ANAC042 Regulates the Biosynthesis of Conserved- and Lineage-Specific Phytoalexins in Arabidopsis
Abstract
1. Introduction
2. Results
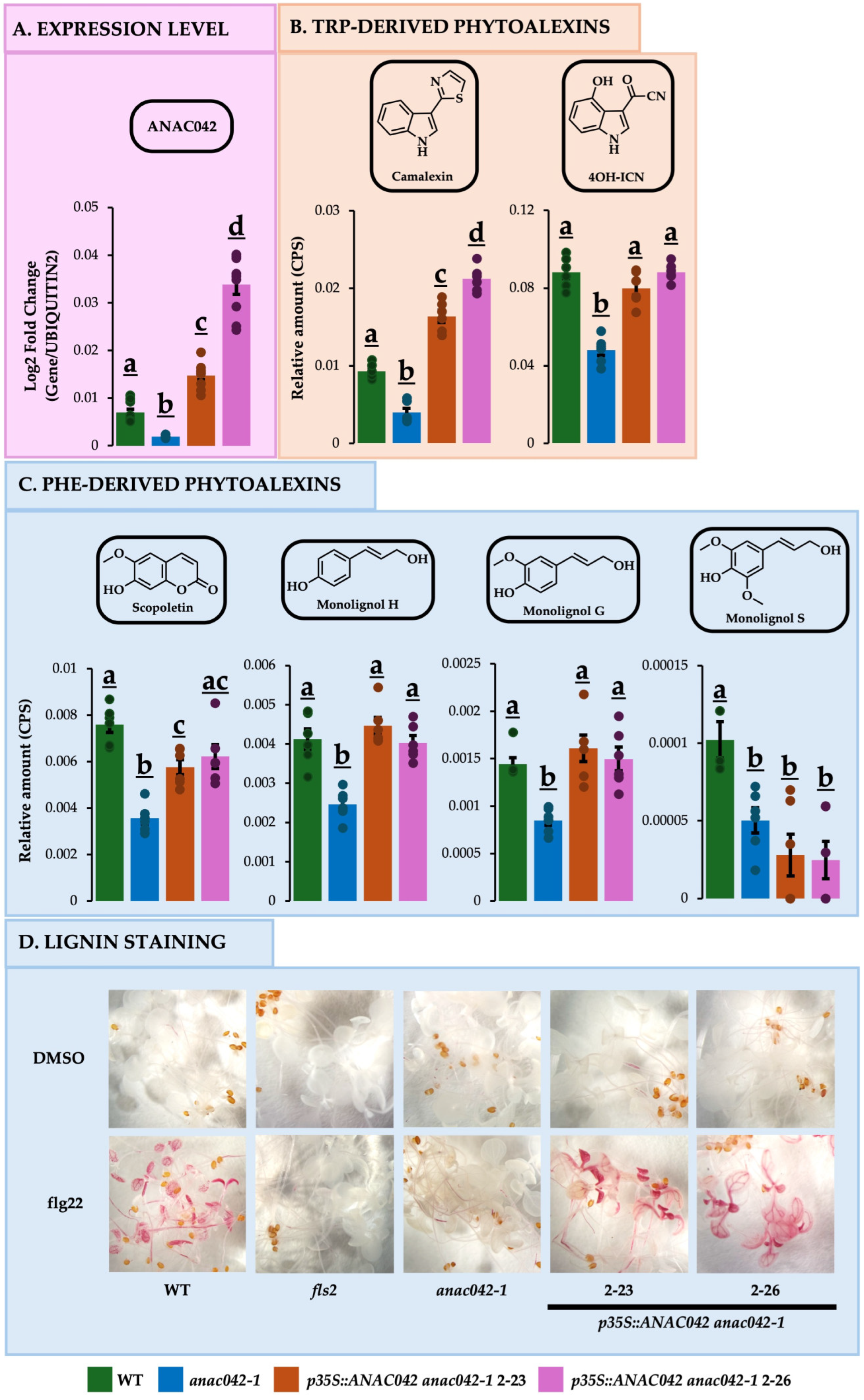
2.1. Arabidopsis Loss-of-Function Mutant anac042-1 Is Deficient in Phe- and Trp-Derived Phytoalexins
2.2. Overexpressing ANAC042 in anac042-1 Background Restored or Exceeded Wildtype Amounts of Phe- and Trp-DERIVED Phytoalexins
2.3. Overexpressing ANAC042 in anac042-1 Background Restored or Exceeded Wildtype Levels of Phe and Trp Phytoalexin Gene Expressions
2.4. ANAC042 Directly Activates the Expression of Scopoletin, Monolignol, 4OH-ICN, and Camalexin Biosynthetic Genes
3. Discussion
3.1. ANAC042 Is a Regulator of Diverse Phytoalexin Biosynthetic Pathways in Arabidopsis
3.2. NAC42-Type Transcription Factors Are Opportunistic Regulators That Coopt Lineage-Specific Genes into Pathogen-Inducible Biochemical Defenses
3.3. ANAC042 as a Member of a Cooperative Network That Regulates Phytoalexin Biosynthesis
3.4. Limitations and Future Directions
4. Materials and Methods
4.1. Chemicals
4.2. Cloning and Plasmid Constructs
4.3. Plant Materials
4.4. Metabolites Analyses
4.5. Lignin Staining
4.6. RNA Extraction and Gene Expression Measurements (qRT-PCR)
4.7. Subcellular Localization
4.8. Y1H
4.9. Luciferase Transactivation Assay
4.10. Statistical Analysis
4.11. Accession Numbers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, L.; Wang, P.; Yan, X.; Li, J.; Ma, L.; Hu, M.; Ge, X.; Li, F.; Hou, Y. Feruloyl-CoA 6′-hydroxylase-mediated scopoletin accumulation enhances cotton resistance to Verticillium dahliae. Plant Physiol. 2024, 196, 3007–3022. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, R.N.; Locci, F.; Wanke, F.; Zhang, L.S.; Saile, S.C.; Joe, A.; Karelina, D.; Hua, C.L.; Fröhlich, K.; Wan, W.L.; et al. The EDS1-PAD4-ADR1 node mediates Arabidopsis pattern-triggered immunity. Nature 2021, 598, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, R.N.; Gust, A.A.; Nürnberger, T. Plant immunity unified. Nat. Plants 2021, 7, 382–383. [Google Scholar] [CrossRef]
- Saijo, Y.; Loo, E.P.; Yasuda, S. Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 2018, 93, 592–613. [Google Scholar] [CrossRef]
- Tsuji, J.; Jackson, E.P.; Gage, D.A.; Hammerschmidt, R.; Somerville, S.C. Phytoalexin accumulation in Arabidopsis thaliana during the hypersensitive reaction to Pseudomonas syringae pv syringae. Plant Physiol. 1992, 98, 1304–1309. [Google Scholar] [CrossRef]
- Glazebrook, J.; Zook, M.; Mert, F.; Kagan, I.; Rogers, E.E.; Crute, I.R.; Holub, E.B.; Hammerschmidt, R.; Ausubel, F.M. Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics 1997, 146, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, J.; Rogers, E.E.; Ausubel, F.M. Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics 1996, 143, 973–982. [Google Scholar] [CrossRef]
- Glazebrook, J.; Ausubel, F.M. Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc. Natl. Acad. Sci. USA 1994, 91, 8955–8959. [Google Scholar] [CrossRef]
- Thomma, B.P.; Nelissen, I.; Eggermont, K.; Broekaert, W.F. Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J. 1999, 19, 163–171. [Google Scholar] [CrossRef]
- Zhou, N.; Tootle, T.L.; Glazebrook, J. Arabidopsis PAD3, a gene required for camalexin biosynthesis, encodes a putative cytochrome P450 monooxygenase. Plant Cell 1999, 11, 2419–2428. [Google Scholar] [CrossRef]
- Nafisi, M.; Goregaoker, S.; Botanga, C.J.; Glawischnig, E.; Olsen, C.E.; Halkier, B.A.; Glazebrook, J. Arabidopsis cytochrome P450 monooxygenase 71A13 catalyzes the conversion of indole-3-acetaldoxime in camalexin synthesis. Plant Cell 2007, 19, 2039–2052. [Google Scholar] [CrossRef] [PubMed]
- Glawischnig, E.; Hansen, B.G.; Olsen, C.E.; Halkier, B.A. Camalexin is synthesized from indole-3-acetaldoxime, a key branching point between primary and secondary metabolism in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Millet, Y.A.; Danna, C.H.; Clay, N.K.; Songnuan, W.; Simon, M.D.; Werck-Reichhart, D.; Ausubel, F.M. Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell 2010, 22, 973–990. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Mu, Q.; Wang, X.; Zhang, J.; Yu, H.; Huang, T.; He, Y.; Dai, S.; Meng, X. Multilayered synergistic regulation of phytoalexin biosynthesis by ethylene, jasmonate, and MAPK signaling pathways in Arabidopsis. Plant Cell 2022, 34, 3066–3087. [Google Scholar] [CrossRef]
- Ferrari, S.; Plotnikova, J.M.; De Lorenzo, G.; Ausubel, F.M. Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J. 2003, 35, 193–205. [Google Scholar] [CrossRef]
- Ren, D.; Liu, Y.; Yang, K.-Y.; Han, L.; Mao, G.; Glazebrook, J.; Zhang, S. A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2008, 105, 5638–5643. [Google Scholar] [CrossRef]
- Xu, J.; Meng, J.; Meng, X.Z.; Zhao, Y.T.; Liu, J.M.; Sun, T.F.; Liu, Y.D.; Wang, Q.M.; Zhang, S.Q. Pathogen-Responsive MPK3 and MPK6 Reprogram the Biosynthesis of Indole Glucosinolates and Their Derivatives in Arabidopsis Immunity. Plant Cell 2016, 28, 1144–1162. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.; Tong, G.; Xi, C.; Liu, J.; Zhao, H.; Wang, Y.; Ren, D.; Han, S. MPK3/MPK6-mediated phosphorylation of ERF72 positively regulates resistance to Botrytis cinerea through directly and indirectly activating the transcription of camalexin biosynthesis enzymes. J. Exp. Bot. 2022, 73, 413–428. [Google Scholar] [CrossRef]
- Zhou, J.G.; Wang, X.Y.; He, Y.X.; Sang, T.; Wang, P.C.; Dai, S.J.; Zhang, S.Q.; Meng, X.Z. Differential Phosphorylation of the Transcription Factor WRKY33 by the Protein Kinases CPK5/CPK6 and MPK3/MPK6 Cooperatively Regulates Camalexin Biosynthesis in Arabidopsis. Plant Cell 2020, 32, 2621–2638. [Google Scholar] [CrossRef]
- Wang, W.H.; Zhang, H.F.; Wei, X.Y.; Yang, L.; Yang, B.; Zhang, L.; Li, J.; Jiang, Y.Q. Functional characterization of calcium-dependent protein kinase (CPK) 2 gene from oilseed rape (Brassica napus L.) in regulating reactive oxygen species signaling and cell death control. Gene 2018, 651, 49–56. [Google Scholar] [CrossRef]
- Cheval, C.; Perez, M.; Leba, L.-J.; Ranty, B.; Perochon, A.; Reichelt, M.; Mithöfer, A.; Robe, E.; Mazars, C.; Galaud, J.-P. PRR2, a pseudo-response regulator, promotes salicylic acid and camalexin accumulation during plant immunity. Sci. Rep. 2017, 7, 6979. [Google Scholar] [CrossRef]
- Saga, H.; Ogawa, T.; Kai, K.; Suzuki, H.; Ogata, Y.; Sakurai, N.; Shibata, D.; Ohta, D. Identification and Characterization of ANAC042, a Transcription Factor Family Gene Involved in the Regulation of Camalexin Biosynthesis in Arabidopsis. Mol. Plant-Microbe Interact. 2012, 25, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Birkenbihl, R.P.; Diezel, C.; Somssich, I.E. Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection. Plant Physiol. 2012, 159, 266–285. [Google Scholar] [CrossRef]
- Jeandet, P.; Hébrard, C.; Deville, M.A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the Role of Phytoalexins in Plant-Microorganism Interactions and Human Health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef] [PubMed]
- Monsalvo, I.; Lin, J.; Kovinich, N. Phytoalexin Gene Regulation in Arabidopsis thaliana-On the Verge of a Paradigm Shift? Curr. Plant Biol. 2024, 39, 100367. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, F.; Wang, Y.; Li, S.; Zhang, D.; Liang, W.; Yang, Q. Scopoletin negatively regulates the HOG pathway and exerts antifungal activity against Botrytis cinerea by interfering with infection structures, cell wall, and cell membrane formation. Phytopathol. Res. 2024, 6, 1. [Google Scholar] [CrossRef]
- Antika, L.D.; Tasfiyati, A.N.; Hikmat, H.; Septama, A.W. Scopoletin: A review of its source, biosynthesis, methods of extraction, and pharmacological activities. Z. Für Naturforschung C 2022, 77, 303–316. [Google Scholar] [CrossRef]
- Chezem, W.R.; Memon, A.; Li, F.S.; Weng, J.K.; Clay, N.K. SG2-type R2R3-MYB transcription factor MYB15 controls defense-induced lignification and basal immunity in Arabidopsis. Plant Cell 2017, 29, 1907–1926. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Ren, J.; Jia, F.; Zeng, H.; Li, G.; Yang, X. Ethylene-responsive factor ERF114 mediates fungal pathogen effector PevD1-induced disease resistance in Arabidopsis thaliana. Mol. Plant Pathol. 2022, 23, 819–831. [Google Scholar] [CrossRef]
- Ninkuu, V.; Yan, J.; Fu, Z.; Yang, T.; Ziemah, J.; Ullrich, M.S.; Kuhnert, N.; Zeng, H. Lignin and its pathway-associated phytoalexins modulate plant defense against fungi. J. Fungi 2022, 9, 52. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Liu, H.; Zhang, D.; Shi, Q.-S.; Zhong, X.-Q.; Guo, Y.; Xie, X.-B. High value valorization of lignin as environmental benign antimicrobial. Mater. Today Bio 2023, 18, 100520. [Google Scholar] [CrossRef]
- Kai, K.; Shimizu, B.-i.; Mizutani, M.; Watanabe, K.; Sakata, K. Accumulation of coumarins in Arabidopsis thaliana. Phytochemistry 2006, 67, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Miao, H.; Chen, L.; Wang, M.; Xia, C.; Zeng, W.; Sun, B.; Zhang, F.; Zhang, S.; Li, C. WRKY33-mediated indolic glucosinolate metabolic pathway confers resistance against Alternaria brassicicola in Arabidopsis and Brassica crops. J. Integr. Plant Biol. 2022, 64, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Rajniak, J.; Barco, B.; Clay, N.K.; Sattely, E.S. A new cyanogenic metabolite in Arabidopsis required for inducible pathogen defence. Nature 2015, 525, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Kovinich, N. Regulation of phytoalexin biosynthesis for agriculture and human health. Phytochem. Rev. 2021, 20, 483–505. [Google Scholar] [CrossRef]
- Barco, B.; Kim, Y.; Clay, N.K. Expansion of a core regulon by transposable elements promotes Arabidopsis chemical diversity and pathogen defense. Nat. Commun. 2019, 10, 3444. [Google Scholar] [CrossRef]
- Zheng, Z.Y.; Abu Qamar, S.; Chen, Z.X.; Mengiste, T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 2006, 48, 592–605. [Google Scholar] [CrossRef]
- Qiu, J.L.; Fiil, B.K.; Petersen, K.; Nielsen, H.B.; Botanga, C.J.; Thorgrimsen, S.; Palma, K.; Suarez-Rodriguez, M.C.; Sandbech-Clausen, S.; Lichota, J. Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J. 2008, 27, 2214–2221. [Google Scholar] [CrossRef]
- Barco, B.; Clay, N.K. Hierarchical and Dynamic Regulation of Defense-Responsive Specialized Metabolism by WRKY and MYB Transcription Factors. Front. Plant Sci. 2020, 10, 1775. [Google Scholar] [CrossRef]
- Wu, A.H.; Allu, A.D.; Garapati, P.; Siddiqui, H.; Dortay, H.; Zanor, M.I.; Asensi-Fabado, M.A.; Munné-Bosch, S.; Antonio, C.; Tohge, T.; et al. JUNGBRUNNEN1, a Reactive Oxygen Species-Responsive NAC Transcription Factor, Regulates Longevity in Arabidopsis. Plant Cell 2012, 24, 482–506. [Google Scholar] [CrossRef]
- Ogawa, S.; Miyamoto, K.; Nemoto, K.; Sawasaki, T.; Yamane, H.; Nojiri, H.; Okada, K. OsMYC2, an essential factor for JA-inductive sakuranetin production in rice, interacts with MYC2-like proteins that enhance its transactivation ability. Sci. Rep. 2017, 7, 40175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, J.; Li, L.; Zhang, X.; Chen, L.; Zhou, Z.; Wang, J.; Sheng, Q.; Liang, Z.; Hong, G. Single-repeat MYB transcription factor, OsMYB1R, enhanced phytoalexin sakuranetin accumulation and Magnaporthe oryzae resistance. Curr. Plant Biol. 2024, 38, 100351. [Google Scholar] [CrossRef]
- Jahan, M.A.; Harris, B.; Lowery, M.; Infante, A.M.; Percifield, R.J.; Kovinich, N. Glyceollin Transcription Factor GmMYB29A2 Regulates Soybean Resistance to Phytophthora sojae. Plant Physiol. 2020, 183, 530–546. [Google Scholar] [CrossRef]
- Jahan, M.A.; Harris, B.; Lowery, M.; Coburn, K.; Infante, A.M.; Percifield, R.J.; Ammer, A.G.; Kovinich, N. The NAC family transcription factor GmNAC42–1 regulates biosynthesis of the anticancer and neuroprotective glyceollins in soybean. BMC Genom. 2019, 20, 149. [Google Scholar] [CrossRef]
- Liu, S.A.; Kracher, B.; Ziegler, J.; Birkenbihl, R.P.; Somssich, I.E. Negative regulation of ABA signaling by WRKY33 is critical for Arabidopsis immunity towards Botrytis cinerea 2100. eLife 2015, 4, e07295. [Google Scholar] [CrossRef]
- Lin, J.; Monsalvo, I.; Ly, M.; Jahan, M.A.; Wi, D.; Martirosyan, I.; Kovinich, N. RNA-Seq Dissects Incomplete Activation of Phytoalexin Biosynthesis by the Soybean Transcription Factors GmMYB29A2 and GmNAC42-1. Plants 2023, 12, 545. [Google Scholar] [CrossRef]
- Lin, J.; Monsalvo, I.; Jahan, M.A.; Ly, M.; Wi, D.; Martirosyan, I.; Jahan, I.; Kovinich, N. ABA-regulated JAZ1 Suppresses Phytoalexin Biosynthesis by Binding GmNAC42-1 in Soybean. Curr. Plant Biol. 2025, 42, 100453. [Google Scholar] [CrossRef]
- Singh, D.; Singh, N.; Dwivedi, S.; Trivedi, P.K. Transcriptional regulation of secondary plant product biosynthesis: Insights into flavonoid, alkaloid, and terpenoid pathways. Plant Cell Tissue Organ Cult. (PCTOC) 2025, 160, 6. [Google Scholar] [CrossRef]
- Zhang, X.; Henriques, R.; Lin, S.-S.; Niu, Q.-W.; Chua, N.-H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef]
- Denoux, C.; Galletti, R.; Mammarella, N.; Gopalan, S.; Werck, D.; De Lorenzo, G.; Ferrari, S.; Ausubel, F.M.; Dewdney, J. Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol. Plant 2008, 1, 423–445. [Google Scholar] [CrossRef]
- Parasecolo, L.; Monsalvo, I.M.; Kovinich, N.; Ifa, D.R. Development of a Matrix-Assisted Laser Desorption Ionization High Resolution Mass Spectrometry Method for the Quantification of Camalexin and Scopoletin in Arabidopsis thaliana. Rapid Commun. Mass Spectrom. 2025, 39, e9973. [Google Scholar] [CrossRef] [PubMed]
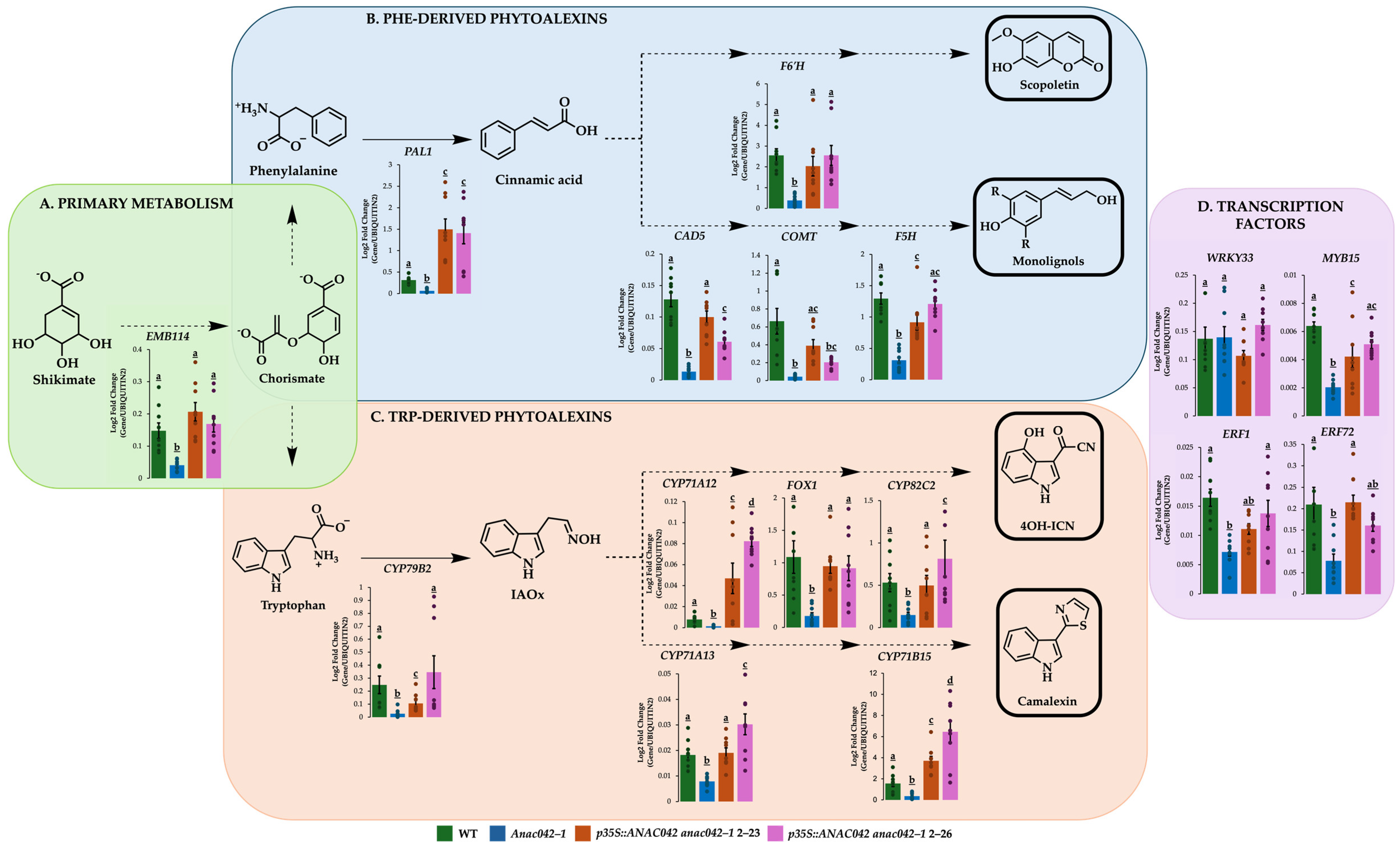

 ) indicate the direction of genes involved in phytoalexin biosynthesis starting at PAL1 (Phe-derived phytoalexins) and CYP79B2 (Trp-derived phytoalexins); Purple arrows (
) indicate the direction of genes involved in phytoalexin biosynthesis starting at PAL1 (Phe-derived phytoalexins) and CYP79B2 (Trp-derived phytoalexins); Purple arrows ( ) indicate direct regulation of the gene; Green dotted arrows (
) indicate direct regulation of the gene; Green dotted arrows ( ) indicated regulation of the gene by qRT-PCR, but that it remains unknown whether the corresponding genes are regulated directly or indirectly. Gene names: GSTF11, GLUTATHIONE S–TRANSFERASE F11; GGP1, Γ–GLUTAMYL PEPTIDASE 1; C4H, CINNAMIC ACID 4–HYDROXYLASE; 4CL, 4–COUMARATE–COENZYME A LIGASE; CCR, CINNAMOYL–COA REDUCTASE; SGT, SCOPOLETIN–GLUCOSYLTRANSFERASE.
) indicated regulation of the gene by qRT-PCR, but that it remains unknown whether the corresponding genes are regulated directly or indirectly. Gene names: GSTF11, GLUTATHIONE S–TRANSFERASE F11; GGP1, Γ–GLUTAMYL PEPTIDASE 1; C4H, CINNAMIC ACID 4–HYDROXYLASE; 4CL, 4–COUMARATE–COENZYME A LIGASE; CCR, CINNAMOYL–COA REDUCTASE; SGT, SCOPOLETIN–GLUCOSYLTRANSFERASE.
 ) indicate the direction of genes involved in phytoalexin biosynthesis starting at PAL1 (Phe-derived phytoalexins) and CYP79B2 (Trp-derived phytoalexins); Purple arrows (
) indicate the direction of genes involved in phytoalexin biosynthesis starting at PAL1 (Phe-derived phytoalexins) and CYP79B2 (Trp-derived phytoalexins); Purple arrows ( ) indicate direct regulation of the gene; Green dotted arrows (
) indicate direct regulation of the gene; Green dotted arrows ( ) indicated regulation of the gene by qRT-PCR, but that it remains unknown whether the corresponding genes are regulated directly or indirectly. Gene names: GSTF11, GLUTATHIONE S–TRANSFERASE F11; GGP1, Γ–GLUTAMYL PEPTIDASE 1; C4H, CINNAMIC ACID 4–HYDROXYLASE; 4CL, 4–COUMARATE–COENZYME A LIGASE; CCR, CINNAMOYL–COA REDUCTASE; SGT, SCOPOLETIN–GLUCOSYLTRANSFERASE.
) indicated regulation of the gene by qRT-PCR, but that it remains unknown whether the corresponding genes are regulated directly or indirectly. Gene names: GSTF11, GLUTATHIONE S–TRANSFERASE F11; GGP1, Γ–GLUTAMYL PEPTIDASE 1; C4H, CINNAMIC ACID 4–HYDROXYLASE; 4CL, 4–COUMARATE–COENZYME A LIGASE; CCR, CINNAMOYL–COA REDUCTASE; SGT, SCOPOLETIN–GLUCOSYLTRANSFERASE.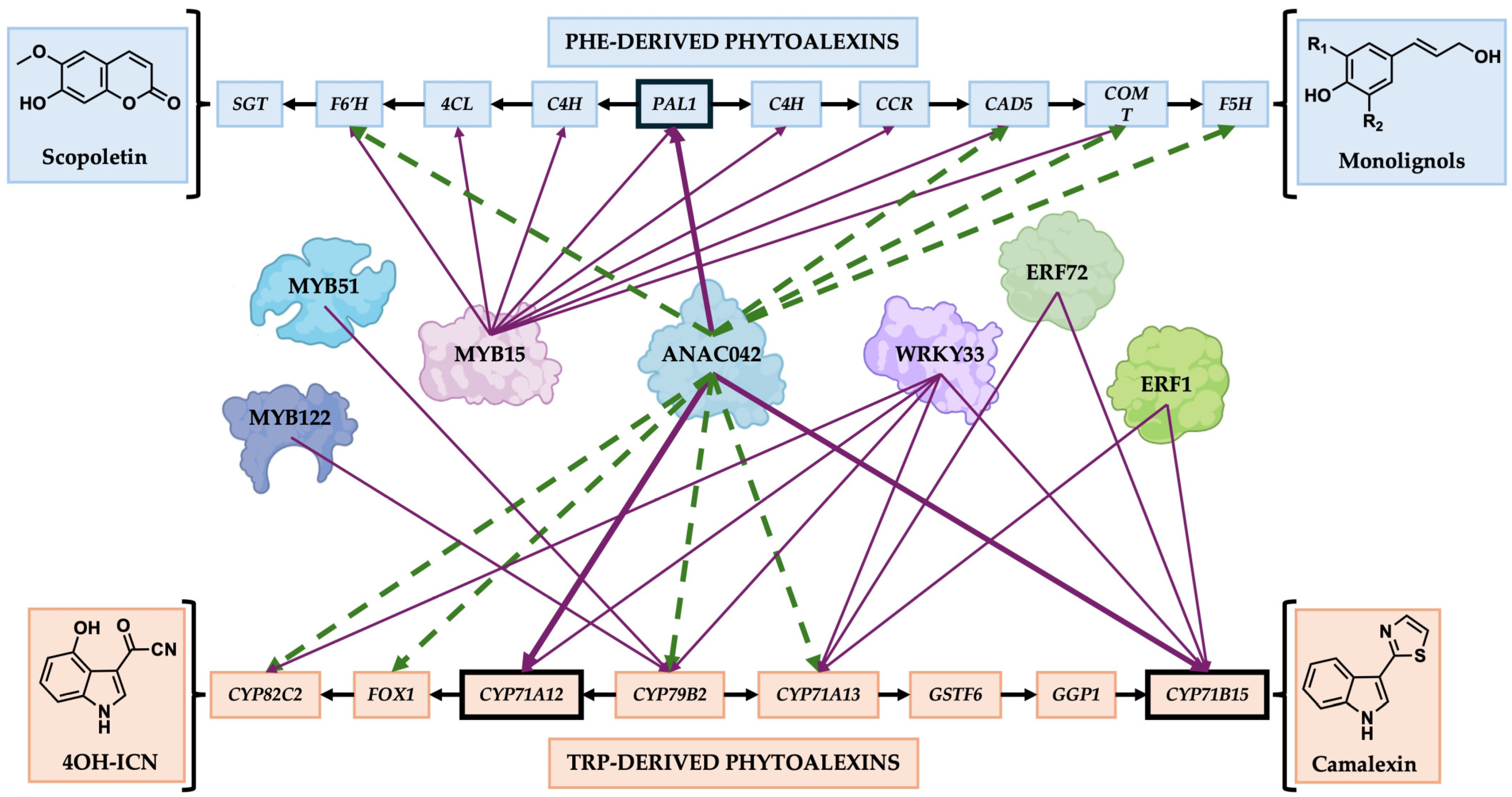
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monsalvo, I.; Parasecolo, L.; Pullano, S.; Lin, J.; Shahabi, A.; Ly, M.; Kwon, H.; Mathur, K.; Rodrillo, K.A.M.; Ifa, D.R.; et al. ANAC042 Regulates the Biosynthesis of Conserved- and Lineage-Specific Phytoalexins in Arabidopsis. Int. J. Mol. Sci. 2025, 26, 3683. https://doi.org/10.3390/ijms26083683
Monsalvo I, Parasecolo L, Pullano S, Lin J, Shahabi A, Ly M, Kwon H, Mathur K, Rodrillo KAM, Ifa DR, et al. ANAC042 Regulates the Biosynthesis of Conserved- and Lineage-Specific Phytoalexins in Arabidopsis. International Journal of Molecular Sciences. 2025; 26(8):3683. https://doi.org/10.3390/ijms26083683
Chicago/Turabian StyleMonsalvo, Ivan, Leonardo Parasecolo, Sarah Pullano, Jie Lin, Aida Shahabi, Melissa Ly, Hyejung Kwon, Khushi Mathur, Karl Angelo M. Rodrillo, Demian R. Ifa, and et al. 2025. "ANAC042 Regulates the Biosynthesis of Conserved- and Lineage-Specific Phytoalexins in Arabidopsis" International Journal of Molecular Sciences 26, no. 8: 3683. https://doi.org/10.3390/ijms26083683
APA StyleMonsalvo, I., Parasecolo, L., Pullano, S., Lin, J., Shahabi, A., Ly, M., Kwon, H., Mathur, K., Rodrillo, K. A. M., Ifa, D. R., & Kovinich, N. (2025). ANAC042 Regulates the Biosynthesis of Conserved- and Lineage-Specific Phytoalexins in Arabidopsis. International Journal of Molecular Sciences, 26(8), 3683. https://doi.org/10.3390/ijms26083683






