Expression of Estrogen Receptors in Main Immune Organs in Sheep During Early Pregnancy
Abstract
1. Introduction
2. Results
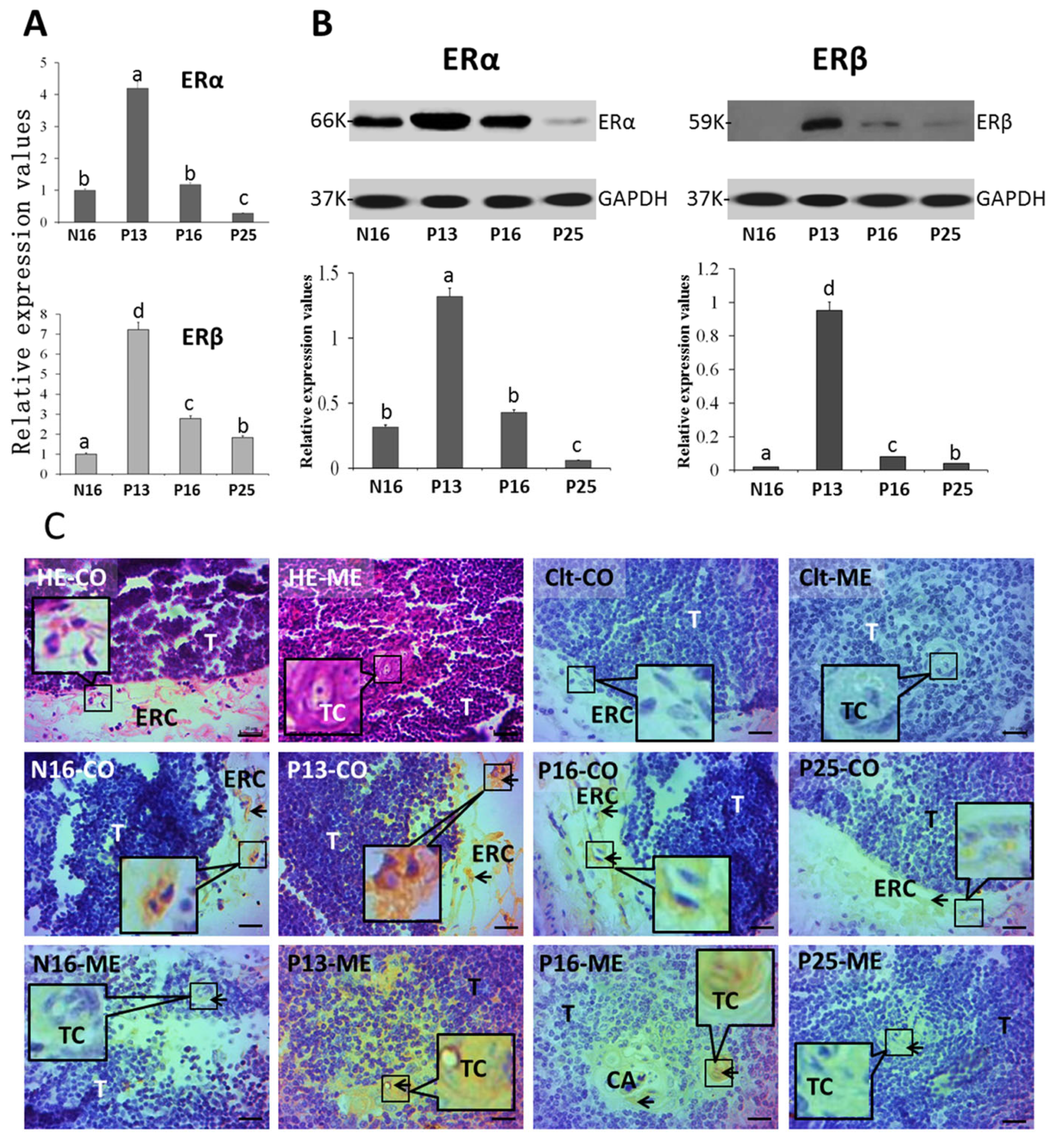
2.1. ERα and ERβ in the Thymus
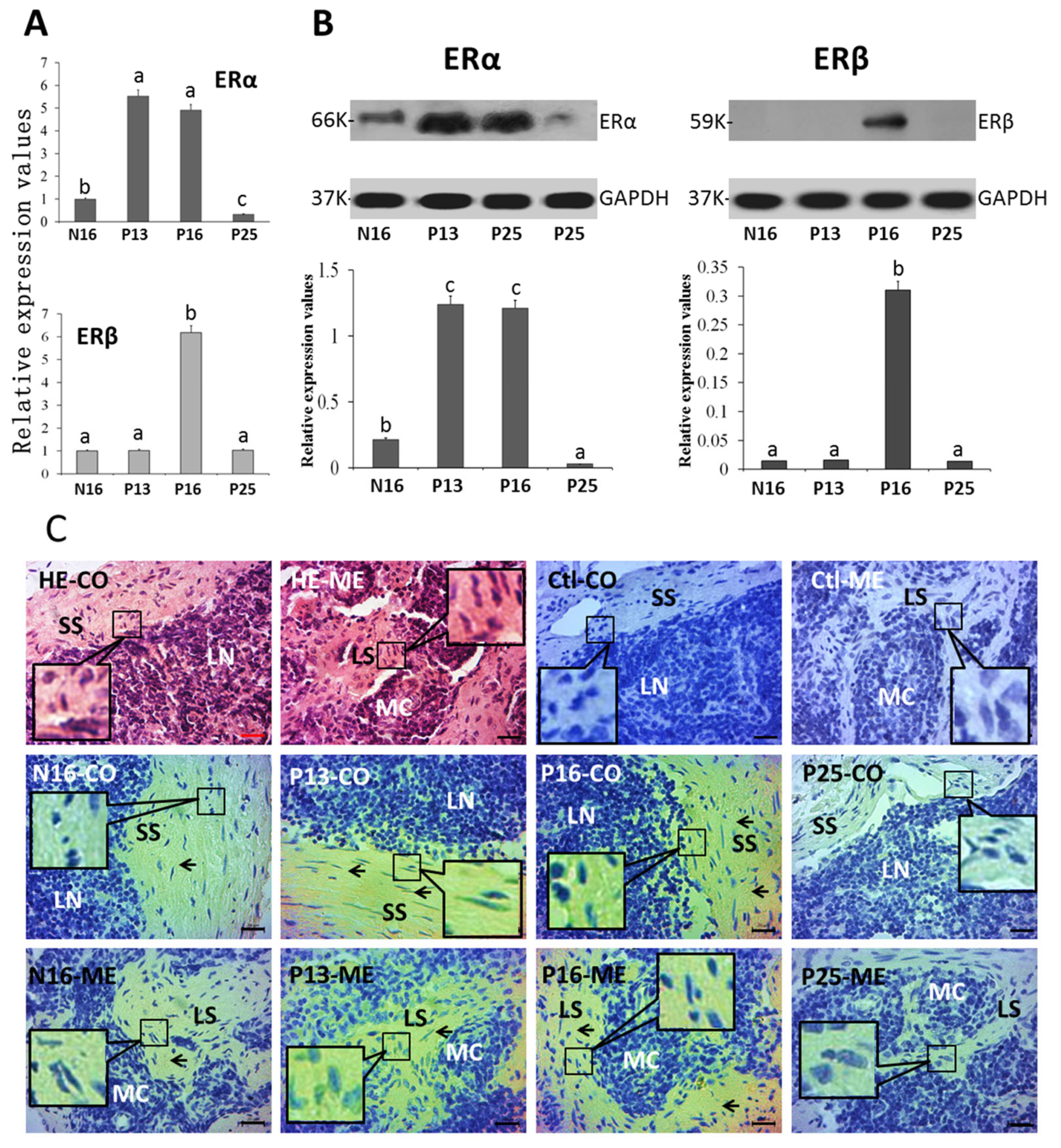
2.2. ERα and ERβ in Lymph Nodes
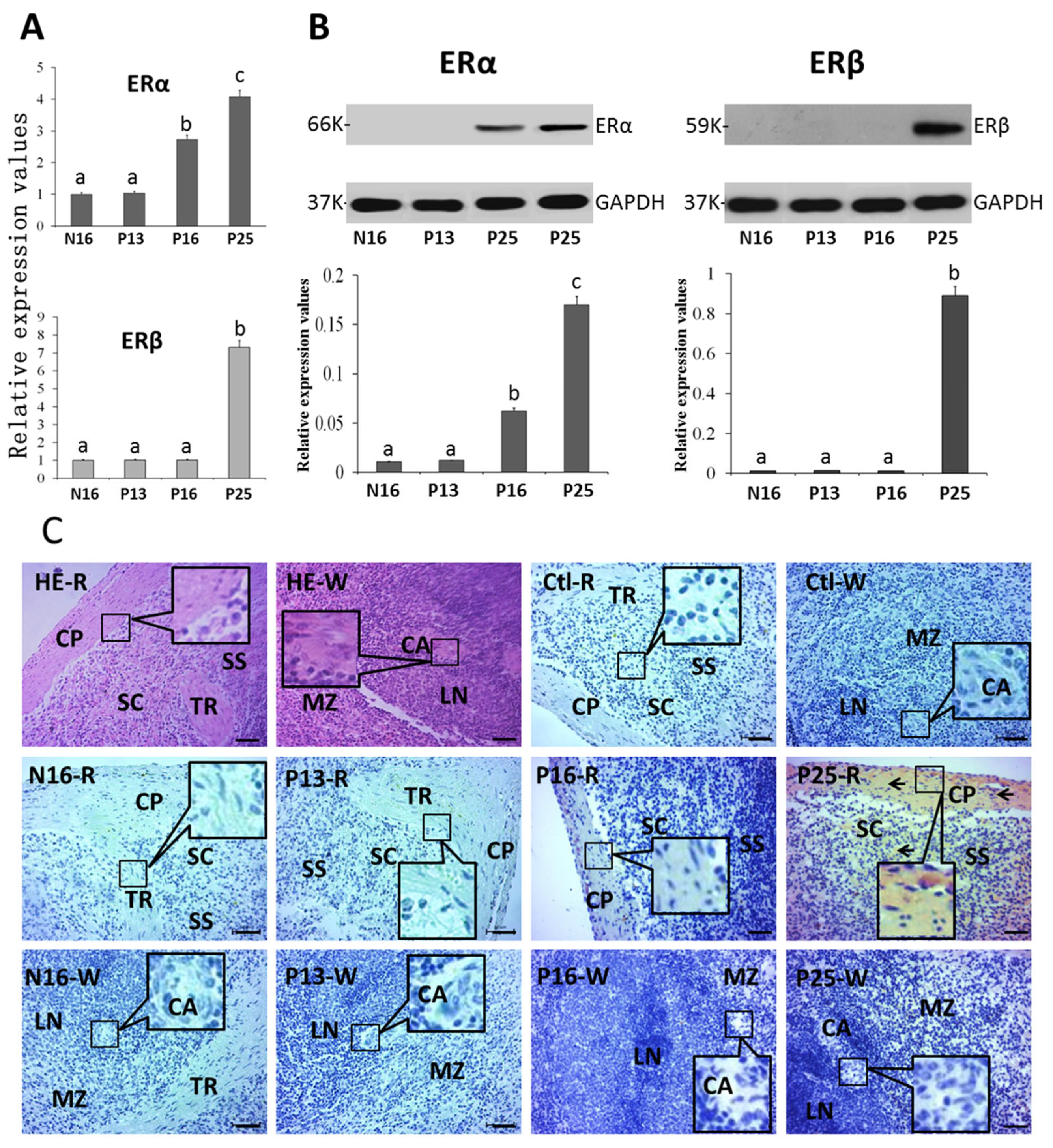
2.3. ERα and ERβ in the Spleen
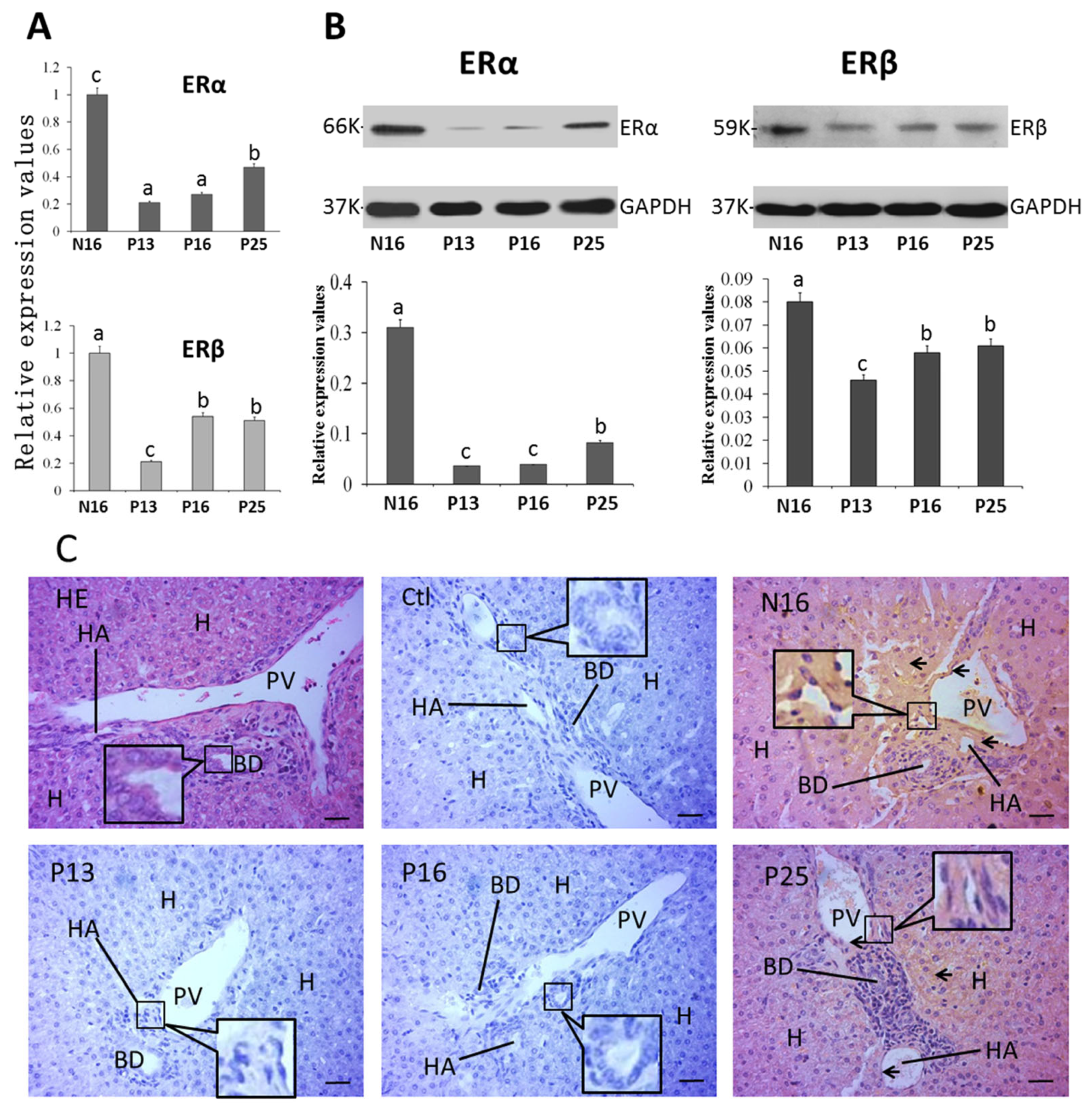
2.4. ERα and ERβ in the Liver
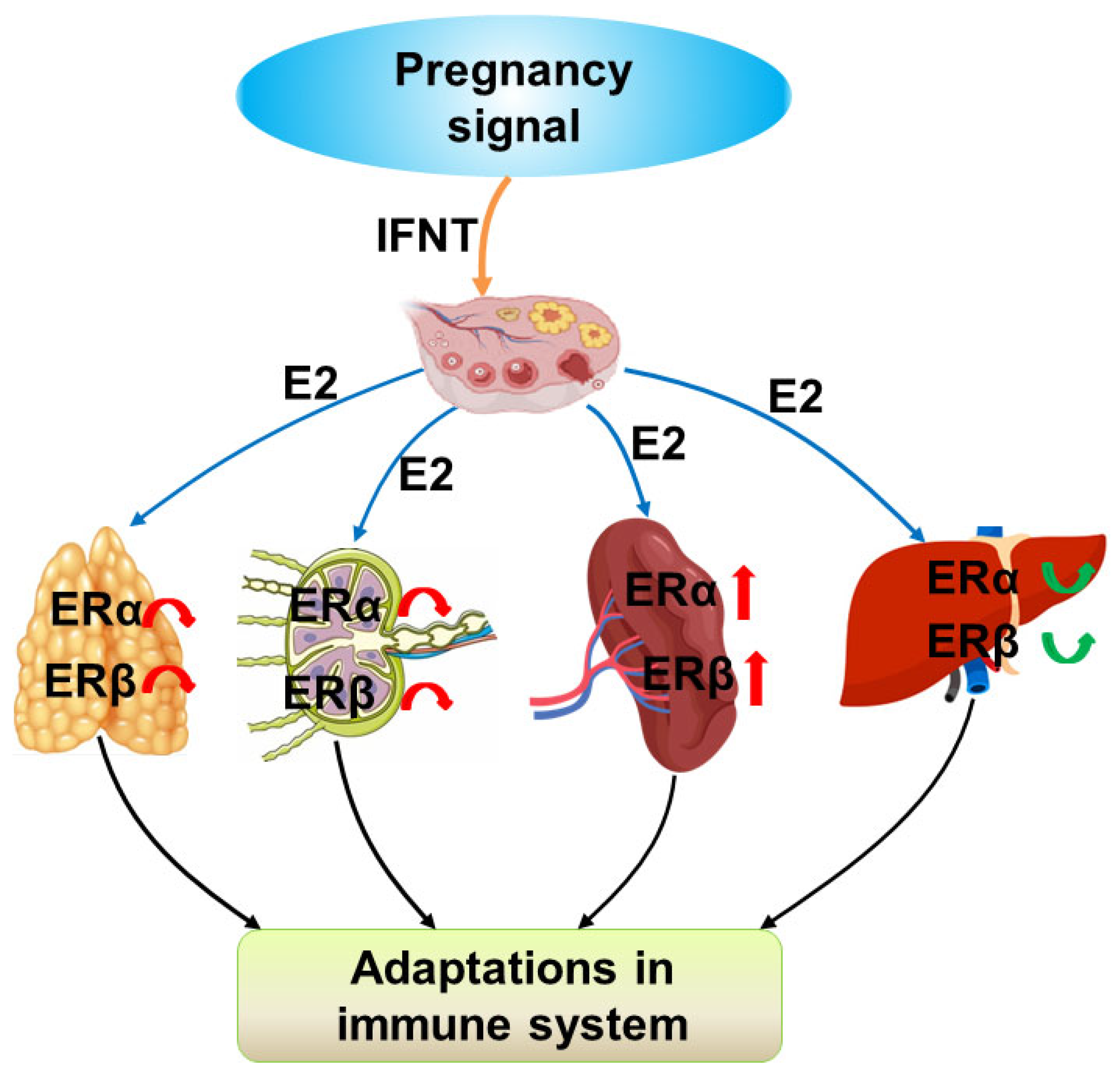
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. RNA Extraction and RT-qPCR Assay
4.3. Western Blot Analysis
4.4. Immunohistochemistry Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parisi, F.; Fenizia, C.; Introini, A.; Zavatta, A.; Scaccabarozzi, C.; Biasin, M.; Savasi, V. The pathophysiological role of estrogens in the initial stages of pregnancy: Molecular mechanisms and clinical implications for pregnancy outcome from the periconceptional period to end of the first trimester. Hum. Reprod. Update 2023, 29, 699–720. [Google Scholar] [CrossRef] [PubMed]
- Robertshaw, I.; Bian, F.; Das, S.K. Mechanisms of uterine estrogen signaling during early pregnancy in mice: An update. J. Mol. Endocrinol. 2016, 56, 127–138. [Google Scholar] [CrossRef]
- Bai, J.; Qi, Q.R.; Li, Y.; Day, R.; Makhoul, J.; Magness, R.R.; Chen, D.B. Estrogen receptors and estrogen-induced uterine vasodilation in pregnancy. Int. J. Mol. Sci. 2020, 21, 4349. [Google Scholar] [CrossRef]
- Yu, K.; Huang, Z.Y.; Xu, X.L.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen receptor function: Impact on the human endometrium. Front. Endocrinol. 2022, 13, 827724. [Google Scholar] [CrossRef]
- Xiong, Y.H.; Yuan, Z.; He, L. Effects of estrogen on CD4(+) CD25(+) regulatory T cell in peripheral blood during pregnancy. Asian. Pac. J. Trop. Med. 2013, 6, 748–752. [Google Scholar] [CrossRef]
- Spencer, T.E.; Bazer, F.W. Temporal and spatial alterations in uterine estrogen receptor and progesterone receptor gene expression during the estrous cycle and early pregnancy in the ewe. Biol. Reprod. 1995, 53, 1527–1543. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal immunological adaptation during normal pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef]
- Gullo, G.; Scaglione, M.; Cucinella, G.; Riva, A.; Coldebella, D.; Cavaliere, A.F.; Signore, F.; Buzzaccarini, G.; Spagnol, G.; Laganà, A.S.; et al. Congenital Zika Syndrome: Genetic avenues for diagnosis and therapy, possible management and long-term outcomes. J. Clin. Med. 2022, 11, 1351. [Google Scholar] [CrossRef]
- Maranto, M.; Zaami, S.; Restivo, V.; Termini, D.; Gangemi, A.; Tumminello, M.; Culmone, S.; Billone, V.; Cucinella, G.; Gullo, G. Symptomatic COVID-19 in pregnancy: Hospital cohort data between May 2020 and April 2021, risk factors and medicolegal implications. Diagnostics. 2023, 13, 1009. [Google Scholar] [CrossRef]
- Montan, G.; Carollo, M.; Torres, L.; Buzzaccarini, G.; Giannini, A.; Etrusco, A.; Cosmi, E.; Rigano, M.; Chiantera, V.; Laganà, A.S.; et al. Androgens and female sexuality: Molecular insights, neuroendocrine crosstalk and future therapeutic directions. Clin. Exp. Obstet. Gynecol. 2023, 50, 141. [Google Scholar] [CrossRef]
- Quatrini, L.; Ricci, B.; Ciancaglini, C.; Tumino, N.; Moretta, L. Regulation of the immune system development by glucocorticoids and sex hormones. Front. Immunol. 2021, 12, 672853. [Google Scholar] [CrossRef]
- Komijani, E.; Parhizkar, F.; Abdolmohammadi-Vahid, S.; Ahmadi, H.; Nouri, N.; Yousefi, M.; Aghebati-Maleki, L. Autophagy-mediated immune system regulation in reproductive system and pregnancy-associated complications. J. Reprod. Immunol. 2023, 158, 103973. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B.; Byemerwa, J.; Krebs, T.; Lim, F.; Chang, C.Y.; McDonnell, D.P. Estrogen receptor signaling in the immune system. Endocr. Rev. 2023, 44, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Ott, T.L. Immunological detection of pregnancy: Evidence for systemic immune modulation during early pregnancy in ruminants. Theriogenology. 2020, 150, 498–503. [Google Scholar] [CrossRef]
- Rocha, C.C.; Da-Silveira, J.C.; Forde, N.; Binelli, M.; Pugliesi, G. Conceptus-modulated innate immune function during early pregnancy in ruminants: A review. Anim. Reprod. 2021, 18, e20200048. [Google Scholar] [CrossRef]
- Feng, P.; Wu, J.; Ren, Y.; Zhang, L.; Cao, J.; Yang, L. Early pregnancy regulates the expression of prolactin and its receptor in the thymus, the liver, the spleen and lymph nodes in sheep. Domest. Anim. Endocrinol. 2022, 81, 106731. [Google Scholar] [CrossRef]
- Dinges, S.S.; Amini, K.; Notarangelo, L.D.; Delmonte, O.M. Primary and secondary defects of the thymus. Immunol. Rev. 2024, 322, 178–211. [Google Scholar] [CrossRef]
- Laan, M.; Haljasorg, U.; Kisand, K.; Salumets, A.; Peterson, P. Pregnancy-induced thymic involution is associated with suppression of chemokines essential for T-lymphoid progenitor homing. Eur. J. Immunol. 2016, 46, 2008–2017. [Google Scholar] [CrossRef]
- Meng, Y.; Yang, Z.; Quan, Y.; Zhao, S.; Zhang, L.; Yang, L. Regulation of IkappaB protein expression by early gestation in the thymus of ewes. Vet. Sci. 2023, 10, 462. [Google Scholar] [CrossRef]
- Taves, M.D.; Ashwell, J.D. Effects of sex steroids on thymic epithelium and thymocyte development. Front. Immunol. 2022, 13, 975858. [Google Scholar] [CrossRef]
- Merrheim, J.; Villegas, J.; Van-Wassenhove, J.; Khansa, R.; Berrih-Aknin, S.; Le-Panse, R.; Dragin, N. Estrogen, estrogen-like molecules and autoimmune diseases. Autoimmun. Rev. 2020, 19, 102468. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.; Chung, Y.; Kim, J.; Yang, H. Thymocyte differentiation is regulated by a change in estradiol levels during the estrous cycle in mouse. Dev. Reprod. 2013, 17, 441–449. [Google Scholar] [CrossRef]
- Shirshev, S.V.; Orlova, E.G.; Loginova, O.A.; Nekrasova, I.V.; Gorbunova, O.L.; Maslennikova, I.L. Hormonal regulation of dendritic cell differentiation in the thymus. Bull. Exp. Biol. Med. 2018, 165, 230–234. [Google Scholar] [CrossRef]
- Zoller, A.L.; Schnell, F.J.; Kersh, G.J. Murine pregnancy leads to reduced proliferation of maternal thymocytes and decreased thymic emigration. Immunology. 2007, 121, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.M.; Lou, M.; Yao, L.; Germain, R.N.; Radtke, A.J. The lymph node at a glance—How spatial organization optimizes the immune response. J. Cell Sci. 2020, 133, jcs241828. [Google Scholar] [CrossRef]
- Quirke, L.D.; Maclean, P.H.; Haack, N.A.; Edwards, S.J.; Heiser, A.; Juengel, J.L. Characterization of local and peripheral immune system in pregnant and nonpregnant ewes. J. Anim. Sci. 2021, 99, skab208. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Cai, C.; Bai, Y.; Zhang, L.; Yang, L. Early pregnancy regulates expression of IkappaB family in ovine spleen and lymph nodes. Int. J. Mol. Sci. 2023, 24, 5156. [Google Scholar] [CrossRef]
- Sapino, A.; Cassoni, P.; Ferrero, E.; Bongiovanni, M.; Righi, L.; Fortunati, N.; Crafa, P.; Chiarle, R.; Bussolati, G. Estrogen receptor alpha is a novel marker expressed by follicular dendritic cells in lymph nodes and tumor-associated lymphoid infiltrates. Am. J. Pathol. 2003, 163, 1313–1320. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Jiang, B.; Sun, L.; Hao, S.; Li, X.; Hou, Y. Estrogen distinctively modulates spleen DC from (NZB x NZW) F1 female mice in various disease development stages. Cell Immunol. 2007, 248, 95–102. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, H.; Li, X.; Sun, L.; Hou, Y. 17β-Estradiol enhances response of mice spleen B cells elicited by TLR9 agonist. Cell Immunol. 2012, 278, 125–135. [Google Scholar] [CrossRef]
- Wang, P.; Jiang, L.N.; Wang, C.; Li, Y.; Yin, M.; Du, H.B.; Zhang, H.; Fan, Z.H.; Liu, Y.X.; Zhao, M.; et al. Estradiol-induced inhibition of endoplasmic reticulum stress normalizes splenic CD4 + T lymphocytes following hemorrhagic shock. Sci. Rep. 2021, 11, 7508. [Google Scholar] [CrossRef]
- Piesta, A.; Maj, T.; Chełmońska-Soyta, A. The influence of mating on estrogen receptor alpha protein level in spleen and uterine macrophages in female mice. Reprod. Biol. 2009, 9, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Fang, H.; Fang, S.; Zhang, T.; Zhang, L.; Yang, L. Changes in nuclear factor kappa B components expression in the ovine spleen during early pregnancy. J. Anim. Feed. Sci. 2022, 31, 3–11. [Google Scholar] [CrossRef]
- Yang, L.; Meng, Y.; Shi, Y.; Fang, H.; Zhang, L. Maternal hepatic immunology during pregnancy. Front. Immunol. 2023, 14, 1220323. [Google Scholar] [CrossRef]
- Varas, S.M.; Jahn, G.A. The expression of estrogen, prolactin, and progesterone receptors in mammary gland and liver of female rats during pregnancy and early postpartum: Regulation by thyroid hormones. Endocr. Res. 2005, 31, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Benslimane, Y.; Amalfi, K.; Lapin, S.; Perrino, S.; Brodt, P. Estrogen receptor blockade potentiates immunotherapy for liver metastases by altering the liver immunosuppressive microenvironment. Cancer. Res. Commun. 2024, 4, 1963–1977. [Google Scholar] [CrossRef]
- Tian, Y.; Hong, X.; Xie, Y.; Guo, Z.; Yu, Q. 17β-Estradiol (E2) Upregulates the ERα/SIRT1/PGC-1α signaling pathway and protects mitochondrial function to prevent bilateral oophorectomy (OVX)-induced nonalcoholic fatty liver disease (NAFLD). Antioxidants. 2023, 12, 2100. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Gene | Primer | Sequence | Size (bp) | Accession Numbers |
|---|---|---|---|---|
| ERα | Forward | CTGCTGCTGGAGATGCTGGATG | 88 | XM_042253635.1 |
| Reverse | GCTGGCTCTGATTCACGTCTTCC | |||
| ERβ | Forward | TGCTGCTGGAGATGCTGAATGC | 112 | NM_001009737.1 |
| Reverse | GGTTTCTGGGAGCCCTCTTTGC | |||
| GAPDH | Forward | GGGTCATCATCTCTGCACCT | 176 | NM_001190390.1 |
| Reverse | GGTCATAAGTCCCTCCACGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Zhang, Y.; Cao, Z.; Li, Z.; Zhang, L.; Yang, L. Expression of Estrogen Receptors in Main Immune Organs in Sheep During Early Pregnancy. Int. J. Mol. Sci. 2025, 26, 3528. https://doi.org/10.3390/ijms26083528
Yang Z, Zhang Y, Cao Z, Li Z, Zhang L, Yang L. Expression of Estrogen Receptors in Main Immune Organs in Sheep During Early Pregnancy. International Journal of Molecular Sciences. 2025; 26(8):3528. https://doi.org/10.3390/ijms26083528
Chicago/Turabian StyleYang, Zhen, Yaqi Zhang, Zhihong Cao, Zhouyuan Li, Leying Zhang, and Ling Yang. 2025. "Expression of Estrogen Receptors in Main Immune Organs in Sheep During Early Pregnancy" International Journal of Molecular Sciences 26, no. 8: 3528. https://doi.org/10.3390/ijms26083528
APA StyleYang, Z., Zhang, Y., Cao, Z., Li, Z., Zhang, L., & Yang, L. (2025). Expression of Estrogen Receptors in Main Immune Organs in Sheep During Early Pregnancy. International Journal of Molecular Sciences, 26(8), 3528. https://doi.org/10.3390/ijms26083528








