A R2R3-MYB Transcription Factor of GmMYB62 Regulates Seed-Coat Color and Seed Size in Arabidopsis
Abstract
1. Introduction
2. Results
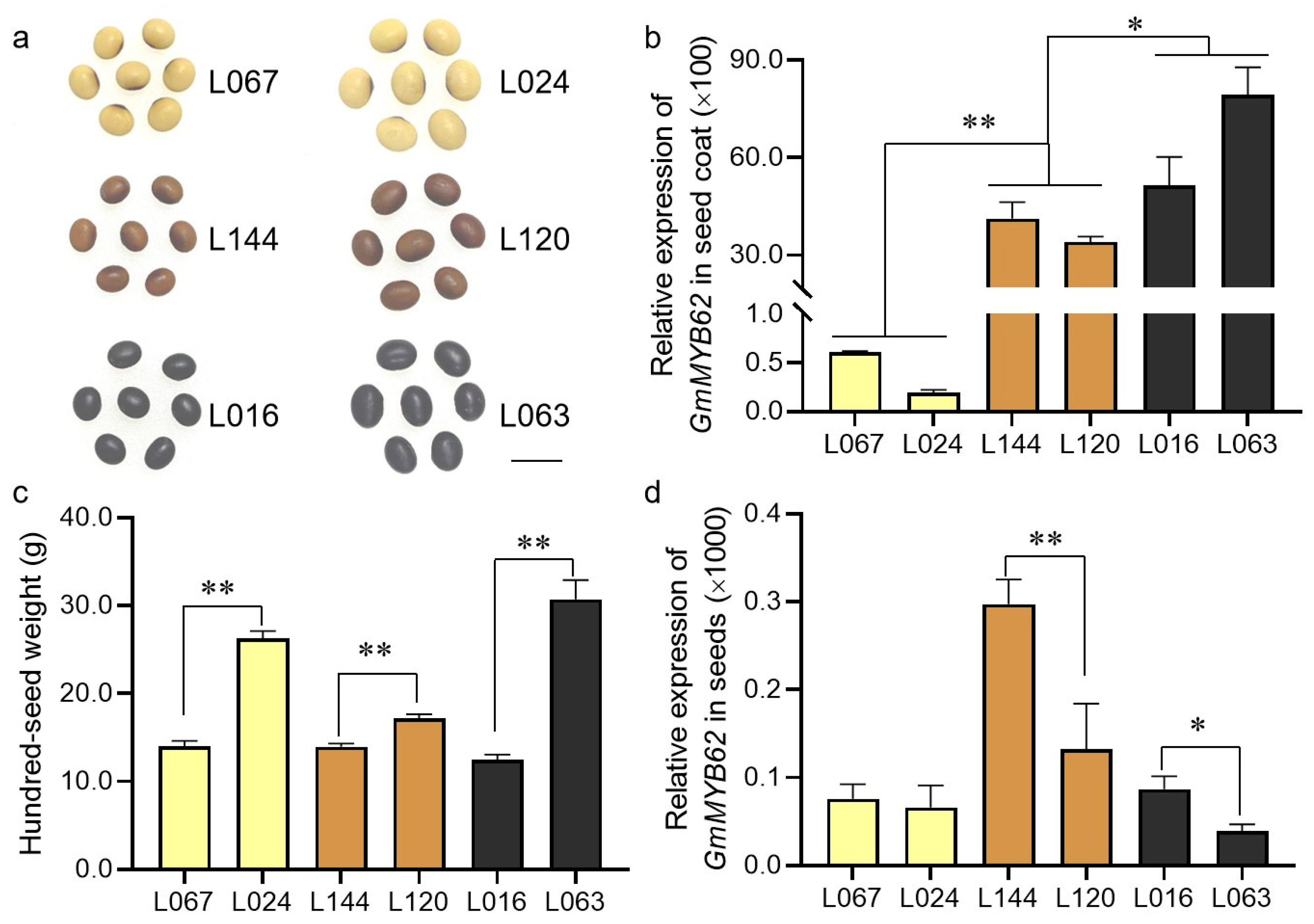
2.1. The Expression of GmMYB62 Continues to Increase in Soybean Seed-Coat from Yellow to Black
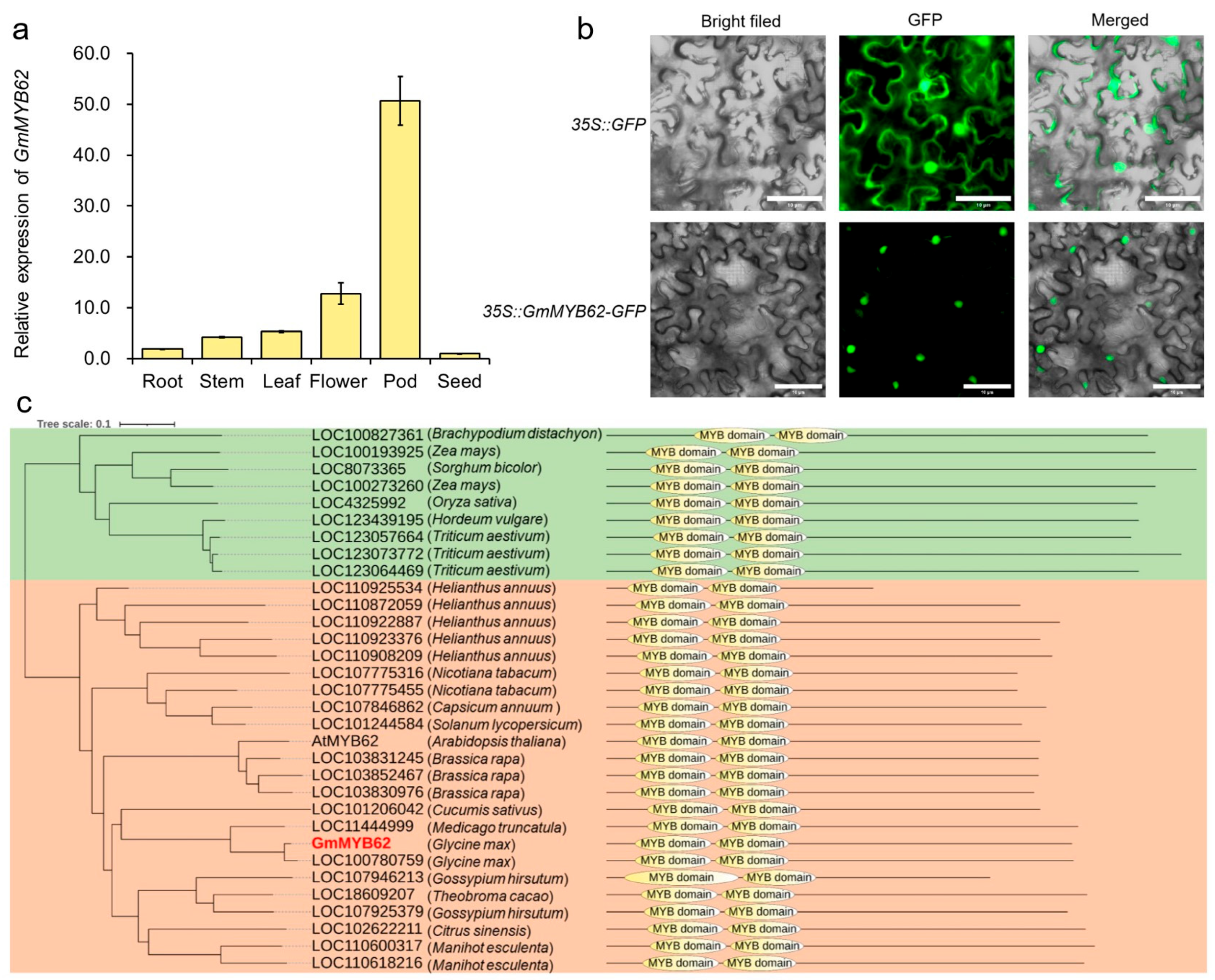
2.2. Expression Pattern and Phylogenetic Analysis of GmMYB62
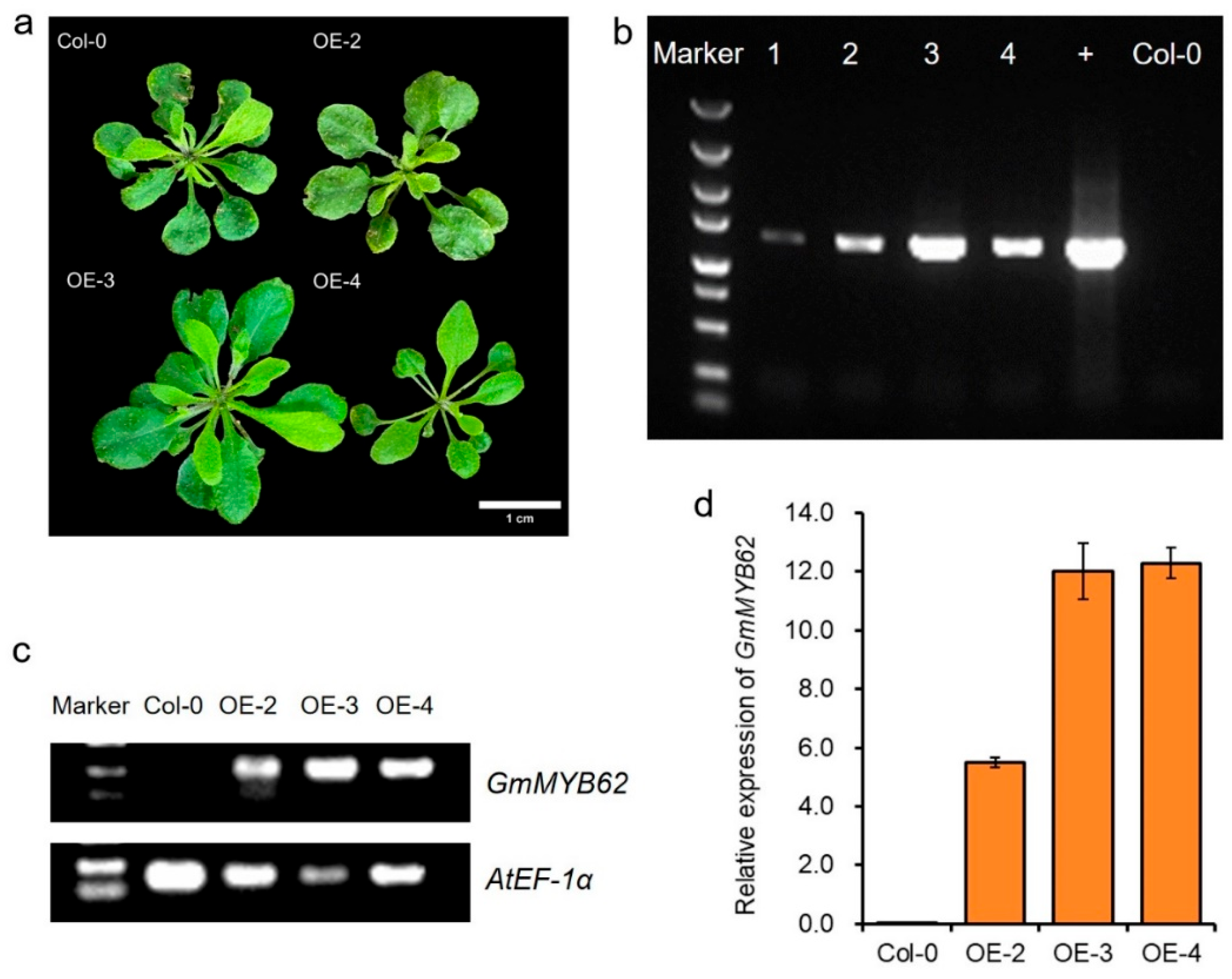
2.3. Generation and Confirmation of GmMYB62-Overexpressing Transgenic Arabidopsis Plants
2.4. Overexpression of GmMYB62 Regulates Seed-Coat Color and Seed Size in Arabidopsis
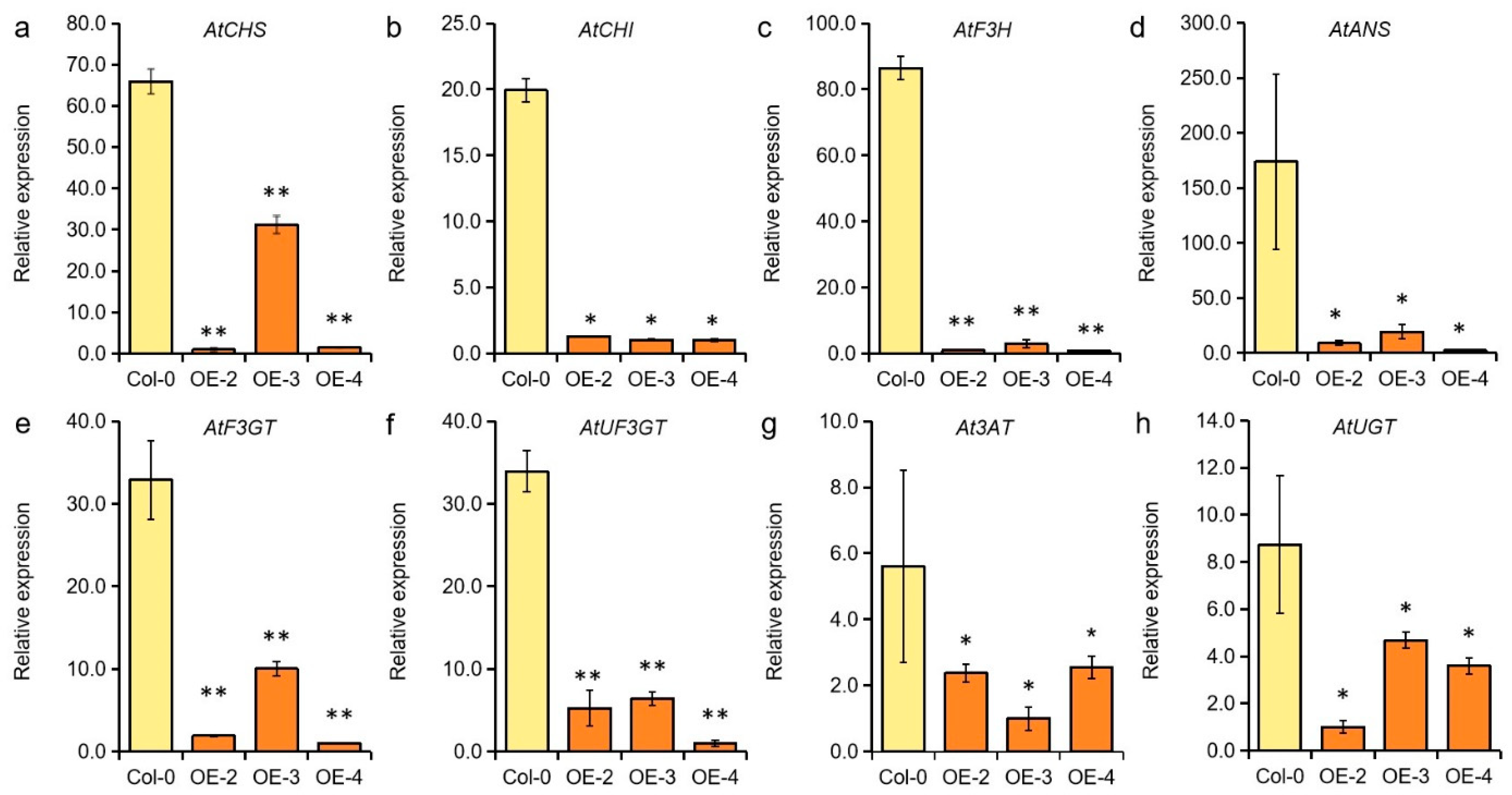
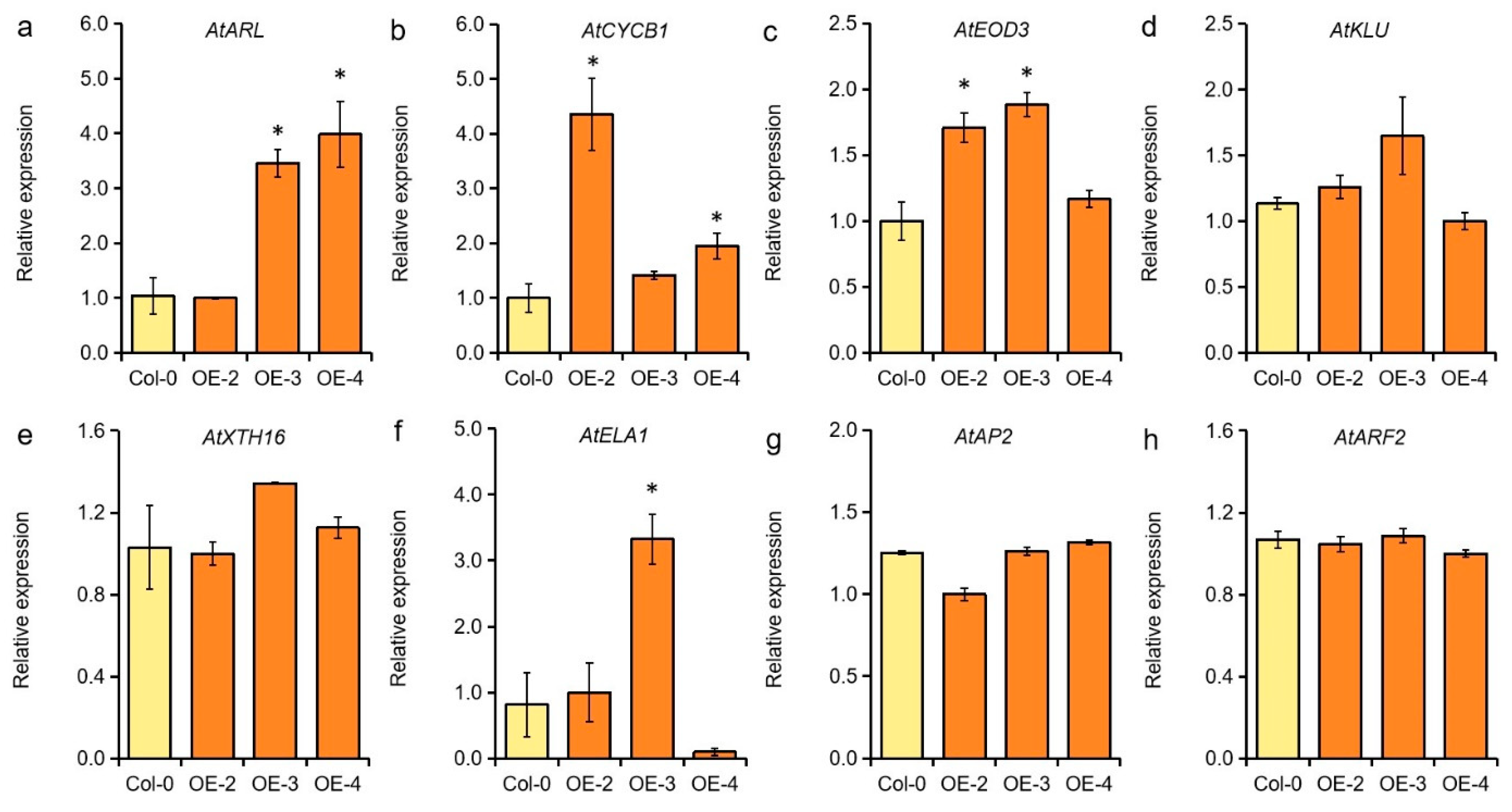
2.5. GmMYB62 Affects the Expression of Genes Related to Anthocyanin Biosynthesis and Seed Size Regulation in Arabidopsis
2.6. GmMYB62 Affects the Expression of Genes Involved in Cell Cycle and Seed Size Regulation in Arabidopsis
3. Discussion
3.1. GmMYB62 Is a R2R3-MYB Transcription Factor That Govern Anthocyanin Accumulation in Plants
3.2. The Transcription Factor of GmMYB62 Specifically Regulates the Expression of Genes in the Pathway of Anthocyanin Synthesis
3.3. R2R3-MYB Transcription Factor of GmMYB62 Was Involved in Seed Size Regulation
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Bioinformatics Analysis of GmMYB62
4.3. Phylogenetic and Domain Analysis of MYB62 in Multispecies
4.4. DNA, RNA Extraction and Quantitative Reverse Transcription PCR (qRT-PCR)
4.5. Subcellular Localization
4.6. Generation and Selection of GmMYB62-Overexpressing Transgenic Arabidopsis Plants
4.7. Seed Size and Weight Measurement
4.8. Relative Anthocyanin Content Measurement
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, B.; Peng, J.; Wu, Y.; Hu, Q.; Huang, W.; Yuan, Z.; Tang, X.; Cao, D.; Xue, Y.; Luan, X.; et al. Identification of an important QTL for seed oil content in soybean. Mol. Breed. 2023, 43, 43. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, L.; Li, S.; Wang, W.; Ding, Y.; Swarm, S.A.; Li, L.; Wang, X.; Tang, X.; Zhang, Z.; et al. Elevation of soybean seed oil content through selection for seed coat shininess. Nat. Plants 2018, 4, 30–35. [Google Scholar]
- Duan, Z.; Li, Q.; Wang, H.; He, X.; Zhang, M. Genetic regulatory networks of soybean seed size, oil and protein contents. Front. Plant Sci. 2023, 14, 1160418. [Google Scholar]
- Xu, M.; Kong, K.; Miao, L.; He, J.; Liu, T.; Zhang, K.; Yue, X.; Jin, T.; Gai, J.; Li, Y. Identification of major quantitative trait loci and candidate genes for seed weight in soybean. Theor. Appl. Genet. 2023, 136, 22. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Hofmann, N.; Li, S.; Ferreira, M.E.; Song, B.; Jiang, G.; Ren, S.; Quigley, C.; Fickus, E.; Cregan, P.; et al. Identification of QTL with large effect on seed weight in a selective population of soybean with genome-wide association and fixation index analyses. Bmc Genom. 2017, 18, 529. [Google Scholar] [CrossRef]
- Yuan, B.; Yuan, C.; Wang, Y.; Liu, X.; Qi, G.; Wang, Y.; Dong, L.; Zhao, H.; Li, Y.; Dong, Y. Identification of genetic loci conferring seed coat color based on a high-density map in soybean. Front. Plant Sci. 2022, 13, 968618. [Google Scholar]
- Cao, Y.; Jia, S.; Chen, L.; Zeng, S.; Zhao, T.; Karikari, B. Identification of major genomic regions for soybean seed weight by genome-wide association study. Mol. Breed. 2022, 42, 38. [Google Scholar]
- Karikari, B.; Wang, Z.; Zhou, Y.; Yan, W.; Feng, J.; Zhao, T. Identification of quantitative trait nucleotides and candidate genes for soybean seed weight by multiple models of genome-wide association study. Bmc Plant Biol. 2020, 20, 404. [Google Scholar]
- Nguyen, C.X.; Paddock, K.J.; Zhang, Z.; Stacey, M.G. GmKIX8-1 regulates organ size in soybean and is the causative gene for the major seed weight QTL qSw17-1. New Phytol. 2021, 229, 920–934. [Google Scholar]
- Chen, X.; Liu, C.; Guo, P.; Hao, X.; Pan, Y.; Zhang, K.; Liu, W.; Zhao, L.; Luo, W.; He, J.; et al. Differential SW16.1 allelic effects and genetic backgrounds contributed to increased seed weight after soybean domestication. J. Integr. Plant Biol. 2023, 65, 1734–1752. [Google Scholar]
- Duan, Z.; Zhang, M.; Zhang, Z.; Liang, S.; Fan, L.; Yang, X.; Yuan, Y.; Pan, Y.; Zhou, G.; Liu, S.; et al. Natural allelic variation of GmST05 controlling seed size and quality in soybean. Plant Biotechnol. J. 2022, 20, 1807–1818. [Google Scholar] [CrossRef] [PubMed]
- Goettel, W.; Zhang, H.; Li, Y.; Qiao, Z.; Jiang, H.; Hou, D.; Song, Q.; Pantalone, V.R.; Song, B.H.; Yu, D.; et al. POWR1 is a domestication gene pleiotropically regulating seed quality and yield in soybean. Nat. Commun. 2022, 13, 3051. [Google Scholar] [CrossRef]
- Song, J.; Xu, R.; Guo, Q.; Wu, C.; Li, Y.; Wang, X.; Wang, J.; Qiu, L.-J. An omics strategy increasingly improves the discovery of genetic loci and genes for seed-coat color formation in soybean. Mol. Breed. 2023, 43, 71. [Google Scholar] [CrossRef]
- Cho, Y.B.; Jones, S.I.; Vodkin, L.O. Nonallelic homologous recombination events responsible for copy number variation within an RNA silencing locus. Plant Direct 2019, 3, e00162. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Li, P.; Shen, Y.; Peng, H.; Liu, S.; Zhou, G.A.; Zhang, H.; Liu, Z.; Shi, M.; et al. Pan-Genome of Wild and Cultivated Soybeans. Cell 2020, 182, 162–176. [Google Scholar] [CrossRef]
- Senda, M.; Jumonji, A.; Yumoto, S.; Ishikawa, R.; Harada, T.; Niizeki, M.; Akada, S. Analysis of the duplicated CHS1 gene related to the suppression of the seed coat pigmentation in yellow soybeans. Theor. Appl. Genet. 2002, 104, 1086–1091. [Google Scholar] [CrossRef]
- Jia, J.; Ji, R.; Li, Z.; Yu, Y.; Nakano, M.; Long, Y.; Feng, L.; Qin, C.; Lu, D.; Zhan, J.; et al. Soybean DICER-LIKE2 Regulates Seed Coat Color via Production of Primary 22-Nucleotide Small Interfering RNAs from Long Inverted Repeats. Plant Cell 2020, 32, 3662–3673. [Google Scholar] [CrossRef]
- Toda, K.; Yang, D.; Yamanaka, N.; Watanabe, S.; Harada, K.; Takahashi, R. A single-base deletion in soybean flavonoid 3′-hydroxylase gene is associated with gray pubescence color. Plant Mol. Biol. 2002, 50, 187–196. [Google Scholar] [CrossRef]
- Kovinich, N.; Saleem, A.; Arnason, J.T.; Miki, B. Combined analysis of transcriptome and metabolite data reveals extensive differences between black and brown nearly-isogenic soybean (Glycine max) seed coats enabling the identification of pigment isogenes. Bmc Genom. 2011, 12, 381. [Google Scholar] [CrossRef]
- Zabala, G.; Vodkin, L.O. Methylation affects transposition and splicing of a large CACTA transposon from a MYB transcription factor regulating anthocyanin synthase genes in soybean seed coats. PLoS ONE 2014, 9, e111959. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S.; Wang, J.; Yokosho, K.; Zhou, B.; Yu, Y.C.; Liu, Z.; Frommer, W.B.; Ma, J.F.; Chen, L.Q.; et al. Simultaneous changes in seed size, oil content and protein content driven by selection of SWEET homologues during soybean domestication. Natl. Sci. Rev. 2020, 7, 1776–1786. [Google Scholar] [PubMed]
- Ge, L.; Yu, J.; Wang, H.; Luth, D.; Bai, G.; Wang, K.; Chen, R. Increasing seed size and quality by manipulating BIG SEEDS1 in legume species. Proc. Natl. Acad. Sci. USA 2016, 113, 12414–12419. [Google Scholar] [PubMed]
- Hu, D.; Li, X.; Yang, Z.; Liu, S.; Hao, D.; Chao, M.; Zhang, J.; Yang, H.; Su, X.; Jiang, M.; et al. Downregulation of a gibberellin 3β-hydroxylase enhances photosynthesis and increases seed yield in soybean. New Phytol. 2022, 235, 502–517. [Google Scholar]
- Li, Y.; Gu, J.; Zhao, B.; Yuan, J.; Li, C.; Lin, Y.; Chen, Y.; Yang, X.; Li, Y.; Wang, Z.-Y. Identification and confirmation of novel genetic loci and domestication gene GmGA20ox1 regulating primary root length in soybean seedling stage. Ind. Crops Prod. 2024, 217, 118814. [Google Scholar]
- LaFountain, A.M.; Yuan, Y.W. Repressors of anthocyanin biosynthesis. New Phytol. 2021, 231, 933–949. [Google Scholar]
- Pratyusha, D.S.; Sarada, D.V.L. MYB transcription factors—Master regulators of phenylpropanoid biosynthesis and diverse developmental and stress responses. Plant Cell Rep. 2022, 41, 2245–2260. [Google Scholar]
- Yin, X.; Zhang, Y.; Zhang, L.; Wang, B.; Zhao, Y.; Irfan, M.; Chen, L.; Feng, Y. Regulation of MYB Transcription Factors of Anthocyanin Synthesis in Lily Flowers. Front. Plant Sci. 2021, 12, 761668. [Google Scholar]
- Nesi, N.; Jond, C.; Debeaujon, I.; Caboche, M.; Lepiniec, L. The Arabidopsis TT2 Gene Encodes an R2R3 MYB Domain Protein That Acts as a Key Determinant for Proanthocyanidin Accumulation in Developing Seed. Plant Cell 2001, 13, 2099–2114. [Google Scholar]
- Zhou, X.; Lei, D.; Yao, W.; Li, S.; Wang, H.; Lu, J.; Zhang, Y.; Lin, Y.; Wang, Y.; He, W.; et al. A novel R2R3-MYB transcription factor PbMYB1L of Pyrus bretschneideri regulates cold tolerance and anthocyanin accumulation. Plant Cell Rep. 2024, 43, 34. [Google Scholar]
- Jiang, L.; Yue, M.; Liu, Y.; Zhang, N.; Lin, Y.; Zhang, Y.; Wang, Y.; Li, M.; Luo, Y.; Zhang, Y.; et al. A novel R2R3-MYB transcription factor FaMYB5 positively regulates anthocyanin and proanthocyanidin biosynthesis in cultivated strawberries (Fragaria × ananassa). Plant Biotechnol. J. 2023, 21, 1140–1158. [Google Scholar]
- Kim, E.; Hyun, T.K. PlgMYBR1, an R2R3-MYB transcription factor, plays as a negative regulator of anthocyanin biosynthesis in Platycodon grandiflorus. 3 Biotech 2023, 13, 75. [Google Scholar]
- Kim, D.; Jeon, S.J.; Yanders, S.; Park, S.C.; Kim, H.S.; Kim, S. MYB3 plays an important role in lignin and anthocyanin biosynthesis under salt stress condition in Arabidopsis. Plant Cell Rep. 2022, 41, 1549–1560. [Google Scholar] [PubMed]
- Deng, G.M.; Zhang, S.; Yang, Q.S.; Gao, H.J.; Sheng, O.; Bi, F.C.; Li, C.Y.; Dong, T.; Yi, G.J.; He, W.D.; et al. MaMYB4, an R2R3-MYB Repressor Transcription Factor, Negatively Regulates the Biosynthesis of Anthocyanin in Banana. Front. Plant Sci. 2020, 11, 600704. [Google Scholar]
- Huang, Y.F.; Vialet, S.; Guiraud, J.L.; Torregrosa, L.; Bertrand, Y.; Cheynier, V.; This, P.; Terrier, N. A negative MYB regulator of proanthocyanidin accumulation, identified through expression quantitative locus mapping in the grape berry. New Phytol. 2014, 201, 795–809. [Google Scholar]
- Naing, A.H.; Kim, C.K. Roles of R2R3-MYB transcription factors in transcriptional regulation of anthocyanin biosynthesis in horticultural plants. Plant Mol. Biol. 2018, 98, 1–18. [Google Scholar]
- Ma, R.; Huang, W.; Hu, Q.; Tian, G.; An, J.; Fang, T.; Liu, J.; Hou, J.; Zhao, M.; Sun, L. Tandemly duplicated MYB genes are functionally diverged in the regulation of anthocyanin biosynthesis in soybean. Plant Physiol. 2024, 194, 2549–2563. [Google Scholar] [PubMed]
- Jia, L.; Xu, H.; Xu, X.; Gao, K.; Zhao, K.; Dong, J.; Su, N. GmMYB114 Facilitates the Synthesis of Anthocyanins in Soybean Sprouts under Blue Light. Plants 2024, 13, 1107. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Li, J.; Muhammad, A.; Feng, Y.; Qi, J.; Sha, D.; Hao, Y.; Li, B.; Sun, J. An R2R3-type MYB transcription factor, GmMYB77, negatively regulates isoflavone accumulation in soybean [Glycine max (L.) Merr.]. Plant Biotechnol. J. 2025, 23, 824–838. [Google Scholar]
- Gao, H.; Ma, J.; Zhao, Y.; Zhang, C.; Zhao, M.; He, S.; Sun, Y.; Fang, X.; Chen, X.; Ma, K.; et al. The MYB Transcription Factor GmMYB78 Negatively Regulates Phytophthora sojae Resistance in Soybean. Int. J. Mol. Sci. 2024, 25, 4247. [Google Scholar] [CrossRef]
- Wei, Y.; Han, R.; Yu, Y. GmMYB183, a R2R3-MYB Transcription Factor in Tamba Black Soybean (Glycine max. cv. Tamba), Conferred Aluminum Tolerance in Arabidopsis and Soybean. Biomolecules 2024, 14, 724. [Google Scholar] [CrossRef]
- Cappellini, F.; Marinelli, A.; Toccaceli, M.; Tonelli, C.; Petroni, K. Anthocyanins: From Mechanisms of Regulation in Plants to Health Benefits in Foods. Front. Plant Sci. 2021, 12, 748049. [Google Scholar]
- Hu, Y.; Xie, Q.; Chua, N.-H. The Arabidopsis Auxin-Inducible Gene ARGOS Controls Lateral Organ Size. Plant Cell 2003, 15, 1951–1961. [Google Scholar] [PubMed]
- Hu, Y.; Poh, H.M.; Chua, N.-H. The Arabidopsis ARGOS-LIKE gene regulates cell expansion during organ growth. Plant J. 2006, 47, 1–9. [Google Scholar]
- Ren, D.; Wang, X.; Yang, M.; Yang, L.; He, G.; Deng, X.W. A new regulator of seed size control in Arabidopsis identified by a genome-wide association study. New Phytol. 2019, 222, 895–906. [Google Scholar]
- Fang, W.; Wang, Z.; Cui, R.; Li, J.; Li, Y. Maternal control of seed size by EOD3/CYP78A6 in Arabidopsis thaliana. Plant J. 2012, 70, 929–939. [Google Scholar]
- Lim, Y.J.; Kwon, S.J.; Qu, S.; Kim, D.G.; Eom, S.H. Antioxidant Contributors in Seed, Seed Coat, and Cotyledon of γ-ray-Induced Soybean Mutant Lines with Different Seed Coat Colors. Antioxidants 2021, 10, 353. [Google Scholar] [CrossRef]
- Song, J.; Liu, Z.; Hong, H.; Ma, Y.; Tian, L.; Li, X.; Li, Y.H.; Guan, R.; Guo, Y.; Qiu, L.J. Identification and Validation of Loci Governing Seed Coat Color by Combining Association Mapping and Bulk Segregation Analysis in Soybean. PLoS ONE 2016, 11, e0159064. [Google Scholar]
- Yang, J.; Chen, Y.; Xiao, Z.; Shen, H.; Li, Y.; Wang, Y. Multilevel regulation of anthocyanin-promoting R2R3-MYB transcription factors in plants. Front. Plant Sci. 2022, 13, 1008829. [Google Scholar]
- Zhou, H.; Lin-Wang, K.; Wang, F.; Espley, R.V.; Ren, F.; Zhao, J.; Ogutu, C.; He, H.; Jiang, Q.; Allan, A.C.; et al. Activator-type R2R3-MYB genes induce a repressor-type R2R3-MYB gene to balance anthocyanin and proanthocyanidin accumulation. New Phytol. 2019, 221, 1919–1934. [Google Scholar] [CrossRef]
- Song, Q.J.; Marek, L.F.; Shoemaker, R.C.; Lark, K.G.; Concibido, V.C.; Delannay, X.; Specht, J.E.; Cregan, P.B. A new integrated genetic linkage map of the soybean. Theor. Appl. Genet. 2004, 109, 122–128. [Google Scholar] [CrossRef]
- Yan, F.; Di, S.; Takahashi, R. CACTA-superfamily transposable element is inserted in MYB transcription factor gene of soybean line producing variegated seeds. Genome 2015, 58, 365–374. [Google Scholar] [PubMed]
- Lu, N.; Rao, X.; Li, Y.; Jun, J.H.; Dixon, R.A. Dissecting the transcriptional regulation of proanthocyanidin and anthocyanin biosynthesis in soybean (Glycine max). Plant Biotechnol. J. 2021, 19, 1429–1442. [Google Scholar] [PubMed]
- Yan, J.; Wang, B.; Zhong, Y.; Yao, L.; Cheng, L.; Wu, T. The soybean R2R3 MYB transcription factor GmMYB100 negatively regulates plant flavonoid biosynthesis. Plant Mol. Biol. 2015, 89, 35–48. [Google Scholar] [PubMed]
- García-Fernández, C.; Campa, A.; Ferreira, J.J. Dissecting the genetic control of seed coat color in a RIL population of common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2021, 134, 3687–3698. [Google Scholar]
- Herniter, I.A.; Muñoz-Amatriaín, M.; Lo, S.; Guo, Y.N.; Close, T.J. Identification of Candidate Genes Controlling Black Seed Coat and Pod Tip Color in Cowpea (Vigna unguiculata [L.] Walp). G3 Genes Genomes Genet. 2018, 8, 3347–3355. [Google Scholar]
- Yeom, W.; Kim, H.; Lee, J.; Jeong, J.; Choi, H.; Jung, H.; Heo, J.; Kim, C.; Chung, Y. Overexpression of R2R3-MYB IbMYB1a induces anthocyanin pigmentation in soybean cotyledon. Plant Cell Rep. 2024, 43, 56. [Google Scholar]
- Zhang, Y.; Liang, W.; Shi, J.; Xu, J.; Zhang, D. MYB56 encoding a R2R3 MYB transcription factor regulates seed size in Arabidopsis thaliana. J. Integr. Plant Biol. 2013, 55, 1166–1178. [Google Scholar]
- Wang, X.; Wu, R.; Shen, T.; Li, Z.; Li, C.; Wu, B.; Jiang, H.; Zhao, G. An R2R3-MYB Transcription Factor OsMYBAS1 Promotes Seed Germination under Different Sowing Depths in Transgenic Rice. Plants 2022, 11, 139. [Google Scholar] [CrossRef]
- Lee, H.G.; Lee, K.; Seo, P.J. The Arabidopsis MYB96 transcription factor plays a role in seed dormancy. Plant Mol. Biol. 2015, 87, 371–381. [Google Scholar]
- Zhao, B.; Zhou, M.; Ren, W.; Li, H.; Zhang, Q.; He, G.; Liu, Y.; He, H. The B-Type Cyclin CYCB1-1 Regulates Embryonic Development and Seed Size in Maize. Int. J. Mol. Sci. 2022, 23, 5907. [Google Scholar] [CrossRef]
- Guo, J.; Wang, F.; Song, J.; Sun, W.; Zhang, X. The expression of Orysa;CycB1;1 is essential for endosperm formation and causes embryo enlargement in rice. Planta 2010, 231, 293–303. [Google Scholar] [PubMed]
- Wang, X.; Li, Y.; Zhang, H.; Sun, G.; Zhang, W.; Qiu, L. Evolution and association analysis of GmCYP78A10 gene with seed size/weight and pod number in soybean. Mol. Biol. Rep. 2015, 42, 489–496. [Google Scholar] [PubMed]
- Zhao, B.; Dai, A.; Wei, H.; Yang, S.; Wang, B.; Jiang, N.; Feng, X. Arabidopsis KLU homologue GmCYP78A72 regulates seed size in soybean. Plant Mol. Biol. 2016, 90, 33–47. [Google Scholar] [PubMed]
- Dai, A.; Yang, S.; Zhou, H.; Tang, K.; Li, G.; Leng, J.; Yu, H.; Zhang, Y.; Gao, J.; Yang, X.; et al. Evolution and Expression Divergence of the CYP78A Subfamily Genes in Soybean. Genes 2018, 9, 611. [Google Scholar] [CrossRef]
- Ohto, M.A.; Fischer, R.L.; Goldberg, R.B.; Nakamura, K.; Harada, J.J. Control of seed mass by APETALA2. Proc. Natl. Acad. Sci. USA 2005, 102, 3123–3128. [Google Scholar]
- Zhang, Y.; Zhang, B.; Yan, D.; Dong, W.; Yang, W.; Li, Q.; Zeng, L.; Wang, J.; Wang, L.; Hicks, L.M.; et al. Two Arabidopsis cytochrome P450 monooxygenases, CYP714A1 and CYP714A2, function redundantly in plant development through gibberellin deactivation. Plant J. 2011, 67, 342–353. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar]
- Li, Y.; Zhao, W.; Tang, J.; Yue, X.; Gu, J.; Zhao, B.; Li, C.; Chen, Y.; Yuan, J.; Lin, Y.; et al. Identification of the domestication gene GmCYP82C4 underlying the major quantitative trait locus for the seed weight in soybean. Theor. Appl. Genet. 2024, 137, 62. [Google Scholar]
- Liu, J.G.; Li, Y.; Wang, W.; Gai, J.Y.; Li, Y. Genome-wide analysis of MATE transporters and expression patterns of a subgroup of MATE genes in response to aluminum toxicity in soybean. Bmc Genom. 2016, 17, 223. [Google Scholar]
- Wu, Y.; Zhao, C.; Zhao, X.; Yang, L.; Liu, C.; Jiang, L.; Liu, G.; Liu, P.; Luo, L. Multi-omics-based identification of purple acid phosphatases and metabolites involved in phosphorus recycling in stylo root exudates. Int. J. Biol. Macromol. 2023, 241, 124569. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [PubMed]
- Jin, T.; Sun, Y.; Shan, Z.; He, J.; Wang, N.; Gai, J.; Li, Y. Natural variation in the promoter of GsERD15B affects salt tolerance in soybean. Plant Biotechnol. J. 2021, 19, 1155–1169. [Google Scholar] [PubMed]
- Jin, T.; An, J.; Xu, H.; Chen, J.; Pan, L.; Zhao, R.; Wang, N.; Gai, J.; Li, Y. A soybean sodium/hydrogen exchanger GmNHX6 confers plant alkaline salt tolerance by regulating Na+/K+ homeostasis. Front. Plant Sci. 2022, 13, 938635. [Google Scholar]
- Wu, M.; Lv, X.; Zhou, Y.; Zeng, Y.; Liu, D. High anthocyanin accumulation in an Arabidopsis mutant defective in chloroplast biogenesis. Plant Growth Regul. 2019, 87, 433–444. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.-Y.; Yuan, J.-B.; Gu, J.-B.; Li, C.; Lin, Y.; Zhang, Y.-H.; Zhang, B.-H.; Wang, Y.-H.; Ye, X.; Li, Y.; et al. A R2R3-MYB Transcription Factor of GmMYB62 Regulates Seed-Coat Color and Seed Size in Arabidopsis. Int. J. Mol. Sci. 2025, 26, 3457. https://doi.org/10.3390/ijms26083457
Zhao B-Y, Yuan J-B, Gu J-B, Li C, Lin Y, Zhang Y-H, Zhang B-H, Wang Y-H, Ye X, Li Y, et al. A R2R3-MYB Transcription Factor of GmMYB62 Regulates Seed-Coat Color and Seed Size in Arabidopsis. International Journal of Molecular Sciences. 2025; 26(8):3457. https://doi.org/10.3390/ijms26083457
Chicago/Turabian StyleZhao, Bi-Yao, Jian-Bo Yuan, Jin-Bao Gu, Cong Li, Yan Lin, Yu-Hang Zhang, Bai-Hong Zhang, Yin-Hua Wang, Xing Ye, Yang Li, and et al. 2025. "A R2R3-MYB Transcription Factor of GmMYB62 Regulates Seed-Coat Color and Seed Size in Arabidopsis" International Journal of Molecular Sciences 26, no. 8: 3457. https://doi.org/10.3390/ijms26083457
APA StyleZhao, B.-Y., Yuan, J.-B., Gu, J.-B., Li, C., Lin, Y., Zhang, Y.-H., Zhang, B.-H., Wang, Y.-H., Ye, X., Li, Y., Wang, Z.-Y., & Zhong, T.-X. (2025). A R2R3-MYB Transcription Factor of GmMYB62 Regulates Seed-Coat Color and Seed Size in Arabidopsis. International Journal of Molecular Sciences, 26(8), 3457. https://doi.org/10.3390/ijms26083457





