Formulation and In Vitro Characterization of Granulocyte-Colony-Stimulating-Factor-Loaded Liposomes: Future Prospective in Reproductive Medicine
Abstract
1. Introduction
2. Results
2.1. Obtaining Granulocyte-Colony-Stimulating-Factor-Loaded Liposomes
2.2. Evaluation of Granulocyte-Colony-Stimulating-Factor-Loaded Liposome Morphology by Cryogenic Electron Microscopy
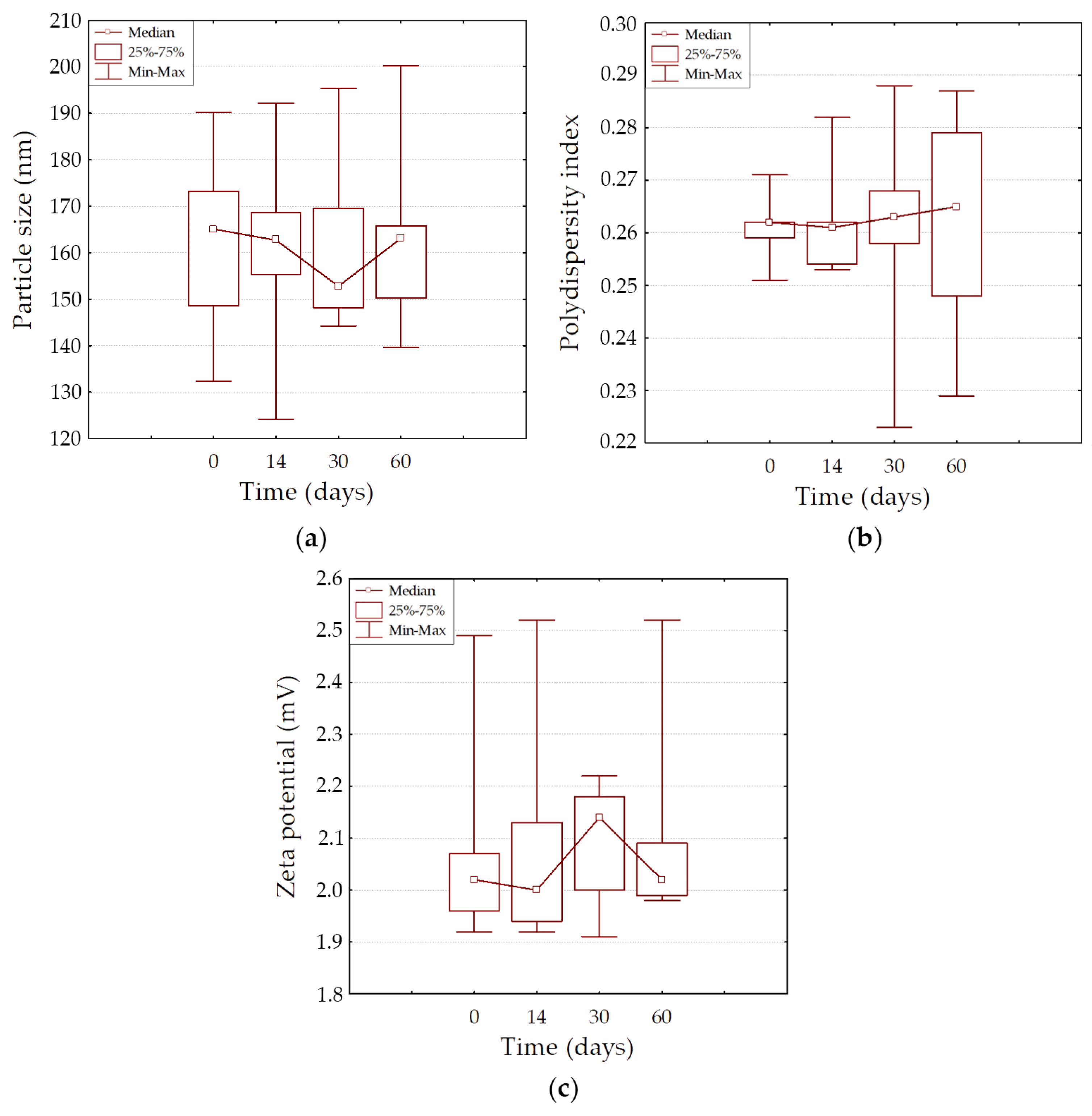
2.3. The Physical Stability of Granulocyte-Colony-Stimulating-Factor-Loaded Liposomes During Long-Term Storage
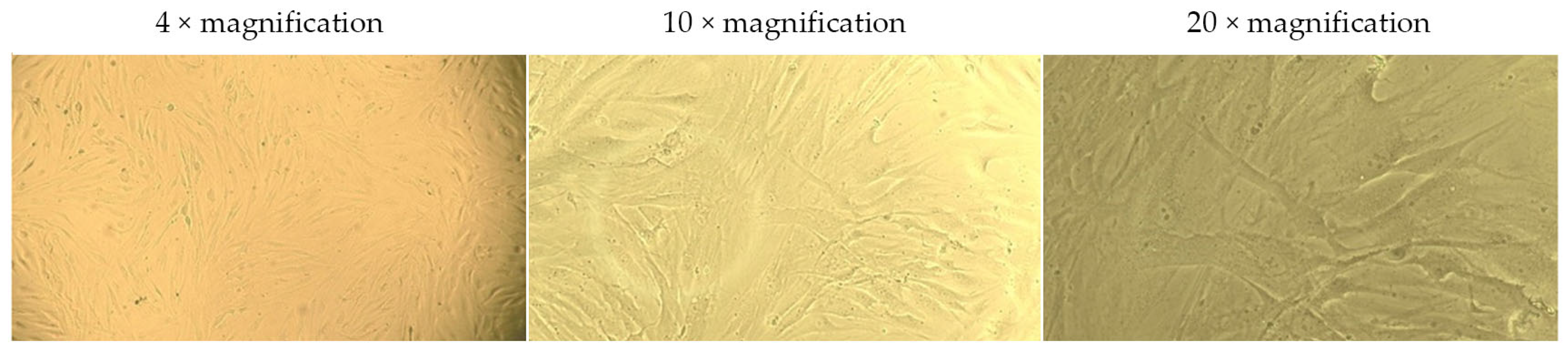
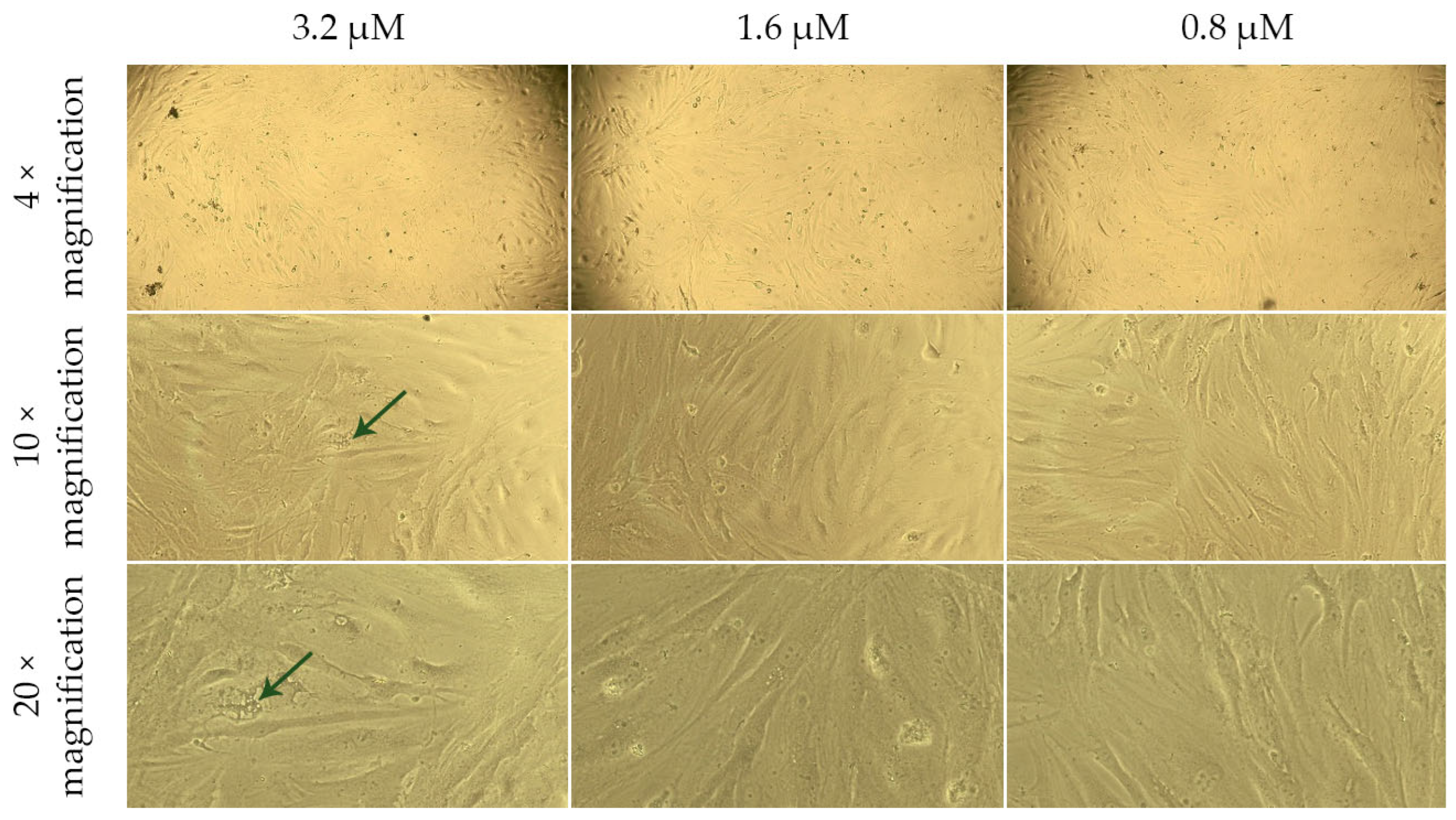
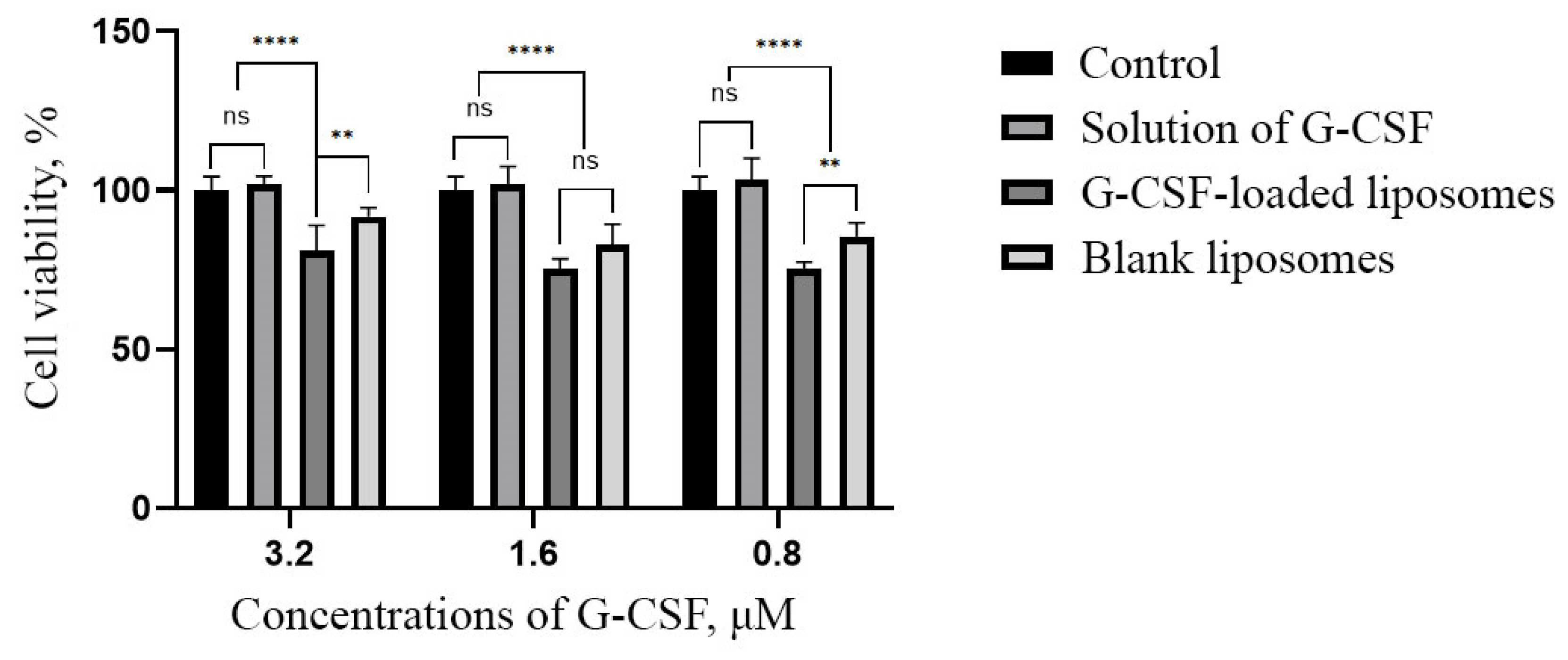
2.4. Assessment of the Effect of G-CSF-Loaded Liposomes on the Metabolic Activity of Human Dermal Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Granulocyte Colony-Stimulating Factor in Phosphate-Buffered Saline Solution and Granulocyte-Colony-Stimulating-Factor-Loaded Liposomes
4.2.1. Granulocyte Colony-Stimulating Factor Solution
4.2.2. Granulocyte-Colony-Stimulating-Factor-Loaded Liposomes
4.3. Determination of Particle Size, Polydispersity Index, and Zeta Potential
4.4. Determination of the Encapsulation Efficiency of Granulocyte Colony-Stimulating Factor
4.5. Determination of the Morphology of Nanoparticle by Cryogenic Transmission Electron Microscopy
4.6. Physical Stability Assessment of Granulocyte-Colony-Stimulating-Factor-Loaded Liposomes
4.7. Assessment of Cell Viability
4.8. Statistical Analysis
5. Conclusions
Future Direction in Reproductive Medicine
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medenica, S.; Abazovic, D.; Ljubić, A.; Vukovic, J.; Begovic, A.; Cucinella, G.; Zaami, S.; Gullo, G. The Role of Cell and Gene Therapies in the Treatment of Infertility in Patients with Thyroid Autoimmunity. Int. J. Endocrinol. 2022, 2022, 4842316. [Google Scholar] [CrossRef] [PubMed]
- Obedkova, K.V.; Ryzhov, J.R.; Storozheva, K.V.; Khalenko, V.V.; Safarian, G.K.; Savicheva, A.M.; Budilovskaya, O.V.; Krysanova, A.A.; Gzgzyan, A.M.; Bespalova, O.N.; et al. Clinical efficacy of peripheral blood mononuclear cell secretome application in patients with repeated implantation failure. Acta Biomed. 2024, 95, e2024174. [Google Scholar] [CrossRef]
- Sarvi, F.; Arabahmadi, M.; Alleyassin, A.; Aghahosseini, M.; Ghasemi, M. Effect of Increased Endometrial Thickness and Implantation Rate by Granulocyte Colony-Stimulating Factor on Unresponsive Thin Endometrium in Fresh In Vitro Fertilization Cycles: A Randomized Clinical Trial. Obstet. Gynecol. Int. 2017, 2017, 3596079. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as drug delivery systems: A review of the implication of nanoparticles’ Physicochemical properties on responses in biological systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N.; et al. Nanoparticles in drug delivery: From history to therapeutic applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef]
- Shan, X.; Gong, X.; Li, J.; Wen, J.; Li, Y.; Zhang, Z. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm. Sin. B 2022, 12, 3028–3048. [Google Scholar] [CrossRef]
- Muppidi, K.; Pumerantz, A.S.; Wang, J.; Betageri, G. Development and stability studies of novel liposomal vancomycin formulations. ISRN Pharm. 2012, 2012, 636743. [Google Scholar] [CrossRef]
- Meyer, J.; Whitcomb, L.; Collins, D. Efficient encapsulation of proteins within liposomes for slow release in vivo. Biochem. Biophys. Res. Commun. 1994, 199, 433–438. [Google Scholar] [CrossRef]
- Salade, L.; Wauthoz, N.; Deleu, M.; Vermeersch, M.; De Vriese, C.; Amighi, K.; Goole, J. Development of coated liposomes loaded with ghrelin for nose-to-brain delivery for the treatment of cachexia. Int. J. Nanomed. 2017, 12, 8531–8543. [Google Scholar] [CrossRef] [PubMed]
- Kiafar, F.; Siahi Shadbad, M.R.; Valizadeh, H. Filgrastim (G-CSF) loaded liposomes: Mathematical modeling and optimization of encapsulation efficiency and particle size. Bioimpacts 2016, 6, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Lewicki, S.; Leśniak, M.; Sobolewska-Ruta, A.; Lewicka, A.; Grodzik, M.; Machaj, E.K.; Saracyn, M.; Kubiak, J.Z.; Pojda, Z. Encapsulation of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in liposomes prepared by thin film hydration and their transfer to mesenchymal stem cells and cord blood hematopoietic stem cells. Arch. Med. Sci. 2020, 18, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Theyab, A.; Algahtani, M.; Alsharif, K.F.; Hawsawi, Y.M.; Alghamdi, A.; Alghamdi, A.; Akinwale, J. New insight into the mechanism of granulocyte colony-stimulating factor (G-CSF) that induces the mobilization of neutrophils. Hematology 2021, 26, 628–636. [Google Scholar] [CrossRef]
- Michailov, Y.; AbuMadighem, A.; Lunenfeld, E.; Kapelushnik, J.; Huleihel, M. Granulocyte Colony-Stimulating Factor Restored Impaired Spermatogenesis and Fertility in an AML-Chemotherapy Mice Model. Int. J. Mol. Sci. 2021, 22, 11157. [Google Scholar] [CrossRef]
- Toghraie, F.S.; Yazdanpanah-Samani, M.; Mahmoudi Maymand, E.; Hosseini, A.; Asgari, A.; Ramezani, A.; Ghaderi, A. Molecular Cloning, Expression and Purification of G-CSF Isoform D, an Alternative Splice Variant of Human G-CSF. Iran. J. Allergy Asthma Immunol. 2019, 18, 419–426. [Google Scholar] [CrossRef]
- Theyab, A.; Alsharif, K.F.; Alzahrani, K.J.; Oyouni, A.A.A.; Hawsawi, Y.M.; Algahtani, M.; Alghamdi, S.; Alshammary, A.F. New insight into strategies used to develop long-acting G-CSF biologics for neutropenia therapy. Front. Oncol. 2023, 12, 1026377. [Google Scholar] [CrossRef]
- Vanz, A.L.; Renard, G.; Palma, M.S.; Chies, J.M.; Dalmora, S.L.; Basso, L.A.; Santos, D.S. Human granulocyte colony stimulating factor (hG-CSF): Cloning, overexpression, purification and characterization. Microb. Cell Factories 2008, 7, 13. [Google Scholar] [CrossRef]
- Link, H. Current state and future opportunities in granulocyte colony-stimulating factor (G-CSF). Support. Care Cancer 2022, 30, 7067–7077. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, Y.; Zhou, D.; Zhang, W. Effects of pegylated recombinant human granulocyte colony-stimulating factor on lymphocytes and white blood cells of patients with malignant tumor. Open Life Sci. 2023, 18, 20220590. [Google Scholar] [CrossRef]
- Crawford, J.; Glaspy, J.A.; Stoller, R.G.; Tomita, D.K.; Vincent, M.E.; McGuire, B.W.; Ozer, H. Final results of a placebo-controlled study of filgrastim in small-cell lung cancer: Exploration of risk factors for febrile neutropenia. Support. Cancer Ther. 2005, 3, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Fruehauf, S.; Klaus, J.; Huesing, J.; Veldwijk, M.R.; Buss, E.C.; Topaly, J.; Seeger, T.; Zeller, L.W.; Moehler, T.; Ho, A.D.; et al. Efficient mobilization of peripheral blood stem cells following CAD chemotherapy and a single dose of pegylated G-CSF in patients with multiple myeloma. Bone Marrow Transplant. 2007, 39, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Jantunen, E.; Partanen, A.; Turunen, A.; Varmavuo, V.; Silvennoinen, R. Mobilization strategies in myeloma patients intended for autologous hematopoietic cell transplantation. Transfus. Med. Hemother. 2023, 50, 438–447. [Google Scholar] [CrossRef] [PubMed]
- DiCarlo, A.L.; Horta, Z.P.; Aldrich, J.T.; Jakubowski, A.A.; Skinner, W.K.; Case, C.M., Jr. Use of growth factors and other cytokines for treatment of injuries during a radiation public health emergency. Radiat. Res. 2019, 192, 99–120. [Google Scholar] [CrossRef]
- Singh, R.; Singh, M.; Jindal, A.; Jindal, P.C. A prospective randomized controlled study (RCT) of Intra-uterine administration of Granulocyte Colony-Stimulating Factor (G-CSF) before embryo-transfer on resistant thin endometrium in IVF cycles. Hum. Reprod. 2015, 30, i280. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01098495/full (accessed on 13 March 2025).
- Rahmati, M.; Petitbarat, M.; Dubanchet, S.; Bensussan, A.; Chaouat, G.; Ledee, N. Granulocyte-Colony stimulating factor related pathways tested on an endometrial ex-vivo model. PLoS ONE 2014, 9, e102286. [Google Scholar] [CrossRef]
- Kunicki, M.; Łukaszuk, K.; Liss, J.; Skowrońska, P.; Szczyptańska, J. Granulocyte colony stimulating factor treatment of resistant thin endometrium in women with frozen-thawed blastocyst transfer. Syst. Biol. Reprod. Med. 2017, 63, 49–57. [Google Scholar] [CrossRef]
- Narayana, N.; Khurana, A.; Rai, S.; Poddar, S.D.; Sharma, P.; Abhisheka, K.; Goyal, B.K. Intrauterine G-CSF in IUI cycles for women with thin endometrium may improve endometrial thickness and pregnancy rates: A randomized controlled study. Med. J. Armed Forces India 2024. [Google Scholar] [CrossRef]
- Jinno, M.; Tamaoka, Y.; Teruya, K.; Watanabe, A.; Hatakeyama, N.; Goda, T.; Kimata, H.; Jinno, Y. Granulocyte colony-stimulating factor priming improves embryos and pregnancy rate in patients with poor ovarian reserve: A randomized controlled trial. Reprod. Biol. Endocrinol. 2023, 21, 29. [Google Scholar] [CrossRef]
- Shen, G.Y.; Park, I.H.; Song, Y.S.; Joo, H.W.; Lee, Y.; Shin, J.H.; Kim, K.S.; Kim, H. Local injection of granulocyte-colony stimulating factor accelerates wound healing in a rat excisional wound model. Tissue Eng. Regen. Med. 2016, 13, 297–303. [Google Scholar] [CrossRef]
- Takagi, S.; Jimi, S.; Oyama, T.; Miyazaki, M.; Suzumiya, J.; Ohjimi, H. Topical administration of G-CSF ameliorates refractory cutaneous ulcers: A clinical case series. Skin Dis. Skin Care 2021, 6, 4537. [Google Scholar]
- Cody, D.T., 2nd; Funk, G.F.; Wagner, D.; Gidley, P.W.; Graham, S.M.; Hoffman, H.T. The use of granulocyte colony stimulating factor to promote wound healing in a neutropenic patient after head and neck surgery. Head Neck 1999, 21, 172–175. [Google Scholar] [CrossRef]
- Fine, J.D.; Manes, B.; Frangoul, H. Systemic granulocyte colony-stimulating factor (G-CSF) enhances wound healing in dystrophic epidermolysis bullosa (DEB): Results of a pilot trial. J. Am. Acad. Dermatol. 2015, 73, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Lapidari, P.; Vaz-Luis, I.; Di Meglio, A. Side effects of using granulocyte-colony stimulating factors as prophylaxis of febrile neutropenia in cancer patients: A systematic review. Crit. Rev. Oncol. Hematol. 2021, 157, 103193. [Google Scholar] [CrossRef]
- Bumbăcea, R.S.; Udrea, M.R.; Ali, S.; Bojincă, V.C. Balancing benefits and risks: A literature review on hypersensitivity reactions to human G-CSF (Granulocyte Colony-Stimulating Factor). Int. J. Mol. Sci. 2024, 25, 4807. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Pisani, S.; Di Martino, D.; Cerri, S.; Genta, I.; Dorati, R.; Bertino, G.; Benazzo, M.; Conti, B. Investigation and Comparison of Active and Passive Encapsulation Methods for Loading Proteins into Liposomes. Int. J. Mol. Sci. 2023, 24, 13542. [Google Scholar] [CrossRef]
- Lee, B.N.; Hong, S.J.; Yu, M.H.; Shin, G.H.; Kim, J.T. Enhancement of Storage Stability and Masking Effect of Curcumin by Turmeric Extract-Loaded Nanoemulsion and Water-Soluble Chitosan Coating. Pharmaceutics 2022, 14, 1547. [Google Scholar] [CrossRef]
- Tseu, G.Y.W.; Kamaruzaman, K.A. A Review of Different Types of Liposomes and Their Advancements as a Form of Gene Therapy Treatment for Breast Cancer. Molecules 2023, 28, 1498. [Google Scholar] [CrossRef]
- Angius, F.; Floris, A. Liposomes and MTT cell viability assay: An incompatible affair. Toxicol. In Vitro 2015, 29, 314–319. [Google Scholar] [CrossRef]
- Sonju, J.J.; Dahal, A.; Singh, S.S.; Jois, S.D. Peptide-functionalized liposomes as therapeutic and diagnostic tools for cancer treatment. J. Control. Release 2021, 329, 624–644. [Google Scholar] [CrossRef] [PubMed]
- Zaman, R.; Islam, R.A.; Ibnat, N.; Othman, I.; Zaini, A.; Lee, C.Y.; Chowdhury, E.H. Current strategies in extending half-lives of therapeutic proteins. J. Control. Release 2019, 301, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Berselli, E.; Coccolini, C.; Tosi, G.; Gökçe, E.H.; Oliveira, M.B.P.P.; Fathi, F.; Krambeck, K.; Souto, E.B. Therapeutic peptides and proteins: Stabilization challenges and biomedical applications by means of nanodelivery systems. Int. J. Pept. Res. Ther. 2024, 30, 15. [Google Scholar] [CrossRef]
- Cui, J.; Wen, Z.; Zhang, W.; Wu, W. Recent Advances in Oral Peptide or Protein-Based Drug Liposomes. Pharmaceuticals 2022, 15, 1072. [Google Scholar] [CrossRef]
- Dmitrieva, M.V.; Timofeeva, T.A.; Oborotova, N.A.; Krasnyuk, I.I.; Stepanova, O.I. Characteristics and stability assessment of liposomal preparations. Drug Dev. Regist. 2018, 3, 36–44. (In Russian) [Google Scholar]
- Burdaev, N.I.; Nikolaeva, L.L.; Kosenko, V.V.; Shprakh, Z.S.; Bunyatyan, N.D. Liposomes as Drug Carriers: Classification, Preparation Methods, and Medicinal Use. Bull. Sci. Cent. Expert Eval. Med. Prod. Regul. Res. Med. Eval. 2023, 13, 316–332. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252, IN26–IN27. [Google Scholar] [CrossRef]
- Colletier, J.P.; Chaize, B.; Winterhalter, M.; Fournier, D. Protein encapsulation in liposomes: Efficiency depends on interactions between protein and phospholipid bilayer. BMC Biotechnol. 2002, 2, 9. [Google Scholar] [CrossRef]
- Khayrani, A.C.; Fahmi, M.; Nurhayati, R.W.; Manas, N.H.A.; Suhaeri, M. Effect of Freeze-Thaw Cycles Method to Transfersome Characteristics for Growth Protein Encapsulation. Int. J. Technol. 2024, 15, 267–278. [Google Scholar] [CrossRef]
- Azumah, J.; Smistad, G.; Hiorth, M. Preparation of stable polymer-liposome complexes by a novel approach employing a one-pot method. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129924. [Google Scholar] [CrossRef]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–265. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 2337. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.S.; Baliga, V.; Londhe, V.Y. Liposomal Formulations: A Recent Update. Pharmaceutics 2025, 17, 36. [Google Scholar] [CrossRef]
- Kalem, Z.; Namli Kalem, M.; Bakirarar, B.; Kent, E.; Makrigiannakis, A.; Gurgan, T. Intrauterine G-CSF Administration in Recurrent Implantation Failure (RIF): An Rct. Sci. Rep. 2020, 10, 5139. [Google Scholar] [CrossRef]
- Nagori, C.B.; Panchal, S.Y.; Patel, H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman’s syndrome. J. Hum. Reprod. Sci. 2011, 4, 43–48. [Google Scholar] [CrossRef]
- Singh, N.; Mohanty, S.; Seth, T.; Shankar, M.; Bhaskaran, S.; Dharmendra, S. Autologous stem cell transplantation in refractory Asherman’s syndrome: A novel cell based therapy. J. Hum. Reprod. Sci. 2014, 7, 93–98. [Google Scholar] [CrossRef]
- Cucinella, G.; Gullo, G.; Catania, E.; Perino, A.; Billone, V.; Marinelli, S.; Napoletano, G.; Zaami, S. Stem Cells and Infertility: A Review of Clinical Applications and Legal Frameworks. J. Pers. Med. 2024, 14, 135. [Google Scholar] [CrossRef]
- Gullo, G.; Scaglione, M.; Cucinella, G.; Chiantera, V.; Perino, A.; Greco, M.E.; Laganà, A.S.; Marinelli, E.; Basile, G.; Zaami, S. Neonatal Outcomes and Long-Term Follow-Up of Children Born from Frozen Embryo, a Narrative Review of Latest Research Findings. Medicina 2022, 58, 1218. [Google Scholar] [CrossRef]
- Gullo, G.; Perino, A.; Cucinella, G. Open vs. closed vitrification system: Which one is safer? Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1065–1067. [Google Scholar] [CrossRef] [PubMed]
- Gullo, G.; Cucinella, G.; Chiantera, V.; Dellino, M.; Cascardi, E.; Török, P.; Herman, T.; Garzon, S.; Uccella, S.; Laganà, A.S. Fertility-Sparing Strategies for Early-Stage Endometrial Cancer: Stepping towards Precision Medicine Based on the Molecular Fingerprint. Int. J. Mol. Sci. 2023, 24, 811. [Google Scholar] [CrossRef] [PubMed]
- Zaami, S.; Melcarne, R.; Patrone, R.; Gullo, G.; Negro, F.; Napoletano, G.; Monti, M.; Aceti, V.; Panarese, A.; Borcea, M.C.; et al. Oncofertility and Reproductive Counseling in Patients with Breast Cancer: A Retrospective Study. J. Clin. Med. 2022, 11, 1311. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tian, Z.; Qi, Q.; Li, Z.; Bi, Y.; Qin, A.; Yang, Y. The therapeutic effects and underlying mechanisms of the intrauterine perfusion of granulocyte colony-stimulating factor on a thin-endometrium rat model. Life Sci. 2020, 260, 118439. [Google Scholar] [CrossRef]

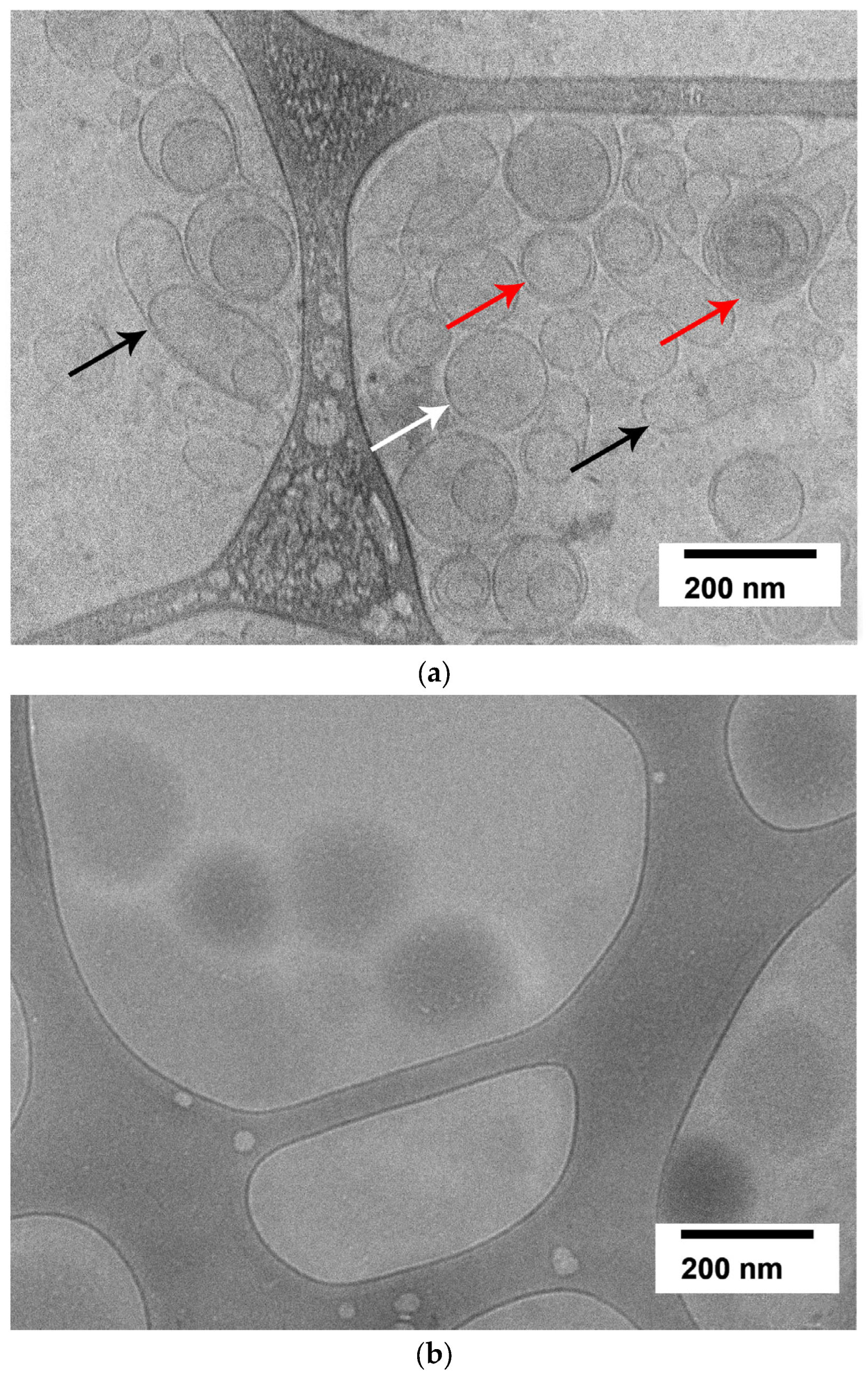
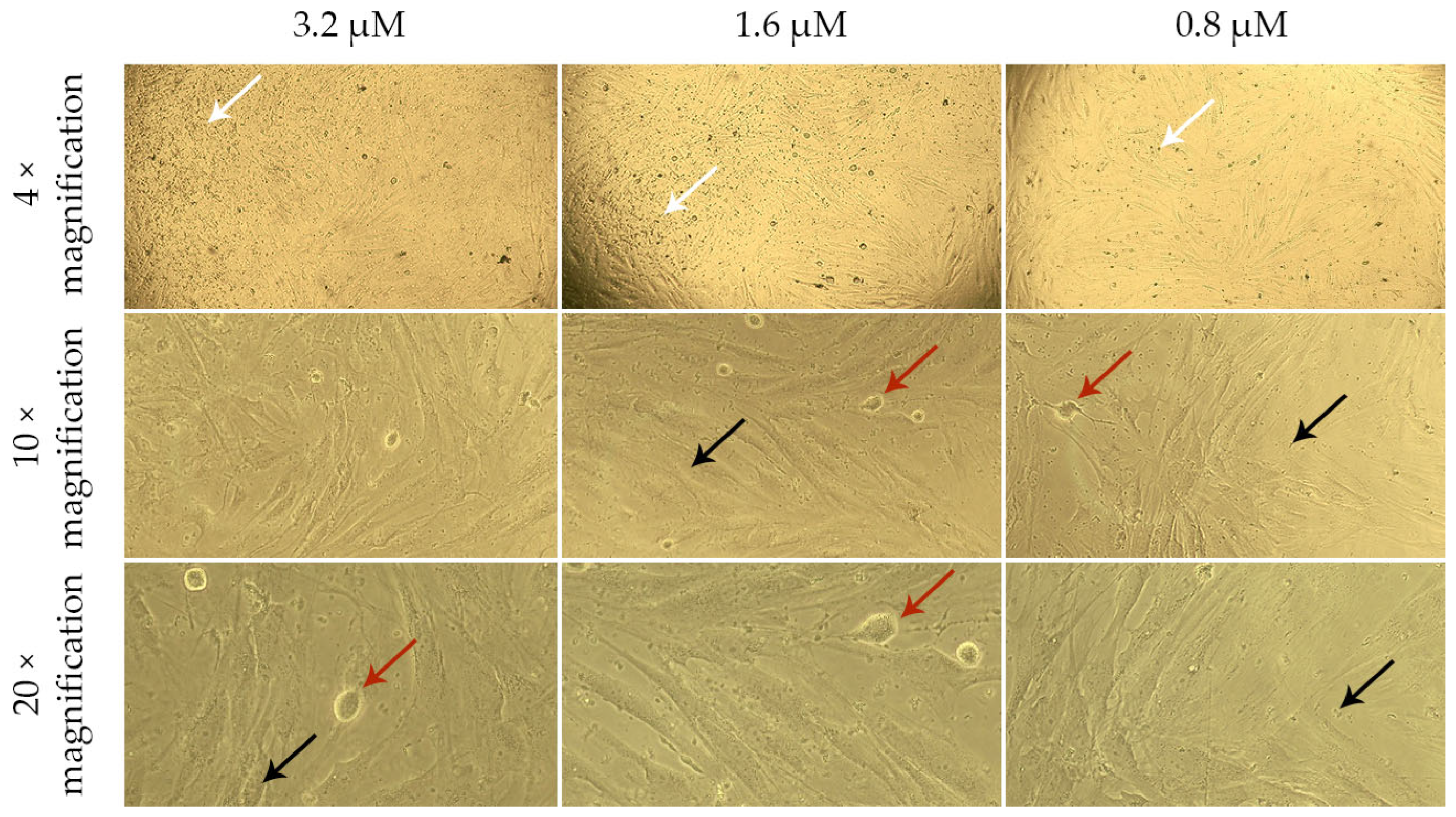
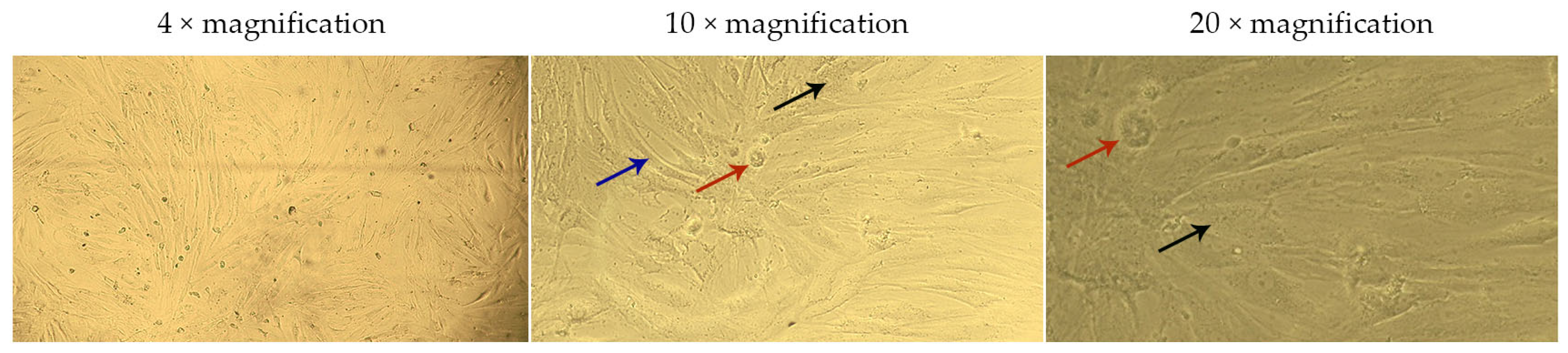
| Liposome’s Type | PC, mg/mL | G-CSF, mg/mL | PDI | Size, nm | ZP, mV | EE, % |
|---|---|---|---|---|---|---|
| Blank liposomes | 50 | – | 0.271 ± 0.05 | 143.7 ± 7.7 | –2.11 ± 0.11 | – |
| G-CSF-loaded liposomes | 50 | 0.5 | 0.261 ± 0.03 | 161.9 ± 9.9 | +2.09 ± 0.10 | 52.37 ± 3.64 |
| Liposome’s Type | Mean | Std. Err. | Std. Dev. | Median | Percentile 25% | Percentile 75% | Minimum | Maximum |
|---|---|---|---|---|---|---|---|---|
| Particle size, nm | ||||||||
| 0 days | 161.9 | ±9.9 | ±22.3 | 165.1 | 148.6 | 173.2 | 132.3 | 190.2 |
| 14 days | 160.6 | ±11.0 | ±24.6 | 162.7 | 155.3 | 168.6 | 124.2 | 192.2 |
| 30 days | 162.0 | ±9.4 | ±20.9 | 152.8 | 148.2 | 169.5 | 144.3 | 195.3 |
| 60 days | 163.8 | ±10.2 | ±22.9 | 163.1 | 150.3 | 165.8 | 139.7 | 200.3 |
| PDI | ||||||||
| 0 days | 0.261 | ±0.003 | ±0.007 | 0.262 | 0.259 | 0.262 | 0.251 | 0.271 |
| 14 days | 0.262 | ±0.005 | ±0.011 | 0.261 | 0.254 | 0.262 | 0.253 | 0.282 |
| 30 days | 0.260 | ±0.011 | ±0.024 | 0.263 | 0.258 | 0.268 | 0.223 | 0.288 |
| 60 days | 0.262 | ±0.010 | ±0.023 | 0.265 | 0.248 | 0.279 | 0.229 | 0.287 |
| Zeta potential, mV | ||||||||
| 0 days | 2.09 | ±0.10 | ±0.23 | 2.02 | 1.96 | 2.07 | 1.92 | 2.49 |
| 14 days | 2.10 | ±0.11 | ±0.25 | 2.00 | 1.94 | 2.13 | 1.92 | 2.52 |
| 30 days | 2.09 | ±0.06 | ±0.13 | 2.14 | 2.00 | 2.18 | 1.91 | 2.22 |
| 60 days | 2.12 | ±0.10 | ±0.23 | 2.02 | 1.99 | 2.09 | 1.98 | 2.52 |
| Group | Cell Viability, % | ||||
|---|---|---|---|---|---|
| 3.2 μM | 1.6 μM | 0.8 μM | Control | Blank Liposomes | |
| Solution of G-CSF | 101.75 ± 2.67 | 101.87 ± 5.56 | 103.22 ± 6.79 | 100.00 ± 4.29 | 82.81 ± 6.53 |
| G-CSF-loaded liposomes | 80.99 ± 7.98 | 75.44 ± 2.95 | 75.15 ± 2.31 | ||
| Time, min | Mobile Phase A, % v/v | Mobile Phase B, % v/v |
|---|---|---|
| 0–4 | 92 | 8 |
| 4–19 | 92 to 72 | 8 to 28 |
| 19–19.1 | 72 to 0 | 28 to 100 |
| 19.1–21 | 0 | 100 |
| 21–21.1 | 0 to 92 | 100 to 8 |
| 21.1–30 | 92 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obedkova, K.V.; Khalenko, V.V.; Tovpeko, D.V.; Ryzhov, J.R.; Bespalova, O.N.; Tapilskaya, N.I. Formulation and In Vitro Characterization of Granulocyte-Colony-Stimulating-Factor-Loaded Liposomes: Future Prospective in Reproductive Medicine. Int. J. Mol. Sci. 2025, 26, 2689. https://doi.org/10.3390/ijms26062689
Obedkova KV, Khalenko VV, Tovpeko DV, Ryzhov JR, Bespalova ON, Tapilskaya NI. Formulation and In Vitro Characterization of Granulocyte-Colony-Stimulating-Factor-Loaded Liposomes: Future Prospective in Reproductive Medicine. International Journal of Molecular Sciences. 2025; 26(6):2689. https://doi.org/10.3390/ijms26062689
Chicago/Turabian StyleObedkova, Kseniia V., Vladislava V. Khalenko, Dmitry V. Tovpeko, Julian R. Ryzhov, Olesya N. Bespalova, and Natalya I. Tapilskaya. 2025. "Formulation and In Vitro Characterization of Granulocyte-Colony-Stimulating-Factor-Loaded Liposomes: Future Prospective in Reproductive Medicine" International Journal of Molecular Sciences 26, no. 6: 2689. https://doi.org/10.3390/ijms26062689
APA StyleObedkova, K. V., Khalenko, V. V., Tovpeko, D. V., Ryzhov, J. R., Bespalova, O. N., & Tapilskaya, N. I. (2025). Formulation and In Vitro Characterization of Granulocyte-Colony-Stimulating-Factor-Loaded Liposomes: Future Prospective in Reproductive Medicine. International Journal of Molecular Sciences, 26(6), 2689. https://doi.org/10.3390/ijms26062689









