Abstract
Zinc-based MOFs exhibit significant advantages in ion detection due to their unique structure and chemical properties. They can efficiently and selectively recognize and detect specific ions, making them powerful analytical tools for applications in environmental monitoring, biomedical fields, and more. In this work, we used a simple ligand to improve the coordination environment of Zn2+ ions and successfully synthesized a 3D coordination compound Zn(all-bdc)(Py) MOF through a straightforward hydrothermal method at low temperature. Additionally, we explored the potential of this MOF as a bifunctional ion fluorescence probe for both cationic and anionic recognition. The results showed that this 3D porous MOF exhibited excellent recognition ability for trivalent iron ions (Fe3+) and potassium permanganate (KMnO4−) ions due to its highly porous structures and efficient ion recognition. When iron ions were added to 500 μL and potassium permanganate ions were added to 100 μL, the fluorescence of the compound was effectively quenched, and the detection limits for these two ions were 0.95 μM and 0.13 μM, respectively. The mixed-ion experiments also demonstrated that even in the presence of similar ions, this 3D MOF still maintained good selective recognition ability, specifically identifying Fe3+ and KMnO4− ions. This work provides a novel synthetic strategy for the design of MOFs capable of mixed-ion recognition and detection, expanding their application potential in ion sensing and analysis.
1. Introduction
Metal-Organic Frameworks (MOFs) are compounds formed by the self-assembly of metal ions or clusters with organic ligands, offering high surface areas and porous structures. Due to their high tunability, ultra-high surface area, and excellent chemical stability, these materials have shown immense potential in areas like gas storage, catalyst design, drug delivery, and environmental remediation [1,2,3]. One of their key advantages is the ability to adsorb and concentrate trace pollutants, improving detection sensitivity and accuracy [4,5]. In atmospheric pollution monitoring, they can capture and concentrate low concentrations of volatile organic compounds or greenhouse gases such as carbon dioxide and methane [6,7]. In aquatic environments, these materials can be designed with specific pore sizes and chemical functionalities to selectively adsorb heavy metal ions or organic pollutants from water [8,9]. Their chemical functionality can be adjusted by altering metal ions, organic ligands, or synthesis conditions, enabling the selective recognition and adsorption of various pollutants. For example, certain types are highly efficient at adsorbing heavy metals such as lead, mercury, and cadmium, reducing their impact on ecosystems and human health [10,11,12]. Additionally, they can be used to remove dyes, pesticides, and other organic pollutants through specific interactions with pollutants, allowing for selective capture and separation [13,14,15]. Overall, their versatility and specificity make them highly effective for environmental monitoring, pollution control, and the development of efficient filtration systems [16,17].
Traditional detection methods typically rely on high-precision instruments with specific functions to perform qualitative or quantitative analyses of target substances. However, these methods are undeniably costly due to the high expense of specialized equipment and materials, and they often have a limited detection range. In contrast, the use of MOFs in detection offers clear advantages [18,19,20]. They are cost-effective, easy to operate, and involve relatively simple physical changes during detection, typically involving energy transfer without altering the framework structure [21,22]. This makes it easier to separate and recycle the materials after detection. Additionally, MOF-based probes have a broad range of applications, including metal ions, small organic molecules, and various pollutants [23,24]. MOFs have shown good performance in detecting these diverse targets [25,26,27]. These benefits have made MOF-based detection a popular and rapidly developing research direction, offering an efficient alternative to traditional detection methods [28,29,30]. Among them, transition metal-based MOFs have the widest range of applications and the best performance [31,32]. Their high compatibility with organic ligands, stable coordination structures, multidimensional frameworks, and precisely sized pores enable efficient capture and enrichment of target ions [33]. Additionally, the organic ligands and metal ions in transition metal-based MOFs can interact specifically with target ions through mechanisms such as intermolecular hydrogen bonding and covalent bonding, allowing for highly selective ion detection [34]. This high sensitivity and selectivity give transition metal-based MOFs significant advantages in trace ion detection [35]. Omer et al. synthesized a novel Mn-based MOF that functions as a cold/hot-adapted and recyclable oxidase nanozyme (Km 0.085 mM), further developed for the ratiometric-based, colorimetric, and color tonality visual-mode detection of nitrite in water and food [36]. Similarly, Zhang et al. successfully synthesized a series of ratiometric fluorescent probes based on Zr-based MOFs using a one-pot method. These dual-responsive RhB-doped Zr-based MOF probes, attributed to fluorescence resonance energy transfer (FRET), photoinduced electron transfer (PET), and competitive absorption mechanisms, effectively detect Cu2+ and Fe3+ ions [37]. Bifunctional MOF-based fluorescent probes have attracted considerable attention due to their ability to detect both anions and cations [38]. These multifunctional probes significantly improve detection efficiency, making them particularly suitable for complex systems where multiple ions coexist, such as biological fluids and environmental water samples. However, most of the highly effective bifunctional fluorescent probes reported to date are based on lanthanide metal MOFs [39,40]. Fluorescent probes based on transition metal MOFs with detection limits below 1.0 μM are still rare [41]. Therefore, the development of efficient bifunctional fluorescent probes based on transition metal MOFs remains highly necessary.
In this study, we synthesized a Zn-based MOF by using a simple cost-effective method, used it as a cation–cation dual-ion detector to selectively detect Fe3+ and KMnO4− in water, and achieved good results. After adding a solution containing ferric ions or potassium permanganate ions, the fluorescence intensity of the Zn(all-bdc)(Py) MOF was significantly weakened. Based on our calculations, the detection limits for Fe3+ and MnO4− ions were both below 1.0 μM, specifically 0.95 μM and 0.13 μM, respectively, which means they are at the forefront of those reported in the literature [41]. At the same time, we also tested the ability of Zn(all-bdc)(Py) to selectively detect dual ions by mixing with other types of cations or anions. The results also show that in the presence of other cations and anions, the MOF probe we synthesized can still play a good role and can selectively detect Fe3+ and KMnO4−. This has a good reference role in the identification of ferric ions and potassium permanganate ions in the fields of medicine, the environment, materials, etc.
2. Results and Discussion
By adjusting the ligands involved in coordination, the structure and porosity of MOFs can be well adjusted, which is a major feature of MOF design and synthesis. Therefore, when we replaced bipyridine with a smaller coordination angle with straight-chain pyridine with a larger coordination angle, we successfully adjusted the spatial structure of Zn-based MOF from one dimension to three dimensions. The strategy is shown in Figure 1.
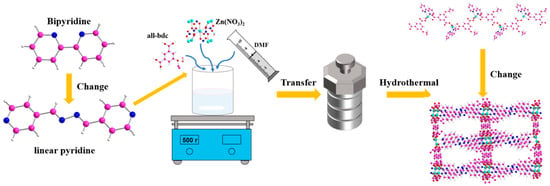
Figure 1.
Schematic diagram of the construction of three-dimensional Zn-based MOF.
The results from crystal structure analysis indicate that this compound belongs to the monoclinic crystal system with the P21/c space group. The overall structure exhibits C2h symmetry, featuring a 2-fold symmetry axis and a horizontal mirror plane (Figure 2). From the crystallographic perspective, the ZnII ion in the basic unit of this Zn-based MOF adopts a distorted octahedral coordination geometry with six coordinating atoms. Four of these coordinating atoms are oxygen atoms from the carboxyl groups of three different all-bdc ligands. One ligand contributes both oxygen atoms from its carboxyl group, while the other two carboxyl groups each contribute one oxygen atom. The remaining two coordination sites are occupied by nitrogen atoms from two different linear pyridine (Py) molecules.
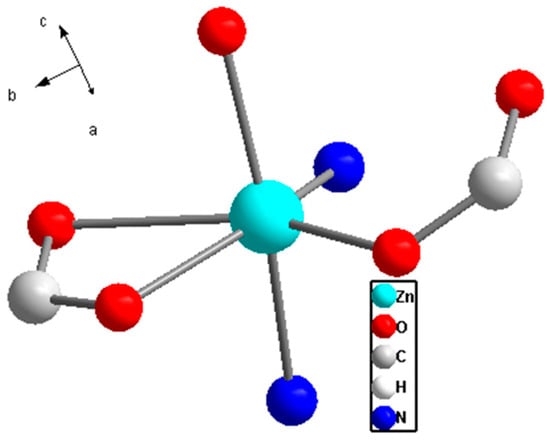
Figure 2.
Coordination environment of the ZnII in complex Zn(all-bdc)(Py).
Thus, the minimum asymmetric unit of the Zn(all-bdc)(Py) complex consists of one Zn²⁺ ion, one deprotonated all-bdc ligand, and one linear Py molecule (Figure 3a). The Zn-O (All-bdc) bond lengths range from 2.027 to 2.351 Å, while the Zn-N (Py) bond lengths range from 2.140 to 2.163 Å. In terms of spatial configuration, by replacing the original 1,4-bipyridine with linear pyridine, we overcame the issue of close coordination sites, which typically leads to a structure with excessive coordination to a single metal ion and insufficient additional coordination sites for metal–metal interactions. The all-bdc ligand connects with Zn2+ ions to receive a one-dimensional chain and connects with other all-bdc ligands to obtain a 2D layer (Figure 3b,c). This adjustment resulted in the formation of a three-dimensional layered structure for the synthesized Zn(all-bdc)(Py) complex, where the layers are connected by linear pyridine molecules (Figure 3d). This design resolves the problem of the one-dimensional chain structure that would have been observed if 1, 4-bipyridine were used. Meanwhile, the designed three-dimensional MOF features a porous structure with pore sizes of approximately 1.2 nm (Figure 3e). This porous framework facilitates the free diffusion of target ions, providing favorable conditions for interaction.
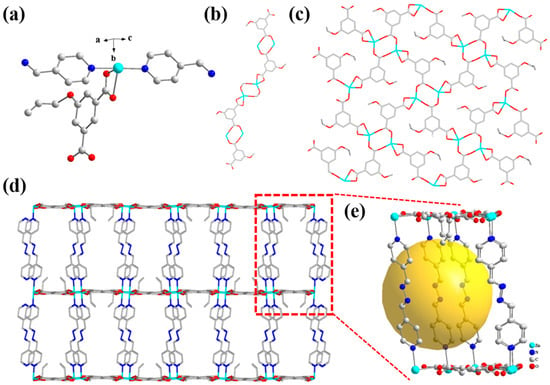
Figure 3.
(a) The asymmetric structure of the Zn-based MOF. (b) View of 1D chain (only all-bdc is considered). (c) The 2D layer structure of the Zn-based MOF. (d) The 3D structure of the Zn-based MOF viewed along different views and (e) its porous structure.
X-ray diffraction (XRD) was used to analyze the purity of the complex, and the results are presented in Figure 4a. By comparing the experimental pattern with the simulated pattern, we observed that the characteristic peaks align well with those of the simulated curve. Therefore, it can be concluded that the synthesized Zn-based MOF is predominantly pure-phase. In the practical application of MOF materials, the stability determines whether the material can maintain long-term performance and be recycled. Therefore, in order to test the thermal stability of the Zn(all-bdc)(Py) complex, thermogravimetric analysis (TGA) was used, and the results are shown in Figure 4b. The Zn-based MOF was placed in a thermal analyzer, and the weight loss of the material was tested from 25 °C to 800 °C, so as to obtain the thermal stability of the complex. From the figure, we can see that the first weight loss process of Zn(all-bdc)(Py) MOF is before 154 °C, and the weight loss is about 29.14%, which is the loss of solvent DMF molecules. The second process occurs between 154 °C and 500 °C, which is the collapse process of Zn-based MOF. Starting from 500 °C, the collapse begins to accelerate. At 600 °C, the collapse is complete, with a weight loss of 90.2%, and the remaining 9.8%, which is zinc oxide, and the theoretical value is 12.1%. These results indicate that the synthesized Zn-based MOF maintains structural stability at room temperature and even under relatively high temperatures, supporting its long-term performance stability and potential for sustainable reuse.
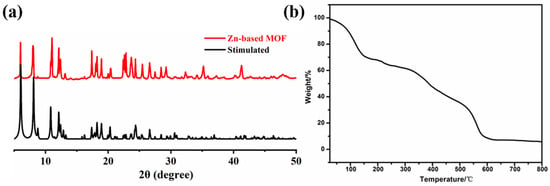
Figure 4.
(a) XRD patterns and (b) thermogravimetric analyses of the Zn-based MOF.
When the Zn-MOF is photoexcited, its internal electrons transition from the ground state to the excited state and then generate fluorescence during the de-excitation process [42]. Therefore, using Zn-based MOFs as fluorescent probes to detect target substances through fluorescence quenching is one of their key applications [43,44]. Based on this principle, we conducted ion sensitivity tests on the Zn(all-bdc)(Py) MOF. As a pre-designed zwitterionic probe, we first examined its recognition of cations. A 4.0 mg sample of the ground complex was placed in a vial, and 5.0 mL of distilled water was added, followed by 30 min of ultrasonication to ensure thorough dispersion. During the experiment, metal nitrate solutions (1.0 × 10−2 mol/L) were gradually added separately, with cations including Sr2+, Cr3+, Mn2+, Ni2+, Co2+, Zn2+, Ba2+, Ag+, Fe3+, K+, Cu2+, Cd2+, Na+, Fe2+, and Ca2+. From the results, after adding a certain concentration of iron ions to the solution of the Zn-based MOF, the fluorescence intensity of the complex decreased significantly (Figure 5a). As Fe3+ was incrementally added to the Zn(all-bdc)(Py) MOF solution, the fluorescence intensity gradually weakened. When the added volume reached 500 μL, the fluorescence was completely quenched (Figure 5b). This indicates that the Zn-based MOF exhibits good selectivity toward Fe3+. As shown in Figure 5c, there is a good linear correlation between the quenching efficiency of the Zn-based MOF and the amount of Fe3+ in a concentration range of 0–500 µM (R2 = 0.995). The detection limit was calculated to be 0.95 µM based on 3σ/k (σ: standard error; k: slope), which indicates that the MOF fluorescent probe we synthesized can be used as a potential detection agent for detecting Fe3+ in aqueous solution. The comparison with other fluorescent probes is shown in Table 1, which also proves the superior level of detection of our probe.
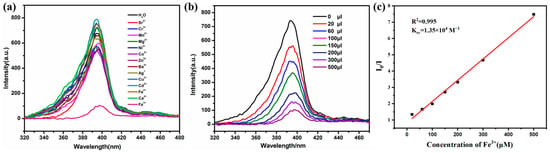
Figure 5.
(a) Comparison of the luminescence intensity of the Zn(all-bdc)(Py) MOF upon adding 500 μL of different metal ions. (b) The luminescence intensity of the Zn(all-bdc)(Py) MOF in the presence of various contents of Fe3+. (c) Stern–Volmer plots of Fe3+ ion. I is the luminescence intensity with Fe3+, and I0 is the luminescence intensity without Fe3+.

Table 1.
Performance comparison between various MOF fluorescent probes.
At the same time, we conducted an anion sensitivity test for the Zn(all-bdc)(Py) MOF. In the experiment, 4.0 mg of Zn(all-bdc)(Py) MOF was ground and placed in a vial, followed by the addition of 5.0 mL of distilled water. The mixture was sonicated for 30 min to ensure thorough dispersion. Gradually, sodium salt solutions of different anions (1.0 × 10−2 mol/L) were separately added to the Zn-based MOF aqueous solution. The tested anions included Br−, SeO42−, I−, CrO3−, SO32−, HCO3−, CO32−, S2−, CrO42−, Cl−, and MnO4−. The experimental results indicate that when a certain concentration of permanganate ions (MnO4−) was introduced into the Zn-based MOF solution, the fluorescence intensity of the MOF significantly decreased (Figure 6a). As MnO4− solution was gradually added, its fluorescence intensity progressively weakened. When the added amount reached 100 μL, a clear fluorescence quenching effect was observed (Figure 6b). This demonstrates that the Zn-based MOF exhibits good selectivity in recognizing anion of MnO4−. As shown in Figure 6c, the quenching efficiency of Zn-based MOF also exhibits a good linear correlation with the MnO4− concentration (R² = 0.997). The calculated detection limit is 0.13 μM, which is lower than that of most reported MOFs. A detailed comparison of detection limits with other reported MOF probes is also provided in Table 1.
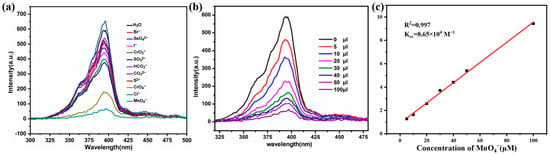
Figure 6.
(a) Comparison of the luminescence intensity of the Zn(all-bdc)(Py) MOF upon adding 100 μL of different anions. (b) The luminescence intensity of the Zn(all-bdc)(Py) MOF in the presence of various contents of MnO4−. (c) Stern–Volmer plots of MnO4− ion. I is the luminescence intensity with MnO4− ion, and I0 is the luminescence intensity without MnO4− ion.
In practical applications, MOF materials used as probes often encounter complex environments, and ion detection must have good anti-interference ability, especially to avoid interference from similar ions, in order to achieve accurate identification and efficient detection. Therefore, we conducted an experiment to evaluate the anti-interference ability of the Zn(all-bdc)(Py) MOF in detecting Fe3+. We simultaneously added 500 μL of Fe3+ and other metal ions of equal concentration to the complex solution to evaluate the recognition ability of the Zn-based MOF as a fluorescent probe in complex conditions. The experimental results showed that even in the presence of other metal ions, the fluorescence intensity of Zn(all-bdc)(Py) still exhibited significant quenching (Figure 7a), demonstrating its excellent anti-interference performance in Fe3+ detection. In addition, we further tested the anti-interference performance of the Zn(all-bdc)(Py) MOF in detecting MnO4− anions. Similarly, we added 100 μL of MnO4− along with other anions of equal concentration to the MOF solution to investigate its selective recognition ability for MnO4−. The experimental results revealed that despite the presence of other anions, the fluorescence intensity of the Zn(all-bdc)(Py) MOF still showed a significant decline (Figure 7b), indicating that this fluorescent probe also exhibits excellent anti-interference ability when detecting target anions. In summary, these two anti-interference experiments strongly confirm that the synthesized Zn(all-bdc)(Py) MOF, as a dual-functional fluorescent probe, can effectively recognize specific target ions in complex environments, demonstrating outstanding selectivity and stability.
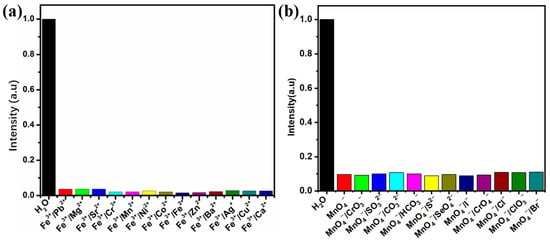
Figure 7.
Comparison of the luminescence intensity of the Zn(all-bdc)(Py) MOF immersed in the presence of (a) 500ul Fe3+ and other metal cations and (b) 100 μL MnO4− and other anions.
For a fluorescent probe to be reusable, it is essential to ensure structural stability before and after experiments. To confirm that the structure of the Zn-based MOF remained unchanged, we performed XRD analysis on the samples after detection. As shown in Figure 8, after detecting Fe3+ and MnO4− ions and subsequent separation, the main peaks of the sample exhibited no significant changes. This demonstrates that the synthesized MOF maintains excellent structural stability.
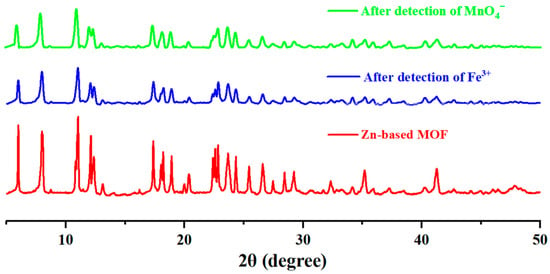
Figure 8.
XRD patterns of Zn-based MOF before and after ion detection.
For the fluorescence quenching mechanism of our synthesized Zn-based MOF toward Fe3+ and MnO4−, we analyzed it in conjunction with previous studies [49,53]. First, the XRD pattern of the Zn-based MOF after Fe3+ detection showed no significant changes in the main peaks compared to the original pattern, indicating that the fluorescence quenching was not caused by structural collapse or ion exchange. Therefore, the interaction between Fe3+ ions and the organic ligands of the Zn-based MOF is likely the primary reason for fluorescence quenching. Additionally, the uncoordinated nitrogen atoms from the linear pyridine moieties provide open Lewis base sites, which facilitate binding with external metal ions, leading to an enhanced quenching effect. Similarly, the XRD pattern of the Zn-based MOF after MnO4− detection also showed no significant changes in the main peaks, suggesting that the fluorescence quenching was not due to structural collapse or ion exchange. Instead, the host–guest interaction is likely the dominant factor for fluorescence quenching [54]. This is most likely due to the strong absorption of MnO4− in the 350–500 nm range, which overlaps with the excitation wavelength of Zn-MOF, thereby leading to fluorescence quenching [50].
To further confirm the fluorescence quenching mechanism of Zn-based MOF detection, we performed DFT theoretical calculations, and the results are shown in Figure 9. For the Zn-based MOF monomer, the HOMO electron density is mainly distributed on the carboxyl oxygen and pyridine rings of the ligand molecule, while the LUMO electron density is located on the pyridine rings adjacent to the metal coordination sites [55]. After forming the (Zn-based MOF)-Fe3+, the HOMO shifts to the all-bdc ligand, while the LUMO is primarily located on Fe3+ and the pyridine rings of the ligand. These results indicate that Fe3+ inhibits charge transfer in the excited state of the Zn-based MOF, ultimately leading to fluorescence quenching. The Zn-based MOF exhibits a strong absorption band at 395 nm, corresponding to the HOMO-LUMO bandgap transition with ΔE = 3.38 eV. Meanwhile, the (Zn-based MOF)-Fe3+ has a lower energy level (ΔE = 2.683 eV), indicating that the (Zn-based MOF)-Fe3+ structure is more stable [56]. Therefore, the interaction between the Zn-based MOF probe and Fe3+ can be attributed to the coordination of Fe3+ with the uncoordinated nitrogen sites, forming a relatively stable state that prevents intramolecular charge transfer, ultimately causing fluorescence quenching. For the (Zn-based MOF)-MnO4−, the HOMO and LUMO electron density distributions remain largely unchanged, while the bandgap is significantly smaller (ΔE = 1.21 eV). This suggests that MnO4− absorbs energy in the same spectral range as the MOF excitation, leading to competitive energy absorption and fluorescence quenching of the Zn-based MOF.
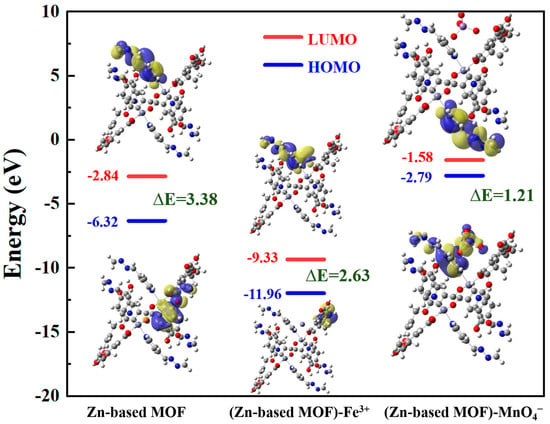
Figure 9.
LUMO and HOMO orbitals of Zn-based MOF, (Zn-based MOF)-Fe3+, and (Zn-based MOF)- MnO4−.
Our results demonstrate that Zn-based MOFs have significant potential as fluorescent probes for ion detection. With continuous advancements in synthesis techniques and the emergence of novel Zn-based MOFs, the sensitivity and accuracy of these materials in ion detection are expected to improve further. By optimizing pore structures, introducing more efficient functional groups, and developing new detection strategies, Zn-based MOFs will be capable of detecting ions at even lower concentrations with high precision. Moreover, by tuning the structure and functional groups of Zn-based MOFs, selective detection of different types of ions can be achieved, thereby expanding their application range. As detection technologies continue to evolve, Zn-based MOFs are also expected to enable the simultaneous detection of multiple ions, greatly enhancing detection efficiency and meeting the demands of complex systems. Despite their promising prospects, Zn-based MOFs still face several challenges. For instance, improving their stability and recyclability remains crucial for extending their lifespan and reducing detection costs. Additionally, optimizing the synthesis process to enable large-scale production while maintaining cost-effectiveness is another key challenge. To address these issues, several strategies can be considered. Further research should focus on enhancing the stability and recyclability of Zn-based MOFs by exploring new methods and technologies. Refining synthesis conditions can also improve production efficiency and reduce costs. Furthermore, interdisciplinary collaborations between chemistry, materials science, and environmental science will be essential in driving the development of Zn-based MOFs for ion detection applications.
3. Materials and Methods
3.1. Materials
All the chemicals were of analytical grade and used as received without further purification. Zinc nitrate (≥99%), 1, 4-bis(4-pyridyl)-2, 3-diaza-1, 3-butadiene (Py) (≥99%), N, N-Dimethylformamide (DMF) (≥99%), ethanol (≥99%), dimethylacetamide (≥99%), dimethyl5-hydroxyisophthalate (≥99%), potassium carbonate (≥99%), allyl bromide (≥99%), anhydrous sodium sulfate (≥99%), and sodium hydroxide (≥99%) were purchased from Sigma-Aldrich Co. LLC., St. Louis, MO, USA.
3.2. Measurements
FT-IR spectra were obtained by using a Bio-Rad FTS6000 spectrophotometer, and the wavelength range was from 4000 to 400 cm−1 (KBr pellets). Powder Xray diffraction (PXRD) was performed on a Rigaku D/max-IIIA diffractometer (CuKa, λ = 1.54056 Å). Thermogravimetric analysis (TGA) curves were obtained by using a NETZSCH TG 209 analyzer, and the temperature range was from 25 to 800 °C under nitrogen flow with a heating rate of 10 °C min−1. As for the theoretical calculation methods, structural optimization and orbitals were performed using DFT/Lanl2dz with the quantum chemical package Gaussian 09.
3.3. X-Ray Structure Determination and Structure Refinement
Single-crystal X-ray diffraction data for the Zn-based MOF were collected with a Bruker SMART APEX CCD instrument with graphite monochromatic Mo Ka radiation (λ = 0.71073 Å). Absorption corrections were made by multi-scan methods. The structures were solved with the program Olex2-1.5 and refined by full matrix least squares methods on all F2 data with Shelxtl-2019. The SQUEEZE subroutine of the PLATON-241123 software suite was applied to remove the scattering from highly disordered solvent molecules. All fluorescence measurements were performed on an MPF-4 fluorescence spectrofluorometer.
3.4. Synthesis of Ligand of All-bdc
The ligand of 5-(allyloxy)isophthalic acid (C11H10O5) (all-bdc) was synthesized according to a previous study [57]. We dissolved 1.015 g (0.0406 mol) of 5-propyleneoxyisophthalic acid dimethyl in 50.0 mL of 95% ethanol aqueous solution. Then, we mixed this solution with 50 mL of an aqueous solution containing 0.649 g (0.162 mol) of NaOH. Once the mixture was a solution, it was reacted at 65 °C for 15 h. After the reaction solution was cooled to room temperature, the ethanol was removed by rotary evaporation at 40 °C in a water bath. Then, 2.0 mol·L−¹ hydrochloric acid solution was added to adjust the pH to 6.5, ultimately yielding a white solid product with a yield of 78.7%. NMR data analysis: (300 MHz, CDCl3): δ = 8.26 (s, 1 H); 7.75 (s, 2 H); 6.02 (ddt, 3J(H-3, H-1) = 17.4 Hz, 3J(H-3, H-2) = 10.4 Hz, 3J(H-3, H-4) = 5.2 Hz, 1 H, H-3); 5.36 (dd, 3J(H-1, H-3) = 17.4 Hz,3J(H-2, H-3) = 10.4 Hz, 2 H, H-1, H-2); 4.61 (d, 3J(H-4, H-3) = 5.2 Hz, 2 H, H-4); 3.92 (s, 6 H).
3.5. Synthesis of Zn-Based MOF
A total of 0.0226 g (0.10 mmol) all-bdc, 0.0200 g (0.10 mmol) Py, and 0.0297 g (0.10 mmol) Zn(NO3)2·6H2O were mixed with 10.0 mL DMF and stirred for 30 min until the solution was clear. The mixture was stirred for 30 min until the solution became clear. The solution was transferred to a 25 mL PTFE reaction vessel with a stainless-steel protective outer jacket, sonicated for 30 min to ensure thorough mixing, and then placed in a blast drying oven. The program was set to heat the mixture to 80 °C for 1.5 h, maintaining the temperature at 80 °C for 96 h, followed by cooling to room temperature over 48 h. After the reaction, the product was filtered and washed with distilled water. FT-IR (KBr pellets, cm−1): 3431m, 3076w, 2935w, 2349w, 1684s, 1604s, 1453s, 1382s, 1249s, 1143m, 1099s, 1037s, 930s, 779s, 726s, 691s, 531s.
Yellow cubic transparent crystals were selected from the product and fixed on a single-crystal diffraction apparatus. Diffraction data were collected at a temperature of 113 K. Intensity correction was performed using the SADABS-8.40B and SAINT-2016 software packages. The crystallographic data of the Zn-MOF are stored in the Cambridge Crystallographic Data Centre. The CCDC number of the Zn-MOF is 2427350. The crystallographic data are reported in Table 2.

Table 2.
Crystallographic data of Zn-based MOF.
4. Conclusions
We used a dicarboxylic acid ligand combined with a long-chain pyridine to improve the coordination environment of zinc metal ions, successfully synthesizing a three-dimensional structure of the complex Zn(all-bdc)(Py) MOF. The thermal stability of the Zn-based MOF was significantly improved after the coordination environment was improved. At the same time, the practical effect of this MOF synthesized by a simple hydrothermal method as a novel type of zwitterionic fluorescence detection probe has been well verified. It can detect potassium permanganate ions as an anion detection probe and trivalent iron ions as a cation detection probe. After adding the target ions, the fluorescence intensity of the aqueous solution of the Zn(all-bdc)(Py) MOF changed significantly. When 500 μL cation of Fe3+ and 100 μL anion of MnO4− were added, the fluorescence of the complex almost disappeared, indicating quenching. Through calculations, the detection limits for these two ions were determined as 0.95 μM and 0.13 μM. At the same time, we also conducted ion sensitivity experiments on the detection of the complex. The results showed that the complex could selectively detect Fe3+ in the presence of other cations and selectively detect MnO4− in the presence of other anions. This demonstrated that the Zn-based MOF, as a bifunctional fluorescent probe, can effectively identify specific target ions in complex environments and show good selectivity and stability. This provides valuable reference for the application of MOFs used as probes in the selective detection of specific ions in complex environments.
Author Contributions
Methodology, H.L. (Hongbin Liu), Y.Z. and B.H.; Software, H.L. (Hongbin Liu) and H.L. (Hui Liu); Validation, Y.Z. and W.G.; Formal analysis, H.L. (Hongbin Liu) and P.Z.; Investigation, Y.Z., B.H., H.L. (Hui Liu) and W.G.; Resources, P.Z. and W.G.; Data curation, B.H., H.L. (Hui Liu) and P.Z.; Writing—original draft, H.L. (Hongbin Liu) and Y.Z.; Writing—review & editing, H.L. (Hongbin Liu), W.G. and T.M.; Project administration, T.M.; Funding acquisition, W.G. and T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Nanxun Scholars Program for Young Scholars of ZJWEU (No. RC2023021315), the National Natural Science Foundation of China (Grant No. 62304070; No. 51972293), the Hangzhou Key Research Program Project (2023SZD0099), the LingYan project (2024C01090), and the start-up funding for scientific research for high-level talents (No. 88106324004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mohanty, B.; Kumari, S.; Yadav, P.; Kanoo, P.; Chakraborty, A. Metal-organic frameworks (MOFs) and MOF composites based biosensors. Coord. Chem. Rev. 2024, 519, 216102. [Google Scholar] [CrossRef]
- Snyder, B.E.R.; Turkiewicz, A.B.; Furukawa, H.; Paley, M.V.; Velasquez, E.O.; Dods, M.N.; Long, J.R. A ligand insertion mechanism for cooperative NH3 capture in metal–organic frameworks. Nature 2023, 613, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-W.; Nguyen, Q.; Dieng, A.B.; Gómez-Gualdrón, D.A. Diversity-driven, efficient exploration of a MOF design space to optimize MOF properties. Chem. Sci. 2024, 15, 18903–18919. [Google Scholar] [CrossRef]
- Jin, R.; Li, R.; Ma, M.-L.; Chen, D.-Y.; Zhang, J.-Y.; Xie, Z.-H.; Ding, L.-F.; Xie, Y.; Li, J.-R. Beyond Tradition: A MOF-On-MOF Cascade Z-Scheme Heterostructure for Augmented CO2 Photoreduction. Small 2025, 2409759. [Google Scholar] [CrossRef]
- Razavi, S.A.A.; Morsali, A. Metal ion detection using luminescent-MOFs: Principles, strategies and roadmap. Coord. Chem. Rev. 2020, 415, 213299. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, Y.-n.; Li, L.; Chen, W.; Li, F.; Kitagawa, S. Controllable Modular Growth of Hierarchical MOF-on-MOF Architectures. Angew. Chem. Int. Ed. 2017, 56, 15658–15662. [Google Scholar] [CrossRef]
- Gulbalkan, H.C.; Haslak, Z.P.; Altintas, C.; Uzun, A.; Keskin, S. Assessing CH4/N2 separation potential of MOFs, COFs, IL/MOF, MOF/Polymer, and COF/Polymer composites. Chem. Eng. J. 2022, 428, 131239. [Google Scholar] [CrossRef]
- Choi, S.; Kim, T.; Ji, H.; Lee, H.J.; Oh, M. Isotropic and Anisotropic Growth of Metal–Organic Framework (MOF) on MOF: Logical Inference on MOF Structure Based on Growth Behavior and Morphological Feature. J. Am. Chem. Soc. 2016, 138, 14434–14440. [Google Scholar] [CrossRef]
- Devic, T.; Serre, C. High valence 3p and transition metal based MOFs. Chem. Soc. Rev. 2014, 43, 6097–6115. [Google Scholar] [CrossRef]
- Cao, Y.; Wen, Y.; Li, Y.; Cao, M.; Li, B.; Shen, Q.; Gu, W. Doping Ru on FeNi LDH/FeII/III–MOF heterogeneous core–shell structure for efficient oxygen evolution. Dalton Trans. 2024, 53, 5291–5300. [Google Scholar] [CrossRef]
- Feng, X.; Hu, D.-Y.; Liang, Z.-J.; Zhou, M.-Y.; Wang, Z.-S.; Su, W.-Y.; Lin, R.-B.; Zhou, D.-D.; Zhang, J.-P. A metal azolate framework with small aperture for highly efficient ternary benzene/cyclohexene/cyclohexane separation. Chin. J. Struct. Chem. 2025, 44, 100540. [Google Scholar] [CrossRef]
- Yue, Y.; Mohamed, S.A.; Loh, N.D.; Jiang, J. Toward a Generalizable Machine-Learned Potential for Metal–Organic Frameworks. ACS Nano 2025, 19, 933–949. [Google Scholar] [CrossRef]
- Tao, Y.; Tang, Z.; Xie, L.; Xu, X.; Zhao, W.; Xu, W.; Bao, D.; Zhong, Y.; Gao, Z.; Wen, Z.; et al. Surface-enhanced light harvesting over MOF-derived porous ZnO films for highly efficient QDs-based photoelectrochemical hydrogen generation. Green Energy Environ. 2024, in press. [Google Scholar] [CrossRef]
- Ismail, K.M.; Rashidi, F.B.; Hassan, S.S. Ultrasonic synthesis, characterization, DFT and molecular docking of a biocompatible Zn-based MOF as a potential antimicrobial, anti-inflammatory and antitumor agent. Sci. Rep. 2024, 14, 21989. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Xu, X.; Li, H.; Cui, Z.; Chu, B.; Xie, N.; Wang, Z.; Bai, P.; Guo, X.; Lyu, J. Fabrication Methods of Continuous Pure Metal–Organic Framework Membranes and Films: A Review. Molecules 2024, 29, 3885. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Q.; Zhao, N.; Li, Z.; Jiang, L.; Zhang, Z. 2D Conjugated Metal–Organic Framework-Based Composite Membranes for Nanofluidic Ionic Photoelectric Conversion. Adv. Mater. 2025, 37, 2416093. [Google Scholar] [CrossRef]
- Marquardt, N.; von der Haar, F.; Schaate, A. Transition metal and lanthanide modified MOF-808 for barcode design. Dalton Trans. 2024, 53, 8608–8618. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, T.; Iftikhar, N.; Chi, G.; Qiu, W.; Xie, Y.; Liang, Z.; Huang, C.; Su, L. Unlocking the future of brain research: MOFs, TMOs, and MOFs/TMOs for electrochemical NTMs detection and analysis. Talanta 2024, 267, 125146. [Google Scholar] [CrossRef]
- Meng, S.; Li, G.; Wang, P.; He, M.; Sun, X.; Li, Z. Rare earth-based MOFs for photo/electrocatalysis. Mater. Chem. Front. 2023, 7, 806–827. [Google Scholar] [CrossRef]
- Violet, C.; Parkinson, M.; Ball, A.K.; Kulik, H.J.; Fortner, J.D.; Elimelech, M. Tuning Metal–Organic Framework Linker Chemistry for Transition Metal Ion Separations. ACS Appl. Mater. Interfaces 2025, 17, 1911–1921. [Google Scholar] [CrossRef]
- Guo, X.; Feng, S.; Peng, Y.; Li, B.; Zhao, J.; Xu, H.; Meng, X.; Zhai, W.; Pang, H. Emerging insights into the application of metal-organic framework (MOF)-based materials for electrochemical heavy metal ion detection. Food Chem. 2025, 463, 141387. [Google Scholar] [CrossRef]
- Wu, X.; Omagari, S.; Gao, J.; Vacha, M. In Situ Monitoring of Nanocrystal Formation and Ion Migration in Lead Halide Perovskite Metal–Organic Framework Composites. Adv. Opt. Mater. 2024, 12, 2301479. [Google Scholar] [CrossRef]
- Ranjith Kumar, D.; Karthik, R.; Dhakal, G.; Nguyen, V.Q.; Lee, J.; Shim, J.-J. Catechol redox couple functionalized metal-organic framework UiO-66-NH2 as an efficient catalyst for chromium ion sensor in water samples. J. Clean. Prod. 2022, 374, 133731. [Google Scholar] [CrossRef]
- Devaraj, M.; Sasikumar, Y.; Rajendran, S.; Ponce, L.C. Review—Metal Organic Framework Based Nanomaterials for Electrochemical Sensing of Toxic Heavy Metal Ions: Progress and Their Prospects. J. Electrochem. Soc. 2021, 168, 037513. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, J.; Dai, Y.; Chen, L.; Li, W.; Yuan, Z.; Zhou, J.; Tang, E. A cost-effective and innovative detector for iron ions. RSC Adv. 2025, 15, 2645–2650. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, Y.; Gong, Y.; Wei, Y.; Hu, Q.; Yu, L. Hydrodynamic Fluidic Pump Empowered Sensitive Recognition and Active Transport of Hydrogen Peroxide in 1D Channels. Adv. Sci. 2025, 12, 2408755. [Google Scholar] [CrossRef]
- Janáky, C.; Rajeshwar, K. Current Trends in Semiconductor Photoelectrochemistry. ACS Energy Lett. 2017, 2, 1425–1428. [Google Scholar] [CrossRef]
- Ji, Z.; Li, T.; Yaghi, O.M. Sequencing of metals in multivariate metal-organic frameworks. Science 2020, 369, 674–680. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Reddy, A.S.; Panda, A.; Sarkar, D.; Son, Y.; Yoon, M. Reversible Fluorescence Switching of Metal–Organic Framework Nanoparticles for Use as Security Ink and Detection of Pb2+ Ions in Aqueous Media. ACS Appl. Nano Mater. 2020, 3, 3684–3692. [Google Scholar] [CrossRef]
- Jones, C.W. Metal–Organic Frameworks and Covalent Organic Frameworks: Emerging Advances and Applications. JACS Au 2022, 2, 1504–1505. [Google Scholar] [CrossRef]
- Ameen, S.S.M.; Alhasan, H.S.; Khazaal, F.A.; Omer, K.M. Quantitative On-Site Instrument-Free Visual Detection of Ferric Ions in Environmental and Biological Samples Using a Novel Fluorescent Metal–Organic Framework. J. Inorg. Organomet. Polym. Mater. 2024, in press. [Google Scholar] [CrossRef]
- Zhao, H.; Tan, X.; Chai, H.; Hu, L.; Li, H.; Qu, L.; Zhang, X.; Zhang, G. Recent advances in conductive MOF-based electrochemical sensors. Chin. Chem. Lett. 2024, 110571, in press. [Google Scholar] [CrossRef]
- Lv, R.; Wang, J.; Zhang, Y.; Li, H.; Yang, L.; Liao, S.; Gu, W.; Liu, X. An amino-decorated dual-functional metal–organic framework for highly selective sensing of Cr(iii) and Cr(vi) ions and detection of nitroaromatic explosives. J. Mater. Chem. A 2016, 4, 15494–15500. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Xia, C.; Zhu, W.; Hou, Y.; Zeng, X.; Xu, H. Calcination-induced enhancement of Cd2+ and Pb2+ electrochemical detection capabilities of nano-ag-supported CoZn bi-metal ZIFs. Environ. Sci. Nano 2024, 11, 2061–2072. [Google Scholar] [CrossRef]
- Fajal, S.; Mandal, W.; Majumder, D.; Shirolkar, M.M.; More, Y.D.; Ghosh, S.K. Cover Feature: Unfolding the Role of Building Units of MOFs with Mechanistic Insight Towards Selective Metal Ions Detection in Water (Chem. Eur. J. 21/2022). Chem. A Eur. J. 2022, 28, e202200824. [Google Scholar] [CrossRef]
- Mohammad Ameen, S.S.; Omer, K.M. Multifunctional MOF: Cold/hot adapted sustainable oxidase-like MOF nanozyme with ratiometric and color tonality for nitrite ions detection. Food Chem. 2025, 462, 141027. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, R.; Li, J.; Tang, H.; Su, H.; Feng, W.; Zhang, Z. A dual-responsive RhB-doped MOF probe for simultaneous recognition of Cu2+ and Fe3+. Sci. Rep. 2024, 14, 11740. [Google Scholar] [CrossRef]
- Mohan, B.; Ma, S.; Kumar, S.; Yang, Y.; Ren, P. Tactile Sensors: Hydroxyl Decorated Silver Metal–Organic Frameworks for Detecting Cr2O72–, MnO4–, Humic Acid, and Fe3+ Ions. ACS Appl. Mater. Interfaces 2023, 15, 17317–17323. [Google Scholar] [CrossRef]
- He, Q.-Q.; Yao, S.-L.; Zheng, T.-F.; Xu, H.; Liu, S.-J.; Chen, J.-L.; Li, N.; Wen, H.-R. A multi-responsive luminescent sensor based on a stable Eu(iii) metal–organic framework for sensing Fe3+, MnO4−, and Cr2O72− in aqueous solutions. CrystEngComm 2022, 24, 1041–1048. [Google Scholar] [CrossRef]
- Ma, J.-J.; Liu, W.-s. Effective luminescence sensing of Fe3+, Cr2O72−, MnO4− and 4-nitrophenol by lanthanide metal–organic frameworks with a new topology type. Dalton Trans. 2019, 48, 12287–12295. [Google Scholar] [CrossRef]
- Su, F.; Liu, X.-Y.; Li, S.-D.; Sun, L.; Wu, L.-T.; Han, C.; Wang, Z.-J. A water-stable Y(III)-MOF as multi-responsive luminescent sensor for high-efficiency detection of Fe3+, Cu2+, and MnO4- ions in aqueous solutions. J. Mol. Struct. 2024, 1298, 136975. [Google Scholar] [CrossRef]
- Chen, J.-K.; Yang, S.-M.; Li, B.-H.; Lin, C.-H.; Lee, S. Fluorescence Quenching Investigation of Methyl Red Adsorption on Aluminum-Based Metal–Organic Frameworks. Langmuir 2018, 34, 1441–1446. [Google Scholar] [CrossRef]
- Amani, V.; Norouzi, F.; Akrami, Z. A review of UiO-based MOF detection and removal strategies for antibiotics in water. New J. Chem. 2024, 48, 18600–18617. [Google Scholar] [CrossRef]
- Sharma, A.; Kim, D.; Park, J.-H.; Rakshit, S.; Seong, J.; Jeong, G.H.; Kwon, O.-H.; Lah, M.S. Mechanistic insight into the sensing of nitroaromatic compounds by metal-organic frameworks. Commun. Chem. 2019, 2, 39. [Google Scholar] [CrossRef]
- Chen, H.; Fan, P.; Tu, X.; Min, H.; Yu, X.; Li, X.; Zeng, J.-L.; Zhang, S.; Cheng, P. A Bifunctional Luminescent Metal–Organic Framework for the Sensing of Paraquat and Fe3+ Ions in Water. Chem. Asian J. 2019, 14, 3611–3619. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-Q.; Fu, L.; Cui, G.-H. Two chemically robust Cd(ii)-frameworks for efficient sensing of levofloxacin, benzaldehyde, and Fe3+ ions. Dalton Trans. 2021, 50, 15743–15753. [Google Scholar] [CrossRef]
- Wu, Y.-B.; Ren, L.; Dong, G.-Y. Syntheses, crystal structures, luminescent sensing and photocatalytic properties of two 2D cadmium(II) coordination polymers constructed from mixed ligands. Inorganica Chim. Acta 2022, 530, 120703. [Google Scholar] [CrossRef]
- Peng, J.; Zhou, W.; Ding, H.; Du, H.; Li, S.J. Surface-effect on detection ability of fluorescent Eu(btc) metal-organic frameworks to metal ions. J. Rare Earths 2021, 39, 446–452. [Google Scholar] [CrossRef]
- Mao, X.; Li, H.; Shi, Y.; Liu, J.; Kuai, L.; Yang, F.; Wu, C. A multifunctional fluorescence sensor based Zn(II) metal-organic framework for rapid and sensitive detection Fe3+ and Al3+. Polyhedron 2024, 264, 117246. [Google Scholar] [CrossRef]
- Safaei, S.; Wang, J.; Junk, P.C. Incorporation of thiazolothiazole fluorophores into a MOF structure: A highly luminescent Zn(II)-based MOF as a selective and reversible sensor for Cr2O72− and MnO4− anions. J. Solid State Chem. 2021, 294, 121762. [Google Scholar] [CrossRef]
- Su, Y.-Q.; Wang, R.-T.; Blatova, O.A.; Shi, Y.-S.; Cui, G.-H. Two robust Zn(ii)-organic frameworks as dual-functional fluorescent probes for efficient sensing of enrofloxacin and MnO4− anions. CrystEngComm 2022, 24, 182–191. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, J.; Xi, L.; Xie, J.; Wang, X.; Ma, Y.; Li, L. Two Novel Lanthanide Metal–Organic Frameworks: Selective Luminescent Sensing for Nitrobenzene, Cu2+, and MnO4–. Cryst. Growth Des. 2020, 20, 5225–5234. [Google Scholar] [CrossRef]
- Xu, Z.; Su, M.; He, X.; Zhang, B.; Wang, Y.; Li, H. A porous luminescent Zn-MOF for selective probing Fe3+ and nitrophenolic compounds. Inorg. Chem. Commun. 2020, 111, 107644. [Google Scholar] [CrossRef]
- Abdollahi, N.; Morsali, A. Highly sensitive fluorescent metal-organic framework as a selective sensor of MnVII and CrVI anions (MnO4−/Cr2O72−/CrO42−) in aqueous solutions. Anal. Chim. Acta 2019, 1064, 119–125. [Google Scholar] [CrossRef]
- Liu, X.; Shi, T.; Xu, C.; Zhu, M.; Wang, Y. A highly selective and sensitive ICT-based Cu(2+) fluorescent probe and its application in bioimaging. Ecotoxicol. Environ. Saf. 2023, 262, 115127. [Google Scholar] [CrossRef]
- Li, H.; Chen, Q.; Zhang, Z.; Wang, Z.; Gong, Z.; Fan, M. Functionalized fluorescent Zr-MOF based on photoinduced electron transfer for highly sensitive detection of nitroaromatic explosives. Dye. Pigment. 2023, 210, 111035. [Google Scholar] [CrossRef]
- Liu, H.-b.; Tao, X.-m.; Li, H.; Lv, R.; Liu, X.; Gu, W. Exciting high symmetric 24-nuclear copper cluster with excellent selective adsorption capacity for cationic dyes. Inorg. Chem. Commun. 2016, 74, 102–105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).