Comprehending the Role of Metabolic and Hemodynamic Factors Alongside Different Signaling Pathways in the Pathogenesis of Diabetic Nephropathy
Abstract
1. Introduction
2. Epidemiology and Prevalence of DN
3. Risk Factors of DN
3.1. Age and Race
3.2. Obesity
3.3. Gene Susceptibility
3.4. Duration of DM
3.5. Hypertension
3.6. Raised Albuminuria
3.7. Dyslipidemia
3.8. Smoking
4. Progression of DM into DN
5. Developmental Stages in DN
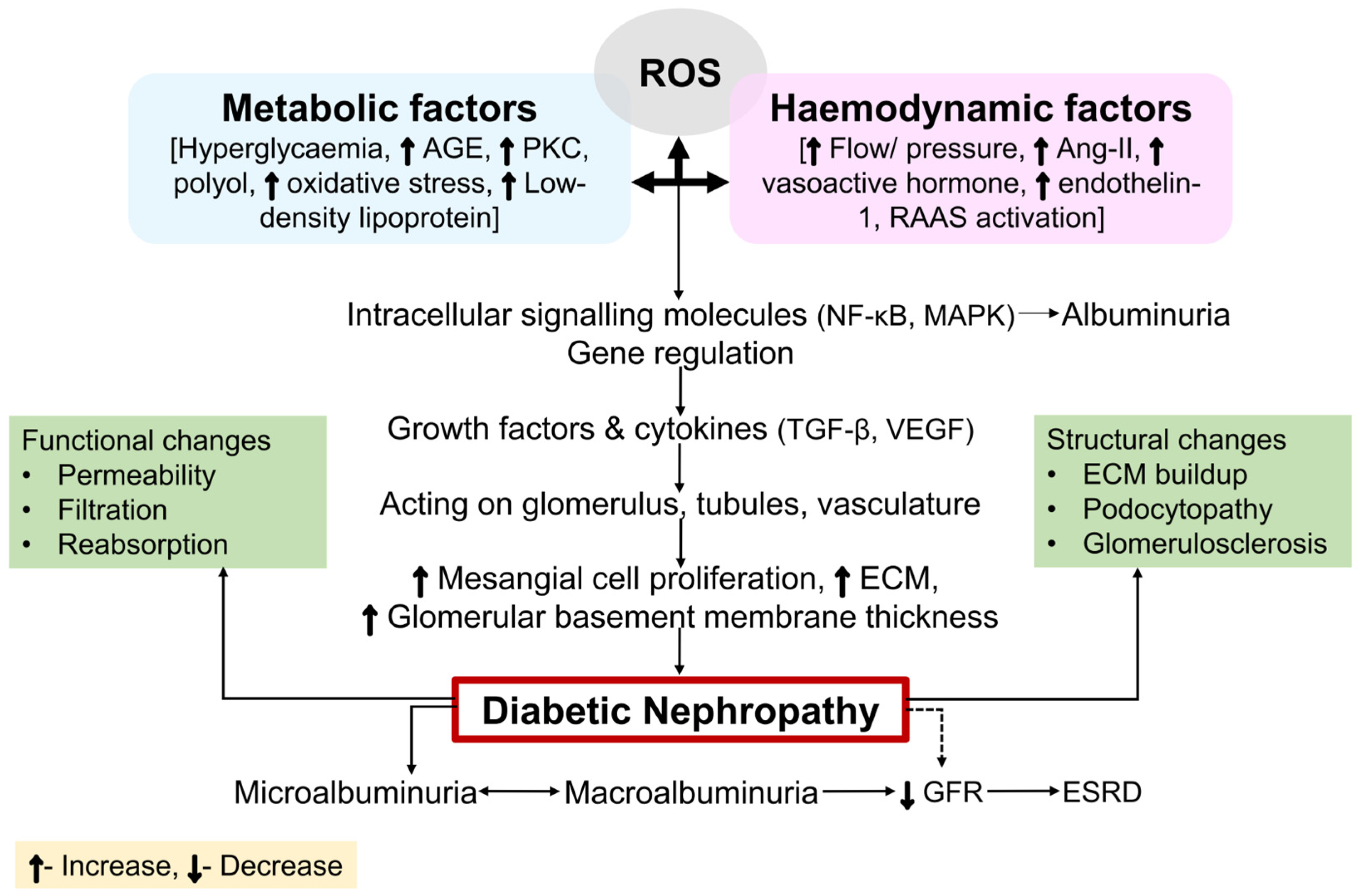
6. Molecular Mechanisms in the Pathogenesis of DN
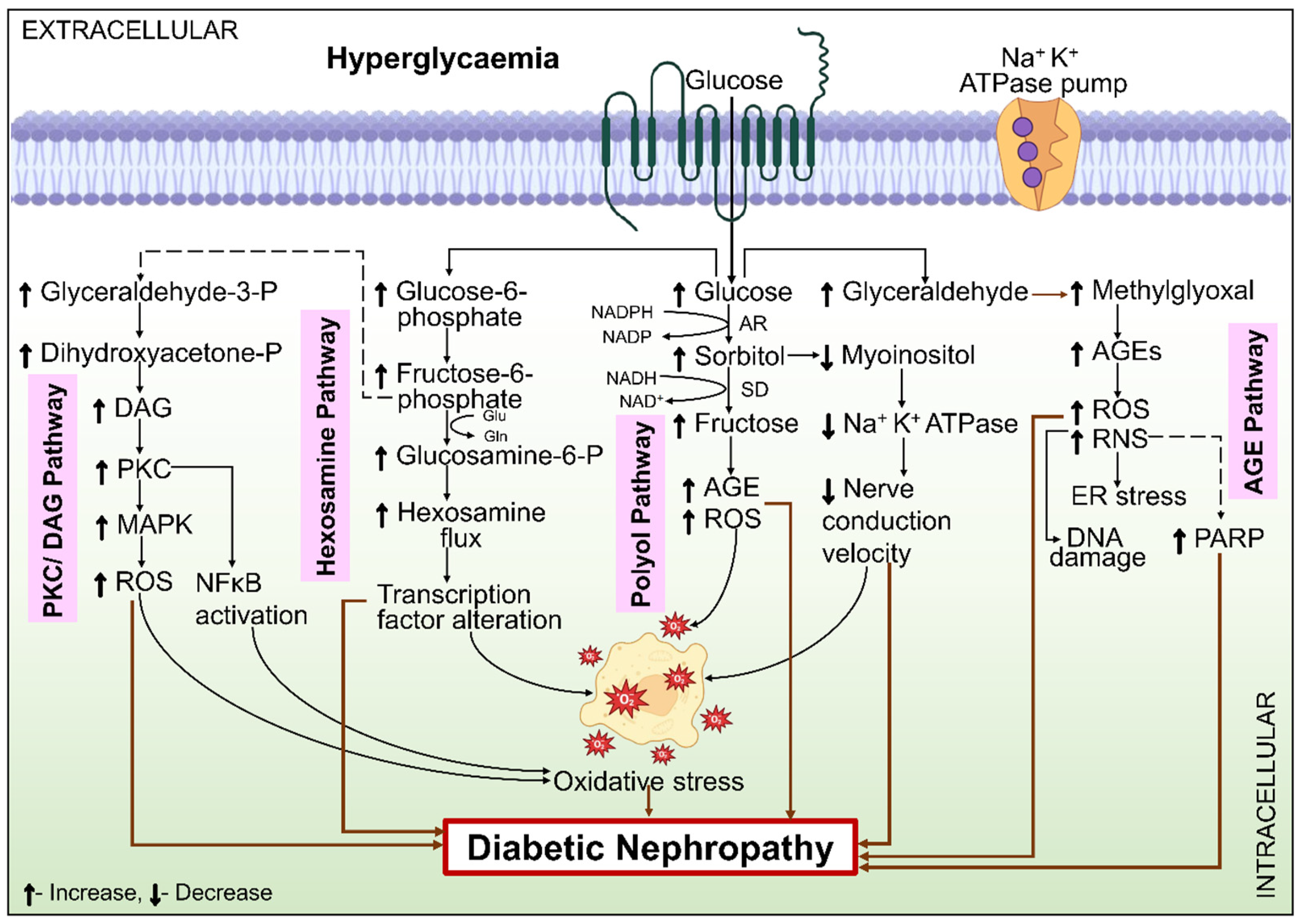
6.1. Metabolic Mechanisms
6.1.1. Polyol Pathway
6.1.2. Hexosamine Pathway
6.1.3. PKC Pathway
6.1.4. AGE
6.1.5. Oxidative Stress
6.1.6. Mitochondrial Dysfunction
6.2. Hemodynamic Factors
6.2.1. RAAS
6.2.2. Vasoactive Hormones
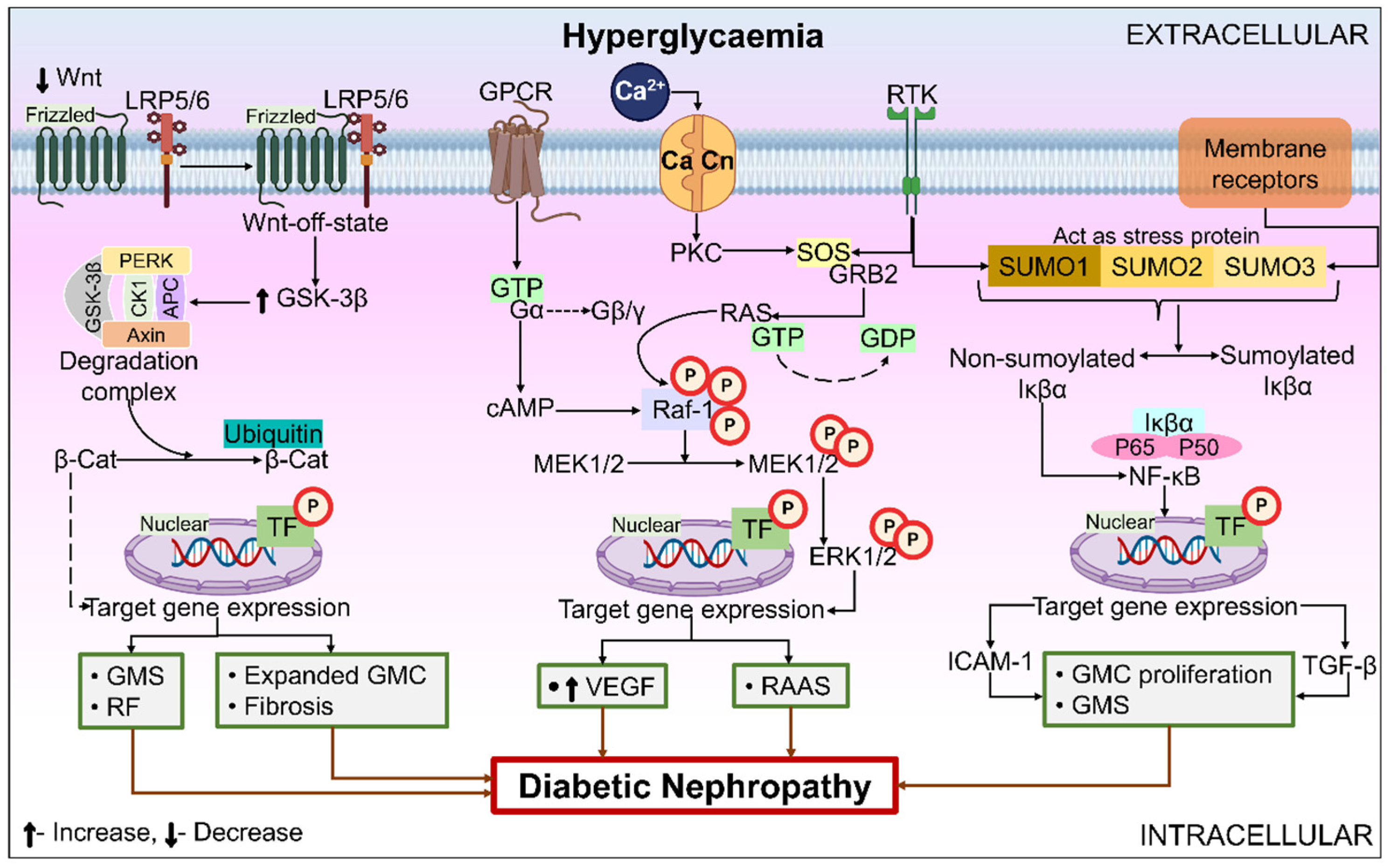
6.3. Role of Miscellaneous Signal Transduction Pathways/Receptors/Enzymes/Processes in DN
6.3.1. Wnt Pathway
6.3.2. Extracellular Signal-Regulated Kinase (ERK) Signaling Pathway
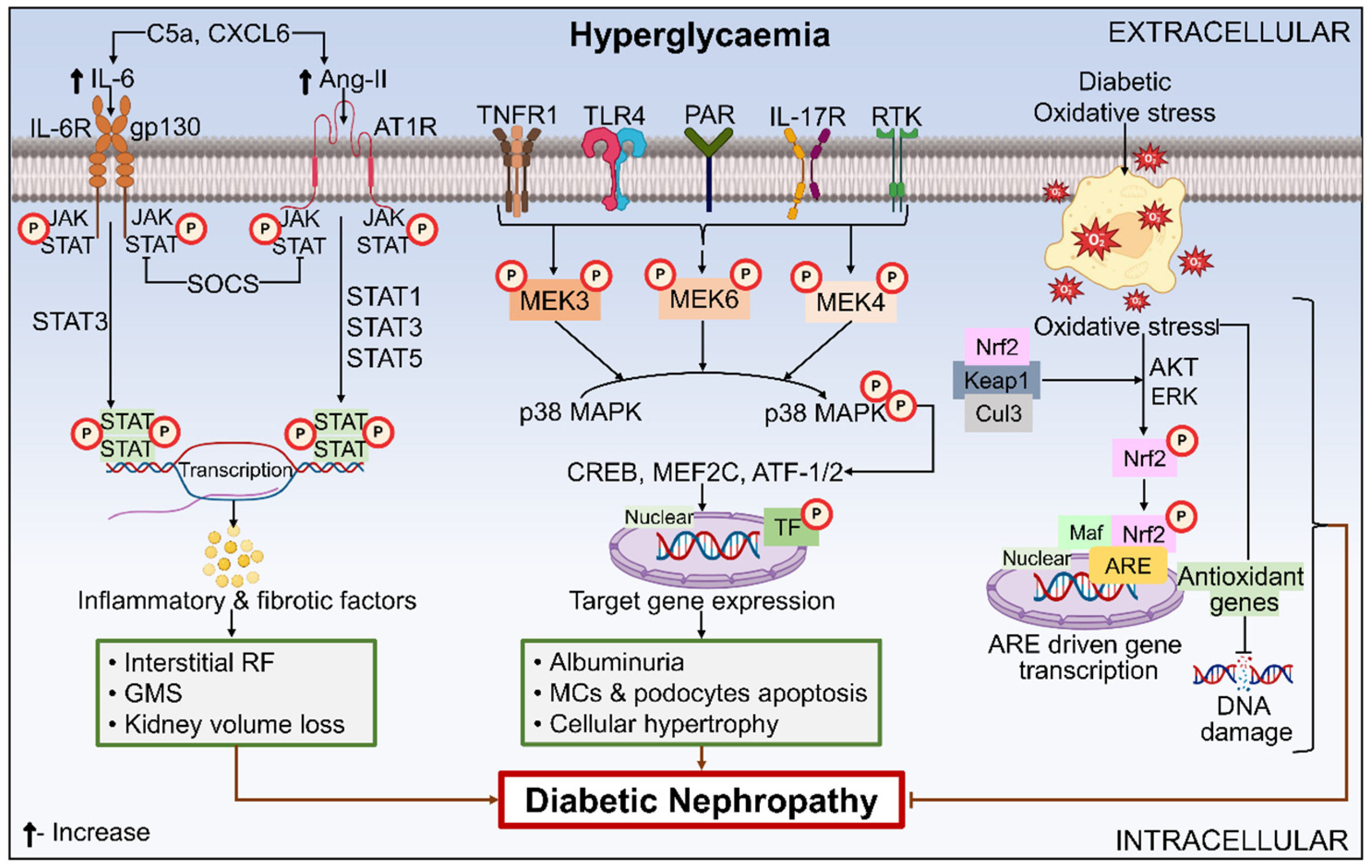
6.3.3. NF-κB
6.3.4. JAK-STAT
6.3.5. MAPK Signaling Pathway
6.3.6. Apoptosis Signal-Regulating Kinase (ASK) 1
6.3.7. Nox
6.3.8. Nrf2
6.3.9. Autophagy
6.3.10. Fibrosis
6.3.11. Epigenetics
7. Role of Blood Stasis in DN and Its Molecular Mechanism
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alsharidah, A.S. Diabetes Mellitus and Diabetic Nephropathy: A Review of the Literature on Hemostatic Changes in Coagulation and Thrombosis. Blood Res. 2022, 57, 101–105. [Google Scholar] [CrossRef]
- He, K.J.; Wang, H.; Xu, J.; Gong, G.; Liu, X.; Guan, H. Global Burden of Type 2 Diabetes Mellitus from 1990 to 2021, with Projections of Prevalence to 2044: A Systematic Analysis across SDI Levels for the Global Burden of Disease Study 2021. Front. Endocrinol. 2024, 15, 1501690. [Google Scholar] [CrossRef] [PubMed]
- Ratan, Y.; Rajput, A.; Pareek, A.; Pareek, A.; Kaur, R.; Sonia, S.; Kumar, R.; Singh, G. Recent Advances in Biomolecular Patho-Mechanistic Pathways behind the Development and Progression of Diabetic Neuropathy. Biomedicines 2024, 12, 1390. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Franzén, S.; Eliasson, B.; Svensson, A.-M.; Miftaraj, M.; McGuire, D.K.; Sattar, N.; Rosengren, A.; Gudbjörnsdottir, S. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N. Engl. J. Med. 2017, 376, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, M.K. Diabetic Nephropathy: Recent Advances in Pathophysiology and Challenges in Dietary Management. Diabetol. Metab. Syndr. 2019, 11, 7. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, W.; Liu, J.; Xie, M.; Liu, Q.; Li, S. Vascular Complications of Diabetes. Medicine 2023, 102, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hahr, A.J.; Molitch, M.E. Management of Diabetes Mellitus in Patients with Chronic Kidney Disease. Clin. Diabetes Endocrinol. 2015, 1, 2. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Mohandes, S.; Doke, T.; Hu, H.; Mukhi, D.; Dhillon, P.; Susztak, K. Molecular Pathways That Drive Diabetic Kidney Disease. J. Clin. Investig. 2023, 133, e165654. [Google Scholar] [CrossRef]
- Wu, T.; Ding, L.; Andoh, V.; Zhang, J.; Chen, L. The Mechanism of Hyperglycemia-Induced Renal Cell Injury in Diabetic Nephropathy Disease: An Update. Life 2023, 13, 539. [Google Scholar] [CrossRef]
- Natesan, V.; Kim, S.J. Diabetic Nephropathy—A Review of Risk Factors, Progression, Mechanism, and Dietary Management. Biomol. Ther. 2021, 29, 365–372. [Google Scholar] [CrossRef]
- Rossing, P.; Frimodt-Møller, M. Clinical Features and Natural Course of Diabetic Nephropathy. In Diabetic Nephropathy: Pathophysiology and Clinical Aspects; Roelofs, J.J., Vogt, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 21–32. [Google Scholar]
- Rossing, P.; Persson, F.; Frimodt-Møller, M. Prognosis and Treatment of Diabetic Nephropathy: Recent Advances and Perspectives. Nephrol. Ther. 2018, 14, S31–S37. [Google Scholar] [PubMed]
- Wu, X.Q.; Zhang, D.D.; Wang, Y.N.; Tan, Y.Q.; Yu, X.Y.; Zhao, Y.Y. AGE/RAGE in Diabetic Kidney Disease and Ageing Kidney. Free Radic. Biol. Med. 2021, 171, 260–271. [Google Scholar] [PubMed]
- Forbes, J.M.; Thorburn, D.R. Mitochondrial Dysfunction in Diabetic Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 291–312. [Google Scholar]
- Yang, M.; Liu, C.; Jiang, N.; Liu, Y.; Luo, S.; Li, C.; Zhao, H.; Han, Y.; Chen, W.; Li, L.; et al. Endoplasmic Reticulum Homeostasis: A Potential Target for Diabetic Nephropathy. Front. Endocrinol. 2023, 14, 1182848. [Google Scholar]
- Sagoo, M.K.; Gnudi, L. Diabetic Nephropathy: Is There a Role for Oxidative Stress? Free Radic. Biol. Med. 2018, 116, 50–63. [Google Scholar]
- Sinha, S.K.; Nicholas, S.B. Pathomechanisms of Diabetic Kidney Disease. J. Clin. Med. 2023, 12, 7349. [Google Scholar] [CrossRef]
- Patel, D.M.; Bose, M.; Cooper, M.E. Glucose and Blood Pressure-Dependent Pathways—The Progression of Diabetic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 2218. [Google Scholar] [CrossRef]
- Srivastava, M.; Rai, B. Molecular Mechanisms of Pathways in Diabetic Nephropathy Development in Patients with T2DM—A Review. Int. J. Appl. Biol. Pharm. Technol. 2021, 12, 380–392. [Google Scholar]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar]
- Hoogeveen, E.K. The Epidemiology of Diabetic Kidney Disease. Kidney Dial. 2022, 2, 433–442. [Google Scholar] [CrossRef]
- Duan, J.; Wang, C.; Liu, D.; Qiao, Y.; Pan, S.; Jiang, D.; Zhao, Z.; Liang, L.; Tian, F.; Yu, P.; et al. Prevalence and Risk Factors of Chronic Kidney Disease and Diabetic Kidney Disease in Chinese Rural Residents: A Cross-Sectional Survey. Sci. Rep. 2019, 9, 10408. [Google Scholar]
- Al-Shamsi, S.; Regmi, D.; Govender, R.D. Chronic Kidney Disease in Patients at High Risk of Cardiovascular Disease in the United Arab Emirates: A Population-Based Study. PLoS ONE 2018, 13, e0199920. [Google Scholar]
- Moradi-Lakeh, M.; El Bcheraoui, C.; Khalil, I.; Charara, R.; Afshin, A.; Wang, H.; Collison, M.; Krohn, K.J.; Chew, A.; Daoud, F.; et al. Diabetes Mellitus and Chronic Kidney Disease in the Eastern Mediterranean Region: Findings from the Global Burden of Disease 2015 Study. Int. J. Public Health 2018, 63, 177–186. [Google Scholar]
- de Boer, I.H.; Rue, T.C.; Hall, Y.N.; Heagerty, P.J.; Weiss, N.S.; Himmelfarb, J. Temporal Trends in the Prevalence of Diabetic Kidney Disease in the United States. JAMA 2011, 305, 2532–2539. [Google Scholar] [CrossRef]
- Roy, S.; Schweiker-Kahn, O.; Jafry, B.; Masel-Miller, R.; Raju, R.S.; O’Neill, L.M.O.; Correia, C.R.; Trivedi, A.; Johnson, C.; Pilot, C.; et al. Risk Factors and Comorbidities Associated with Diabetic Kidney Disease. J. Prim. Care Community Health 2021, 12, 21501327211048556. [Google Scholar]
- Hameed, I.; Masoodi, S.R.; Malik, P.A.; Mir, S.A.; Ghazanfar, K.; Ganai, B.A. Genetic Variations in Key Inflammatory Cytokines Exacerbates the Risk of Diabetic Nephropathy by Influencing the Gene Expression. Gene 2018, 661, 51–59. [Google Scholar] [PubMed]
- Kato, M.; Natarajan, R. Diabetic Nephropathy-Emerging Epigenetic Mechanisms. Nat. Rev. Nephrol. 2014, 10, 517–530. [Google Scholar]
- Zheng, Z.; Zheng, F. Immune Cells and Inflammation in Diabetic Nephropathy. J. Diabetes Res. 2016, 2016, 1841690. [Google Scholar]
- Ni, W.J.; Tang, L.Q.; Wei, W. Research Progress in Signalling Pathway in Diabetic Nephropathy. Diabetes. Metab. Res. Rev. 2015, 31, 221–233. [Google Scholar]
- Svensson, M.; Nyström, L.; Schön, S.; Dahlquist, G. Age at Onset of Childhood-Onset Type 1 Diabetes and the Development of End-Stage Renal Disease: A Nationwide Population-Based Study. Diabetes Care 2006, 29, 538–542. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hill, C.J.; Cardwell, C.R.; Maxwell, A.P.; Young, R.J.; Matthews, B.; O’Donoghue, D.J.; Fogarty, D.G. Obesity and Kidney Disease in Type 1 and 2 Diabetes: An Analysis of the National Diabetes Audit. QJM 2013, 106, 933–942. [Google Scholar] [CrossRef]
- Feodoroff, M.; Harjutsalo, V.; Forsblom, C.; Thorn, L.; Wadén, J.; Tolonen, N.; Lithovius, R.; Groop, P.H. Smoking and Progression of Diabetic Nephropathy in Patients with Type 1 Diabetes. Acta Diabetol. 2016, 53, 525–533. [Google Scholar] [CrossRef]
- Ramanjaneyulu, M.; Ananth Kumar, K.; Shubash Kumar, M.; Reddy, S.; Kara, D.; Mukthala, P.; Raj, R.; Madhu, C. Target Identification and Validation for Diabetic Nephropathy Using Molecular Docking Studies. Der Pharma Chem. 2013, 5, 353–363. [Google Scholar]
- Hussain, S.; Chand Jamali, M.; Habib, A.; Hussain, M.S.; Akhtar, M.; Najmi, A.K. Diabetic Kidney Disease: An Overview of Prevalence, Risk Factors, and Biomarkers. Clin. Epidemiol. Glob. Health 2021, 9, 2–6. [Google Scholar] [CrossRef]
- Wagnew, F.; Eshetie, S.; Kibret, G.D.; Zegeye, A.; Dessie, G.; Mulugeta, H.; Alemu, A. Diabetic Nephropathy and Hypertension in Diabetes Patients of Sub-Saharan Countries: A Systematic Review and Meta-Analysis. BMC Res. Notes 2018, 11, 1–7. [Google Scholar] [CrossRef]
- Persson, F.; Rossing, P. Diagnosis of Diabetic Kidney Disease: State of the Art and Future Perspective. Kidney Int. Suppl. 2018, 8, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Chehade, J.M.; Gladysz, M.; Mooradian, A.D. Dyslipidemia in Type 2 Diabetes: Prevalence, Pathophysiology, and Management. Drugs 2013, 73, 327–339. [Google Scholar] [CrossRef]
- Caramori, M.L.; Fioretto, P.; Mauer, M. The Need for Early Predictors of Diabetic Nephropathy Risk. Diabetes 2000, 49, 1399–1408. [Google Scholar] [CrossRef]
- Martin, M.; Edrisa, M.; SSinabulya, I.; Samuel, K.; Frank, M.; Kiiza, M.C. Microalbuminuria among Newly Diagnosed Diabetic Patients at Mulago National Referral Hospital in Uganda: A Cross Sectional Study. J. Obes. Weight Loss Medicat. 2018, 4, 021. [Google Scholar]
- Jansson, F.J.; Forsblom, C.; Harjutsalo, V.; Thorn, L.M.; Wadén, J.; Elonen, N.; Ahola, A.J.; Saraheimo, M.; Groop, P.H. Regression of Albuminuria and Its Association with Incident Cardiovascular Outcomes and Mortality in Type 1 Diabetes: The FinnDiane Study. Diabetologia 2018, 61, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Zelmanovitz, T.; Gerchman, F.; Balthazar, A.P.S.; Thomazelli, F.C.S.; Matos, J.D.; Canani, L.H. Diabetic Nephropathy. Diabetol. Metab. Syndr. 2009, 1, 1–17. [Google Scholar]
- Kim, Y.; Kim, W.; Kim, J.K.; Moon, J.Y.; Park, S.; Park, C.W.; Park, H.S.; Song, S.H.; Yoo, T.H.; Lee, S.Y.; et al. Blood Pressure Control in Patients with Diabetic Kidney Disease. Electrolyte Blood Press. 2022, 20, 39–48. [Google Scholar] [PubMed]
- De Boer, I.H.; Afkarian, M.; Rue, T.C.; Cleary, P.A.; Lachin, J.M.; Molitch, M.E.; Steffes, M.W.; Sun, W.; Zinman, B. Renal Outcomes in Patients with Type 1 Diabetes and Macroalbuminuria. J. Am. Soc. Nephrol. 2014, 25, 2342–2350. [Google Scholar] [CrossRef]
- Hovind, P.; Tarnow, L.; Rossing, P.; Jensen, B.R.; Graae, M.; Torp, I.; Binder, C.; Parving, H.H. Predictors for the Development of Microalbuminuria and Macroalbuminuria in Patients with Type 1 Diabetes: Inception Cohort Study. Br. Med. J. 2004, 328, 1105–1108. [Google Scholar]
- Retnakaran, R.; Cull, C.A.; Thorne, K.I.; Adler, A.I.; Holman, R.R. Risk Factors for Renal Dysfunction in Type 2 Diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006, 55, 1832–1839. [Google Scholar]
- Pavkov, M.E.; Knowler, W.C.; Bennett, P.H.; Looker, H.C.; Krakoff, J.; Nelson, R.G. Increasing Incidence of Proteinuria and Declining Incidence of End-Stage Renal Disease in Diabetic Pima Indians. Kidney Int. 2006, 70, 1840–1846. [Google Scholar]
- Mogensen, C.E. Microalbuminuria, Blood Pressure and Diabetic Renal Disease. Diabetologia 1999, 42, 263–285. [Google Scholar]
- Pérez-Morales, R.E.; Del Pino, M.D.; Valdivielso, J.M.; Ortiz, A.; Mora-Fernández, C.; Navarro-González, J.F. Inflammation in Diabetic Kidney Disease. Nephron 2019, 143, 12–16. [Google Scholar] [CrossRef]
- Kopel, J.; Pena-Hernandez, C.; Nugent, K. Evolving Spectrum of Diabetic Nephropathy. World J. Diabetes 2019, 10, 269–279. [Google Scholar]
- Tavafi, M. Diabetic Nephropathy and Antioxidants. J. Nephropathol. 2013, 2, 20–27. [Google Scholar] [PubMed]
- Singh, M.; Kapoor, A.; Bhatnagar, A. Physiological and Pathological Roles of Aldose Reductase. Metabolites 2021, 11, 655. [Google Scholar] [CrossRef] [PubMed]
- Brosius, F.C.; Tuttle, K.R.; Kretzler, M. JAK Inhibition in the Treatment of Diabetic Kidney Disease. Diabetologia 2016, 59, 1624–1627. [Google Scholar] [PubMed]
- Sharma, V. Role of Different Molecular Pathways in the Development of Diabetes-Induced Nephropathy. J. Diabetes Metab. 2013, 01, 14–15. [Google Scholar]
- Gamal, H.; Munusamy, S. Aldose Reductase as a Drug Target for Treatment of Diabetic Nephropathy: Promises and Challenges. Protein Pept. Lett. 2016, 24, 71–77. [Google Scholar]
- Yan, L. jun Redox Imbalance Stress in Diabetes Mellitus: Role of the Polyol Pathway. Anim. Model. Exp. Med. 2018, 1, 7–13. [Google Scholar]
- Paneque, A.; Fortus, H.; Zheng, J.; Werlen, G.; Jacinto, E. The Hexosamine Biosynthesis Pathway: Regulation and Function. Genes 2023, 14, 933. [Google Scholar] [CrossRef]
- Forbes, J.M.; Fukami, K.; Cooper, M.E. Diabetic Nephropathy: Where Hemodynamics Meets Metabolism. Exp. Clin. Endocrinol. Diabetes 2007, 115, 69–84. [Google Scholar]
- Agarwal, R. Pathogenesis of Diabetic Nephropathy. ADA Clin. Compendia 2021, 2021, 2–7. [Google Scholar]
- Pan, D.; Xu, L.; Guo, M. The Role of Protein Kinase C in Diabetic Microvascular Complications. Front. Endocrinol. 2022, 13, 973058. [Google Scholar]
- Noh, H.; King, G.L. The Role of Protein Kinase C Activation in Diabetic Nephropathy. Kidney Int. 2007, 72, S49–S53. [Google Scholar]
- Geraldes, P.; King, G.L. Activation of Protein Kinase C Isoforms & Its Impact on Diabetic Complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [PubMed]
- Ohshiro, Y.; Ma, R.C.; Yasuda, Y.; Hiraoka-Yamamoto, J.; Clermont, A.C.; Isshiki, K.; Yagi, K.; Arikawa, E.; Kern, T.S.; King, G.L. Reduction of Diabetes-Induced Oxidative Stress, Fibrotic Cytokine Expression, and Renal Dysfunction in Protein Kinase Cβ-Null Mice. Diabetes 2006, 55, 3112–3120. [Google Scholar]
- Tuttle, K.R.; Bakris, G.L.; Toto, R.D.; McGill, J.B.; Hu, K.; Anderson, P.W. The Effect of Ruboxistaurin on Nephropathy in Type 2 Diabetes. Diabetes Care 2005, 28, 2686–2690. [Google Scholar] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [PubMed]
- Brownlee, M.; Cerami, A.; Vlassara, H. Advanced Glycosylation End Products in Tissue and the Biochemical Basis of Diabetic Complications. N. Engl. J. Med. 1988, 318, 1315–1321. [Google Scholar]
- Wendt, T.M.; Tanji, N.; Guo, J.; Kislinger, T.R.; Qu, W.; Lu, Y.; Bucciarelli, L.G.; Ling Rong, L.; Moser, B.; Markowitz, G.S.; et al. RAGE Drives the Development of Glomerulosclerosis and Implicates Podocyte Activation in the Pathogenesis of Diabetic Nephropathy. Am. J. Pathol. 2003, 162, 1123–1137. [Google Scholar]
- Tan, A.L.Y.; Sourris, K.C.; Harcourt, B.E.; Thallas-Bonke, V.; Penfold, S.; Andrikopoulos, S.; Thomas, M.C.; O’Brien, R.C.; Bierhaus, A.; Cooper, M.E.; et al. Disparate Effects on Renal and Oxidative Parameters Following RAGE Deletion, AGE Accumulation Inhibition, or Dietary AGE Control in Experimental Diabetic Nephropathy. Am. J. Physiol.-Ren. Physiol. 2010, 298, 763–771. [Google Scholar]
- Chen, J.L.T.; Francis, J. Pyridoxamine, Advanced Glycation Inhibition, and Diabetic Nephropathy. J. Am. Soc. Nephrol. 2012, 23, 6–8. [Google Scholar]
- Rabbani, N.; Alam, S.S.; Riaz, S.; Larkin, J.R.; Akhtar, M.W.; Shafi, T.; Thornalley, P.J. High-Dose Thiamine Therapy for Patients with Type 2 Diabetes and Microalbuminuria: A Randomised, Double-Blind Placebo-Controlled Pilot Study. Diabetologia 2009, 52, 208–212. [Google Scholar]
- Peppa, M.; Brem, H.; Cai, W.; Zhang, J.G.; Basgen, J.; Li, Z.; Vlassara, H.; Uribarri, J. Prevention and Reversal of Diabetic Nephropathy in Db/Db Mice Treated with Alagebrium (ALT-711). Am. J. Nephrol. 2006, 26, 430–436. [Google Scholar] [CrossRef]
- Bolton, W.K.; Cattran, D.C.; Williams, M.E.; Adler, S.G.; Appel, G.B.; Cartwright, K.; Foiles, P.G.; Freedman, B.I.; Raskin, P.; Ratner, R.E.; et al. Randomized Trial of an Inhibitor of Formation of Advanced Glycation End Products in Diabetic Nephropathy. Am. J. Nephrol. 2004, 24, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Allan, F.; Larry, D.; Bieke, F.S.; Tilton, R.G.; Trine, H.M.; Soren, R.P.; Ruth, R. Long-Term Renal Effects of a Neutralizing RAGE Antibody in Obese Type 2 Diabetic Mice. Diabetes 2004, 53, 166–172. [Google Scholar]
- Galasko, D.; Bell, J.; Mancuso, J.Y.; Kupiec, J.W.; Sabbagh, M.N.; Van Dyck, C.; Thomas, R.G.; Aisen, P.S. Clinical Trial of an Inhibitor of RAGE-Ab Interactions in Alzheimer Disease. Neurology 2014, 82, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Syst. Rev. Pharm. 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Mima, A. Inflammation and Oxidative Stress in Diabetic Nephropathy: New Insights on Its Inhibition as New Therapeutic Targets. J. Diabetes Res. 2013, 2013, 248563. [Google Scholar] [CrossRef]
- Gorin, Y.; Block, K. “Nox4 and Diabetic Nephropathy: With a Friend like This Who Needs Enemies. ” Free Radic. Biol. Med. 2013, 61, 130–142. [Google Scholar] [CrossRef]
- Baynes, J.W. Role of Oxidative Stress in Development of Complications in Diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef]
- Hallan, S.; Sharma, K. The Role of Mitochondria in Diabetic Kidney Disease. Curr. Diab. Rep. 2016, 16, 61. [Google Scholar] [CrossRef]
- Coughlan, M.T.; Sharma, K. Challenging the Dogma of Mitochondrial Reactive Oxygen Species Overproduction in Diabetic Kidney Disease. Kidney Int. 2016, 90, 272–279. [Google Scholar] [CrossRef]
- Wei, P.Z.; Szeto, C.C. Mitochondrial Dysfunction in Diabetic Kidney Disease. Clin. Chim. Acta 2019, 496, 108–116. [Google Scholar] [PubMed]
- Catania, J.M.; Chen, G.; Parrish, A.R. Role of Matrix Metalloproteinases in Renal Pathophysiologies. Am. J. Physiol.-Ren. Physiol. 2007, 292, F905–F911. [Google Scholar]
- Lee, S.R.; Lee, H.E.; Yoo, J.Y.; An, E.J.; Song, S.J.; Han, K.H.; Cha, D.R.; Bae, Y.S. Nox4-SH3YL1 Complex Is Involved in Diabetic Nephropathy. iScience 2024, 27, 108868. [Google Scholar] [CrossRef]
- You, Y.H.; Quach, T.; Saito, R.; Pham, J.; Sharma, K. Metabolomics Reveals a Key Role for Fumarate in Mediating the Effects of NADPH Oxidase 4 in Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 466–481. [Google Scholar] [PubMed]
- Pagliarini, D.J.; Calvo, S.E.; Chang, B.; Sheth, S.A.; Scott, B.; Ong, S.; Walford, G.A.; Sugiana, C.; Boneh, A.; William, K.; et al. A Mitochondrial Protein Compendium Elucidates Complex I Disease Biology. Cell 2008, 134, 112–123. [Google Scholar] [PubMed]
- O’Connor, P.M. Renal Oxygen Delivery: Matching Delivery to Metabolic Demand. Clin. Exp. Pharmacol. Physiol. 2006, 33, 961–967. [Google Scholar]
- Coughlan, M.T.; Nguyen, T.V.; Penfold, S.A.; Higgins, G.C.; Thallas-Bonke, V.; Tan, S.M.; Van Bergen, N.J.; Sourris, K.C.; Harcourt, B.E.; Thorburn, D.R.; et al. Mapping Time-Course Mitochondrial Adaptations in the Kidney in Experimental Diabetes. Clin. Sci. 2016, 130, 711–720. [Google Scholar]
- Flemming, N.B.; Gallo, L.A.; Ward, M.S.; Forbes, J.M. Tapping into Mitochondria to Find Novel Targets for Diabetes Complications. Curr. Drug Targets 2016, 17, 1341–1349. [Google Scholar]
- Lin, Y.C.; Chang, Y.H.; Yang, S.Y.; Wu, K.D.; Chu, T.S. Update of Pathophysiology and Management of Diabetic Kidney Disease. J. Formos. Med. Assoc. 2018, 117, 662–675. [Google Scholar]
- Wennmann, D.O.; Hsu, H.H.; Pavenstädt, H. The Renin-Angiotensin-Aldosterone System in Podocytes. Semin. Nephrol. 2012, 32, 377–384. [Google Scholar] [CrossRef]
- Yacoub, R.; Campbell, K.N. Inhibition of RAS in Diabetic Nephropathy. Int. J. Nephrol. Renovasc. Dis. 2015, 8, 29–40. [Google Scholar] [PubMed]
- Wada, J.; Makino, H. Inflammation and the Pathogenesis of Diabetic Nephropathy. Clin. Sci. 2013, 124, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Alkaaby, A.; Alhelu, S.; Almosewi, A. Nephroprotective Effect of Vitamin E Added to Angiotensin Receptor Blocker in Patients with Diabetic Nephropathy. Kufa Med. J. 2021, 17, 55–61. [Google Scholar]
- Nie, J.M.; Li, H.F. Therapeutic Effects of Salvia Miltiorrhiza Injection Combined with Telmisartan in Patients with Diabetic Nephropathy by Influencing Collagen IV and Fibronectin: A Case-Control Study. Exp. Ther. Med. 2018, 16, 3405–3412. [Google Scholar] [CrossRef]
- Samsu, N.; Soeharto, S.; Rifai, M.; Rudijanto, A. Rosmarinic Acid Monotherapy Is Better than the Combination of Rosmarinic Acid and Telmisartan in Preventing Podocyte Detachment and Inhibiting the Progression of Diabetic Nephropathy in Rats. Biol. Targets Ther. 2019, 13, 179–190. [Google Scholar] [CrossRef]
- Lavoie, J.L.; Sigmund, C.D. Minireview: Overview of the Renin-Angiotensin System—An Endocrine and Paracrine System. Endocrinology 2003, 144, 2179–2183. [Google Scholar] [CrossRef]
- Nishiyama, A.; Seth, D.M.; Navar, L.G. Renal Interstitial Fluid Angiotensin I and Angiotensin II Concentrations during Local Angiotensin-Converting Enzyme Inhibition. J. Am. Soc. Nephrol. 2002, 13, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, A.K.; Alavi, N.; Leehey, D.J. Mechanism of Increased Angiotensin II Levels in Glomerular Mesangial Cells Cultured in High Glucose. J. Am. Soc. Nephrol. 2003, 14, 873–880. [Google Scholar]
- Vidotti, D.B.; Casarini, D.E.; Cristovam, P.C.; Leite, C.A.; Schor, N.; Boim, M.A. High Glucose Concentration Stimulates Intracellular Renin Activity and Angiotensin II Generation in Rat Mesangial Cells. Am. J. Physiol.-Ren. Physiol. 2004, 286, F1039–F1045. [Google Scholar] [CrossRef]
- Navar, L.G.; Inscho, E.W.; Majid, D.S.A.; Imig, J.D.; Harrison-Bernard, L.M.; Mitchell, K.D. Nonendothelial Paracrine Regulation of the Renal Microcirculation. Adv. Organ Biol. 2000, 9, 219–233. [Google Scholar]
- Ribeiro-Oliveira, A.; Nogueira, A.I.; Pereira, R.M.; Vilas Boas, W.W.; Souza dos Santos, R.A.; Simões e Silva, A.C. The Renin-Angiotensin System and Diabetes: An Update. Vasc. Health Risk Manag. 2008, 4, 787–803. [Google Scholar] [PubMed]
- Komers, R.; Anderson, S. Paradoxes of Nitric Oxide in the Diabetic Kidney. Am. J. Physiol.-Ren. Physiol. 2003, 284, F1121–F1137. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.J.; Lee, Y.J.; Tsai, J.H. The Correlation of Plasma and Urine Endothelin-1 with the Severity of Nephropathy in Chinese Patients with Type 2 Diabetes. Scand. J. Clin. Lab. Investig. 1996, 56, 571–576. [Google Scholar]
- Simonson, M.S.; Ismail-Beigi, F. Endothelin-1 Increases Collagen Accumulation in Renal Mesangial Cells by Stimulating a Chemokine and Cytokine Autocrine Signaling Loop. J. Biol. Chem. 2011, 286, 11003–11008. [Google Scholar] [CrossRef]
- Mehta, S.; Hingole, S.; Chaudhary, V. The Emerging Mechanisms of Wnt Secretion and Signaling in Development. Front. Cell Dev. Biol. 2021, 9, 714746. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Liu, Y. New Insights into the Role and Mechanism of Wnt/β-Catenin Signalling in Kidney Fibrosis. Nephrology 2018, 23, 38–43. [Google Scholar] [CrossRef]
- Malik, S.A.; Modarage, K.; Goggolidou, P. The Role of WNT Signalling in Chronic Kidney Disease (CKD). Genes 2020, 11, 496. [Google Scholar] [CrossRef]
- Guo, Q.; Zhong, W.; Duan, A.; Sun, G.; Cui, W.; Zhuang, X.; Liu, L. Protective or Deleterious Role of Wnt/Beta-Catenin Signaling in Diabetic Nephropathy: An Unresolved Issue. Pharmacol. Res. 2019, 144, 151–157. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Wu, X.; Chen, Y.; Ji, W.; Wang, J.; Zhang, Y.; Xia, Y.; Tang, Y.; Yuan, J. The Wnt Signaling Pathway in Diabetic Nephropathy. Front. Cell Dev. Biol. 2022, 9, 701547. [Google Scholar] [CrossRef]
- Ho, C.; Lee, P.H.; Hsu, Y.C.; Wang, F.S.; Huang, Y.T.; Lin, C.L. Sustained Wnt/β-Catenin Signaling Rescues High Glucose Induction of Transforming Growth Factor-Β1-Mediated Renal Fibrosis. Am. J. Med. Sci. 2012, 344, 374–382. [Google Scholar] [CrossRef]
- Cheng, X.; Gao, W.; Dang, Y.; Liu, X.; Li, Y.; Peng, X.; Ye, X. Both ERK/MAPK and TGF-Beta/Smad Signaling Pathways Play a Role in the Kidney Fibrosis of Diabetic Mice Accelerated by Blood Glucose Fluctuation. J. Diabetes Res. 2013, 2013, 463740. [Google Scholar] [PubMed]
- Navarro-González, J.F.; Mora-Fernández, C.; De Fuentes, M.M.; García-Pérez, J. Inflammatory Molecules and Pathways in the Pathogenesis of Diabetic Nephropathy. Nat. Rev. Nephrol. 2011, 7, 327–340. [Google Scholar]
- Ricciardi, C.A.; Gnudi, L. Kidney Disease in Diabetes: From Mechanisms to Clinical Presentation and Treatment Strategies. Metabolism. 2021, 124, 154890. [Google Scholar] [PubMed]
- Zhang, H.; Nair, V.; Saha, J.; Atkins, K.B.; Hodgin, J.B.; Saunders, T.L.; Myers, M.G.; Werner, T.; Kretzler, M.; Brosius, F.C. Podocyte-Specific JAK2 Overexpression Worsens Diabetic Kidney Disease in Mice. Kidney Int. 2017, 92, 909–921. [Google Scholar]
- Berthier, C.C.; Zhang, H.; Schin, M.; Henger, A.; Nelson, R.G.; Yee, B.; Boucherot, A.; Neusser, M.A.; Cohen, C.D.; Carter-Su, C.; et al. Enhanced Expression of Janus Kinase-Signal Transducer and Activator of Transcription Pathway Members in Human Diabetic Nephropathy. Diabetes 2009, 58, 469–477. [Google Scholar] [PubMed]
- Zheng, C.; Huang, L.; Luo, W.; Yu, W.; Hu, X.; Guan, X.; Cai, Y.; Zou, C.; Yin, H.; Xu, Z.; et al. Inhibition of STAT3 in Tubular Epithelial Cells Prevents Kidney Fibrosis and Nephropathy in STZ-Induced Diabetic Mice. Cell Death Dis. 2019, 10, 848. [Google Scholar]
- Tuttle, K.R.; Brosius, F.C.; Adler, S.G.; Kretzler, M.; Mehta, R.L.; Tumlin, J.A.; Tanaka, Y.; Haneda, M.; Liu, J.; Silk, M.E.; et al. JAK1/JAK2 Inhibition by Baricitinib in Diabetic Kidney Disease: Results from a Phase 2 Randomized Controlled Clinical Trial. Nephrol. Dial. Transplant. 2018, 33, 1950–1959. [Google Scholar]
- Müller, R.; Daniel, C.; Hugo, C.; Amann, K.; Mielenz, D.; Endlich, K.; Braun, T.; van der Veen, B.; Heeringa, P.; Schett, G.; et al. The Mitogen-Activated Protein Kinase P38α Regulates Tubular Damage in Murine Anti-Glomerular Basement Membrane Nephritis. PLoS ONE 2013, 8, e56316. [Google Scholar]
- Fujita, H.; Omori, S.; Ishikura, K.; Hida, M.; Awazu, M. ERK and P38 Mediate High-Glucose-Induced Hypertrophy and TGF-β Expression in Renal Tubular Cells. Am. J. Physiol.-Ren. Physiol. 2004, 286, 120–126. [Google Scholar]
- Tesch, G.H.; Ma, F.Y.; Han, Y.; Liles, J.T.; Breckenridge, D.G.; Nikolic-Paterson, D.J. ASK1 Inhibitor Halts Progression of Diabetic Nephropathy in Nos3-Deficient Mice. Diabetes 2015, 64, 3903–3913. [Google Scholar]
- Liles, J.T.; Corkey, B.K.; Notte, G.T.; Budas, G.R.; Lansdon, E.B.; Hinojosa-Kirschenbaum, F.; Badal, S.S.; Lee, M.; Schultz, B.E.; Wise, S.; et al. ASK1 Contributes to Fibrosis and Dysfunction in Models of Kidney Disease. J. Clin. Investig. 2018, 128, 4485–4500. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; An, E.J.; Kim, J.; Bae, Y.S. Function of NADPH Oxidases in Diabetic Nephropathy and Development of Nox Inhibitors. Biomol. Ther. 2020, 28, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.; de Haan, J.B. Combating Oxidative Stress in Diabetic Complications with Nrf2 Activators: How Much Is Too Much? Redox Rep. 2014, 19, 107–117. [Google Scholar] [CrossRef]
- Jiang, T.; Huang, Z.; Lin, Y.; Zhang, Z.; Fang, D.; Zhang, D.D. The Protective Role of Nrf2 in Streptozotocin-Induced Diabetic Nephropathy. Diabetes 2010, 59, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Choi, M.E. Autophagy in Diabetic Nephropathy. J. Endocrinol. 2015, 224, 15–30. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Bergamini, E.; Brunk, U.T.; Dröge, W.; Ffrench, M.; Terman, A. Autophagy and Aging: The Importance of Maintaining “Clean” Cells. Autophagy 2005, 1, 131–140. [Google Scholar] [CrossRef]
- Peng, K.Y.; Horng, L.Y.; Sung, H.C.; Huang, H.C.; Wu, R.T. Hepatocyte Growth Factor Has a Role in the Amelioration of Diabetic Vascular Complications via Autophagic Clearance of Advanced Glycation End Products: Dispo85E, an HGF Inducer, as a Potential Botanical Drug. Metabolism 2011, 60, 888–892. [Google Scholar] [CrossRef]
- Yu, L.; McPhee, C.K.; Lixin, Z.; Mardones, G.A.; Rong, Y.; Peng, J.; Mi, N.; Zhao, Y.; Liu, Z.; Wan, F.; et al. Autophagy Termination and Lysosome Reformation Regulated by MTOR. Nature 2010, 465, 942–946. [Google Scholar] [CrossRef]
- Koszegi, S.; Molnar, A.; Lenart, L.; Hodrea, J.; Balogh, D.B.; Lakat, T.; Szkibinszkij, E.; Hosszu, A.; Sparding, N.; Genovese, F.; et al. RAAS Inhibitors Directly Reduce Diabetes-Induced Renal Fibrosis via Growth Factor Inhibition. J. Physiol. 2019, 597, 193–209. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The Master Regulator of Fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Sato, M.; Muragaki, Y.; Saika, S.; Roberts, A.B.; Ooshima, A. Targeted Disruption of TGF-Β1/Smad3 Signaling Protects against Renal Tubulointerstitial Fibrosis Induced by Unilateral Ureteral Obstruction. J. Clin. Investig. 2003, 112, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Voelker, J.; Berg, P.H.; Sheetz, M.; Duffin, K.; Shen, T.; Moser, B.; Greene, T.; Blumenthal, S.S.; Rychlik, I.; Yagil, Y.; et al. Anti–TGF-Β1 Antibody Therapy in Patients with Diabetic Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 953–962. [Google Scholar] [PubMed]
- Wahab, N.A.; Yevdokimova, N.; Weston, B.S.; Roberts, T.; Li, X.J.; Brinkman, H.; Mason, R.M. Role of Connective Tissue Growth Factor in the Pathogenesis of Diabetic Nephropathy. Biochem. J. 2001, 359, 77–87. [Google Scholar] [CrossRef]
- Reddy, M.A.; Natarajan, R. Epigenetics in Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2011, 22, 2182–2185. [Google Scholar]
- Bansal, A.; Pinney, S.E. DNA Methylation and Its Role in the Pathogenesis of Diabetes. Pediatr Diabetes. 2017, 18, 167–177. [Google Scholar] [CrossRef]
- El-Osta, A.; Brasacchio, D.; Yao, D.; Pocai, A.; Jones, P.L.; Roeder, R.G.; Cooper, M.E.; Brownlee, M. Transient High Glucose Causes Persistent Epigenetic Changes and Altered Gene Expression during Subsequent Normoglycemia. J. Exp. Med. 2008, 205, 2409–2417. [Google Scholar] [PubMed]
- Sun, G.; Reddy, M.A.; Yuan, H.; Lanting, L.; Kato, M.; Natarajan, R. Epigenetic Histone Methylation Modulates Fibrotic Gene Expression. J. Am. Soc. Nephrol. 2010, 21, 2069–2080. [Google Scholar]
- Shen, S.; Zhong, H.; Zhou, X.; Li, G.; Zhang, C.; Zhu, Y.; Yang, Y. Advances in Traditional Chinese Medicine Research in Diabetic Kidney Disease Treatment. Pharm. Biol. 2024, 62, 222–232. [Google Scholar]
- Choi, T.Y.; Jun, J.H.; Park, B.; Lee, J.A.; You, S.; Jung, J.; Lee, M.S. Concept of Blood Stasis in Chinese Medical Textbooks: A Systematic Review. Eur. J. Integr. Med. 2016, 8, 158–164. [Google Scholar]
- Li, X.; Liu, W.; Wang, X.; Huang, M.; Xue, Q.; Wang, T.; Cao, Y.; Li, W.; Cao, Z.; Han, M.; et al. Basic Research Progress of Promoting Blood Circulation and Removing Blood Stasis Jingfang on Diabetic Nephropathy. IOP Conf. Ser. Mater. Sci. Eng. 2019, 677, 022127. [Google Scholar]
- Wang, R.; Tian, C.; Liu, Y.; Lou, Q.; Yang, M.; Si, G.; Cai, R.; Wang, R.; Cui, Y.; Fang, Y.; et al. Clinical Overview of Prescription of Promoting Blood Circulation and Removing Blood Stasis in Treating Diabetic Nephropathy. IOP Conf. Ser. Earth Environ. Sci. 2021, 714, 032059. [Google Scholar]
- Liu, Y.; Shang, X.; Wu, H. Efficacy and Safety of “Jiedu Tongluo Therapy” for Diabetic Kidney Disease: A Systematic Review and Meta-Analysis. Chin. J. Exp. Tradit. Med. Formulae 2023, 29, 165–171. [Google Scholar]
- Guo, C.; Li, S.; Rao, X. rong New Goals and Strategies of Chinese Medicine in Prevention and Treatment of Chronic Kidney Disease. Chin. J. Integr. Med. 2019, 25, 163–167. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratan, Y.; Rajput, A.; Pareek, A.; Pareek, A.; Singh, G. Comprehending the Role of Metabolic and Hemodynamic Factors Alongside Different Signaling Pathways in the Pathogenesis of Diabetic Nephropathy. Int. J. Mol. Sci. 2025, 26, 3330. https://doi.org/10.3390/ijms26073330
Ratan Y, Rajput A, Pareek A, Pareek A, Singh G. Comprehending the Role of Metabolic and Hemodynamic Factors Alongside Different Signaling Pathways in the Pathogenesis of Diabetic Nephropathy. International Journal of Molecular Sciences. 2025; 26(7):3330. https://doi.org/10.3390/ijms26073330
Chicago/Turabian StyleRatan, Yashumati, Aishwarya Rajput, Ashutosh Pareek, Aaushi Pareek, and Gurjit Singh. 2025. "Comprehending the Role of Metabolic and Hemodynamic Factors Alongside Different Signaling Pathways in the Pathogenesis of Diabetic Nephropathy" International Journal of Molecular Sciences 26, no. 7: 3330. https://doi.org/10.3390/ijms26073330
APA StyleRatan, Y., Rajput, A., Pareek, A., Pareek, A., & Singh, G. (2025). Comprehending the Role of Metabolic and Hemodynamic Factors Alongside Different Signaling Pathways in the Pathogenesis of Diabetic Nephropathy. International Journal of Molecular Sciences, 26(7), 3330. https://doi.org/10.3390/ijms26073330





