Gut Microbiota and Its Metabolite Taurine-β-Muricholic Acid Contribute to Antimony- and/or Copper-Induced Liver Inflammation
Abstract
1. Introduction
2. Results
2.1. Identification of Liver Inflammation Phenotype Induced by Antimony and/or Copper
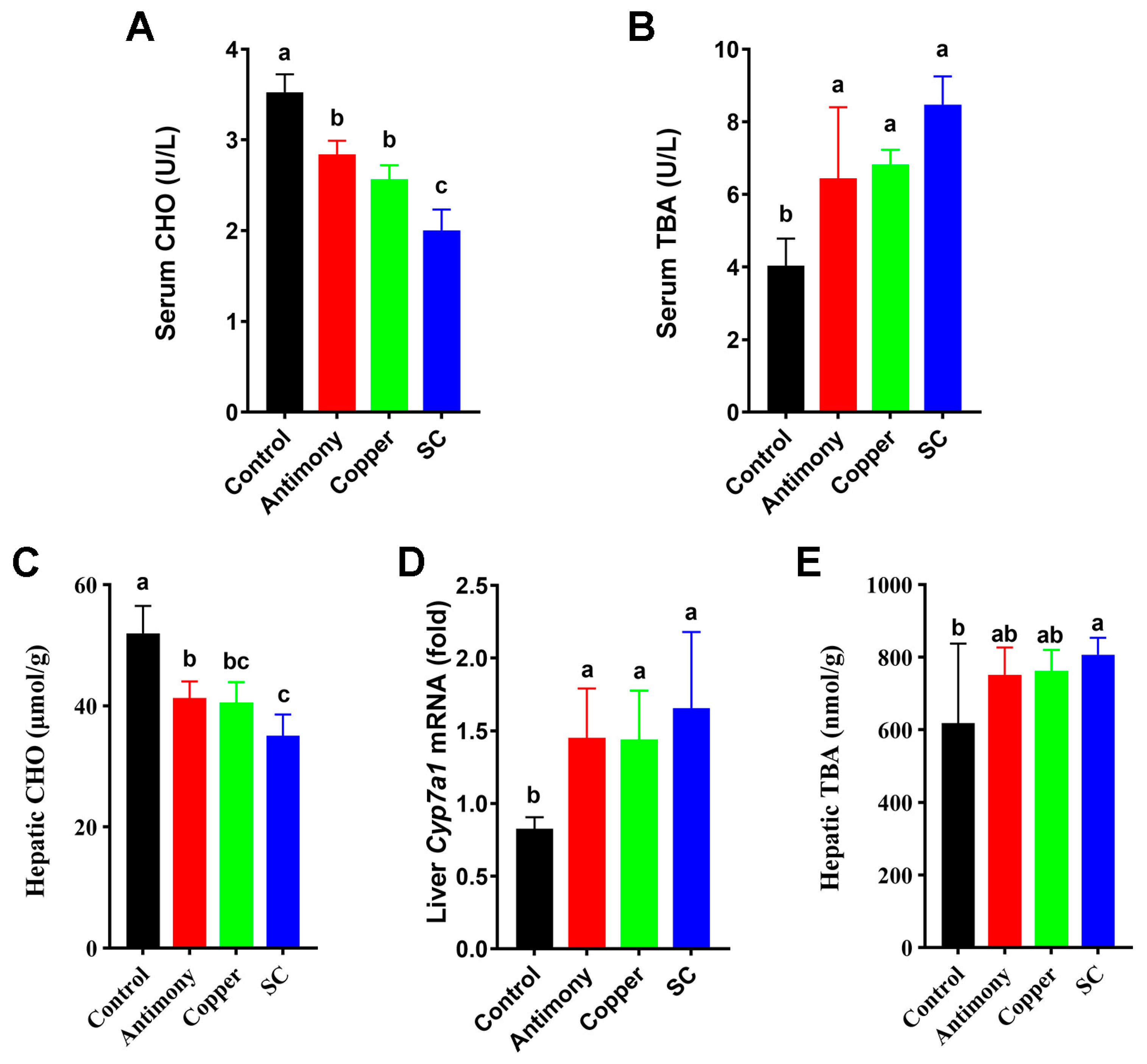
2.2. Disrupted BA Pools in Serum and Liver
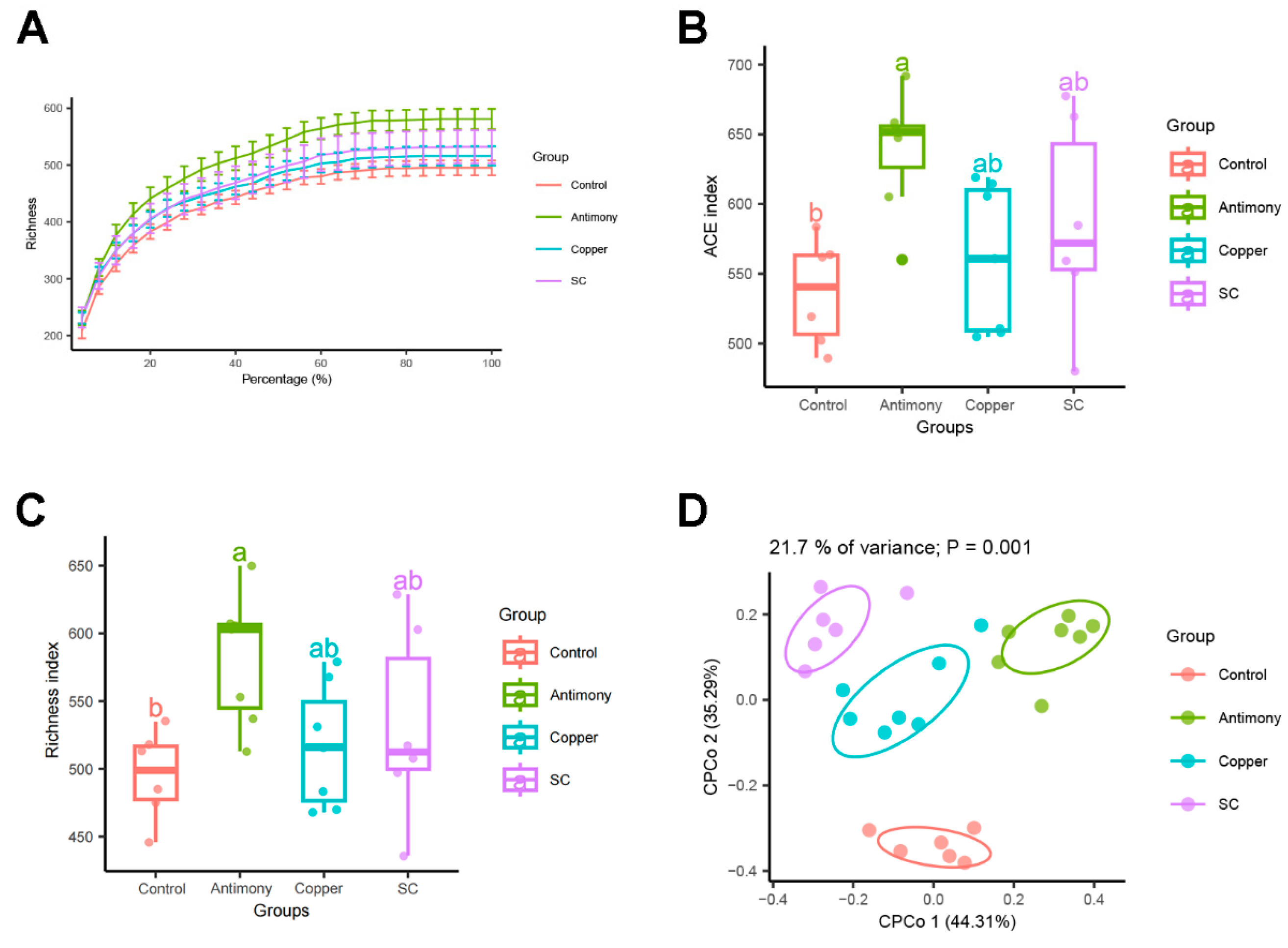
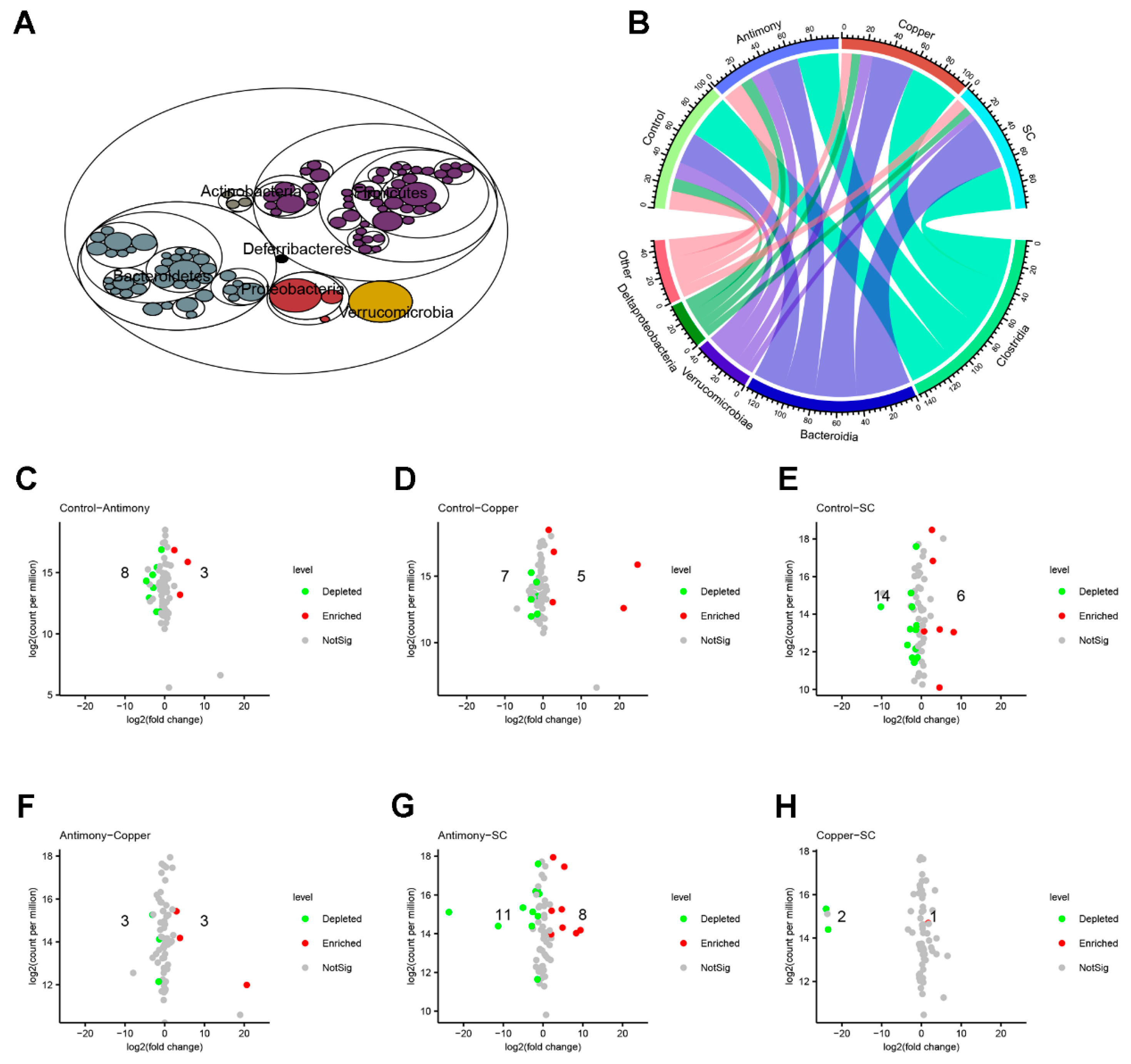
2.3. Gut Microbiota Analysis
2.4. Differential Bacterial Genera and the Relationships Between Lactobacillus and BAs
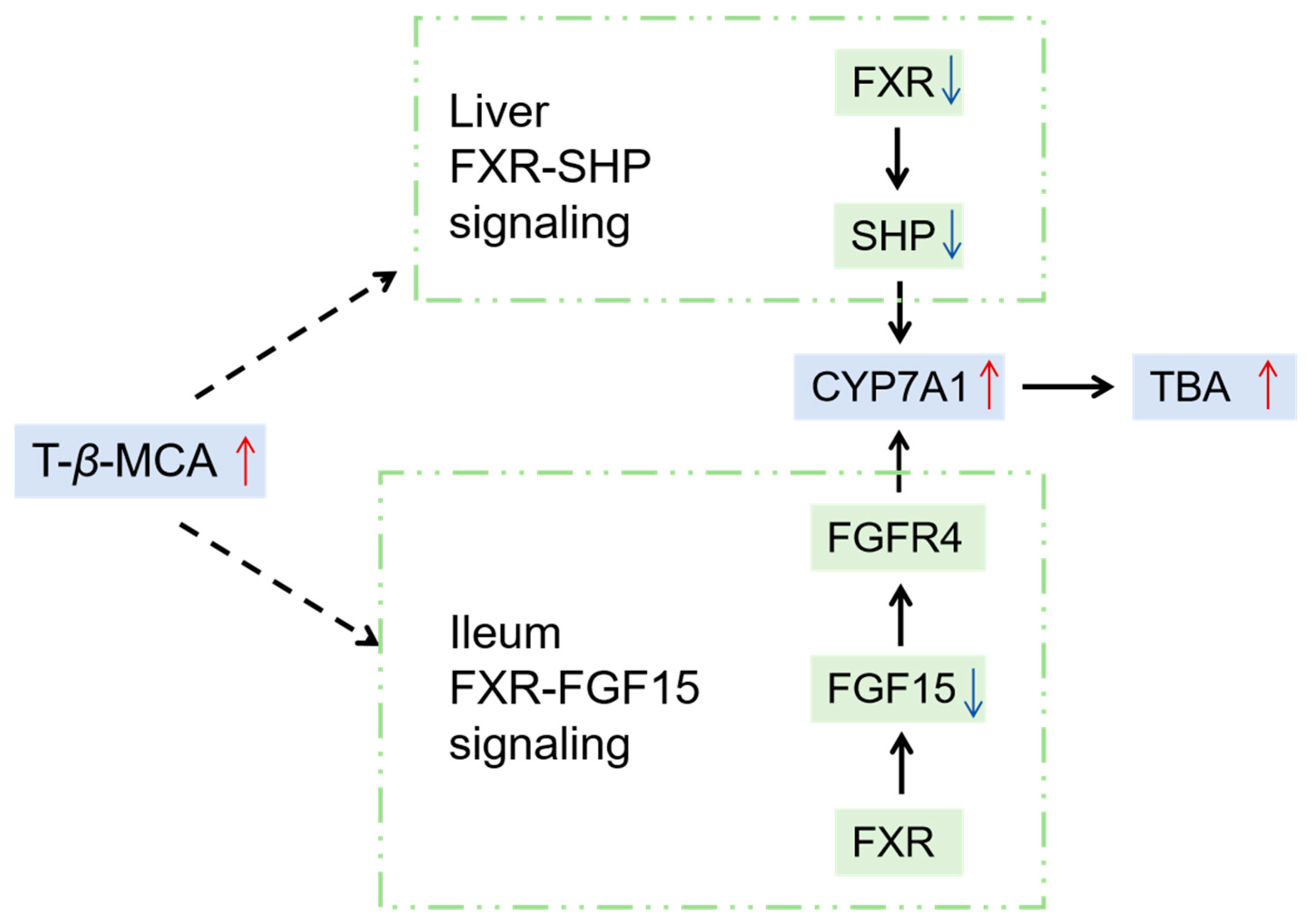
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Animal Experiments
4.3. Serum Biochemical Indicators and Histopathology of the Liver
4.4. Quantitative Real-Time PCR Analysis
4.5. Quantification of BA Metabolites
4.6. Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
4.7. High-Throughput Sequencing of Gut Microbiota
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, R.; Samanta, S.; Pandit, S.; Naaz, T.; Banerjee, S.; Rawat, J.M.; Chaubey, K.K.; Saha, R.P. An Overview of Bacteria-Mediated Heavy Metal Bioremediation Strategies. Appl. Biochem. Biotechnol. 2024, 196, 1712–1751. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Q.; Deng, M.; Japenga, J.; Li, T.; Yang, X.; He, Z. Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in southeast China. J. Environ. Manag. 2018, 207, 159–168. [Google Scholar] [CrossRef]
- Khan, Z.I.; Ahmad, K.; Rehman, S.; Siddique, S.; Bashir, H.; Zafar, A.; Sohail, M.; Ali, S.A.; Cazzato, E.; De Mastro, G. Health risk assessment of heavy metals in wheat using different water qualities: Implication for human health. Environ. Sci. Pollut. Res. Int. 2017, 24, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.M.; Shannon, M.C. Copper and Zinc Nutritional Issues for Agricultural Animal Production. Biol. Trace Elem. Res. 2019, 188, 148–159. [Google Scholar] [CrossRef]
- Pierart, A.; Shahid, M.; Séjalon-Delmas, N.; Dumat, C. Antimony bioavailability: Knowledge and research perspectives for sustainable agricultures. J. Hazard. Mater. 2015, 289, 219–234. [Google Scholar] [CrossRef]
- Duan, H.; Yu, L.; Tian, F.; Zhai, Q.; Fan, L.; Chen, W. Gut microbiota: A target for heavy metal toxicity and a probiotic protective strategy. Sci. Total Environ. 2020, 742, 140429. [Google Scholar] [CrossRef]
- Periferakis, A.; Caruntu, A.; Periferakis, A.T.; Scheau, A.E.; Badarau, I.A.; Caruntu, C.; Scheau, C. Availability, Toxicology and Medical Significance of Antimony. Int. J. Environ. Res. Public Health 2022, 19, 4669. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef]
- Zhong, G.; Wan, F.; Wu, S.; Jiang, X.; Tang, Z.; Zhang, X.; Huang, R.; Hu, L. Arsenic or/and antimony induced mitophagy and apoptosis associated with metabolic abnormalities and oxidative stress in the liver of mice. Sci. Total Environ. 2021, 777, 146082. [Google Scholar] [CrossRef]
- Wu, H.; Guo, H.; Liu, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Copper sulfate-induced endoplasmic reticulum stress promotes hepatic apoptosis by activating CHOP, JNK and caspase-12 signaling pathways. Ecotoxicol. Environ. Saf. 2020, 191, 110236. [Google Scholar] [CrossRef]
- Portincasa, P.; Di Ciaula, A.; Wang, H.H.; Palasciano, G.; van Erpecum, K.J.; Moschetta, A.; Wang, D.Q. Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology 2008, 47, 2112–2126. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Karpen, S.J. Intestinal transport and metabolism of bile acids. J. Lipid Res. 2015, 56, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Vatsalya, V.; Ma, X.; Zhang, J.; Yin, X.; Kim, S.; Feng, W.; McClain, C.J.; Zhang, X. Metabolic Profiling of Bile Acids in the Urine of Patients with Alcohol-Associated Liver Disease. Hepatol. Commun. 2021, 5, 798–811. [Google Scholar] [CrossRef]
- Ding, L.; Yang, L.; Wang, Z.; Huang, W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm. Sin. B 2015, 5, 135–144. [Google Scholar] [CrossRef]
- Claudel, T.; Staels, B.; Kuipers, F. The Farnesoid X receptor: A molecular link between bile acid and lipid and glucose metabolism. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2020–2030. [Google Scholar] [CrossRef]
- Li, T.; Chiang, J.Y. Bile acid signaling in metabolic disease and drug therapy. Pharmacol. Rev. 2014, 66, 948–983. [Google Scholar] [CrossRef]
- Li, Y.; Jadhav, K.; Zhang, Y. Bile acid receptors in non-alcoholic fatty liver disease. Biochem. Pharmacol. 2013, 86, 1517–1524. [Google Scholar] [CrossRef]
- Fu, T.; Coulter, S.; Yoshihara, E.; Oh, T.G.; Fang, S.; Cayabyab, F.; Zhu, Q.; Zhang, T.; Leblanc, M.; Liu, S.; et al. FXR Regulates Intestinal Cancer Stem Cell Proliferation. Cell 2019, 176, 1098–1112.e18. [Google Scholar] [CrossRef]
- Anakk, S.; Watanabe, M.; Ochsner, S.A.; McKenna, N.J.; Finegold, M.J.; Moore, D.D. Combined deletion of Fxr and Shp in mice induces Cyp17a1 and results in juvenile onset cholestasis. J. Clin. Investig. 2011, 121, 86–95. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, W.; Wu, Z.; Tian, X.; Xiang, J.; Li, L.; Li, Z.; Peng, X.; Wei, S.; Ma, X.; et al. Baicalin and the liver-gut system: Pharmacological bases explaining its therapeutic effects. Pharmacol. Res. 2021, 165, 105444. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, J.; Honda, A.; Miyazaki, T.; Monma, T.; Ueda, H.; Morishita, Y.; Yara, S.I.; Hirayama, T.; Ikegami, T. Western Diet Changes Gut Microbiota and Ameliorates Liver Injury in a Mouse Model with Human-Like Bile Acid Composition. Hepatol. Commun. 2021, 5, 2052–2067. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Trauner, M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L.; Zhong, Y.; Wang, M.; Ai, Y.; Hou, Y.; Chen, H.; Lin, X.; Zhang, Y.; Ding, M.; et al. Gut microbiota-bile acids-glucagon like peptide-1 axis contributes the resistance to high fat diet-induced obesity in mice. J. Nutr. Biochem. 2023, 117, 109358. [Google Scholar] [CrossRef]
- Kwiringira, J.; Mohling, E.W.P.; Mathias, A.; Ariho, P.; Mugisha, J.; Zakumumpa, H.; Rujumba, J.; Tumwebaze, I.K. Urban agriculture as an evolved sustainable urban livelihood: Evidence from Kampala city, Uganda. Agric. Food Secur. 2024, 13, 53. [Google Scholar] [CrossRef]
- Dala-Paula, B.M.; Custódio, F.B.; Knupp, E.A.N.; Palmieri, H.E.L.; Silva, J.B.B.; Glória, M.B.A. Cadmium, copper and lead levels in different cultivars of lettuce and soil from urban agriculture. Environ. Pollut. 2018, 242 Pt A, 383–389. [Google Scholar] [CrossRef]
- Fu, X.; Song, X.; Zheng, Q.; Liu, C.; Li, K.; Luo, Q.; Chen, J.; Wang, Z.; Luo, J. Frontier Materials for Adsorption of Antimony and Arsenic in Aqueous Environments: A Review. Int. J. Environ. Res. Public Health 2022, 19, 10824. [Google Scholar] [CrossRef]
- Teschke, R. Copper, Iron, Cadmium, and Arsenic, All Generated in the Universe: Elucidating Their Environmental Impact Risk on Human Health Including Clinical Liver Injury. Int. J. Mol. Sci. 2024, 25, 6662. [Google Scholar] [CrossRef]
- Hamamah, S.; Iatcu, O.C.; Covasa, M. Dietary Influences on Gut Microbiota and Their Role in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Nutrients 2024, 17, 143. [Google Scholar] [CrossRef]
- Soldán, M.; Argalášová, Ľ.; Hadvinová, L.; Galileo, B.; Babjaková, J. The Effect of Dietary Types on Gut Microbiota Composition and Development of Non-Communicable Diseases: A Narrative Review. Nutrients 2024, 16, 3134. [Google Scholar] [CrossRef] [PubMed]
- Ballway, J.W.; Song, B.J. Translational Approaches with Antioxidant Phytochemicals against Alcohol-Mediated Oxidative Stress, Gut Dysbiosis, Intestinal Barrier Dysfunction, and Fatty Liver Disease. Antioxidants 2021, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M.; et al. Gut Microbiota Mediates the Protective Effects of Dietary Capsaicin against Chronic Low-Grade Inflammation and Associated Obesity Induced by High-Fat Diet. mBio 2017, 8, 3. [Google Scholar] [CrossRef]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Perino, A.; Demagny, H.; Velazquez-Villegas, L.; Schoonjans, K. Molecular Physiology of Bile Acid Signaling in Health, Disease, and Aging. Physiol. Rev. 2021, 101, 683–731. [Google Scholar] [CrossRef]
- Lim, M.Y.C.; Ho, H.K. Pharmacological modulation of cholesterol 7α-hydroxylase (CYP7A1) as a therapeutic strategy for hypercholesterolemia. Biochem. Pharmacol. 2024, 220, 115985. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Jia, A.; Wang, Y.; Bi, Y.; Liu, G. The crosstalk between gut bacteria and host immunity in intestinal inflammation. J. Cell. Physiol. 2021, 236, 2239–2254. [Google Scholar] [CrossRef]
- Fan, X.; Mai, C.; Zuo, L.; Huang, J.; Xie, C.; Jiang, Z.; Li, R.; Yao, X.; Fan, X.; Wu, Q.; et al. Herbal formula BaWeiBaiDuSan alleviates polymicrobial sepsis-induced liver injury via increasing the gut microbiota Lactobacillus johnsonii and regulating macrophage anti-inflammatory activity in mice. Acta Pharm. Sin. B 2023, 13, 1164–1179. [Google Scholar] [CrossRef]
- Xia, J.; Lv, L.; Liu, B.; Wang, S.; Zhang, S.; Wu, Z.; Yang, L.; Bian, X.; Wang, Q.; Wang, K.; et al. Akkermansia muciniphila Ameliorates Acetaminophen-Induced Liver Injury by Regulating Gut Microbial Composition and Metabolism. Microbiol. Spectr. 2022, 10, e0159621. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, H.; Jeong, Y.; Kang, C.H. Lactic Acid Bacteria Exert a Hepatoprotective Effect against Ethanol-Induced Liver Injury in HepG2 Cells. Microorganisms 2021, 9, 1844. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Y.; Zhang, Y.N.; Wang, H.; Ga, Y.; Fan, Y.; Wang, Q.; Gu, J.H.; Zhang, X.Y.; Gong, X.H.; Hao, Z.H. Mori fructus aqueous extracts attenuate carbon tetrachloride-induced renal injury via the Nrf2 pathway and intestinal flora. Ecotoxicol. Environ. Saf. 2022, 245, 114118. [Google Scholar] [CrossRef] [PubMed]
- Gräfe, C.; Graf, H.; Wustrow, V.; Liebchen, U.; Conter, P.; Paal, M.; Habler, K.; Scharf, C. Correlation of bilirubin and toxic bile acids in critically ill patients with cholestatic liver dysfunction and adsorber application. Sci. Rep. 2024, 14, 21762. [Google Scholar] [CrossRef]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef]
- Fu, K.; Chen, X.; Shou, N.; Wang, Z.; Yuan, X.; Wu, D.; Wang, Q.; Cheng, Y.; Ling, N.; Shi, Z. Swainsonine Induces Liver Inflammation in Mice via Disturbance of Gut Microbiota and Bile Acid Metabolism. J. Agric. Food Chem. 2023, 71, 1758–1767. [Google Scholar] [CrossRef]
- Gonzalez, F.J.; Jiang, C.; Xie, C.; Patterson, A.D. Intestinal Farnesoid X Receptor Signaling Modulates Metabolic Disease. Dig. Dis. 2017, 35, 178–184. [Google Scholar] [CrossRef]
- Yan, M.; Man, S.; Liang, Y.; Ma, L.; Guo, L.; Huang, L.; Gao, W. Diosgenin alleviates nonalcoholic steatohepatitis through affecting liver-gut circulation. Pharmacol. Res. 2023, 187, 106621. [Google Scholar] [CrossRef]
- Yan, M.; Man, S.; Ma, L.; Gao, W. Comprehensive molecular mechanisms and clinical therapy in nonalcoholic steatohepatitis: An overview and current perspectives. Metabolism 2022, 134, 155264. [Google Scholar] [CrossRef]
- Gillard, J.; Clerbaux, L.A.; Nachit, M.; Sempoux, C.; Staels, B.; Bindels, L.B.; Tailleux, A.; Leclercq, I.A. Bile acids contribute to the development of non-alcoholic steatohepatitis in mice. JHEP Rep. 2022, 4, 100387. [Google Scholar] [CrossRef]
- Inagaki, T.; Choi, M.; Moschetta, A.; Peng, L.; Cummins, C.L.; McDonald, J.G.; Luo, G.; Jones, S.A.; Goodwin, B.; Richardson, J.A.; et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005, 2, 217–225. [Google Scholar] [CrossRef]
- Simbrunner, B.; Trauner, M.; Reiberger, T. Review article: Therapeutic aspects of bile acid signalling in the gut-liver axis. Aliment. Pharmacol. Ther. 2021, 54, 1243–1262. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Lin, Q.; Hou, S.; Cui, X.; Shou, N.; Yuan, X.; Xu, W.; Fu, K.; Wang, Q.; Shi, Z. Gut Microbiota and Its Metabolite Taurine-β-Muricholic Acid Contribute to Antimony- and/or Copper-Induced Liver Inflammation. Int. J. Mol. Sci. 2025, 26, 3332. https://doi.org/10.3390/ijms26073332
Wu D, Lin Q, Hou S, Cui X, Shou N, Yuan X, Xu W, Fu K, Wang Q, Shi Z. Gut Microbiota and Its Metabolite Taurine-β-Muricholic Acid Contribute to Antimony- and/or Copper-Induced Liver Inflammation. International Journal of Molecular Sciences. 2025; 26(7):3332. https://doi.org/10.3390/ijms26073332
Chicago/Turabian StyleWu, Dandan, Qiwen Lin, Senao Hou, Xiaorui Cui, Na Shou, Xuefeng Yuan, Wenqian Xu, Keyi Fu, Qi Wang, and Zunji Shi. 2025. "Gut Microbiota and Its Metabolite Taurine-β-Muricholic Acid Contribute to Antimony- and/or Copper-Induced Liver Inflammation" International Journal of Molecular Sciences 26, no. 7: 3332. https://doi.org/10.3390/ijms26073332
APA StyleWu, D., Lin, Q., Hou, S., Cui, X., Shou, N., Yuan, X., Xu, W., Fu, K., Wang, Q., & Shi, Z. (2025). Gut Microbiota and Its Metabolite Taurine-β-Muricholic Acid Contribute to Antimony- and/or Copper-Induced Liver Inflammation. International Journal of Molecular Sciences, 26(7), 3332. https://doi.org/10.3390/ijms26073332







