | (2R)-7-hydroxy-5-methoxy-2-phenylchroman-4-one | 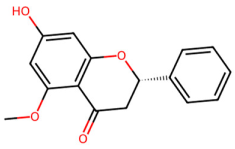 | 270.3 | 2.82 | 1 | 4 | 55.23 | 0.2 |
| MOL002714 | Baicalein | 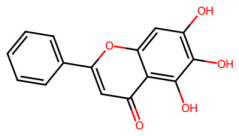 | 270.25 | 2.33 | 3 | 5 | 33.52 | 0.21 |
| MOL002908 | 5,8,2′-Trihydroxy-7-methoxyflavone | 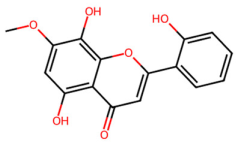 | 300.28 | 2.32 | 3 | 6 | 37.01 | 0.27 |
| MOL002909 | 5,7,2,5-tetrahydroxy-8,6-dimethoxyflavone |  | 376.34 | 2.02 | 4 | 9 | 33.82 | 0.45 |
| MOL002910 | Carthamidin | 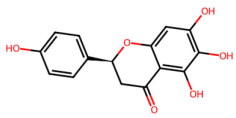 | 288.27 | 2.03 | 4 | 6 | 41.15 | 0.24 |
| MOL002911 | 2,6,2′,4′-tetrahydroxy-6′-methoxychaleone | 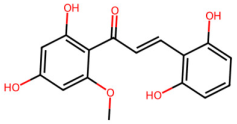 | 302.3 | 2.62 | 4 | 6 | 69.04 | 0.22 |
| MOL002913 | Dihydrobaicalin_qt | 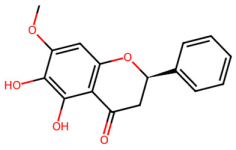 | 272.27 | 2.3 | 3 | 5 | 40.04 | 0.21 |
| MOL002914 | Eriodyctiol | 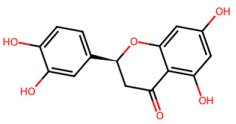 | 288.27 | 2.03 | 4 | 6 | 41.35 | 0.24 |
| MOL002915 | Salvigenin | 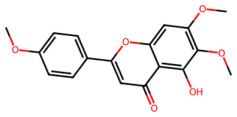 | 328.34 | 2.82 | 1 | 6 | 49.07 | 0.33 |
| MOL002917 | 5,2′,6′-Trihydroxy-7,8-dimethoxyflavone | 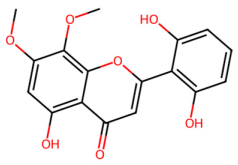 | 330.31 | 2.3 | 3 | 7 | 45.05 | 0.33 |
| MOL002925 | 5,7,2′,6′-Tetrahydroxyflavone | 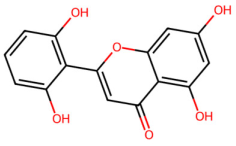 | 286.25 | 2.07 | 4 | 6 | 37.01 | 0.24 |
| MOL002926 | Dihydrooroxylin A |  | 286.3 | 2.55 | 2 | 5 | 38.72 | 0.23 |
| MOL002927 | Skullcapflavone II | 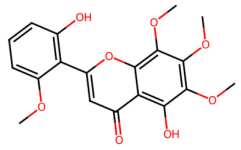 | 374.37 | 2.54 | 2 | 8 | 69.51 | 0.44 |
| MOL002928 | Oroxylin a | 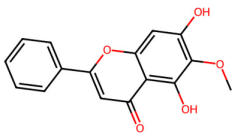 | 284.28 | 2.59 | 2 | 5 | 41.37 | 0.23 |
| MOL002932 | Panicolin | 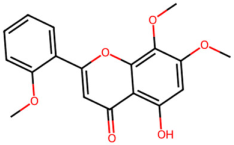 | 314.31 | 2.57 | 2 | 6 | 76.26 | 0.29 |
| MOL002933 | 5,7,4′-Trihydroxy-8-methoxyflavone | 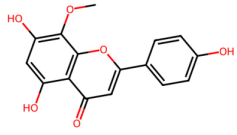 | 300.28 | 2.32 | 3 | 6 | 36.56 | 0.27 |
| MOL002934 | Neobaicalein | 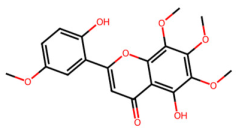 | 374.37 | 2.54 | 2 | 8 | 104.34 | 0.44 |
| MOL002937 | Dihydrooroxylin | 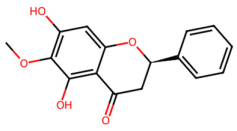 | 286.3 | 2.55 | 2 | 5 | 66.06 | 0.23 |
| MOL000525 | Norwogonin | 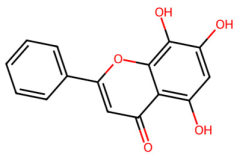 | 270.25 | 2.33 | 3 | 5 | 39.4 | 0.21 |
| MOL000552 | 5,2′-Dihydroxy-6,7,8-trimethoxyflavone |  | 344.34 | 2.55 | 2 | 7 | 31.71 | 0.35 |
| MOL000073 | Epicatechin | 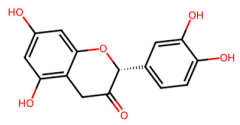 | 290.29 | 1.92 | 5 | 6 | 48.96 | 0.24 |
| MOL001458 | Coptisine |  | 320.34 | 3.25 | 0 | 4 | 30.67 | 0.86 |
| MOL002897 | Epiberberine | 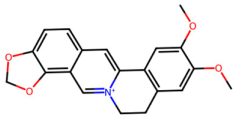 | 336.39 | 3.45 | 0 | 4 | 43.09 | 0.78 |
| MOL008206 | Moslosooflavone |  | 298.31 | 2.84 | 1 | 5 | 44.09 | 0.25 |
| MOL012245 | 5,7,4′-trihydroxy-6-methoxyflavanone | 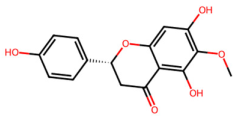 | 302.3 | 2.28 | 3 | 6 | 36.63 | 0.27 |
| MOL012246 | 5,7,4′-trihydroxy-8-methoxyflavanone |  | 302.3 | 2.28 | 3 | 6 | 74.24 | 0.26 |
| MOL012266 | Rivularin | 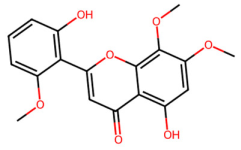 | 344.34 | 2.55 | 2 | 7 | 37.94 | 0.37 |
| Compound | Antibacterial Activity | Effective Concentration/MIC | Related Mechanism | Reference |
|---|---|---|---|---|
| Baicalin | ETEC | 1 µg/mL | Inhibits bacterial adhesion and activates NF-κB signaling pathway | [19] |
| Baicalin | H. parasuis | 12.5–100 μg/mL | Suppresses NLRP3 inflammasome and NF-κB signaling | [57] |
| SBE (≥ 12% baicalin) | E. coli K88 | 1000 mg/kg BW | Inhibits NF-κB and P38 signaling pathways | [58] |
| GQD | Lactobacillus | 1.22 g/kg | Inhibits TLR2/MyD88/ NF-κB pathway | [60] |
| Baicalin | ExPEC | 1600 µg/mL | Inhibits LuxS/AI-2 quorum-sensing system | [61] |
| Baicalin | ExPEC | 1600 µg/mL | Inhibits the expression of NF-κB/MAPK signaling pathways and reduces NLRP3 inflammasome activation | [62] |
| Baicalin | G. parasuis | 25–100 µM | Inhibits PANX-1/P2Y6 signaling pathway | [63] |
| Baicalin | H. parasuis | 12.5–100 μg/mL | Modulates apoptosis via RAGE, MAPK, and AP-1 | [64] |
| Baicalin | G. parasuis | 25–100 mg/kg BW | Protects vascular tight junctions | [65] |
| Baicalin | S.aureus | 760 μg/mL | Attenuates biofilm formation and downregulates expression of virulence-related factors | [66] |
| Baicalin | G. parasuis | 50 μg/mL | Changes lncRNA expression | [67] |
| Baicalin | H. parasuis | 50 μg/mL | Changes microRNA expression profiles | [68] |
| Baicalin | H. parasuis | 50 μg/mL | Changes expression profiles of long non-coding RNAs and mRNAs | [69] |
| Baicalin | H. parasuis | 80 mg/kg·BW | Reverses apoptosis through regulating PKC-MAPK signaling pathway | [70] |
| Baicalin | G. parasuis | 25–100 mg/kg BW | Inhibits activation of HMGB1, cell apoptosis, and MAPK signaling pathway | [71] |
| Compound | Effective Concentration | Type of Animal | Age | Main Effects | References |
|---|---|---|---|---|---|
| Mixed herbs containing S. baicalensis | 1000 mg/kg | Sows and suckling piglets | 80–107 d of gestation | Improves antioxidant capacity, liver function, colostrum quality, and immunity in sows; improves growth performance and immunity in piglets | [4] |
| Mixed fermented plant containing S. baicalensis | - | Weaned piglets | 25 d | Modifies gut microbial composition | [5] |
| Scutellaria baicalensis extracts | 1000 mg/kg diet | Weaned piglets | 24 ± 2 d | Attenuates diarrhea | [58] |
| Baicalin–aluminum complexes | 1.36 g/d, 3 d | Piglets | 10 d | Modulates gut microbiome composition of piglets with diarrhea | [64] |
| Baicalin | 5–20 μg/mL | MARC145 cells and PAMs | - | Inhibits PRRSV infection in vitro | [77] |
| Fermented S. baicalensi | 1.5 mg/kg diet | Weaned piglets | 28–56 d | Enhances appetite, increases average daily intake, reduces feed-to-weight ratio and diarrhea rate, and improves feed reward | [85] |
| Mixed fermented plant containing S. baicalensis | - | Growing pigs | 25.50 ± 2.50 kg BW | Improves growth performance and nutrient digestibility; reduces noxious gas emissions of excret | [86] |
| Mixed herbs containing S. baicalensis | 0.025% and 0.05% | Finishing pigs | 44.2 ± 2.23 kg BW | Improves growth performance and nutrient digestibility; decreases serum cortisol levels; benefits meat quality | [87] |
| Mixed herbs containing S. baicalensis | - | Pregnant and lactating sows | - | Decreases weight loss and improves litter performance | [88] |
| Mixed herbs containing Baicalin | 89.74 mg/g | Weaned piglets | 28 d | Prevents infection, enhances immunity, and adjusts intestinal microbial composition | [89] |
| Baicalin | 1 g/kg for week and 500 mg/kg feed for 2 weeks | Weaned piglets | 21 d | Alleviates intestinal injury | [90] |
| Baicalin | 100 μg/mL | Pig intestinal epithelial cell line J2 | - | Attenuates intestinal inflammatory injury | [91] |
| Mixed herbs containing S. baicalensis | 10 g/d | Lactating sows under heat stress and piglets | - | Improves feed intake, digestibility of dry matter, piglets’ weaning weight, and ADG; decreases backfat loss, serum cortisol levels, and diarrhea | [92] |
| S. baicalensis extract | - | Weaned piglets | - | Improves growth performance and nutrient digestibility; decreases fecal noxious gas emissions and alleviates diarrhea | [93] |
| Baicalin | 10 mg/kg BW, 1 time/d, 5 d; intramuscular injection | Weaned piglets | 28–35 d | Prevents swine edema disease | [94] |
| Baicalin | 500 mg/kg | Weaned piglets | - | Restores intestinal health | [95] |
| Mixed herbs containing S. baicalensis | 1000 mg/kg | Sows and suckling piglets | - | Improves growth performance, maternal metabolism, and transmission of antibodies | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Qiu, Y.; Tian, M.; Wang, L.; Gao, K.; Yang, X.; Jiang, Z. Flavonoids from Scutellaria baicalensis: Promising Alternatives for Enhancing Swine Production and Health. Int. J. Mol. Sci. 2025, 26, 3703. https://doi.org/10.3390/ijms26083703
Wu J, Qiu Y, Tian M, Wang L, Gao K, Yang X, Jiang Z. Flavonoids from Scutellaria baicalensis: Promising Alternatives for Enhancing Swine Production and Health. International Journal of Molecular Sciences. 2025; 26(8):3703. https://doi.org/10.3390/ijms26083703
Chicago/Turabian StyleWu, Jing, Yueqin Qiu, Min Tian, Li Wang, Kaiguo Gao, Xuefen Yang, and Zongyong Jiang. 2025. "Flavonoids from Scutellaria baicalensis: Promising Alternatives for Enhancing Swine Production and Health" International Journal of Molecular Sciences 26, no. 8: 3703. https://doi.org/10.3390/ijms26083703
APA StyleWu, J., Qiu, Y., Tian, M., Wang, L., Gao, K., Yang, X., & Jiang, Z. (2025). Flavonoids from Scutellaria baicalensis: Promising Alternatives for Enhancing Swine Production and Health. International Journal of Molecular Sciences, 26(8), 3703. https://doi.org/10.3390/ijms26083703







