Protect Effects of Perilla Seed Extract and Its Active Ingredient Luteolin Against Inflammatory Bowel Disease Model via the PI3K/AKT Signal Pathway In Vivo and In Vitro
Abstract
1. Introduction
2. Results
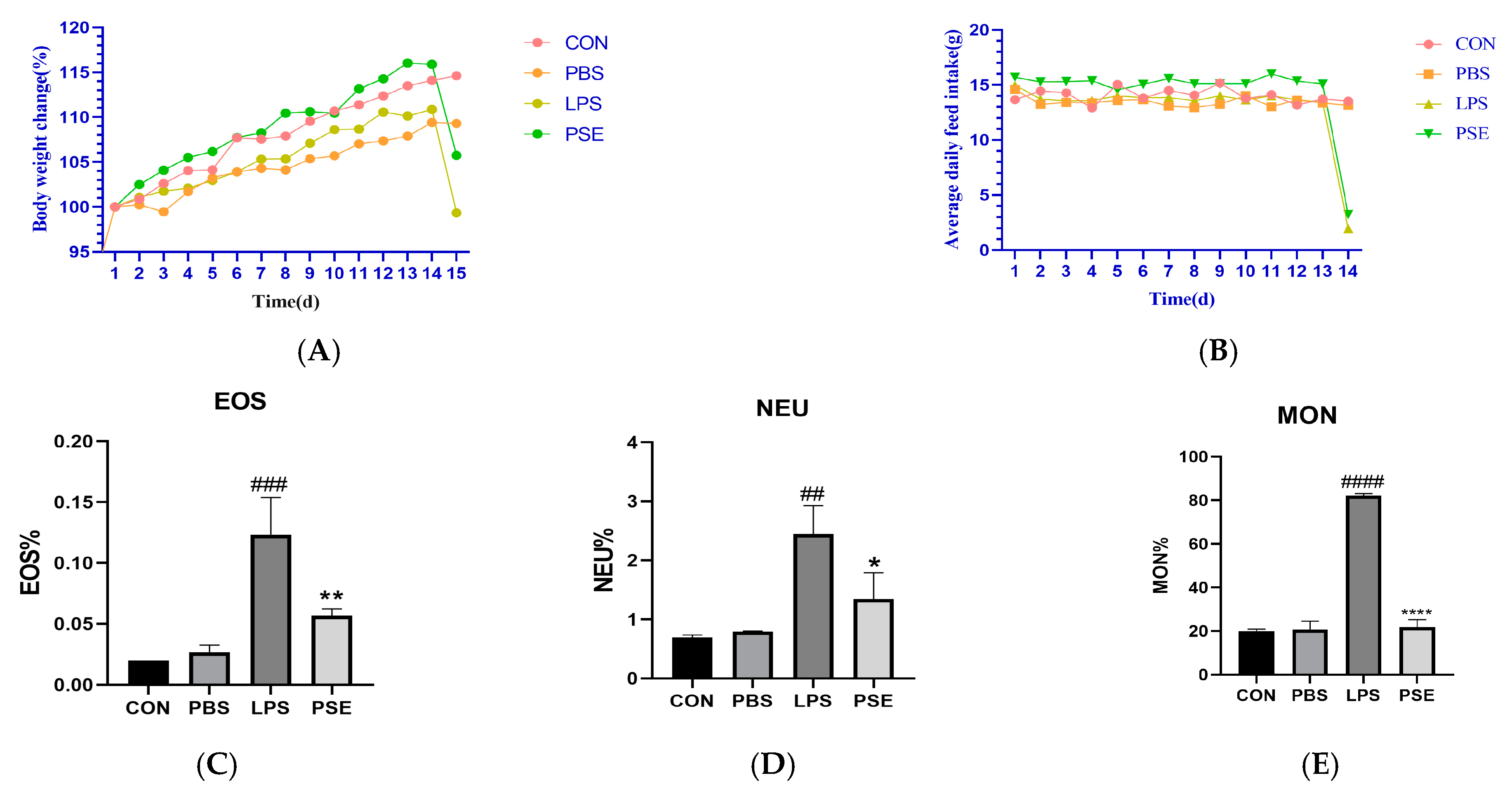
2.1. The Effect of PSE on LPS-Induced IBD in Mice
2.1.1. Effect of PSE on Blood Indices of Mice
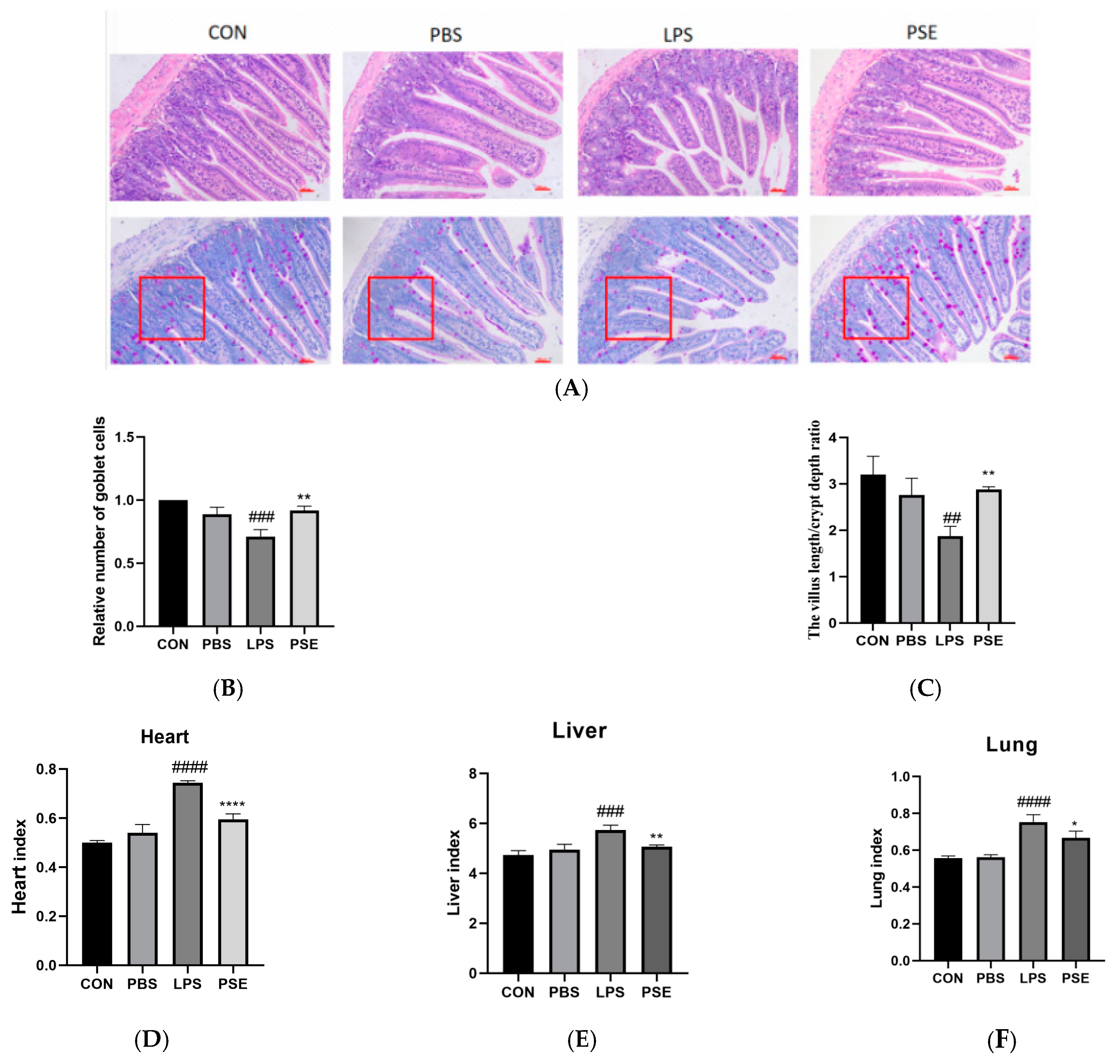
2.1.2. Effects of PSE on Organ Index and Jejunal Histopathology in LPS-Induced Colitis Mice
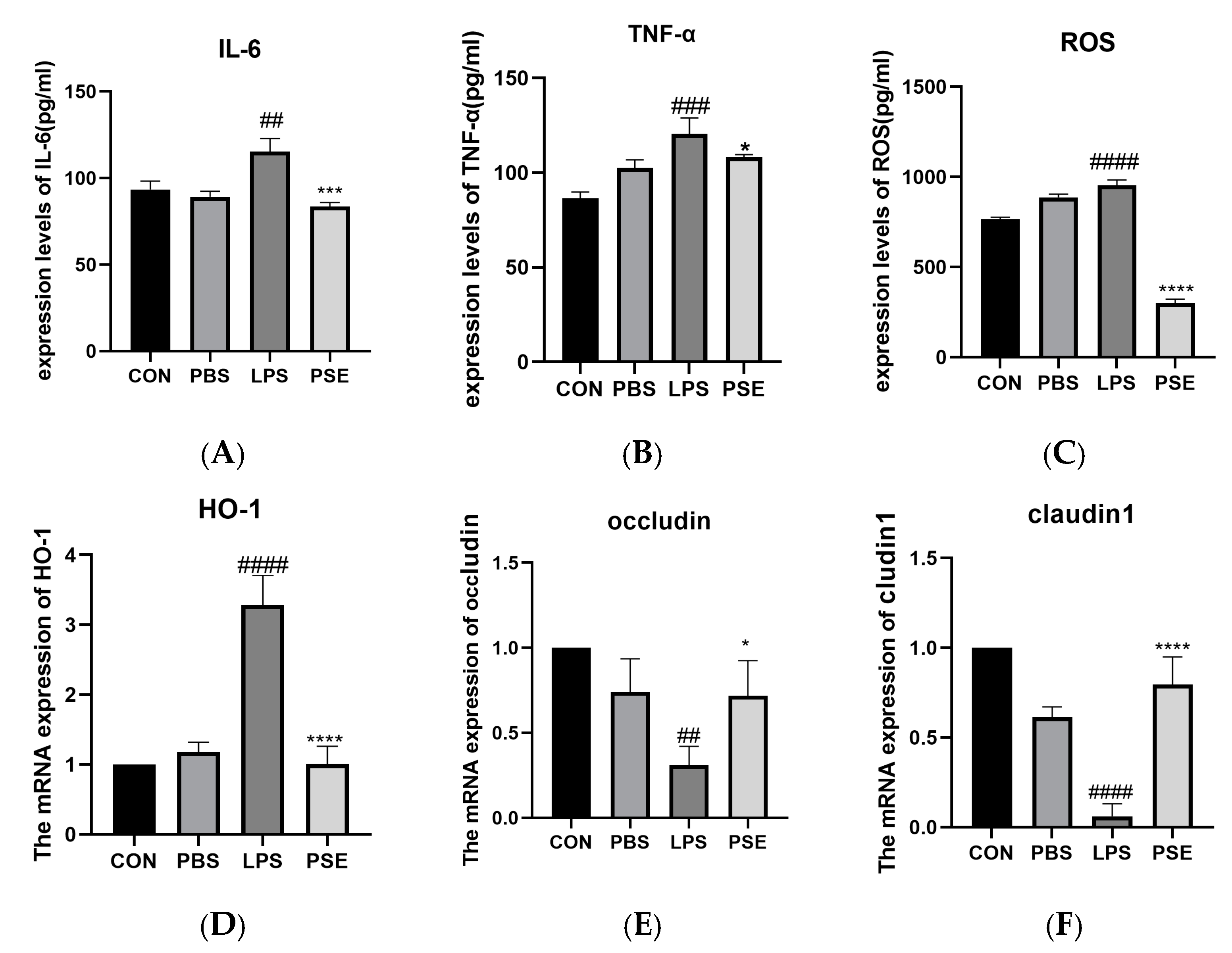
2.1.3. Effects of PSE on Inflammation, Oxidation, and Tight Junction–Related Factors in LPS-Induced Colitis Mice
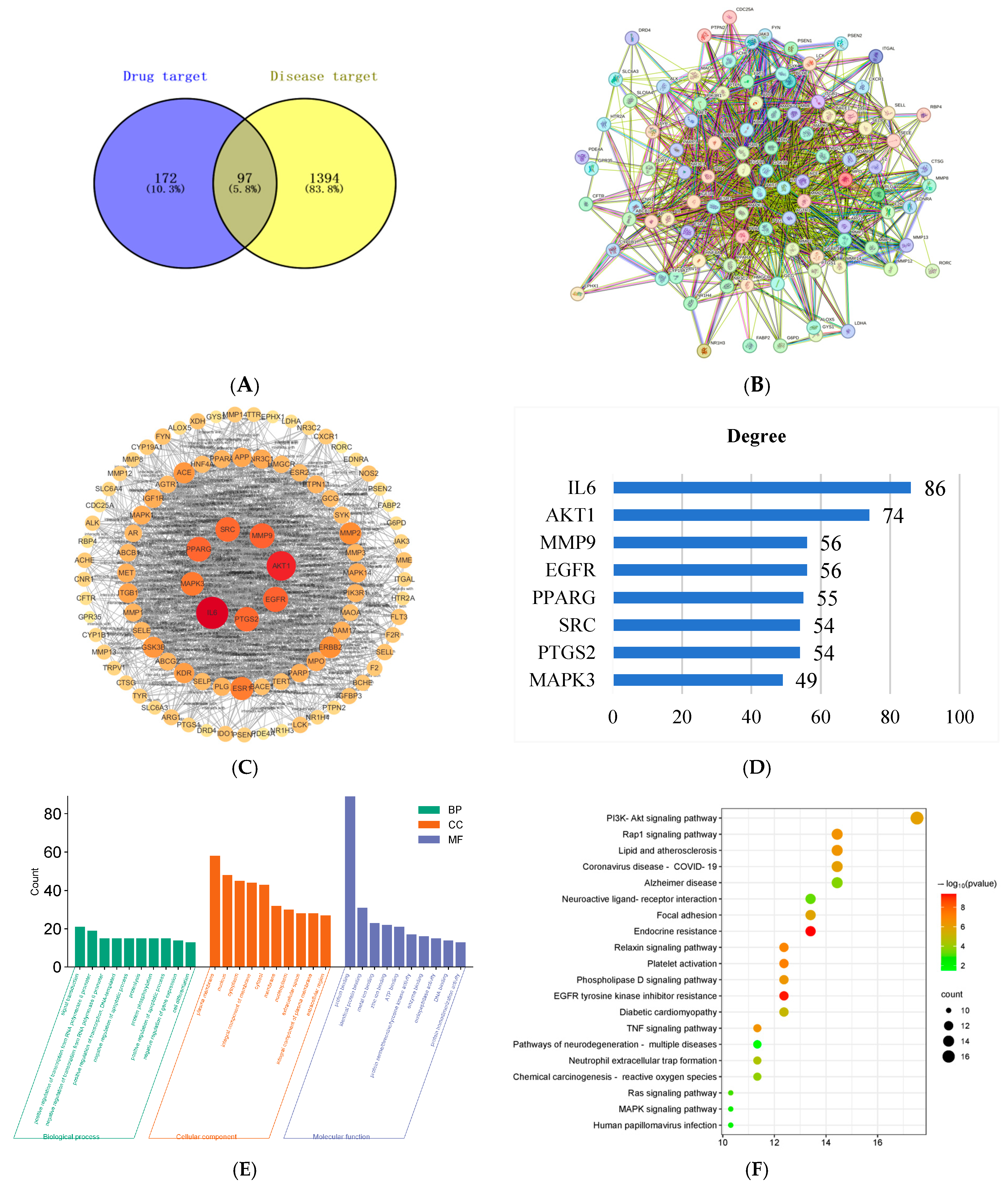
2.2. Network Pharmacology Analysis of Potential Targets for PSE in IBD
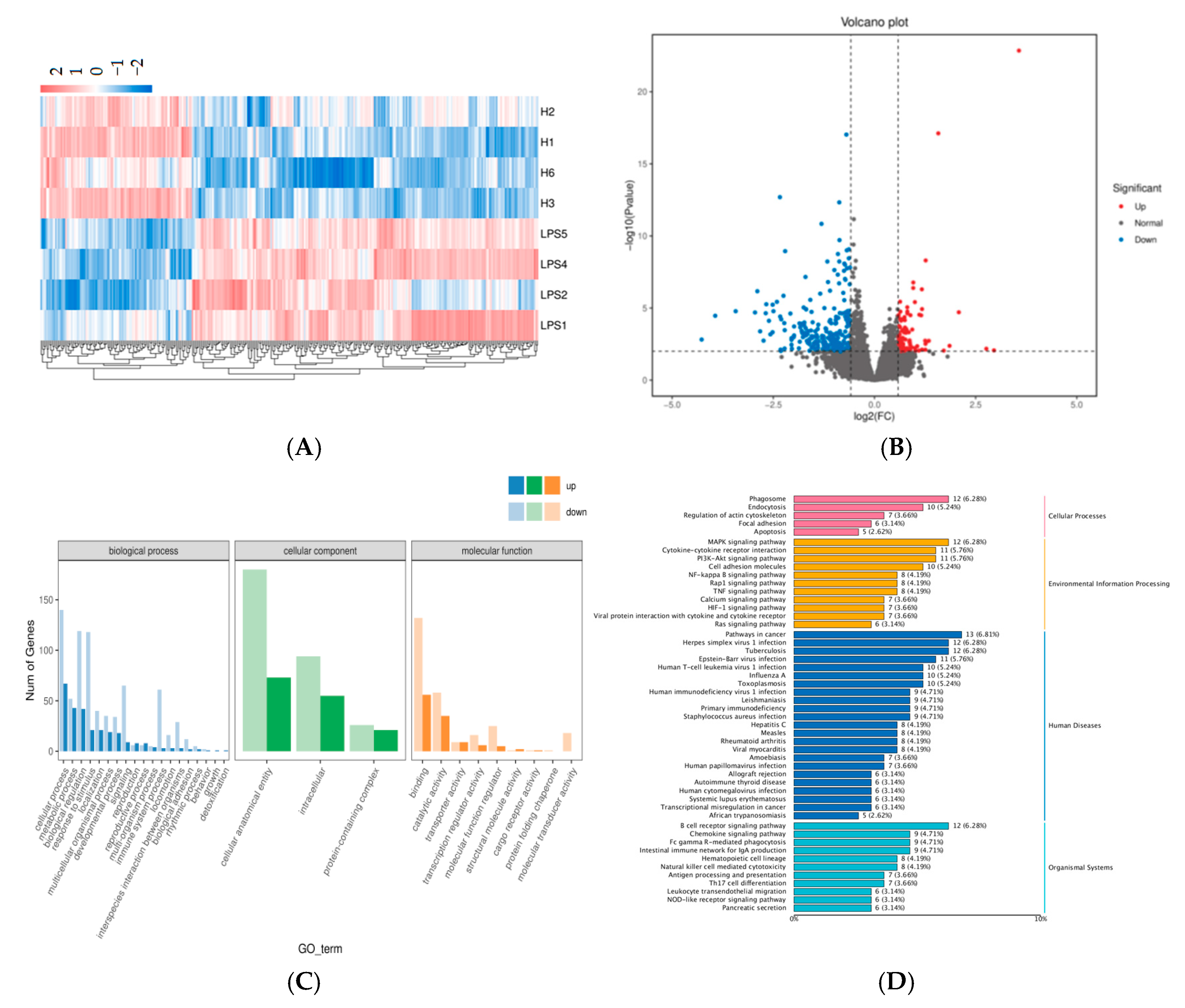
2.3. Transcriptomics Analysis of the DEGs
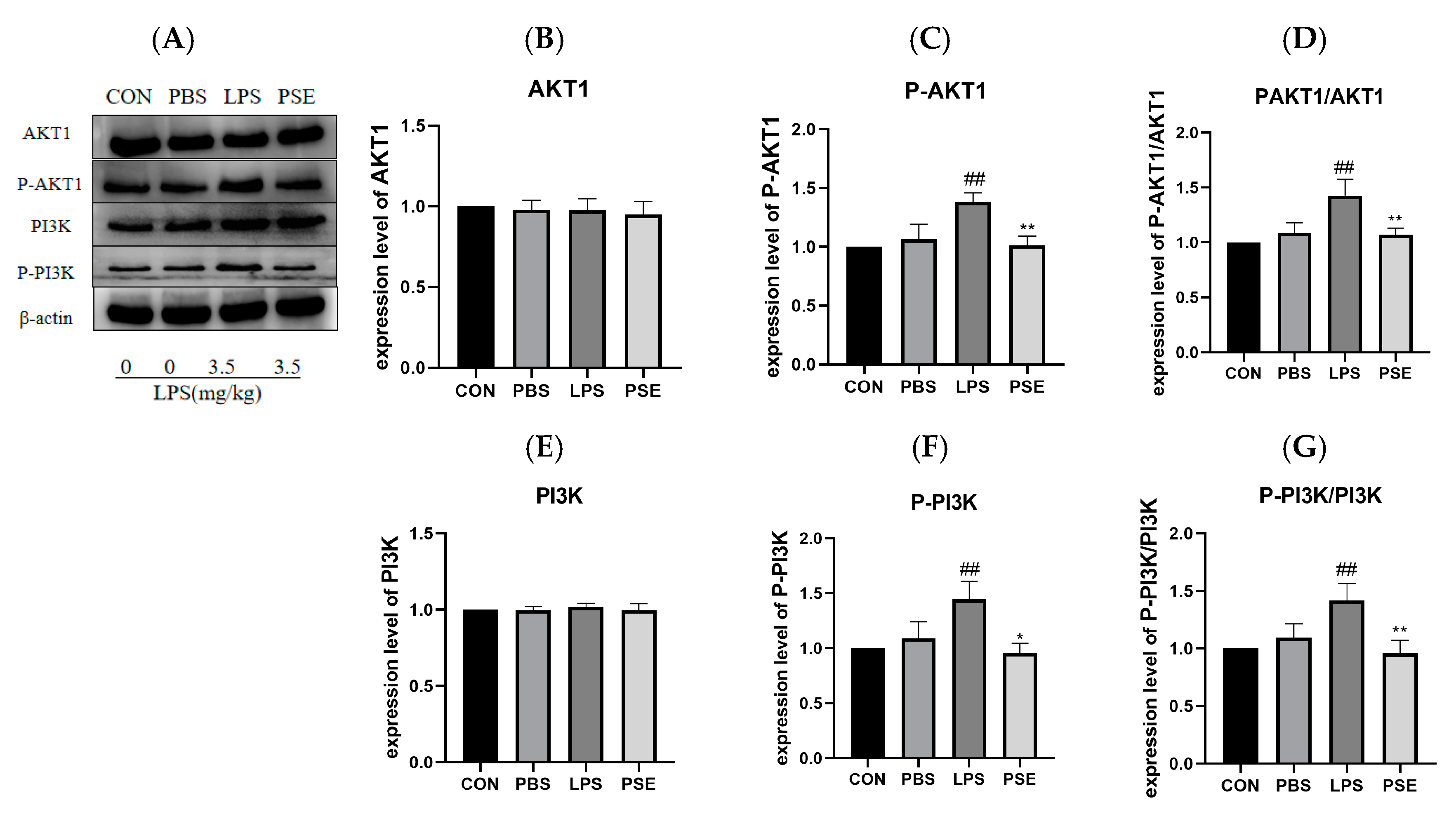
2.4. The Effect of PSE on PI3K/AKT1 Pathway Protein in Intestinal Tissue
2.5. In Vitro Validation Results
2.5.1. The Effects of PSE on LPS-Induced Inflammation, Oxidation, and Tight Junction–Related Factors in MODE-K Cells
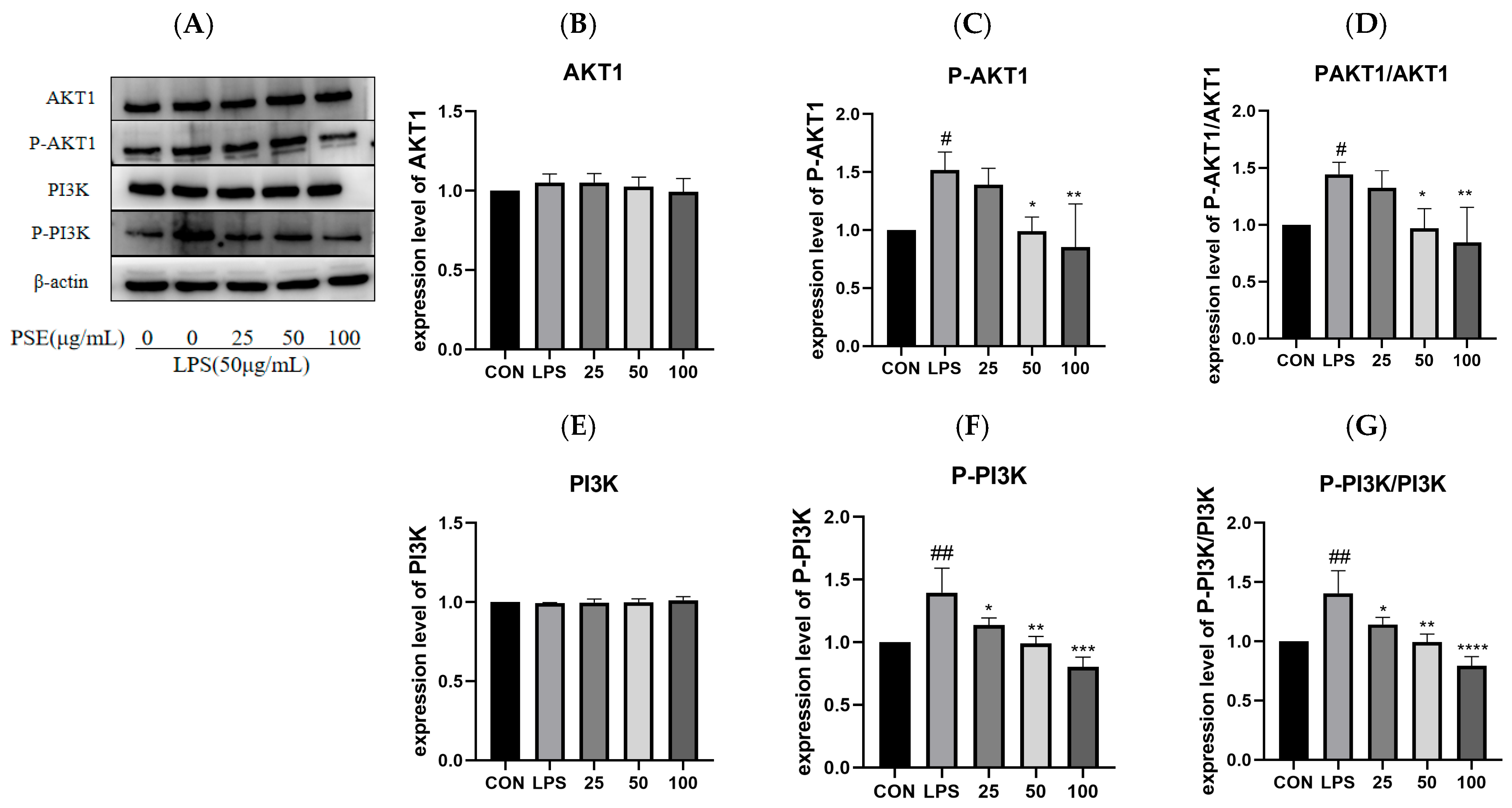
2.5.2. The Effect of PSE on LPS-Induced PI3K/AKT1 Pathway Proteins in MODE-K Cells
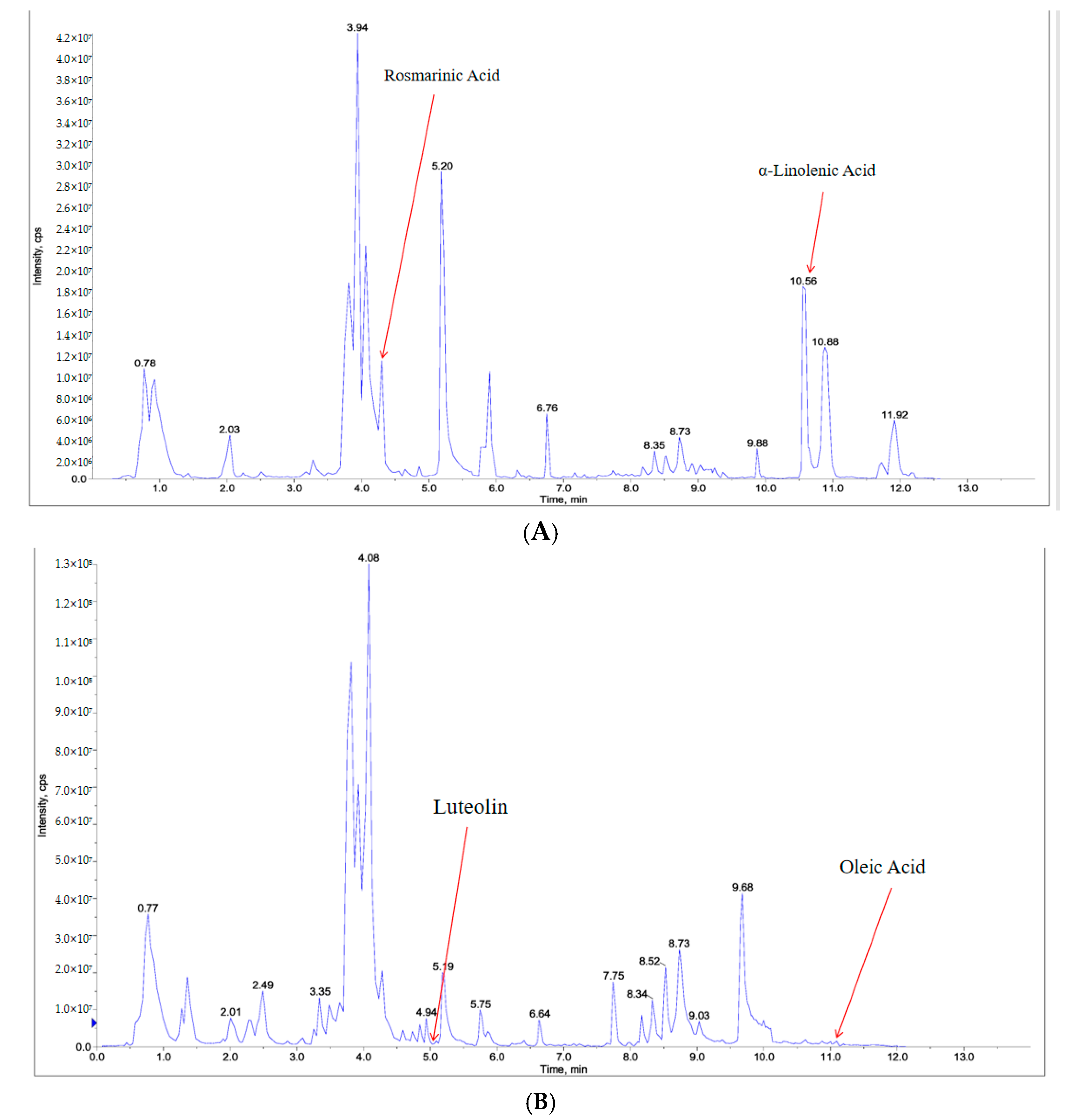
2.6. Component Analysis of PSE
2.7. Molecular Docking Verification
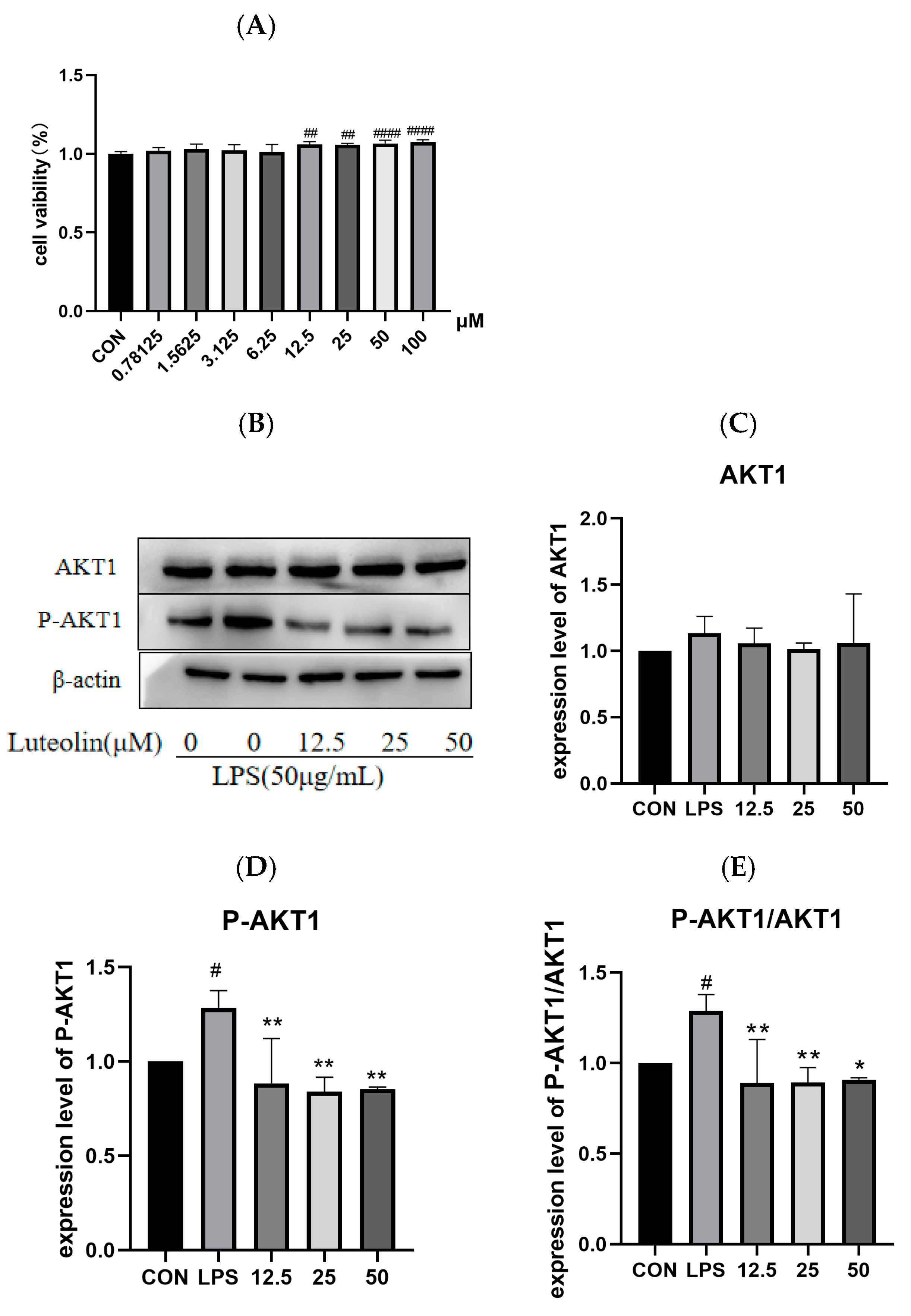
2.8. The Effect of Luteolin on AKT1 Protein
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animal Experiment
4.3. Cell Culture and Model Establishment
4.4. HE and PAS Staining
4.5. RNA Extraction, Reverse Transcription, and Real-Time PCR
4.6. Western Blotting
4.7. ELISA
4.8. Network Pharmacology
4.8.1. Screening of PSE for Active Components and Targets of Action
4.8.2. IBD Target Screening
4.8.3. Protein–Single-Bond Protein Interaction (PPI) Network Construction
4.8.4. GO and KEGG Analysis
4.9. Transcriptomics Study
4.10. UPLC-MS/MS Analysis
4.10.1. Dry Sample Extraction
4.10.2. UPLC Conditions
4.10.3. ESI-Q TRAP-MS/MS
4.11. Molecular Docking
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PSE | Perilla seed extract |
| UPLC-MS/MS | Ultra-performance liquid chromatography–tandem mass spectrometry |
| TCMSP | Traditional Chinese medicine systems pharmacology |
| PPI | Protein–protein interaction |
| H&E | Hematoxylin and eosin |
| PAS | Periodic acid–Schiff stain |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| BP | Biological process |
| CC | Cellular component |
| MF | Molecular function |
| LPS | Lipopolysaccharide |
| OB | Oral bioavailability |
| DL | Drug-likeness |
| DEGs | Differentially expressed genes |
References
- Ren, Y.; Geng, Y.; Du, Y.; Li, W.; Lu, Z.-M.; Xu, H.-Y.; Xu, G.-H.; Shi, J.-S.; Xu, Z.-H. Polysaccharide of hericium erinaceus attenuates colitis in c57bl/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J. Nutr. Biochem. 2018, 57, 67–76. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, W.; Li, Y.; Cong, Y. Enteroendocrine cells: Sensing gut microbiota and regulating inflammatory bowel diseases. Inflamm. Bowel Dis. 2020, 26, 11–20. [Google Scholar] [CrossRef]
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of ibd: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Peng, F.; Shan, Y.; Jiang, J. Berberine reduces the occurrence of neonatal necrotizing enterocolitis by reducing the inflammatory response. Exp. Ther. Med. 2018, 16, 5280–5285. [Google Scholar] [CrossRef]
- Alshahrani, A.; Ali, A.; Abdelwahab, S.F. Chemoprofiling and antimicrobial activity of medicinal herbs used in the treatment of inflammatory bowel disease. Cell. Mol. Biol. 2023, 69, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Chen, H.; Guo, Y.; Bai, A.-P. Advances in treatment of ulcerative colitis with herbs: From bench to bedside. World J. Gastroenterol. 2014, 20, 14099–14104. [Google Scholar] [CrossRef]
- Cao, S.Y.; Ye, S.J.; Wang, W.W.; Wang, B.; Zhang, T.; Pu, Y.-Q. Progress in active compounds effective on ulcerative colitis from chinese medicines. Chin. J. Nat. Med. 2019, 17, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Guo, S.; Liu, L.; Lei, J.; Qu, X.; He, C.; Tang, S.; Xiao, B.; Li, P.; Gao, Q.; Lan, F.; et al. Modulation of intestinal morphology and microbiota by dietary macleaya cordata extract supplementation in xuefeng black-boned chicken. Animal 2021, 15, 100399. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xin, E.; Zhang, Z.; Ma, D.; Liu, T.; Gao, F.; Bian, T.; Sun, Y.; Wang, M.; et al. Network pharmacology and experimental verification reveal the mechanism of hedysari radix and curcumae rhizoma with the optimal compatibility ratio against colitis-associated colorectal cancer. J. Ethnopharmacol. 2024, 322, 117555. [Google Scholar] [CrossRef]
- Dai, S.; Wu, R.; Fu, K.; Li, Y.; Yao, C.; Liu, Y.; Zhang, F.; Zhang, S.; Guo, Y.; Yao, Y.; et al. Exploring the effect and mechanism of cucurbitacin b on cholestatic liver injury based on network pharmacology and experimental verification. J. Ethnopharmacol. 2024, 322, 117584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luan, Y.; Ding, X.; Yang, C.; Xing, L.; Zhang, H.; Liu, Z. Integration of network pharmacology and transcriptomics to explore the mechanism of isoliquiritigenin in treating heart failure induced by myocardial infarction. Toxicol. Appl. Pharmacol. 2024, 492, 117114. [Google Scholar] [CrossRef]
- Wu, X.; Dong, S.; Chen, H.; Guo, M.; Sun, Z.; Luo, H. Perilla frutescens: A traditional medicine and food homologous plant. Chin. Herb. Med. 2023, 15, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, N.; Guo, G.; Lu, S.; Li, Z.; Mu, Y.; Xia, X.; Xu, Z.; Hu, Y.; Xiang, X. Perilla seed oil: A review of health effects, encapsulation strategies and applications in food. Foods 2024, 13, 3615. [Google Scholar] [CrossRef]
- Guan, L.; Zhu, L.; Zhang, X.; Han, Y.; Wang, K.; Ji, N.; Lu, S. Perilla seed oil and protein: Composition, health benefits, and potential applications in functional foods. Molecules 2024, 29, 5258. [Google Scholar] [CrossRef] [PubMed]
- Park, D.D.; Yum, H.W.; Zhong, X.; Kim, S.H.; Kim, S.H.; Kim, D.H.; Surh, Y.J. Perilla frutescens extracts protects against dextran sulfate sodium-induced murine colitis: Nf-kappab, stat3, and nrf2 as putative targets. Front. Pharmacol. 2017, 8, 482. [Google Scholar] [CrossRef]
- Chantana, W.; Hu, R.; Buddhasiri, S.; Thiennimitr, P.; Tantipaiboonwong, P.; Chewonarin, T. The extract of perilla frutescens seed residue attenuated the progression of aberrant crypt foci in rat colon by reducing inflammatory processes and altered gut microbiota. Foods 2023, 12, 988. [Google Scholar] [CrossRef]
- Alqudah, A.; Qnais, E.; Gammoh, O.; Bseiso, Y.; Wedyan, M.; Alqudah, M.; Hatahet, T. Cirsimaritin alleviates dextran sodium sulfate-induced acute colitis in experimental animals: A therapeutic approach for inflammatory bowel disease. Prev. Nutr. Food Sci. 2024, 29, 31–39. [Google Scholar] [CrossRef]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of disease: Inflammatory bowel diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Wahnou, H.; Hmimid, F.; Errami, A.; Nait Irahal, I.; Limami, Y.; Oudghiri, M. Integrating admet, enrichment analysis, and molecular docking approach to elucidate the mechanism of artemisia herba alba for the treatment of inflammatory bowel disease-associated arthritis. J. Toxicol. Environ. Health Part A 2024, 87, 836–854. [Google Scholar] [CrossRef]
- Gassler, N.; Rohr, C.; Schneider, A.; Kartenbeck, J.; Bach, A.; Obermüller, N.; Otto, H.F.; Autschbach, F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G216–G228. [Google Scholar] [CrossRef]
- Liang, M.; Sun, X.; Guo, M.; Wu, H.; Zhao, L.; Zhang, J.; He, J.; Ma, X.; Yu, Z.; Yong, Y.; et al. Baicalin methyl ester prevents the lps—Induced mice intestinal barrier damage in vivo and in vitro via p65/tnf-alpha/mlck/zo-1 signal pathway. Biomed. Pharmacother. 2024, 180, 117417. [Google Scholar] [CrossRef]
- Li, B.; Alli, R.; Vogel, P.; Geiger, T. Il-10 modulates dss-induced colitis through a macrophage-ros-no axis. Mucosal Immunol. 2014, 7, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; De Castro, C.; Silipo, A.; Molinaro, A. Lipopolysaccharide structures of gram-negative populations in the gut microbiota and effects on host interactions. FEMS Microbiol. Rev. 2019, 43, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, Y.; Wang, Y.; Li, Z.; Wang, Z.; Miao, Y.; Du, H.; Liu, D. The water-soluble subfraction from artemisia argyi alleviates lps-induced inflammatory responses via multiple pathways and targets in vitro and in vivo. J. Ethnopharmacol. 2024, 319, 117364. [Google Scholar] [CrossRef]
- Miao, F.; Shan, C.; Shah, S.A.H.; Akhtar, R.W.; Geng, S.; Ning, D.; Wang, X. The protective effect of walnut oil on lipopolysaccharide-induced acute intestinal injury in mice. Food Sci. Nutr. 2021, 9, 711–718. [Google Scholar] [CrossRef]
- Miao, F.; Shan, C.; Ning, D. Walnut oil alleviates lps-induced intestinal epithelial cells injury by inhibiting tlr4/myd88/nf-kappab pathway activation. J. Food Biochem. 2021, 45, e13955. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Yan, W. Treatment effects of natural products on inflammatory bowel disease in vivo and their mechanisms: Based on animal experiments. Nutrients 2023, 15, 1031. [Google Scholar] [CrossRef]
- Kangwan, N.; Pintha, K.; Khanaree, C.; Kongkarnka, S.; Chewonarin, T.; Suttajit, M. Anti-inflammatory effect of perilla frutescens seed oil rich in omega-3 fatty acid on dextran sodium sulfate-induced colitis in mice. Res. Pharm. Sci. 2021, 16, 464–473. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, H.; Zeng, Y.; Xu, F.; Zhao, S.; Zhang, L.; An, Z.; Li, H.; Li, J.; Song, J.; et al. Short-term exposure to air pollution on peripheral white blood cells and inflammation biomarkers: A cross-sectional study on rural residents. BMC Public Health 2024, 24, 1702. [Google Scholar] [CrossRef]
- Wu, H.; Guo, M.; Zhao, L.; Zhang, J.; He, J.; Xu, A.; Yu, Z.; Ma, X.; Yong, Y.; Li, Y.; et al. Siraitia grosvenorii extract protects lipopolysaccharide-induced intestinal inflammation in mice via promoting m2 macrophage polarization. Pharmaceuticals 2024, 17, 1023. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liang, J.; Guo, Z.; Bai, X.; Zhang, H.; Zhang, N.; Wang, H.; Chen, Q.; Li, W.; Dong, R.; et al. Network pharmacology and transcriptomics to determine danggui yifei decoction mechanism of action for the treatment of chronic lung injury. J. Ethnopharmacol. 2024, 318, 116873. [Google Scholar] [CrossRef]
- Burke, J.E. Structural basis for regulation of phosphoinositide kinases and their involvement in human disease. Mol. Cell 2018, 71, 653–673. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. Akt/pkb signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.R.; Degterev, A. Small-molecule inhibitors of the pi3k signaling network. Future Med. Chem. 2011, 3, 549–565. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Chen, B.; Li, B.; Huang, G.; Huang, Y.; Hou, Y.; Zhong, P.; Jin, J.; Li, D.; et al. Parabacteroides goldsteinii enriched by pericarpium citri reticulatae ‘chachiensis’ polysaccharides improves colitis via the inhibition of lipopolysaccharide-involved pi3k-akt signaling pathway. Int. J. Biol. Macromol. 2024, 277, 133726. [Google Scholar] [CrossRef]
- Qin, X.; Liu, Z.; Nong, K.; Fang, X.; Chen, W.; Zhang, B.; Wu, Y.; Wang, Z.; Shi, H.; Wang, X.; et al. Porcine-derived antimicrobial peptide pr39 alleviates dss-induced colitis via the nf-kappab/mapk pathway. Int. Immunopharmacol. 2024, 127, 111385. [Google Scholar] [CrossRef]
- Yang, S.; Li, W.; Bai, X.; Di Nunzio, G.; Fan, L.; Zhao, Y.; Ren, L.; Zhao, R.; Bian, S.; Liu, M.; et al. Ginseng-derived nanoparticles alleviate inflammatory bowel disease via the tlr4/mapk and p62/nrf2/keap1 pathways. J. Nanobiotechnol. 2024, 22, 48. [Google Scholar] [CrossRef]
- Gesiorowski, A.; Ettich, J.; Werner, J.; Wittich, C.; Pieper, S.; Padrini, G.; Scheller, J. Bispecific soluble cytokine receptor-nanobody fusions inhibit interleukin (il-)6 trans-signaling and il-12/23 or tumor necrosis factor (tnf) signaling. J. Biol. Chem. 2023, 299, 105343. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, W.; Joseph, A.M.; Chu, C.; Putzel, G.G.; Fang, B.; Teng, F.; Lyu, M.; Yano, H.; Andreasson, K.I.; et al. Group 3 innate lymphoid cells produce the growth factor hb-egf to protect the intestine from tnf-mediated inflammation. Nat. Immunol. 2022, 23, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hu, Q.; Jiang, T.; Chen, Y.; Zhang, W.; Gao, P.; Zeng, J.; Efferth, T. Dehydroevodiamine alleviates ulcerative colitis by inhibiting the pi3k/akt/nf-kappab signaling pathway via targeting akt1 and regulating gut microbes and serum metabolism. Molecules 2024, 29, 4031. [Google Scholar] [CrossRef] [PubMed]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The pi3k/akt/mtor interactive pathway. Mol. BioSyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Feng, C.; Huang, P.; Li, Y.; Liu, R.; Liu, C.; Han, Y.; Chen, L.; Ding, Y.; Shao, C.; et al. Tnfalpha and ifngamma rapidly activate pi3k-akt signaling to drive glycolysis that confers mesenchymal stem cells enhanced anti-inflammatory property. Stem Cell Res. Ther. 2022, 13, 491. [Google Scholar] [CrossRef]
- Zhou, M.; Zhi, J.; Zhi, J.; Xiong, Z.; Wu, F.; Lu, Y.; Hu, Q. Polysaccharide from strongylocentrotus nudus eggs regulates intestinal epithelial autophagy through cd36/pi3k-akt pathway to ameliorate inflammatory bowel disease. Int. J. Biol. Macromol. 2023, 244, 125373. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, F.; Li, Y.; Fan, N.; Zhao, K.; Zhang, A.; Jiang, X. Blockade of pi3k/akt signaling pathway by astragaloside iv attenuates ulcerative colitis via improving the intestinal epithelial barrier. J. Transl. Med. 2024, 22, 406. [Google Scholar] [CrossRef]
- Jin, B.-R.; Chung, K.-S.; Cheon, S.-Y.; Lee, M.; Hwang, S.; Hwang, S.N.; Rhee, K.-J.; An, H.-J. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (dss)-induced mice via dual inhibition of nf-kappab and stat3 activation. Sci. Rep. 2017, 7, srep46252. [Google Scholar]
- Boeing, T.; de Souza, P.; Speca, S.; Somensi, L.B.; Mariano, L.N.B.; Cury, B.J.; dos Anjos, M.F.; Quintão, N.L.M.; Dubuqoy, L.; Desreumax, P.; et al. Luteolin prevents irinotecan-induced intestinal mucositis in mice through antioxidant and anti-inflammatory properties. Br. J. Pharmacol. 2020, 177, 2393–2408. [Google Scholar] [CrossRef]
- Xue, L.; Jin, X.; Ji, T.; Li, R.; Zhuge, X.; Xu, F.; Quan, Z.; Tong, H.; Yu, W. Luteolin ameliorates dss-induced colitis in mice via suppressing macrophage activation and chemotaxis. Int. Immunopharmacol. 2023, 124, 110996. [Google Scholar] [CrossRef]
- Yi, D.; Wang, M.; Liu, X.; Qin, L.; Liu, Y.; Zhao, L.; Peng, Y.; Liang, Z.; He, J. Rosmarinic acid attenuates salmonella enteritidis-induced inflammation via regulating tlr9/nf-kappab signaling pathway and intestinal microbiota. Antioxidants 2024, 13, 1265. [Google Scholar] [CrossRef]
- Gaillard, T. Evaluation of autodock and autodock vina on the casf-2013 benchmark. J. Chem. Inf. Model. 2018, 58, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Song, Y.S. Luteolin and luteolin-7-o-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of nf-kappab/ap-1/pi3k-akt signaling cascades in raw 264.7 cells. Nutr. Res. Pract. 2013, 7, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-L.; Yu, X.-J.; Feng, Y.-Q.; Yang, Y.; Hu, H.-B.; Zhao, Y.-Y.; Zhang, J.-H.; Liu, K.-L.; Zhang, Y.; Fu, L.-Y.; et al. Luteolin attenuates hypertension via inhibiting nf-kappab-mediated inflammation and pi3k/akt signaling pathway in the hypothalamic paraventricular nucleus. Nutrients 2023, 15, 502. [Google Scholar] [CrossRef]
- Sabarathinam, S.; Jayaraman, A.; Venkatachalapathy, R. Gut microbiome-derived metabolites and their impact on gene regulatory networks in gestational diabetes. J. Steroid Biochem. Mol. Biol. 2025, 247, 106674. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Guan, J.; Tian, Y.-H.; Su, G.-Y.; Zhao, Y.-Q. Acute and sub-chronic 90-day oral toxicity study of perilla seed oil in rodents and beagle dogs. Regul. Toxicol. Pharmacol. 2019, 103, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Tian, Y.H.; Guan, J.; Xie, Q.M.; Zhao, Y.Q. The anti-tussive, anti-inflammatory effects and sub-chronic toxicological evaluation of perilla seed oil. J. Sci. Food Agric. 2021, 101, 1419–1427. [Google Scholar] [CrossRef]
- De Leo, E.; Taranta, A.; Raso, R.; Pezzullo, M.; Piccione, M.; Matteo, V.; Emma, F. Long-term effects of luteolin in a mouse model of nephropathic cystinosis. Biomed. Pharmacother. 2024, 178, 117236. [Google Scholar] [CrossRef]

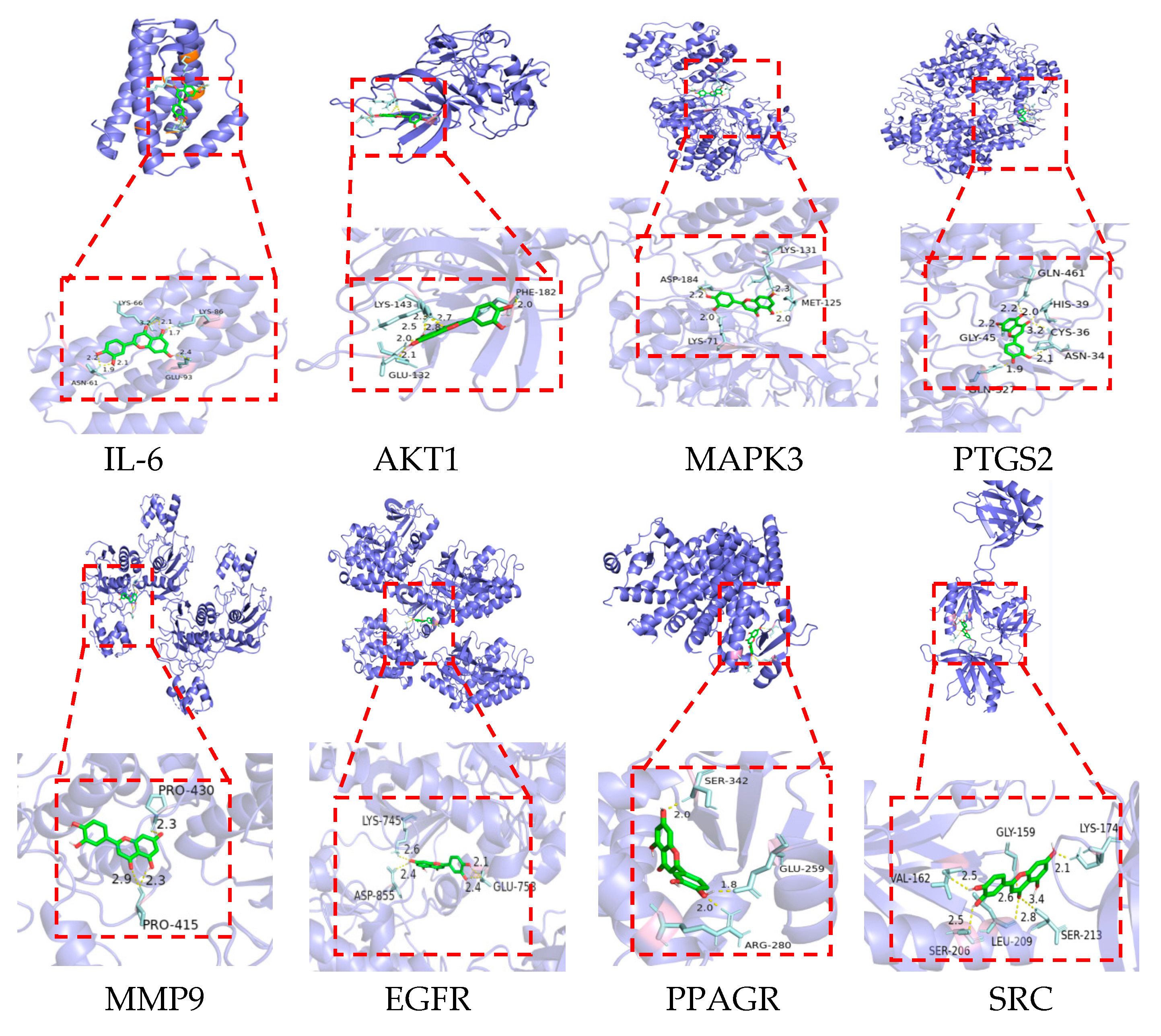

| MOL ID | Name | Formula | OB% | DL |
|---|---|---|---|---|
| MOL012893 | (E)-(4-methylbenzylidene)-(4-phenyltriazol-1-yl)amine | C16H14N4 | 57.87 | 0.19 |
| MOL001439 | arachidonic acid | C20H32O2 | 45.57 | 0.2 |
| MOL000006 | Luteolin | C15H10O6 | 36.16 | 0.25 |
| MOL011865 | Rosmarinic Acid | C18H16O8 | 1.38 | 0.35 |
| MOL000432 | α-Linolenic Acid | C18H30O2 | 45.01 | 1.14 |
| MOL000675 | Oleic Acid | C18H34O2 | 33.13 | 0.14 |
| Target | IL-6 | AKT1 | SRC | PTGS2 | MMP9 | PPARG | EGFR | MAPK3 |
|---|---|---|---|---|---|---|---|---|
| Name | ||||||||
| Rosmarinic Acid | −4.42 | −5.22 | −3.07 | −6.29 | −4.93 | −4.29 | −4.38 | −5.82 |
| Luteolin | −5.87 | −6.96 | −5.2 | −7.46 | −6.59 | −5.47 | −6.81 | −6.9 |
| alpha-Linolenic Acid | −4.5 | −4.7 | −2.76 | −4.8 | −4.07 | −5.12 | −5.83 | −4.29 |
| Oleic Acid | −3.6 | −4.87 | −1.82 | −4.44 | −4.7 | −4.29 | −5.9 | −4.95 |
| Arachidonic acid | −4.3 | −4.58 | −1.5 | −5.42 | −4.15 | −5.77 | −4.99 | −4.67 |
| (E)-(4-methylbenzylidene)-(4-phenyltriazol-1-yl)amine | −6.69 | −7.81 | −4.68 | −7.72 | −9.73 | −6.89 | −8.37 | −7.73 |
| Gene | Primer Sequence (5′ to 3′) | Prodsize | Login Number |
|---|---|---|---|
| β-actin | F:GATCTGGCACCACACCTTCTACAAC | 107 | NC_010445.4 |
| R:TCATCTTCTCACGGTTGGCTTTGG | |||
| HO-1 | F:AAGCCGAGAATGCTGAGTTCA | 106 | NC_000074.7 |
| R:GCCGTGTAGATATGGTACAAGGA | |||
| Claudin1 | F:GCACAGCACTTTACAAGCAACC | 117 | NW_004624730.1 |
| R:TCGTCATCTTCCAAGCACTTCATAC | |||
| Occludin | F:TGGCTATGGCGGATATACAGACC | 86 | NC_000079.7 |
| R:TTACTAAGGAAGCGATGAAGCAGAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhao, L.; He, J.; Wu, H.; Guo, M.; Yu, Z.; Ma, X.; Yong, Y.; Li, Y.; Ju, X.; et al. Protect Effects of Perilla Seed Extract and Its Active Ingredient Luteolin Against Inflammatory Bowel Disease Model via the PI3K/AKT Signal Pathway In Vivo and In Vitro. Int. J. Mol. Sci. 2025, 26, 3564. https://doi.org/10.3390/ijms26083564
Zhang J, Zhao L, He J, Wu H, Guo M, Yu Z, Ma X, Yong Y, Li Y, Ju X, et al. Protect Effects of Perilla Seed Extract and Its Active Ingredient Luteolin Against Inflammatory Bowel Disease Model via the PI3K/AKT Signal Pathway In Vivo and In Vitro. International Journal of Molecular Sciences. 2025; 26(8):3564. https://doi.org/10.3390/ijms26083564
Chicago/Turabian StyleZhang, Jin, Linlu Zhao, Jieyi He, Huining Wu, Mengru Guo, Zhichao Yu, Xingbin Ma, Yanhong Yong, Youquan Li, Xianghong Ju, and et al. 2025. "Protect Effects of Perilla Seed Extract and Its Active Ingredient Luteolin Against Inflammatory Bowel Disease Model via the PI3K/AKT Signal Pathway In Vivo and In Vitro" International Journal of Molecular Sciences 26, no. 8: 3564. https://doi.org/10.3390/ijms26083564
APA StyleZhang, J., Zhao, L., He, J., Wu, H., Guo, M., Yu, Z., Ma, X., Yong, Y., Li, Y., Ju, X., & Liu, X. (2025). Protect Effects of Perilla Seed Extract and Its Active Ingredient Luteolin Against Inflammatory Bowel Disease Model via the PI3K/AKT Signal Pathway In Vivo and In Vitro. International Journal of Molecular Sciences, 26(8), 3564. https://doi.org/10.3390/ijms26083564







