Association Between Pentraxins and Obesity in Prediabetes and Newly Diagnosed Type 2 Diabetes Mellitus Patients
Abstract
1. Introduction
2. Results
2.1. Demographic Characteristics of Patients with Prediabetes and Diabetes
2.2. Laboratory Characteristics of Patients in the Groups Under Study
2.3. Comparing the Inflammatory Biomarker Values Between the Studied Groups
2.4. Comparing the Inflammatory Biomarkers Between the BMI Categories in the Studied Groups
2.5. Comparing the Inflammatory Biomarker Values Between the HbA1c Quartiles in the Studied Groups
2.6. Correlations Between Inflammatory Biomarkers and the Different Obesity-Related Indices in the PreDM Group
2.7. Correlations Between Inflammatory Biomarkers and the Different Obesity-Related Indices in the T2DM Group
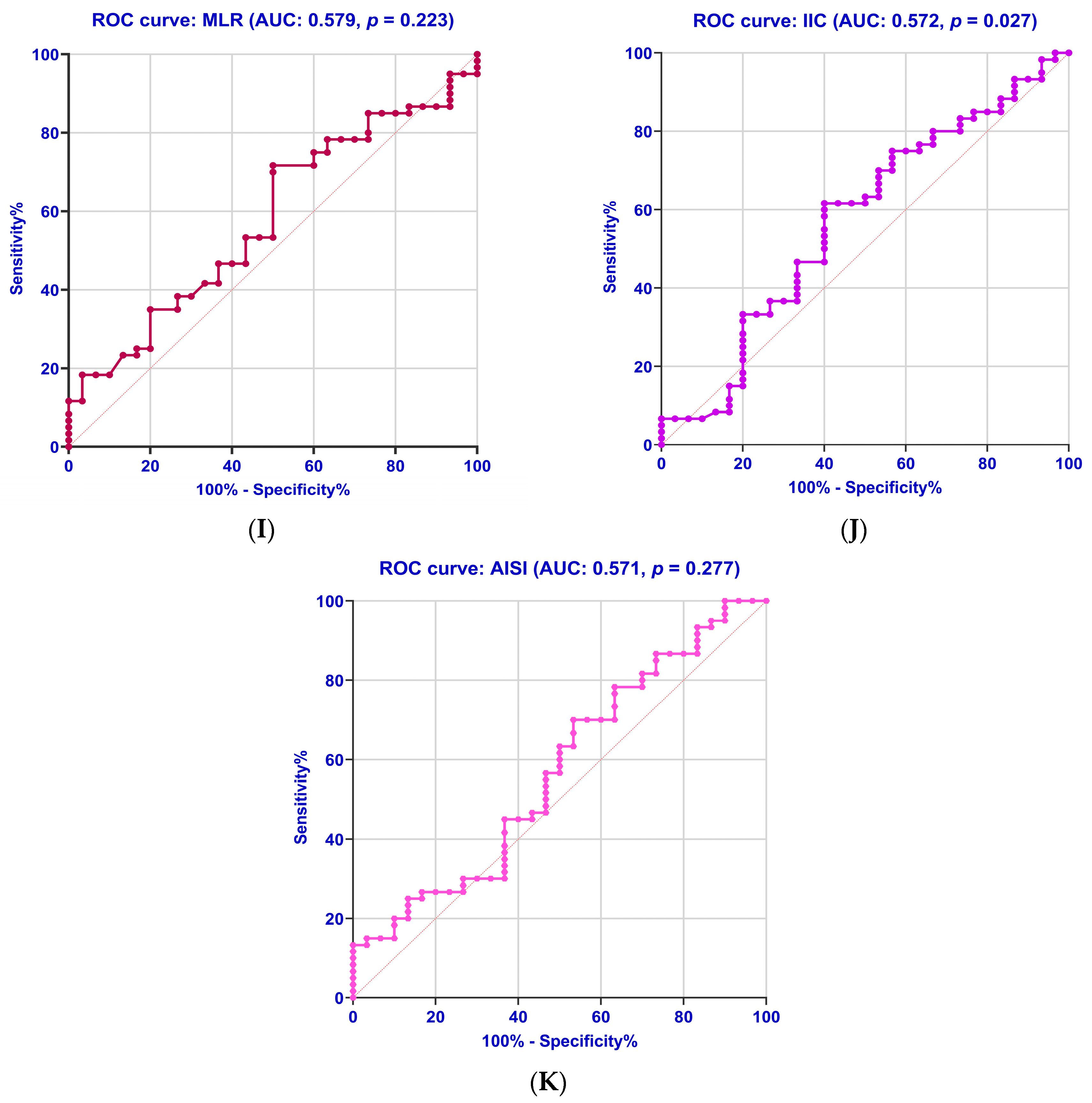
2.8. Diagnostic Accuracy of the Biomarkers
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. Diagnosis of Diabetes and Prediabetes in Laboratories
4.3. Medical Background, Evaluation of Biometric Parameters, and Demographic Information
4.4. Evaluation of Different Obesity-Related Indices (BMI, WHR, WHtR, and BAI)
4.5. Laboratory Investigations
Sample Collection
4.6. Immunological Assessment
4.7. Calculations for the Prognostic Nutritional Index and Glasgow Prognostic Score
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 6 February 2025).
- Available online: https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/ (accessed on 6 February 2025).
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef] [PubMed]
- Bragg, F.; Holmes, M.V.; Iona, A.; Guo, Y.; Du, H.; Chen, Y.; Bian, Z.; Yang, L.; Herrington, W.; Bennett, D.; et al. Association Between Diabetes and Cause-Specific Mortality in Rural and Urban Areas of China. JAMA 2017, 317, 280–289. [Google Scholar] [CrossRef]
- Marques-Vidal, P.; Schmid, R.; Bochud, M.; Bastardot, F.; von Känel, R.; Paccaud, F.; Glaus, J.; Preisig, M.; Waeber, G.; Vollenweider, P. Adipocytokines, hepatic and inflammatory biomarkers and incidence of type 2 diabetes. the CoLaus study. PLoS ONE 2012, 7, e51768. [Google Scholar] [CrossRef]
- Lainampetch, J.; Panprathip, P.; Phosat, C.; Chumpathat, N.; Prangthip, P.; Soonthornworasiri, N.; Puduang, S.; Wechjakwen, N.; Kwanbunjan, K. Association of Tumor Necrosis Factor Alpha, Interleukin 6, and C-Reactive Protein with the Risk of Developing Type 2 Diabetes: A Retrospective Cohort Study of Rural Thais. J. Diabetes Res. 2019, 2019, 9051929. [Google Scholar] [CrossRef] [PubMed]
- Hoshikawa, T.; Okamoto, N.; Natsuyama, T.; Fujii, R.; Ikenouchi, A.; Honma, Y.; Harada, M.; Yoshimura, R. Associations of Serum Cytokines, Growth Factors, and High-Sensitivity C-Reactive Protein Levels in Patients With Major Depression with and Without Type 2 Diabetes Mellitus: An Explanatory Investigation. Neuropsychiatr. Dis. Treat. 2022, 18, 173–186. [Google Scholar] [CrossRef]
- Tahir, A.; Martinez, P.J.; Ahmad, F.; Fisher-Hoch, S.P.; McCormick, J.; Gay, J.L.; Mirza, S.; Chaudhary, S.U. An evaluation of lipid profile and pro-inflammatory cytokines as determinants of cardiovascular disease in those with diabetes: A study on a Mexican American cohort. Sci. Rep. 2021, 11, 2435. [Google Scholar] [CrossRef] [PubMed]
- Alzamil, H. Elevated Serum TNF-α Is Related to Obesity in Type 2 Diabetes Mellitus and Is Associated with Glycemic Control and Insulin Resistance. J. Obes. 2020, 2020, 5076858. [Google Scholar] [CrossRef]
- Velikova, T.V.; Kabakchieva, P.P.; Assyov, Y.S.; Georgiev, T.A. Targeting Inflammatory Cytokines to Improve Type 2 Diabetes Control. Biomed. Res. Int. 2021, 2021, 7297419. [Google Scholar] [CrossRef]
- Bahgat, M.M.; Ibrahim, D.R. Proinflammatory cytokine polarization in type 2 diabetes. Cent. Eur. J. Immunol. 2020, 45, 170–175. [Google Scholar] [CrossRef]
- Elimam, H.; Abdulla, A.M.; Taha, I.M. Inflammatory markers and control of type 2 diabetes mellitus. Diabetes Metab. Syndr. 2019, 13, 800–804. [Google Scholar] [CrossRef]
- Lin, C.C.; Li, C.I.; Liu, C.S.; Liao, L.N.; Yang, C.W.; Lin, C.H.; Yang, S.Y.; Li, T.C. Association of high-sensitivity C-reactive protein and diabetic nephropathy in patients with type 2 diabetes: A Mendelian randomization study. BMJ Open Diabetes Res. Care 2023, 11, e003197. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Matia-Garcia, I.; Vadillo, E.; Pelayo, R.; Muñoz-Valle, J.F.; García-Chagollán, M.; Loaeza-Loaeza, J.; Vences-Velázquez, A.; Salgado-Goytia, L.; García-Arellano, S.; Parra-Rojas, I. Th1/Th2 Balance in Young Subjects: Relationship with Cytokine Levels and Metabolic Profile. J. Inflamm. Res. 2012, 14, 6587–6600. [Google Scholar] [CrossRef]
- Mraz, M.; Haluzik, M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 2014, 222, R113–R127. [Google Scholar] [CrossRef]
- Akalın Ertürk, B.; Gülbahar, Ö.; Kaymak Şahap, S.; Deveci Bulut, T.S.; Çetinkaya, S.; Savaş Erdeve, Ş. The Level of Inflammatory Markers and Their Relationship with Fat Tissue Distribution in Children with Obesity and Type 2 Diabetes Mellitus. Turk. Arch. Pediatr. 2023, 58, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Wu, C.; Cao, L.; Wang, R.; Zhang, T.Y.; He, Z. The association between the neutrophil-to-lymphocyte ratio and type 2 diabetes mellitus: A cross-sectional study. BMC Endocr. Disord. 2024, 24, 107. [Google Scholar] [CrossRef]
- Basaran, E.; Aktas, G. The relationship of vitamin D levels with hemogram indices and metabolic parameters in patients with type 2 diabetes mellitus. AIMS Med. Sci. 2024, 11, 47–57. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Khaleel, M.; RM, P.; Jalily, Q.A.; Dhanekula, K.; Dinesh Eshwar, M. Neutrophil-to-Lymphocyte Ratio as a Potential Biomarker to Managing Type 2 Diabetes Mellitus and Predicting Disease Progression. Cureus 2024, 16, e55227. [Google Scholar] [CrossRef]
- Adane, T.; Melku, M.; Worku, Y.B.; Fasil, A.; Aynalem, M.; Kelem, A.; Getawa, S. The Association between Neutrophil-to-Lymphocyte Ratio and Glycemic Control in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2023, 2023, 3117396. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Babar, M.Z.M.; Akhtar, L.; Hussain, M.S. Neutrophil lymphocyte ratio (NLR): A well assessment tool of glycemic control in type 2 diabetic patients. Pak. J. Med. Sci. 2017, 33, 1366–1370. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, H.; Zhu, X.; Mao, F.; Zhang, S.; Shi, H.; Li, Y.; Lu, B. Neutrophil-to-lymphocyte ratio is associated with diabetic peripheral neuropathy in type 2 diabetes patients. Diabetes Res. Clin. Pract. 2017, 130, 90–97. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Liu, M.; Zhou, H.; Xu, H. Association between neutrophil-to-lymphocyte ratio and diabetic kidney disease in type 2 diabetes mellitus patients: A cross-sectional study. Front. Endocrinol. 2024, 14, 1285509. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Jia, W.; Wang, K.; Wang, W.; Diao, W.; Ou, F.; Ma, J.; Yang, Y. Association of the systemic immuno-inflammation index, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio with diabetic microvascular complications. Front. Endocrinol. 2024, 15, 1367376. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Li, C.; Li, X.; Sun, W.; Wang, Y. Association of systemic immune-inflammation index (SII) and aggregate index of systemic inflammation (AISI) with thyroid nodules in patients with type 2 diabetes mellitus: A retrospective study. BMC Endocr. Disord. 2023, 23, 251. [Google Scholar] [CrossRef]
- Tuzimek, A.; Dziedzic, E.A.; Beck, J.; Kochman, W. Correlations Between Acute Coronary Syndrome and Novel Inflammatory Markers (Systemic Immune-Inflammation Index, Systemic Inflammation Response Index, and Aggregate Index of Systemic Inflammation) in Patients with and without Diabetes or Prediabetes. J. Inflamm. Res. 2024, 17, 2623–2632. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Y.; Shu, Y.; Zhang, L.; Cheng, W.; Wang, L.; Shu, M.; Xue, B.; Wang, R.; Feng, Z.; et al. Combination model of neutrophil to high-density lipoprotein ratio and system inflammation response index is more valuable for predicting peripheral arterial disease in type 2 diabetic patients: A cross-sectional study. Front. Endocrinol. 2023, 14, 1100453. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Zhang, Y.; Dan, X.; Wu, X.; Yang, Y.; Chen, X.; Li, S.; Xu, Y.; Wan, Q.; et al. Increased Systemic Immune-Inflammation Index Was Associated with Type 2 Diabetic Peripheral Neuropathy: A Cross-Sectional Study in the Chinese Population. J. Inflamm. Res. 2023, 16, 6039–6053. [Google Scholar] [CrossRef]
- Yan, P.; Yang, Y.; Zhang, X.; Zhang, Y.; Li, J.; Wu, Z.; Dan, X.; Wu, X.; Chen, X.; Li, S.; et al. Association of systemic immune-inflammation index with diabetic kidney disease in patients with type 2 diabetes: A cross-sectional study in Chinese population. Front. Endocrinol. 2024, 14, 1307692. [Google Scholar] [CrossRef]
- Li, X.; Cui, L.; Xu, H. Association between systemic inflammation response index and chronic kidney disease: A population-based study. Front. Endocrinol. 2024, 15, 1329256. [Google Scholar] [CrossRef] [PubMed]
- Poenariu, I.S.; Boldeanu, L.; Ungureanu, B.S.; Caragea, D.C.; Cristea, O.M.; Pădureanu, V.; Siloși, I.; Ungureanu, A.M.; Statie, R.C.; Ciobanu, A.E.; et al. Interrelation of Hypoxia-Inducible Factor-1 Alpha (HIF-1 α) and the Ratio between the Mean Corpuscular Volume/Lymphocytes (MCVL) and the Cumulative Inflammatory Index (IIC) in Ulcerative Colitis. Biomedicines 2023, 11, 3137. [Google Scholar] [CrossRef]
- Rădulescu, P.M.; Căluianu, E.I.; Traşcă, E.T.; Mercuţ, D.; Georgescu, I.; Georgescu, E.F.; Ciupeanu-Călugăru, E.D.; Mercuţ, M.F.; Mercuţ, R.; Padureanu, V.; et al. The Impact of the COVID-19 Pandemic on Outcomes in Acute Pancreatitis: A Propensity Score Matched Study Comparing before and during the Pandemic. Diagnostics 2023, 13, 2446. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, P.M.; Davitoiu, D.V.; Baleanu, V.D.; Padureanu, V.; Ramboiu, D.S.; Surlin, M.V.; Bratiloveanu, T.C.; Georgescu, E.F.; Streba, C.T.; Mercut, R.; et al. Has COVID-19 Modified the Weight of Known Systemic Inflammation Indexes and the New Ones (MCVL and IIC) in the Assessment as Predictive Factors of Complications and Mortality in Acute Pancreatitis? Diagnostics 2022, 12, 3118. [Google Scholar] [CrossRef]
- Șerban, R.E.; Popescu, D.M.; Boldeanu, M.V.; Florescu, D.N.; Șerbănescu, M.S.; Șandru, V.; Panaitescu-Damian, A.; Forțofoiu, D.; Șerban, R.C.; Gherghina, F.L.; et al. The Diagnostic and Prognostic Role of Inflammatory Markers, Including the New Cumulative Inflammatory Index (IIC) and Mean Corpuscular Volume/Lymphocyte (MCVL), in Colorectal Adenocarcinoma. Cancers 2025, 17, 990. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, P.; Hrycek, A. Long pentraxin 3 (PTX3) in the light of its structure, mechanism of action and clinical implications. Autoimmunity 2012, 45, 119–128. [Google Scholar] [CrossRef]
- Inoue, K.; Kodama, T.; Daida, H. Pentraxin 3: A novel biomarker for inflammatory cardiovascular disease. Int. J. Vasc. Med. 2012, 2012, 657025. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.; Hu, W. Association of serum pentraxin 3 concentrations with diabetic nephropathy. J. Investig. Med. 2016, 64, 1124–1127. [Google Scholar] [CrossRef]
- Dawood, A.A.; Kamel, M.A.; Omar, T.A.; Agaba, A.A.M. Study of serum pentraxin 3 level in patients with diabetic nephropathy. Egypt. J. Intern. Med. 2020, 32, 2–9. [Google Scholar] [CrossRef]
- Li, B.; Tian, X.; Guo, S.; Zhang, M.; Li, J.; Zhai, N.; Wang, H.; Zhang, Y. Pentraxin-3 and adropin as inflammatory markers of early renal damage in type 2 diabetes patients. Int. Urol. Nephrol. 2020, 52, 2145–2152. [Google Scholar] [CrossRef]
- Yang, H.S.; Woo, J.E.; Lee, S.J.; Park, S.H.; Woo, J.M. Elevated plasma pentraxin 3 levels are associated with development and progression of diabetic retinopathy in Korean patients with type 2 diabetes mellitus. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5989–5997. [Google Scholar] [CrossRef] [PubMed]
- Karamfilova, V.; Assyov, Y.; Nedeva, I.; Gateva, A.; Ivanova, I.; Cherkezov, N.; Mateva, L.; Kamenov, Z. Increased Serum Pentraxin 3 Is Associated with Prediabetes and Type 2 Diabetes in Obese Patients with Nonalcoholic Fatty Liver Disease. Metab. Syndr. Relat. Disord. 2022, 20, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Waluś-Miarka, M.; Trojak, A.; Miarka, P.; Kapusta, M.; Kawalec, E.; Idzior-Waluś, B.; Małecki, M.T. Correlates of pentraxin 3 serum concentration in men and women with type 2 diabetes. Innate Immun. 2020, 26, 351–357. [Google Scholar] [CrossRef]
- Prospective Studies Collaboration and Asia Pacific Cohort Studies Collaboration. Sex-specific relevance of diabetes to occlusive vascular and other mortality: A collaborative meta-analysis of individual data from 980,793 adults from 68 prospective studies. Lancet Diabetes Endocrinol. 2018, 6, 538–546. [Google Scholar] [CrossRef]
- Watanabe, Y.; Yamaguchi, T.; Ishihara, N.; Nakamura, S.; Tanaka, S.; Oka, R.; Imamura, H.; Sato, Y.; Ban, N.; Kawana, H.; et al. 7-Ketocholesterol induces ROS-mediated mRNA expression of 12-lipoxygenase, cyclooxygenase-2 and pro-inflammatory cytokines in human mesangial cells: Potential role in diabetic nephropathy. Prostaglandins Other Lipid Mediat. 2018, 134, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Shore, A.C.; Colhoun, H.M.; Natali, A.; Palombo, C.; Khan, F.; Östling, G.; Aizawa, K.; Kennbäck, C.; Casanova, F.; Persson, M.; et al. Use of Vascular Assessments and Novel Biomarkers to Predict Cardiovascular Events in Type 2 Diabetes: The SUMMIT VIP Study. Diabetes Care 2018, 41, 2212–2219. [Google Scholar] [CrossRef]
- Takashi, Y.; Koga, M.; Matsuzawa, Y.; Saito, J.; Omura, M.; Nishikawa, T. Circulating pentraxin 3 is positively associated with chronic hyperglycemia but negatively associated with plasma aldosterone concentration. PLoS ONE 2018, 13, e0196526. [Google Scholar] [CrossRef]
- Uzun, S.; Ozari, M.; Gursu, M.; Karadag, S.; Behlul, A.; Sari, S.; Koldas, M.; Demir, S.; Karaali, Z.; Ozturk, S. Changes in the inflammatory markers with advancing stages of diabetic nephropathy and the role of pentraxin-3. Ren. Fail. 2016, 38, 1193–1198. [Google Scholar] [CrossRef]
- Shindo, A.; Tanemura, H.; Yata, K.; Hamada, K.; Shibata, M.; Umeda, Y.; Asakura, F.; Toma, N.; Sakaida, H.; Fujisawa, T.; et al. Inflammatory biomarkers in atherosclerosis: Pentraxin 3 can become a novel marker of plaque vulnerability. PLoS ONE 2014, 9, e100045. [Google Scholar] [CrossRef]
- Zhou, Y.; Ni, Z.; Zhang, J.; Zhang, W.; Wu, Q.; Shen, G.; Wang, Y.; Qian, J. Plasma pentraxin 3 may be a better marker of peripheral artery disease in hemodialysis patients than C-reactive protein. Vasc. Med. 2013, 18, 85–91. [Google Scholar] [CrossRef]
- Ristagno, G.; Fumagalli, F.; Bottazzi, B.; Mantovani, A.; Olivari, D.; Novelli, D.; Latini, R. Pentraxin 3 in Cardiovascular Disease. Front. Immunol. 2019, 10, 823. [Google Scholar] [CrossRef] [PubMed]
- Ahrițculesei, R.V.; Boldeanu, L.; Vladu, I.M.; Clenciu, D.; Mitrea, A.; Cîmpeanu, R.C.; Mustață, M.L.; Siloși, I.; Boldeanu, M.V.; Vere, C.C. Correlation Between Prognostic Nutritional Index, Glasgow Prognostic Score, and Different Obesity-Related Indices in People with Diabetes or Prediabetes. Diagnostics 2024, 14, 2661. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Aktas, G. Association between the Prognostic Nutritional Index and Chronic Microvascular Complications in Patients with Type 2 Diabetes Mellitus. J. Clin. Med. 2023, 12, 5952. [Google Scholar] [CrossRef] [PubMed]
- Efrem, I.C.; Moța, M.; Vladu, I.M.; Mitrea, A.; Clenciu, D.; Timofticiuc, D.C.P.; Diaconu, I.D.; Turcu, A.; Crișan, A.E.; Geormăneanu, C.; et al. A Study of Biomarkers Associated with Metabolic Dysfunction-Associated Fatty Liver Disease in Patients with Type 2 Diabetes. Diagnostics 2022, 12, 2426. [Google Scholar] [CrossRef]
- Protasiewicz Timofticiuc, D.C.; Vladu, I.M.; Ștefan, A.G.; Clenciu, D.; Mitrea, A.; Pădureanu, V.; Efrem, I.C.; Diaconu, I.D.; Turcu, A.; Țenea-Cojan, T.Ș.; et al. Associations of Chronic Diabetes Complications and Cardiovascular Risk with the Risk of Obstructive Sleep Apnea in Patients with Type 2 Diabetes. J. Clin. Med. 2022, 11, 4403. [Google Scholar] [CrossRef]
- Tang, L.; Xu, G.T.; Zhang, J.F. Inflammation in diabetic retinopathy: Possible roles in pathogenesis and potential implications for therapy. Neural Regen. Res. 2023, 18, 976–982. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, Y.; Li, K.; Liu, S.; Pang, C.; Gao, L.; Xie, J.; Wenjing, L.V.; Yu, H.; Deng, B. How inflammation dictates diabetic peripheral neuropathy: An enlightening review. CNS Neurosci. Ther. 2024, 30, e14477. [Google Scholar] [CrossRef]
- Forrester, J.V.; Kuffova, L.; Delibegovic, M. The Role of Inflammation in Diabetic Retinopathy. Front. Immunol. 2020, 11, 583687. [Google Scholar] [CrossRef]
- Yue, T.; Shi, Y.; Luo, S.; Weng, J.; Wu, Y.; Zheng, X. The role of inflammation in immune system of diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Front. Immunol. 2022, 13, 1055087. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, J.; Xiong, Y.; Wang, L.; Huang, X.; Sun, T.; Zha, B.; Wu, Y.; Yan, C.; Zang, S.; et al. Increased neutrophil count Is associated with the development of chronic kidney disease in patients with diabetes. J. Diabetes 2022, 14, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Wang, Y.; Fang, S.; Chen, Y.; Zhang, W.; Xia, F.; Wang, N.; Lu, Y. Associations Between the Neutrophil-to-Lymphocyte Ratio and Diabetic Complications in Adults with Diabetes: A Cross-Sectional Study. J. Diabetes Res. 2020, 2020, 6219545. [Google Scholar] [CrossRef]
- Wang, J.R.; Chen, Z.; Yang, K.; Yang, H.J.; Tao, W.Y.; Li, Y.P.; Jiang, Z.J.; Bai, C.F.; Yin, Y.C.; Duan, J.M.; et al. Association between neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and diabetic retinopathy among diabetic patients without a related family history. Diabetol. Metab. Syndr. 2020, 12, 55. [Google Scholar] [CrossRef]
- Jaaban, M.; Zetoune, A.B.; Hesenow, S.; Hessenow, R. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as novel risk markers for diabetic nephropathy in patients with type 2 diabetes. Heliyon 2021, 7, e07564. [Google Scholar] [CrossRef]
- Rezaei Shahrabi, A.; Arsenault, G.; Nabipoorashrafi, S.A.; Lucke-Wold, B.; Yaghoobpoor, S.; Meidani, F.Z.; Rahmati, R.; Ghaedi, A.; Khanzadeh, S. Relationship between neutrophil to lymphocyte ratio and diabetic peripheral neuropathy: A systematic review and meta-analysis. Eur. J. Med. Res. 2023, 28, 523. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Sun, L.; Zhang, C.; Wu, L.; Nie, G.; Huang, Z.; Xing, C.; Zhang, B.; Yuan, Y. Association of platelet-to-lymphocyte ratio with kidney clinicopathologic features and renal outcomes in patients with diabetic kidney disease. Int. Immunopharmacol. 2021, 93, 107413. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Song, Y.; Sun, Y.; Du, H.; Cai, Y.; You, Q.; Fu, H.; Shao, L. Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: Evidence from NHANES 2011–2018. Front. Endocrinol. 2022, 13, 1071465. [Google Scholar] [CrossRef]
- Wang, S.; Pan, X.; Jia, B.; Chen, S. Exploring the Correlation Between the Systemic Immune Inflammation Index (SII), Systemic Inflammatory Response Index (SIRI), and Type 2 Diabetic Retinopathy. Diabetes Metab. Syndr. Obes. 2023, 16, 3827–3836. [Google Scholar] [CrossRef]
- Klisic, A.; Scepanovic, A.; Kotur-Stevuljevic, J.; Ninic, A. Novel leukocyte and thrombocyte indexes in patients with prediabetes and type 2 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2775–2781. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, L.; Liu, J.; Li, W.; Bai, X.; Li, R.; Li, B.; Wang, L.; Zhou, J.; Wu, Y.; et al. Prognostic Value of Admission Mean Corpuscular Volume for Major Adverse Cardiovascular Events following Stent Implantation in Nondiabetic and Diabetic Patients with Acute Coronary Syndrome. Dis. Markers 2020, 2020, 7054596. [Google Scholar] [CrossRef]
- Hardikar, P.S.; Joshi, S.M.; Bhat, D.S.; Raut, D.A.; Katre, P.A.; Lubree, H.G.; Jere, A.; Pandit, A.N.; Fall, C.H.; Yajnik, C.S. Spuriously high prevalence of prediabetes diagnosed by HbA(1c) in young indians partly explained by hematological factors and iron deficiency anemia. Diabetes Care 2012, 35, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Ludidi, A.; Baloyi, M.C.; Khathi, A.; Sibiya, N.H.; Ngubane, P.S. The effects of Momordica balsamina methanolic extract on haematological function in streptozotocin-induced diabetic rats: Effects on selected markers. Biomed. Pharmacother. 2019, 116, 108925. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Hu, Z.-D.; Liu, S.-J.; Sun, Y.; Qin, Q.; Qin, B.-D.; Zhang, W.-W.; Zhang, J.-R.; Zhong, R.-Q.; Deng, A.-M. Prognostic value of red blood cell distribution width for patients with heart failure: A systematic review and meta-analysis of cohort studies. PLoS ONE 2014, 9, e104861. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S20–S42. [Google Scholar] [CrossRef]
- World Health Organization. Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Li, W.; Gong, X.; Wang, W.; Xiong, K.; Meng, J.; Li, Y.; Wang, L.; Liang, X.; Jin, L.; Huang, W. Association of different kinds of obesity with diabetic retinopathy in patients with type 2 diabetes. BMJ Open 2022, 12, e056332. [Google Scholar] [CrossRef]
- National Kidney Foundation. CKD-EPI Creatinine Equation 2021. Available online: https://www.kidney.org/professionals/gfr_calculator (accessed on 6 February 2025).
- Available online: https://www.mdcalc.com/calc/76/mdrd-gfr-equation (accessed on 6 February 2025).
- Available online: https://radiologyreviewarticles.com/radiology-calculators/prognostic-nutritional-index/ (accessed on 6 February 2025).
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Angerson, W.J.; Dunlop, D.J. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br. J. Cancer 2004, 90, 1704–1706. [Google Scholar] [CrossRef]

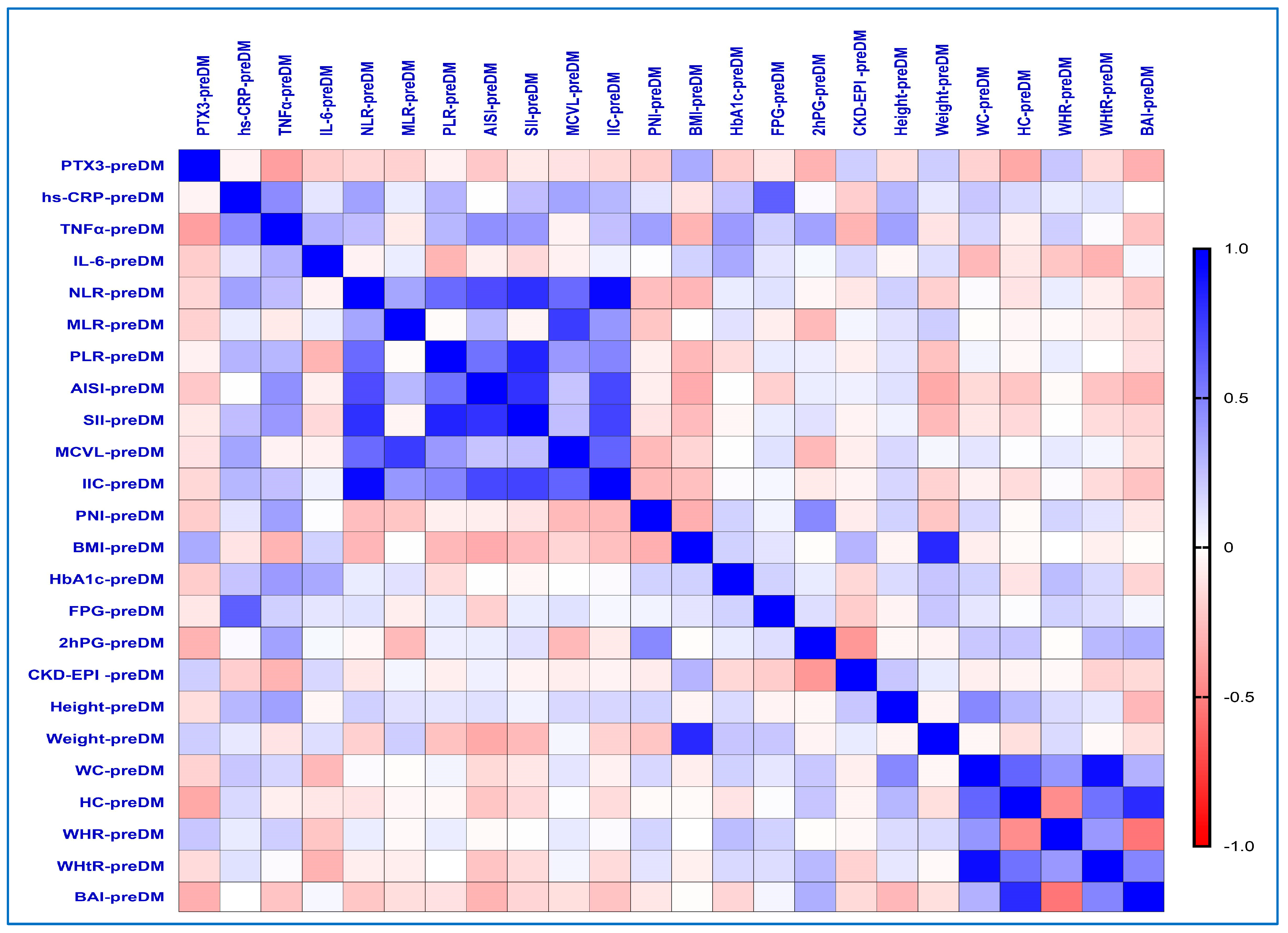
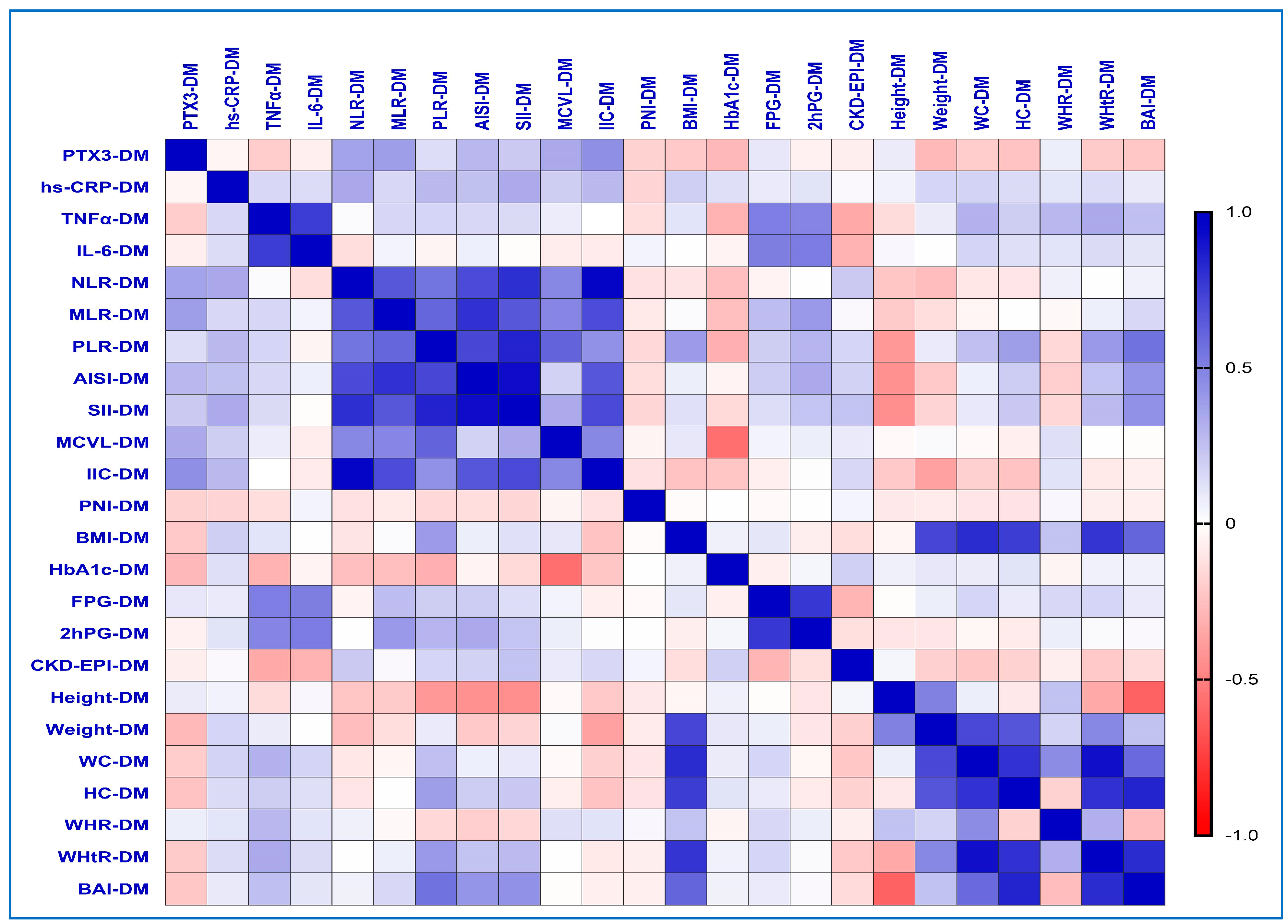


| Variables | PreDM (n = 30) | T2DM (n = 60) | p-Value from Pearson’s Chi-Squared/ Student’s t-Test | |
|---|---|---|---|---|
| Age (years) | Mean | 49.87 | 53.43 | 0.013 * |
| ±SD | 7.68 | 5.46 | ||
| Gender, n (%) | Male | 13 (43.33%) | 28 (46.67%) | 0.765 |
| Female | 17 (56.67%) | 32 (53.33%) | ||
| Residence, n (%) | Urban | 12 (40.00%) | 24 (40.00%) | 0.819 |
| Rural | 18 (60.00%) | 36 (60.00%) | ||
| Smoking habit, n (%) | 14 (46.67%) | 32 (53.33%) | 0.553 | |
| Drinking habit, n (%) | 10 (33.33%) | 24 (40.00%) | 0.541 | |
| Hypertension, n (%) | 23 (76.67%) | 45 (75.00%) | 0.863 | |
| Dyslipidemia, n (%) | 21 (70.00%) | 48 (80.00%) | 0.293 | |
| Hepatosteatosis, n (%) | 16 (53.33%) | 38 (63.33%) | 0.364 | |
| SBP, mmHg | Mean | 131.70 | 135.6 | 0.271 |
| ±SD | 16.84 | 15.18 | ||
| DBP, mmHg | Mean | 78.90 | 83.82 | 0.044 * |
| ±SD | 11.71 | 10.25 | ||
| BMI, kg/m2 | Mean | 30.75 | 31.38 | 0.644 |
| ±SD | 6.99 | 5.57 | ||
| BMI category, n (%) | ||||
| Normal (18.5–24.9 kg/m2) | 9 (30.00%) | 12 (20.00%) | 0.409 | |
| Overweight (25–29.9 kg/m2) | 11 (36.67%) | 14 (23.33%) | 0.325 | |
| Obese (≥30 kg/m2) | 10 (33.33%) | 34 (56.67%) | 0.207 | |
| Height, m | Mean | 1.61 | 1.70 | 0.0001 * |
| ±SD | 0.08 | 0.09 | ||
| Weight, kg | Mean | 84.84 | 87.11 | 0.557 |
| ±SD | 18.98 | 16.21 | ||
| WC, cm | Mean | 97.99 | 104.70 | 0.026 * |
| ±SD | 13.82 | 12.80 | ||
| HC, cm | Mean | 107.10 | 108.90 | 0.513 |
| ±SD | 11.83 | 14.46 | ||
| WHR | Mean | 0.91 | 0.98 | 0.006 * |
| ±SD | 0.16 | 0.08 | ||
| WHtR | Mean | 0.60 | 0.62 | 0.488 |
| ±SD | 0.08 | 0.09 | ||
| BAI | Mean | 30.72 | 35.05 | 0.007 * |
| ±SD | 7.30 | 7.16 | ||
| Variables | PreDM (n = 30) | T2DM (n = 60) | p-Value from Student’s t-Test/ Mann–Whitney Test | |
|---|---|---|---|---|
| FPG (mg/dL) | Median | 108.0 | 212.0 | <0.0001 * |
| range | 100.0–122.0 | 123.0–247.0 | ||
| 2hPG (mg/dL) | Mean | 164.40 | 331.60 | <0.0001 * |
| ±SD | 14.69 | 41.18 | ||
| HbA1c (%) | Median | 5.45 | 9.85 | <0.0001 * |
| range | 4.60–5.86 | 7.40–15.51 | ||
| TC (mg/dL) | Mean | 185.0 | 221.10 | 0.002 * |
| ±SD | 52.26 | 49.27 | ||
| TG (mg/dL) | Mean | 134.8 | 187.4 | 0.016 * |
| ±SD | 71.0 | 106.1 | ||
| LDL-C (mg/dL) | Mean | 102.5 | 137.2 | 0.001 * |
| ±SD | 47.13 | 43.94 | ||
| HDL-C (mg/dL) | Mean | 52.98 | 44.99 | 0.010 * |
| ±SD | 13.23 | 13.60 | ||
| eGFR (CKD-EPI) (mL/min/1.73 m2) | Mean | 86.23 | 85.85 | 0.929 |
| ±SD | 18.21 | 19.79 | ||
| BUN (mg/dL) | Mean | 37.33 | 41.18 | 0.255 |
| ±SD | 14.80 | 15.79 | ||
| Crea (mg/dL) | Median | 0.74 | 0.80 | 0.023 * |
| range | 0.47–1.53 | 0.56–1.67 | ||
| UA (mg/dL) | Mean | 5.01 | 5.17 | 0.657 |
| ±SD | 1.37 | 1.74 | ||
| ALB (g/dL) | Mean | 6.17 | 3.87 | <0.0001 * |
| ±SD | 0.34 | 0.81 |
| Variables | PreDM (n = 30) | T2DM (n = 60) | p-Value from Student’s t-Test/ Mann–Whitney Test | |
|---|---|---|---|---|
| PTX3 (pg/mL) | Mean | 1649.00 | 2826.00 | 0.0009 * |
| ±SD | 494.30 | 1795.00 | ||
| hs-CRP (pg/mL) | Mean | 954.20 | 1193.00 | 0.048 * |
| ±SD | 453.30 | 495.00 | ||
| TNF-α (pg/mL) | Median | 84.23 | 194.00 | 0.019 * |
| range | 30.77–203.90 | 164.70–278.10 | ||
| IL-6 (pg/mL) | Median | 29.96 | 72.34 | <0.0001 * |
| range | 17.52–82.41 | 40.78–185.20 | ||
| ESR (mm/1st h) | Mean | 24.27 | 39.03 | 0.0004 * |
| ±SD | 14.63 | 19.18 | ||
| RBC (×103/μL) | Median | 4.85 | 4.46 | 0.005 * |
| range | 3.43–6.73 | 1.39–5.36 | ||
| WBC (×103/μL) | Mean | 7.84 | 8.70 | 0.036 * |
| ±SD | 1.87 | 1.76 | ||
| NEU (×103/μL) | Mean | 4.73 | 5.17 | 0.759 |
| ±SD | 1.43 | 2.87 | ||
| LYM (×103/μL) | Mean | 2.33 | 2.70 | 0.035 * |
| ±SD | 0.71 | 0.78 | ||
| MON (×103/μL) | Mean | 0.53 | 0.57 | 0.415 |
| ±SD | 0.16 | 0.19 | ||
| PLT (×103/μL) | Mean | 225.70 | 243.70 | 0.254 |
| ±SD | 72.45 | 65.09 | ||
| MCV (fL) | Median | 96.19 | 90.79 | 0.046 * |
| range | 78.60–118.00 | 64.50–98.40 | ||
| RDW (%) | Mean | 13.23 | 12.71 | 0.154 |
| ±SD | 1.17 | 0.99 | ||
| NLR | Mean | 2.15 | 1.90 | 0.120 |
| ±SD | 0.78 | 0.67 | ||
| MLR | Mean | 0.24 | 0.22 | 0.689 |
| ±SD | 0.08 | 0.12 | ||
| PLR | Mean | 113.40 | 89.10 | 0.047 * |
| ±SD | 49.97 | 39.27 | ||
| AISI | Median | 246.30 | 219.70 | 0.038 * |
| range | 93.81–1160.00 | 34.82–823.20 | ||
| SII | Median | 411.80 | 377.90 | 0.017 * |
| range | 240.30–1657.00 | 54.40–1066.00 | ||
| MCVL | Mean | 41.70 | 38.69 | 0.054 ** |
| ±SD | 10.41 | 12.49 | ||
| IIC | Mean | 2.54 | 2.32 | 0.249 |
| ±SD | 0.91 | 0.84 | ||
| PNI | Mean | 61.99 | 38.70 | <0.0001 * |
| ±SD | 3.44 | 8.09 |
| Variables | PreDM | T2DM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal (n = 9) | Overweight (n = 11) | Obese (n = 10) | p-Value from One-Way ANOVA/ Kruskal–Wallis Test | Normal (n = 12) | Overweight (n = 14) | Obese (n = 34) | p-Value from One-Way ANOVA/ Kruskal–Wallis Test | ||
| PTX3 (pg/mL) | Mean | 1318 | 1789 | 1792 | 0.050 * | 2611 | 2826 | 3019 | 0.038 * |
| ±SD | 320 | 425.3 | 568.4 | 2033 | 1795 | 1285 | |||
| hs-CRP (pg/mL) | Mean | 656 | 1079 | 1085 | 0.056 ** | 1152 | 1179 | 1193 | 0.068 |
| ±SD | 378.9 | 363.8 | 497.7 | 483.5 | 532.9 | 495 | |||
| TNF-α (pg/mL) | Median | 102.10 | 218.90 | 432.60 | 0.0001 * | 207.90 | 320.80 | 329.10 | 0.028 * |
| range | 76.92–211.10 | 87.27–463.50 | 176.50–509.80 | 167.20–90.90 | 189.60–709.90 | 172.20–01.70 | |||
| IL-6 (pg/mL) | Median | 25.46 | 29.76 | 69.91 | 0.002 * | 71.82 | 72.34 | 75.93 | 0.463 |
| range | 19.24–62.03 | 19.24–54.56 | 17.52–82.41 | 41.92–185.20 | 40.78–87.04 | 44.22–110.80 | |||
| NLR | Mean | 2.10 | 2.03 | 1.94 | 0.837 | 2.32 | 2.12 | 1.99 | 0.665 |
| ±SD | 0.92 | 0.55 | 0.72 | 0.76 | 0.90 | 0.70 | |||
| MLR | Mean | 0.21 | 0.23 | 0.27 | 0.280 | 0.26 | 0.25 | 0.24 | 0.942 |
| ±SD | 0.06 | 0.05 | 0.11 | 0.18 | 0.15 | 0.14 | |||
| PLR | Mean | 113.8 | 120.7 | 105.2 | 0.788 | 110.2 | 94.0 | 89.1 | 0.308 |
| ±SD | 46.1 | 70.32 | 22.83 | 55.14 | 32.13 | 37.67 | |||
| AISI | Median | 278.90 | 237.50 | 233.30 | 0.036 * | 263.50 | 220.10 | 201.00 | 0.043 * |
| range | 145.5–834.6 | 93.8–1160.0 | 101.2–433.2 | 84.4–436.9 | 51.9–823.2 | 75.1–510.8 | |||
| SII | Median | 460.90 | 398.40 | 391.40 | 0.097 | 421.30 | 394.10 | 387.10 | 0.071 |
| range | 316.2–866.3 | 246.6–1284.0 | 240.30–1657.0 | 165.5–769.0 | 121.6–1066.0 | 83.6–1064.0 | |||
| MCVL | Mean | 42.80 | 41.30 | 40.90 | 0.916 | 44.60 | 40.00 | 37.10 | 0.046 * |
| ±SD | 14.40 | 8.10 | 9.50 | 18.70 | 13.90 | 13.50 | |||
| ICC | Mean | 2.71 | 2.47 | 2.45 | 0.790 | 2.42 | 2.40 | 2.22 | 0.756 |
| ±SD | 0.79 | 0.99 | 0.98 | 1.07 | 0.85 | 0.71 | |||
| PNI | Mean | 63.22 | 61.76 | 59.90 | 0.108 | 41.90 | 38.33 | 37.51 | 0.232 |
| ±SD | 3.724 | 2.790 | 3.383 | 9.04 | 9.05 | 7.19 | |||
| Variables | Quartiles of HbA1c | |||
|---|---|---|---|---|
| Q1 + Q2 (4.60–5.45) (n = 15) | Q3 + Q4 (5.46–5.86) (n = 15) | p-Value from Student’s t-Test/ Mann–Whitney Test | ||
| PTX3 (pg/mL) | Mean | 1576 | 1722 | 0.039 * |
| ±SD | 536.1 | 455.3 | ||
| hs-CRP (pg/mL) | Mean | 944.4 | 963.9 | 0.909 |
| ±SD | 449.2 | 473.0 | ||
| TNF-α (pg/mL) | Median | 210.0 | 218.9 | 0.967 |
| range | 79.9–509.8 | 76.9–489.9 | ||
| IL-6 (pg/mL) | Median | 27.32 | 37.33 | 0.095 |
| range | 19.2–71.4 | 17.5–82.4 | ||
| NLR | Mean | 2.23 | 2.07 | 0.577 |
| ±SD | 0.82 | 0.76 | ||
| MLR | Mean | 0.25 | 0.23 | 0.500 |
| ±SD | 0.11 | 0.05 | ||
| PLR | Mean | 119.5 | 107.4 | 0.517 |
| ±SD | 88.4 | 26.2 | ||
| AISI | Median | 278.9 | 201.2 | 0.029 * |
| range | 93.8–1160.0 | 104.7–433.2 | ||
| SII | Median | 418.8 | 404.9 | 0.480 |
| range | 240.3–866.3 | 246.3–1657.0 | ||
| MCVL | Mean | 44.38 | 39.02 | 0.162 |
| ±SD | 9.54 | 10.87 | ||
| IIC | Mean | 2.62 | 2.46 | 0.661 |
| ±SD | 0.86 | 0.98 | ||
| PNI | Mean | 61.85 | 61.52 | 0.799 |
| ±SD | 3.79 | 3.18 | ||
| Variables | Quartiles of HbA1c | |||||
|---|---|---|---|---|---|---|
| Q1 (7.40–8.31) | Q2 (8.32–9.84) | Q3 (9.85–10.97) | Q4 (10.98–15.51) | p-Value from One-Way ANOVA/ Kruskal–Wallis Test | ||
| PTX3 (pg/mL) | Mean | 2460 | 2639 | 2785 | 3429 | 0.048 * |
| ±SD | 1175 | 1451 | 1494 | 2482 | ||
| hs-CRP (pg/mL) | Mean | 1067 | 1212 | 1203 | 1241 | 0.782 |
| ±SD | 529 | 475 | 340 | 615 | ||
| TNF-α (pg/mL) | Median | 75 | 217 | 227.8 | 399.2 | <0.0001 * |
| range | 57.4–95.4 | 182–699 | 177.4–613.0 | 172.2–801.7 | ||
| IL-6 (pg/mL) | Median | 66.85 | 69.44 | 75.00 | 79.63 | 0.211 |
| range | 41.92–102.80 | 40.78–185.20 | 57.43–95.37 | 44.22–178.60 | ||
| NLR | Mean | 2.28 | 2.04 | 1.96 | 1.81 | 0.159 |
| ±SD | 0.78 | 0.73 | 0.55 | 0.59 | ||
| MLR | Mean | 0.33 | 0.25 | 0.22 | 0.21 | 0.148 |
| ±SD | 0.20 | 0.14 | 0.14 | 0.10 | ||
| PLR | Mean | 107.1 | 106.8 | 85.47 | 78.37 | 0.013 * |
| ±SD | 36.8 | 59.17 | 26.61 | 25.52 | ||
| AISI | Median | 268.3 | 263.5 | 199.8 | 195.8 | 0.020 * |
| range | 51.9–702.4 | 115.5–823.2 | 84.4–432.6 | 53.5–331.2 | ||
| SII | Median | 431.7 | 394.1 | 376.6 | 371.2 | 0.066 |
| range | 116.6–1064.0 | 165.5–839.7 | 121.6–1066.0 | 83.7–714.7 | ||
| MCVL | Mean | 44.92 | 40.27 | 39.64 | 36.08 | 0.056 ** |
| ±SD | 19.44 | 12.56 | 13.77 | 11.85 | ||
| IIC | Mean | 2.74 | 2.43 | 2.29 | 2.03 | 0.027 * |
| ±SD | 0.85 | 0.91 | 0.74 | 0.64 | ||
| PNI | Mean | 39.43 | 39.60 | 38.93 | 36.84 | 0.782 |
| ±SD | 9.35 | 7.14 | 8.39 | 7.87 | ||
| Parameter | AUC | Std. Error | Cut-Off Values | 95% CI | Sensitivity % | Specificity % | Youden Index | p-Value |
|---|---|---|---|---|---|---|---|---|
| IL-6 | 0.866 | 0.045 | 40.30 | 0.778–0.954 | 100.00 | 60.00 | 0.600 | <0.0001 |
| PTX3 | 0.720 | 0.065 | 1888 | 0.593–0.846 | 67.60 | 73.30 | 0.409 | 0.003 |
| MCVL | 0.677 | 0.064 | 39.60 | 0.560–0.795 | 63.30 | 66.70 | 0.300 | 0.047 |
| TNF-α | 0.671 | 0.068 | 222 | 0.538–0.803 | 67.60 | 56.70 | 0.243 | 0.019 |
| hs-CRP | 0.635 | 0.071 | 1025 | 0.497–0.774 | 61.80 | 56.70 | 0.185 | 0.036 |
| PLR | 0.616 | 0.060 | 101 | 0.491–0.740 | 73.30 | 60.00 | 0.333 | 0.006 |
| SII | 0.588 | 0.064 | 397 | 0.463–0.713 | 56.70 | 60.00 | 0.167 | 0.173 |
| NLR | 0.586 | 0.068 | 1.98 | 0.453–0.718 | 65.00 | 60.00 | 0.250 | 0.187 |
| MLR | 0.579 | 0.063 | 0.212 | 0.456–0.702 | 53.30 | 56.70 | 0.100 | 0.223 |
| IIC | 0.572 | 0.066 | 2.35 | 0.442–0.702 | 61.70 | 60.00 | 0.217 | 0.027 |
| AISI | 0.571 | 0.065 | 233 | 0.443–0.698 | 56.70 | 53.30 | 0.100 | 0.277 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahrițculesei, R.-V.; Boldeanu, L.; Caragea, D.C.; Vladu, I.M.; Clenciu, D.; Mitrea, A.; Ungureanu, A.M.; Văduva, C.-C.; Dijmărescu, A.L.; Popescu, A.I.S.; et al. Association Between Pentraxins and Obesity in Prediabetes and Newly Diagnosed Type 2 Diabetes Mellitus Patients. Int. J. Mol. Sci. 2025, 26, 3661. https://doi.org/10.3390/ijms26083661
Ahrițculesei R-V, Boldeanu L, Caragea DC, Vladu IM, Clenciu D, Mitrea A, Ungureanu AM, Văduva C-C, Dijmărescu AL, Popescu AIS, et al. Association Between Pentraxins and Obesity in Prediabetes and Newly Diagnosed Type 2 Diabetes Mellitus Patients. International Journal of Molecular Sciences. 2025; 26(8):3661. https://doi.org/10.3390/ijms26083661
Chicago/Turabian StyleAhrițculesei, Roxana-Viorela, Lidia Boldeanu, Daniel Cosmin Caragea, Ionela Mihaela Vladu, Diana Clenciu, Adina Mitrea, Anca Marilena Ungureanu, Constantin-Cristian Văduva, Anda Lorena Dijmărescu, Alin Iulian Silviu Popescu, and et al. 2025. "Association Between Pentraxins and Obesity in Prediabetes and Newly Diagnosed Type 2 Diabetes Mellitus Patients" International Journal of Molecular Sciences 26, no. 8: 3661. https://doi.org/10.3390/ijms26083661
APA StyleAhrițculesei, R.-V., Boldeanu, L., Caragea, D. C., Vladu, I. M., Clenciu, D., Mitrea, A., Ungureanu, A. M., Văduva, C.-C., Dijmărescu, A. L., Popescu, A. I. S., Assani, M.-Z., Boldeanu, M. V., & Vere, C. C. (2025). Association Between Pentraxins and Obesity in Prediabetes and Newly Diagnosed Type 2 Diabetes Mellitus Patients. International Journal of Molecular Sciences, 26(8), 3661. https://doi.org/10.3390/ijms26083661








