The Wx/SSIIa and GS3/GW7 Alleles, Both Individually and in Combination, Can Significantly Distinguish Rice Germplasm Quality
Abstract
1. Introduction
2. Results
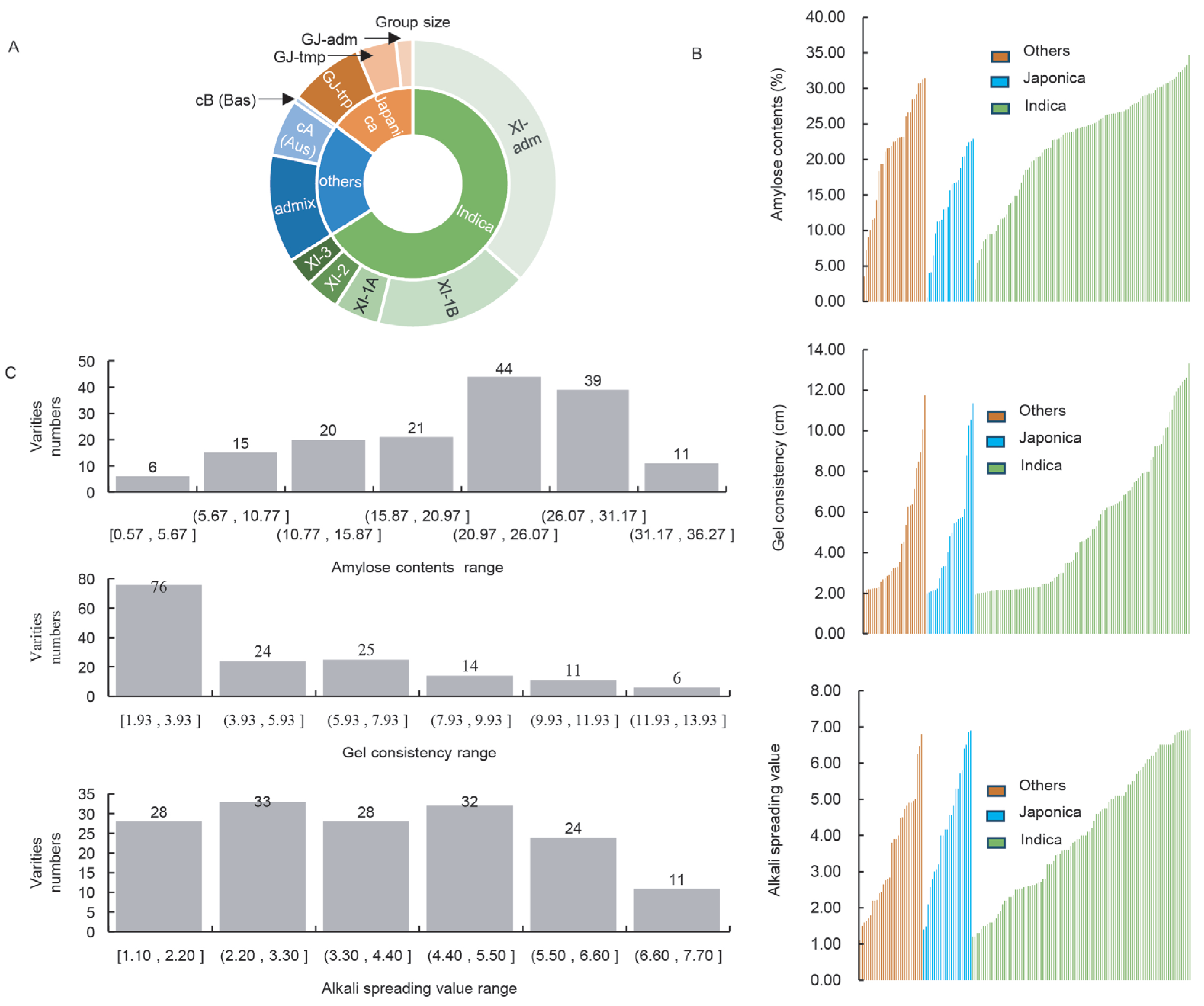
2.1. The CEQ Measured Values of 156 Germplasms Exhibit a Broad Range of Distributions
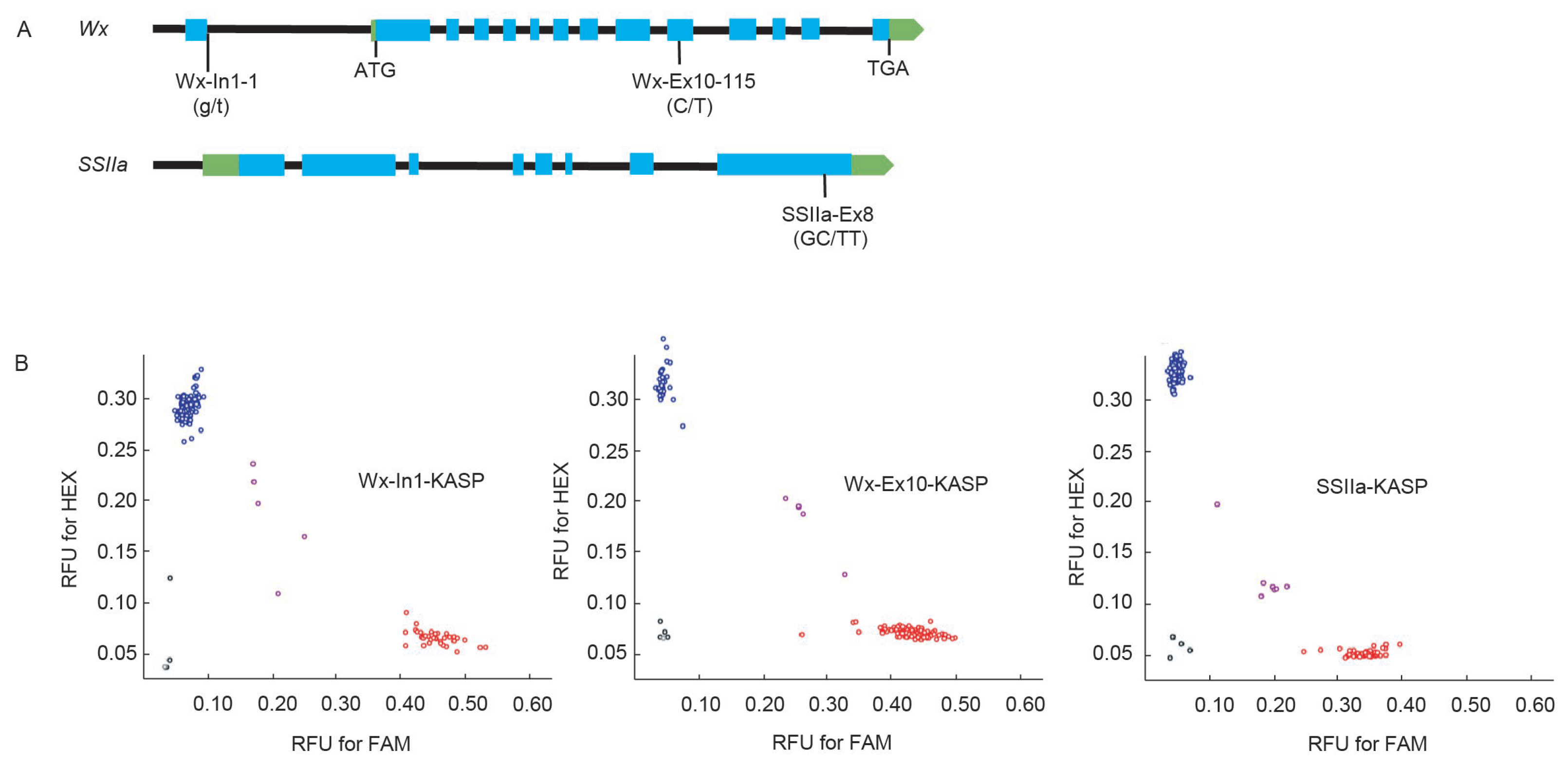
2.2. Development of Functional Markers of Wx-In1, Wx-Ex10, and SSIIa-Ex8
2.3. Wx-In1 Locus Exerts a Major Effect in Controlling Both AC and GC, While the SSIIa-Ex8 Locus Primarily Governs ASV
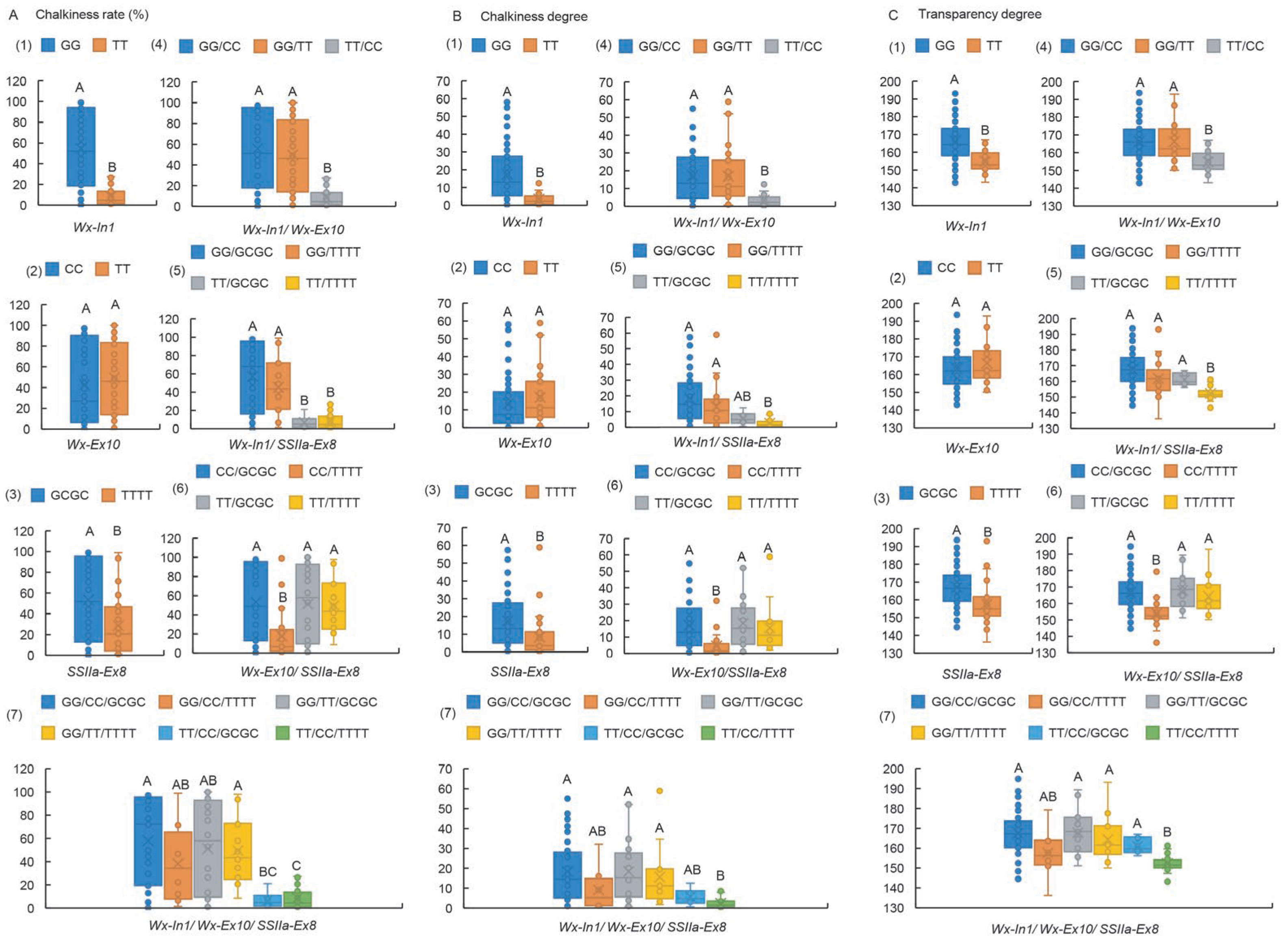
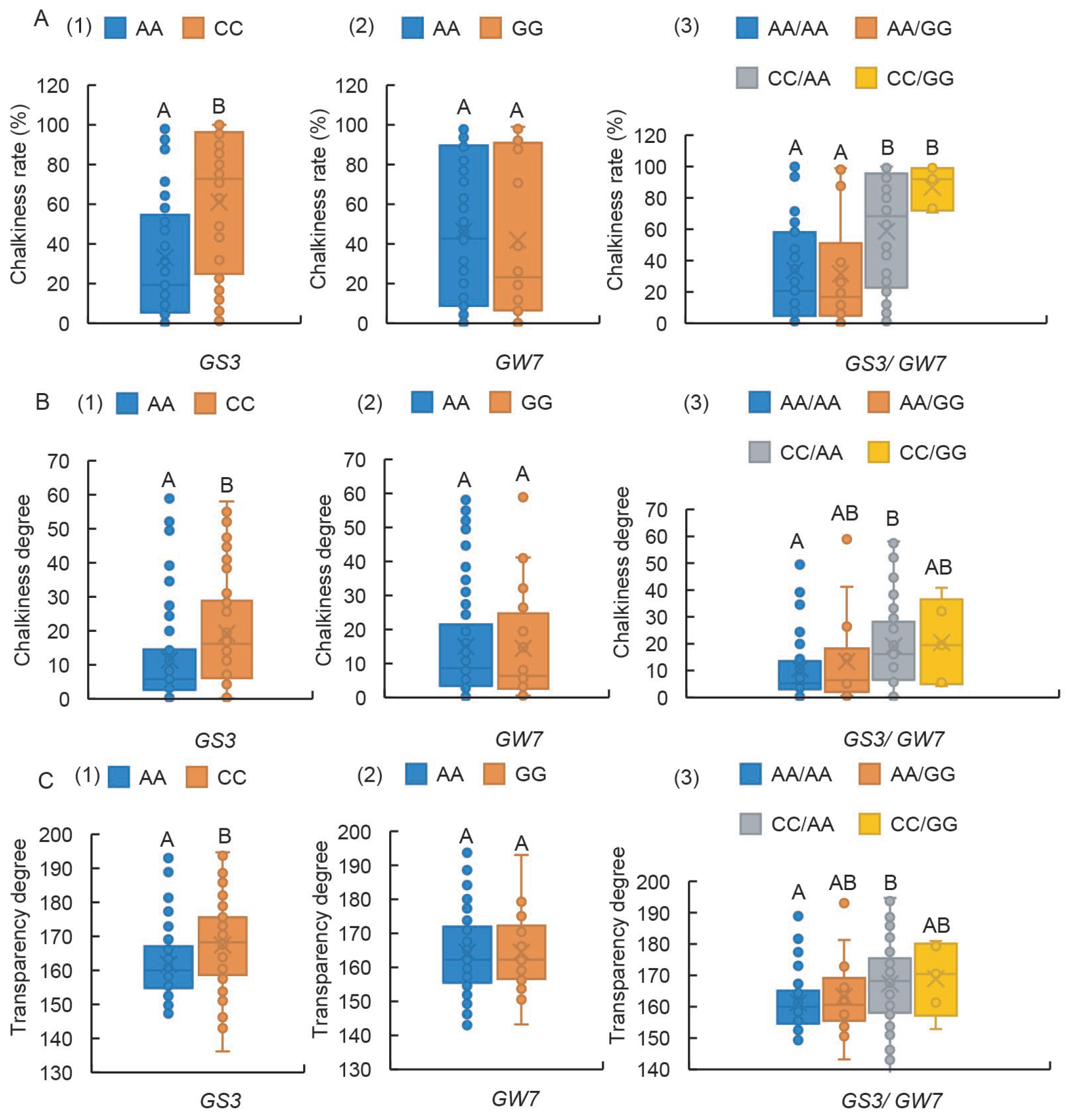
2.4. The Impact on Chalkiness-Related Traits Follows the Hierarchy of Wx-In1 > SSIIa-Ex8 > Wx-Ex10
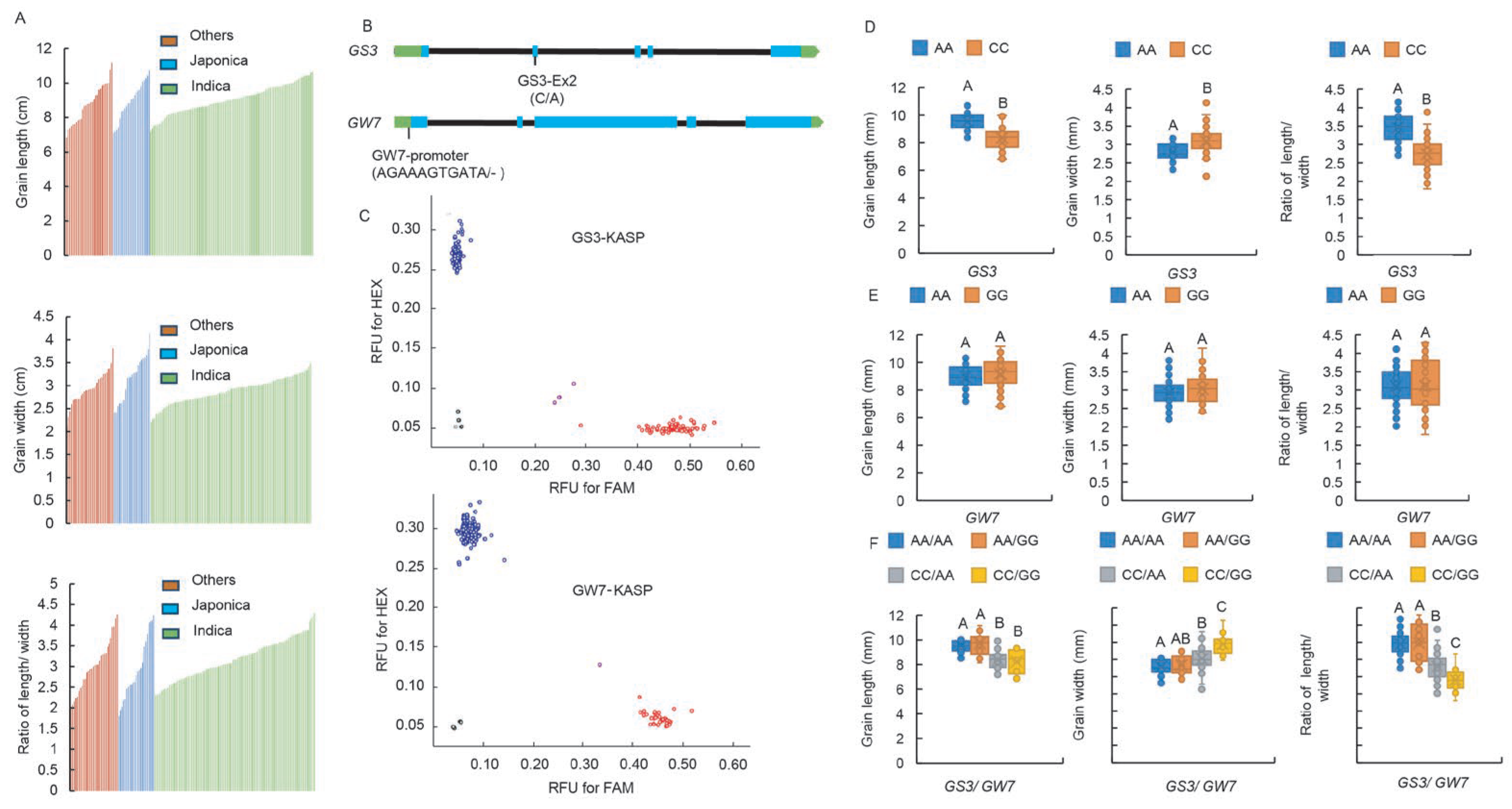
2.5. Development of Functional Markers of GS3-Ex2 and GW7-Pro
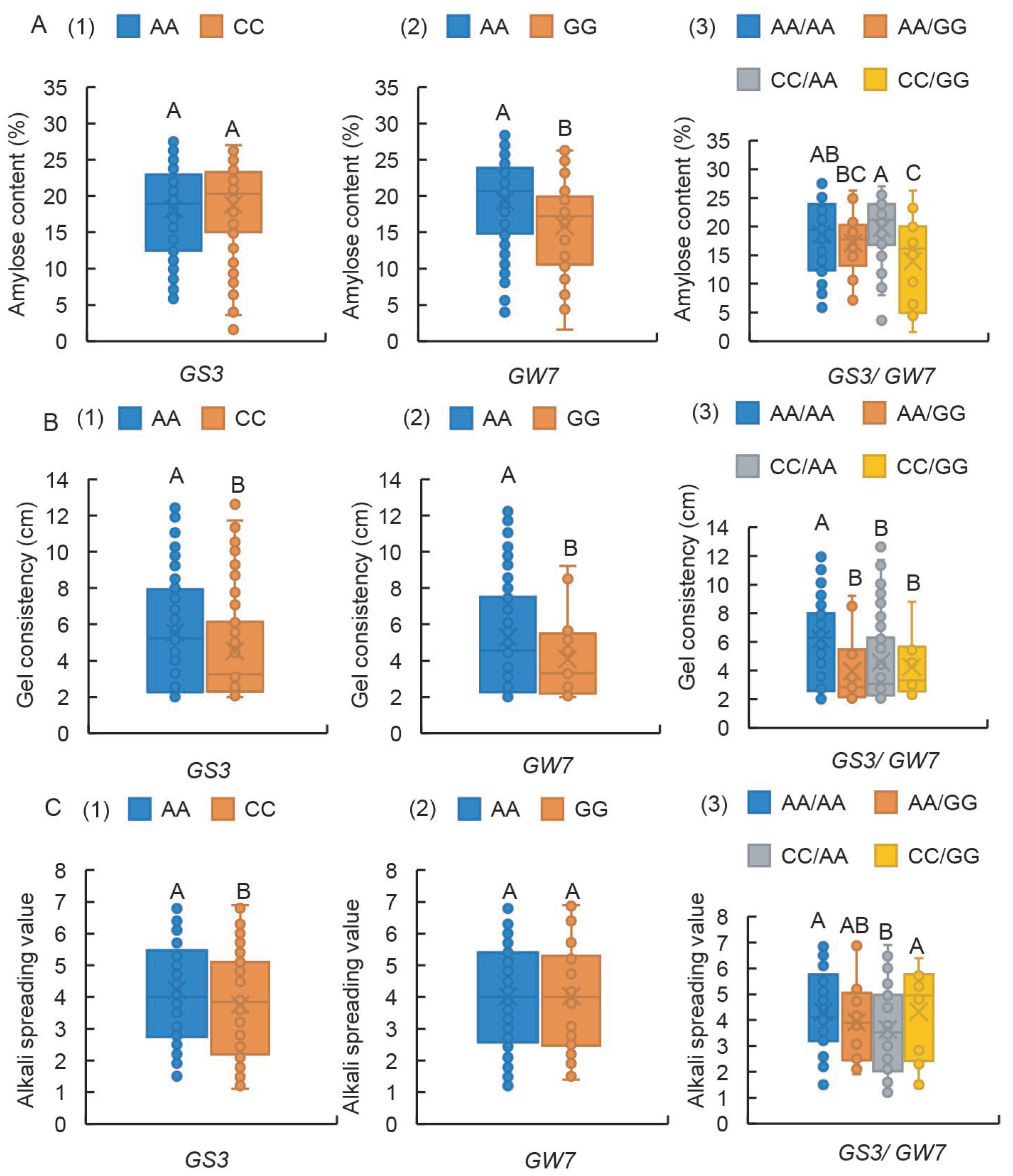
2.6. GS3-Ex2 Has a Highly Significant Impact on Chalkiness-Related Traits, and the GW7-Pro Locus Exerts a Synergistic Effect
2.7. GS3-Ex2 Locus Exerts an Effect in Controlling Both GC and ASV, While the GW7-Pro Locus Governs AC
3. Discussion
3.1. Wx-In1, Wx-Ex10, and SSIIa-Ex8 Loci Exhibit Highly Significant Effects on Rice CEQ
3.2. The Loci of Wx-In1, Wx-Ex10, and SSIIa-Ex8 Exhibit Highly Significant Effects on Chalkiness-Related Traits
3.3. GS3-Ex2 and GW7-Pro Exert Highly Significant Effects on Rice Quality
4. Materials and Methods
4.1. Plant Materials
4.2. DNA Extraction and Kompetitive Allele-Specific PCR (KASP) Marker Assay
4.3. CEQ Measurement
4.4. Grain Shape Measurement
4.5. Measurement of Chalkiness-Related Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sreenivasulu, N.; Zhang, C.Q.; Tiozon, R.N.; Liu, Q.Q. Post-genomics revolution in the design of premium quality rice in a high-yielding background to meet consumer demands in the 21st century. Plant Commun. 2022, 3, 100271. [Google Scholar] [CrossRef] [PubMed]
- Custodio, M.C.; Cuevas, R.P.; Ynion, J.; Laborte, A.G.; Velasco, M.L.; Demont, M. Rice quality: How is it defined by consumers, industry, food scientists, and geneticists? Trends Food Sci. Tech. 2019, 92, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.X.; Qian, Q.; Liu, Q.Q.; Yan, M.X.; Liu, X.F.; Yan, C.J.; Liu, G.F.; Gao, Z.Y.; Tang, S.Z.; Zeng, D.; et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc. Natl. Acad. Sci. USA 2009, 106, 21760–21765. [Google Scholar] [CrossRef] [PubMed]
- Buenafe, R.J.Q.; Kumanduri, V.; Sreenivasulu, N. Deploying viscosity and starch polymer properties to predict cooking and eating quality models: A novel breeding tool to predict texture. Carbohydr. Polym. 2021, 260, 117766. [Google Scholar] [CrossRef]
- Zhu, F. Relationships between amylopectin internal molecular structure and physicochemical properties of starch. Trends Food Sci. Tech. 2018, 78, 234–242. [Google Scholar] [CrossRef]
- Wang, L.Q.; Liu, W.J.; Xu, Y.; He, Y.Q.; Luo, L.J.; Xing, Y.Z.; Xu, C.G.; Zhang, Q.F. Genetic basis of 17 traits and viscosity parameters characterizing the eating and cooking quality of rice grain. Theor. Appl. Genet. 2007, 115, 463–476. [Google Scholar] [CrossRef]
- Sato, H.; Suzuki, Y.; Sakai, M.; Imbe, T. Molecular characterization of Wx-mq, a novel mutant gene for low-amylose content in endosperm of rice (Oryza sativa L.). Breed. Sci. 2002, 52, 131–135. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Zhu, J.H.; Chen, S.J.; Fan, X.L.; Li, Q.F.; Lu, Y.; Wang, M.; Yu, H.X.; Yi, C.D.; Tang, S.Z.; et al. Wxlv, the ancestral allele of rice Waxy gene. Mol. Plant 2019, 12, 1157–1166. [Google Scholar] [CrossRef]
- Zeng, D.L.; Yan, M.X.; Wang, Y.H.; Liu, X.F.; Qian, Q.; Li, J.Y. Du1, encoding a novel Prp1 protein, regulates starch biosynthesis through affecting the splicing of Wxb pre-mRNAs in rice (Oryza sativa L.). Plant Mol. Biol. 2007, 65, 501–509. [Google Scholar] [CrossRef]
- Cai, X.L.; Wang, Z.Y.; Xing, Y.Y.; Zhang, J.L.; Hong, M.M. Aberrant splicing of intron 1 leads to the heterogeneous 5′ UTR and decreased expression of waxy gene in rice cultivars of intermediate amylose content. Plant J. 1998, 14, 459–465. [Google Scholar] [CrossRef]
- Tran, N.A.; Daygon, V.D.; Resurreccion, A.P.; Cuevas, R.P.; Corpuz, H.M.; Fitzgerald, M.A. A single nucleotide polymorphism in the Waxy gene explains a significant component of gel consistency. Theor. Appl. Genet. 2011, 123, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Y.; Lv, Y.; Dong, C.L.; Wang, P.R.; Deng, X.J. Genetic relationship among Wx gene, AC, GC and GT of rice. Acta Agron. Sin. 2005, 31, 535–539. [Google Scholar]
- Zhang, Z.H.; Gao, S.P.; Chu, C.C. Improvement of nutrient use efficiency in rice: Current toolbox and future perspectives. Theor. Appl. Genet. 2020, 133, 1365–1384. [Google Scholar] [CrossRef]
- Umemoto, T.; Yano, M.; Satoh, H.; Shomura, A.; Nakamura, Y. Mapping of a gene responsible for the difference in amylopectin structure between japonica-type and indica-type rice varieties. Theor. Appl. Genet. 2002, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.S.; Corke, H.; Sun, M. Nucleotide diversity in starch synthase IIa and validation of single nucleotide polymorphisms in relation to starch gelatinization temperature and other physicochemical properties in rice (Oryza sativa L.). Theor. Appl. Genet. 2006, 113, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Z.; Lu, Y.; Feng, L.H.; Hao, W.Z.; Li, C.; Yang, Y.; Fan, X.L.; Li, Q.F.; Zhang, C.Q.; Liu, Q.Q. Genetic dissection and functional differentiation of ALKa and ALKb, two natural alleles of the ALK/SSIIa gene, responding to low gelatinization temperature in rice. Rice 2020, 13, 39. [Google Scholar] [CrossRef]
- Zhao, M.C.; Lin, Y.J.; Chen, H. Improving nutritional quality of rice for human health. Theor. Appl. Genet. 2020, 133, 1397–1413. [Google Scholar] [CrossRef]
- Fan, C.C.; Xing, Y.Z.; Mao, H.L.; Lu, T.T.; Han, B.; Xu, C.G.; Li, X.H.; Zhang, Q.F. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 2006, 112, 1164–1171. [Google Scholar] [CrossRef]
- Fan, C.C.; Yu, S.B.; Wang, C.R.; Xing, Y.Z. A causal C-A mutation in the second exon of GS3 highly associated with rice grain length and validated as a functional marker. Theor. Appl. Genet. 2009, 118, 465–472. [Google Scholar] [CrossRef]
- Wang, S.K.; Li, S.; Liu, Q.; Wu, K.; Zhang, J.Q.; Wang, S.S.; Wang, Y.; Chen, X.B.; Zhang, Y.; Gao, C.X.; et al. The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat. Genet. 2015, 47, 949–954. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xiong, G.S.; Hu, J.; Jiang, L.; Yu, H.; Xu, J.; Fang, Y.X.; Zeng, L.J.; Xu, E.B.; Xu, J.; et al. Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat. Genet. 2015, 47, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Misra, G.; Badoni, S.; Parween, S.; Singh, R.K.; Leung, H.; Ladejobi, O.; Mott, R.; Sreenivasulu, N. Genome-wide association coupled gene to gene interaction studies unveil novel epistatic targets among major effect loci impacting rice grain chalkiness. Plant Biotechnol. J. 2021, 19, 910–925. [Google Scholar] [CrossRef]
- Zhou, L.J.; Jiang, L.; Zhai, H.Q.; Wan, J.M. Current status and strategies for improvement of rice grain chalkiness. Yi Chuan Hered. 2009, 31, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.M.; Zhong, L.J.; Wang, F.; Zhang, G.P. Differences in cooking and eating properties between chalky and translucent parts in rice grains. Food Chem. 2005, 90, 39–46. [Google Scholar] [CrossRef]
- Phing Lau, W.C.; Latif, M.A.; Rafii, M.; Ismail, M.R.; Puteh, A. Advances to improve the eating and cooking qualities of rice by marker-assisted breeding. Crit. Rev. Biotechnol. 2016, 36, 87–98. [Google Scholar] [CrossRef]
- Huang, L.C.; Gu, Z.W.; Chen, Z.Z.; Yu, J.W.; Chu, R.; Tan, H.Y.; Zhao, D.S.; Fan, X.L.; Zhang, C.Q.; Li, Q.F.; et al. Improving rice eating and cooking quality by coordinated expression of the major starch synthesis-related genes, SSII and Wx, in endosperm. Plant Mol. Biol. 2021, 106, 419–432. [Google Scholar] [CrossRef]
- Wang, D.W.; Wang, J.L.; Sun, W.Q.; Qiu, X.J.; Yuan, Z.Y.; Yu, S.B. Verifying the breeding value of a rare haplotype of Chalk7, GS3, and Chalk5 to improve grain appearance quality in rice. Plants 2022, 11, 1470. [Google Scholar] [CrossRef]
- Chun, A.; Song, J.; Kim, K.; Lee, H. Quality of head and chalky rice and deterioration of eating quality by chalky rice. J. Crop Sci. Biotechnol. 2009, 12, 239–244. [Google Scholar] [CrossRef]
- Zhao, D.S.; Zhang, C.Q.; Li, Q.F.; Liu, Q.Q. Genetic control of grain appearance quality in rice. Biotechnol. Adv. 2022, 60, 108014. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Cheng, Z.J.; Zhang, X.; Guo, X.P.; Su, N.; Jiang, L.; Mao, L.; Wan, J.M. Double repression of soluble starch synthase genes SSIIa and SSIIIa in rice (Oryza sativa L.) uncovers interactive effects on the physicochemical properties of starch. Genome 2011, 54, 448–459. [Google Scholar] [CrossRef]
- Zou, X.Y.; Deng, H.M.; Fu, J.R.; Peng, X.S.; Zhu, C.L.; He, X.P.; Chen, X.R.; He, H.H.; Liu, Y.B. Genetic and correlation analysis of grain characters in three-line hybrid rice. Agric. Sci. Technol. 2009, 10, 78–81. [Google Scholar]
- Gong, J.Y.; Miao, J.S.; Zhao, Y.; Zhao, Q.; Feng, Q.; Zhan, Q.L.; Cheng, B.Y.; Xia, J.H.; Huang, X.H.; Yang, S.H.; et al. Dissecting the genetic basis of grain shape and chalkiness traits in hybrid rice using multiple collaborative populations. Mol. Plant 2017, 10, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Matsuoka, M. Rice genetics: Control of grain length and quality. Nat. Plants 2015, 1, 15112. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Xu, J.; Wu, M.; Zhu, Y.; Song, J.; Han, Y.; Li, C.; Huang, F. The Wx/SSIIa and GS3/GW7 Alleles, Both Individually and in Combination, Can Significantly Distinguish Rice Germplasm Quality. Int. J. Mol. Sci. 2025, 26, 6726. https://doi.org/10.3390/ijms26146726
Hao Y, Xu J, Wu M, Zhu Y, Song J, Han Y, Li C, Huang F. The Wx/SSIIa and GS3/GW7 Alleles, Both Individually and in Combination, Can Significantly Distinguish Rice Germplasm Quality. International Journal of Molecular Sciences. 2025; 26(14):6726. https://doi.org/10.3390/ijms26146726
Chicago/Turabian StyleHao, Yuanyuan, Junfeng Xu, Mingming Wu, Ying Zhu, Jiayu Song, Yifei Han, Chunshou Li, and Fudeng Huang. 2025. "The Wx/SSIIa and GS3/GW7 Alleles, Both Individually and in Combination, Can Significantly Distinguish Rice Germplasm Quality" International Journal of Molecular Sciences 26, no. 14: 6726. https://doi.org/10.3390/ijms26146726
APA StyleHao, Y., Xu, J., Wu, M., Zhu, Y., Song, J., Han, Y., Li, C., & Huang, F. (2025). The Wx/SSIIa and GS3/GW7 Alleles, Both Individually and in Combination, Can Significantly Distinguish Rice Germplasm Quality. International Journal of Molecular Sciences, 26(14), 6726. https://doi.org/10.3390/ijms26146726





