Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is a significant adverse event with unclear mechanisms and limited treatment alternatives. This study aimed to investigate the efficacy of two alkalizing agents, a mixture of potassium citrate and sodium citrate (K/Na citrate) or sodium bicarbonate (NaHCO3), in preventing and treating paclitaxel (PTX)-induced mechanical allodynia in rodents. The results from rodent models demonstrated that repeated prophylactic administration of K/Na citrate or NaHCO3 could inhibit the development of PTX-induced mechanical allodynia. Moreover, K/Na citrate was effective in preventing the PTX-induced exacerbation of mechanical allodynia, even when treatment was initiated immediately after the onset of allodynia. K/Na citrate also reduced the levels of the plasma complement component anaphylatoxin C3a in a PTX-induced CIPN rat model. Complement activation, resulting in the production of C3a, has been implicated in the pathogenesis of this model. Additionally, pretreatment with Na citrate significantly prevented the reduction in neurite outgrowth caused by PTX. Furthermore, K/Na citrate inhibited spontaneous and mechanical stimuli-induced firing in spinal dorsal horn neurons. These findings indicate that K/Na citrate may regulate the development of PTX-induced mechanical allodynia by modulating complement activation and providing neuroprotection against PTX-induced peripheral nerve injury. This study implies that alkalization could help prevent PTX-induced peripheral neuropathy and mitigate its exacerbation.
1. Introduction
Most cancers can be treated with anticancer drugs, such as platinum-based drugs like oxaliplatin, taxanes like paclitaxel (PTX), vinca alkaloids like vincristine, and proteasome holoenzyme inhibitors like bortezomib. However, these drugs can induce peripheral neuropathy [1,2,3,4].
Chemotherapy-induced peripheral neuropathy (CIPN) can present with sensory neuropathy symptoms, such as numbness, hypoesthesia, and pain in the extremities; motor neuropathy symptoms, such as muscular atrophy, weakness, and flaccid paralysis with impaired intestinal motility and involuntary muscles, dysuria, dyshidrosis, and orthostatic hypotension; and autonomic neuropathy symptoms, such as constipation. PTX, in particular, is associated with sensory and motor neuropathy [5]. CIPN can also present as shooting or burning pain that can be persistent and debilitating, having a significant impact on patients’ quality of life.
CIPN is a well-known adverse event; however, the detailed mechanism underlying its development remains unclear, resulting in a lack of established effective treatment alternatives. CIPN significantly impacts the continuation of cancer treatment; hence, its prevention and treatment are crucial for improving treatment outcomes and enhancing patients’ quality of life. Currently, the discontinuation of anticancer drugs is the only available option to alleviate CIPN symptoms [6].
In terms of CIPN prevention, calcium or magnesium may be administered to prevent neurotoxicity; however, there is no clear evidence to support their effectiveness. Drugs commonly used to manage CIPN-associated numbness or pain may include Goshajinkigan, vitamin B12 preparations, nonsteroidal anti-inflammatory drugs, pregabalin, gabapentin, and opioids. However, there is a lack of evidence behind the recommended specific dosages and their efficacy remains unclear [7,8,9,10,11,12,13].
CIPN’s pathogenesis is multifaceted, with immune cell-mediated neuroinflammation involving microglia, astrocytes, and macrophages playing a significant role [14,15,16]. A previous study suggested that a decrease in the local pH around neurons at CIPN’s onset is a contributing factor, as astrocyte activation can result in excessive lactic acid production [17]. In addition, allodynia may be induced by the excessive excitation of nerve cells.
PTX and oxaliplatin have been reported to decrease the velocity of the peripheral blood flow [18], suggesting that the peripheral neuropathy induced by these drugs contributes to sensory disturbances such as pain and numbness.
Numbness is an unpleasant sensation that can occur spontaneously or in response to external stimuli, often accompanied by pain, paresthesia, hypoesthesia, and a loss of sensation. Although the exact mechanism underlying numbness remains to be completely understood, ischemia–reperfusion resulting from blood flow occlusion and restoration is expected to be involved.
An inadequate oxygen supply, either due to insufficient blood flow or ischemia–reperfusion, can enhance the glycolytic pathway, thus decreasing the pH [19,20,21,22]. Therefore, the local pH may decrease at sites with inadequate blood flow. Such decreases in local pH may occur in the peripheral nerves at CIPN’s onset. However, the effectiveness of alkalization in CIPN remains unknown.
PTX can enhance the therapeutic effect of anticancer agents in pancreatic cancer [23], and the efficacy of alkalinization therapy in oncology has attracted attention. PTX is a standard anticancer drug used in the treatment of pancreatic cancer [24,25]. Thus, evaluating the effect of alkalinization on the development of PTX-induced CIPN is important.
Therefore, this study aimed to investigate the efficacy of alkalization in PTX-induced CIPN.
2. Results
2.1. Preventive Effect of Repeated Oral Administration of a Mixture of Potassium Citrate and Sodium Citrate (K/Na Citrate) on Mechanical Allodynia Induced by PTX in Mice
We investigated the potential preventive effects of the alkalinizing agent K/Na citrate on PTX-induced mechanical allodynia in mice. A single injection of PTX (5 mg/kg; intraperitoneal, i.p.) caused a significant increase in pain-related scores compared with those of sham mice from day 3 until day 16, which peaked on day 14 (Figure 1A, day 14, 0.18 ± 0.06, sham-vehicle group; 1.75 ± 0.07, PTX-vehicle group, p < 0.0001). The repeated administration of K/Na citrate (2 g/kg/day; per os, p.o.) significantly suppressed PTX-induced mechanical allodynia on days 3, 5, 7, 10, 12, 14, and 18 (Figure 1A, day 14, 0.69 ± 0.11, PTX-K/Na citrate group, p < 0.0001). This antiallodynic effect of K/Na citrate was found to be dose-dependent over the course of PTX-induced allodynia (Figure 1B). PTX injection or K/Na citrate administration did not alter the body weights of mice (Figure 1C). In addition, previous studies have demonstrated that K/Na citrate does not have a sedative effect [26]. Therefore, the observed analgesic effect of K/Na citrate is unlikely to be attributed to sedation or the body weight loss induced by its administration. Thus, in subsequent experiments, the dose of 2 g/kg/day, which most effectively suppressed mechanical allodynia, was employed.
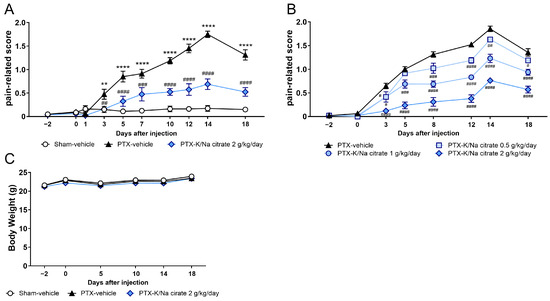
Figure 1.
Preventive effect of repeated K/Na citrate oral administration on PTX-induced mechanical allodynia in mice. (A,B) Time course of the pain-related scores in PTX-treated mice orally administered K/Na citrate or without K/Na citrate at doses of 2 g/kg/day (A) or at 0.5, 1, or 2 g/kg/day (B). (C) Body weights of mice from each group in (A). Data are presented as mean ± standard error of the mean (SEM) (n = 7–8). ** p < 0.01, **** p < 0.0001 vs. sham-vehicle. # p < 0.05, ## p < 0.01, ### p < 0.001, #### p < 0.0001 vs. PTX-vehicle (two-way repeated measures analysis of variance (ANOVA) with post hoc Holm–Sidak multiple comparison test).
2.2. Blood Bicarbonate (HCO3−) Concentrations and pH After the Administration of K/Na Citrate
We investigated whether K/Na citrate has an alkalinization effect in the PTX mouse model that is similar to that in naive mice. In naive mice, an increase in blood HCO3− concentration was observed after the oral administration of K/Na citrate, leading to a concomitant increase in blood pH. In particular, a single oral dose of K/Na citrate significantly increased the blood’s HCO3− concentration (Figure 2A, at 1.5, 3, and 5.5 h, p < 0.01) and pH (Figure 2B, 3 h, p < 0.05) in naive mice. In the PTX-induced CIPN mice, the repeated oral administration of K/Na citrate also increased blood HCO3− concentrations on day 14 at 2 h after the administration of K/Na citrate (Figure 2C, p < 0.05); however, it did not upregulate the blood pH significantly (Figure 2D).
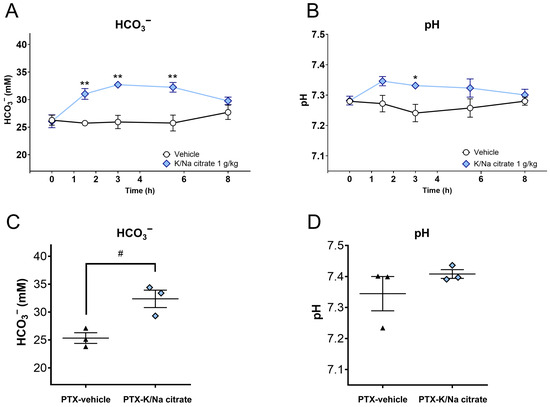
Figure 2.
Blood HCO3− concentrations and pH after oral administration of K/Na citrate. (A,B) Blood HCO3− concentrations (A) and blood pH (B) in healthy C57BL/6 mice after a single administration of K/Na citrate (1 g/kg, p.o.), measured at different time points post administration (0, 1.5, 3, 5.5, and 8 h). Data are presented as mean ± SEM (n = 3–4). * p < 0.05, ** p < 0.01 vs. vehicle (two-way ANOVA with post hoc Holm–Sidak multiple comparison test). (C,D) Blood HCO3− concentrations (C) and pH (D) in PTX-induced CIPN mice after repeated administration of K/Na citrate (2 g/kg/day, p.o.), measured on day 14 at 2 h post administration. Data are presented as mean ± SEM (n = 3). # p < 0.05 vs. PTX-vehicle (Student’s t-test).
2.3. Comparison of the Effect of Repeated Oral Administration of K/Na Citrate and Sodium Bicarbonate (NaHCO3) on PTX-Induced Mechanical Allodynia
The antiallodynic effect of K/Na citrate may be mediated through the upregulation of blood HCO3−. Therefore, we investigated the effect of NaHCO3 at a dose of 1.5 g/kg/day, which has an equivalent blood alkalizing effect to K/Na citrate at 2 g/kg/day, on PTX-induced mechanical allodynia as a comparative study. NaHCO3 also suppressed PTX-induced mechanical allodynia (Figure 3, day 14, 1.36 ± 0.11, PTX-NaHCO3 group; 1.73 ± 0.07, PTX-vehicle group, p < 0.05); however, the antiallodynic effect of NaHCO3 was significantly weaker than that of K/Na citrate (Figure 3, day 14, 0.71 ± 0.11, PTX-K/Na citrate group, p < 0.01).
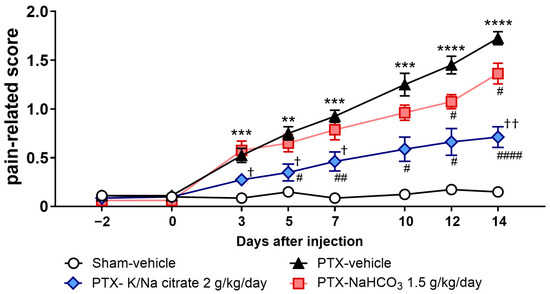
Figure 3.
Comparison of the effects of repeated oral administration of K/Na citrate and NaHCO3 on PTX-induced mechanical allodynia. Time course of the pain-related scores in PTX-treated mice administered K/Na citrate (2 g/kg/day, p.o.) or NaHCO3 (1.5 g/kg/day, p.o.) and those without either administered. Data are presented as mean ± SEM (n = 8). ** p < 0.01, *** p < 0.001, **** p < 0.0001 vs. sham-vehicle. # p < 0.05, ## p < 0.01, #### p < 0.0001 vs. PTX-vehicle. † p < 0.05, †† p < 0.01 vs. PTX NaHCO3 (two-way repeated measures ANOVA with post hoc Holm–Sidak multiple comparison test).
2.4. Effect of Repeated Oral Administration of K/Na Citrate on Spontaneous Firing and Von Frey Filament (vFF)-Evoked Firing Induced by PTX in Superficial Dorsal Horn Neurons
On day 13, the pain-related scores increased with the PTX treatment (Figure 4A, day 13, 1.64 ± 0.06, PTX-vehicle group; 0.14 ± 0.06, sham-vehicle group; p < 0.0001) and reduced with K/Na citrate administration (Figure 4A, day 13, 0.70 ± 0.05, PTX-K/Na citrate group, p < 0.0001), consistent with the abovementioned results. Spontaneous firing in superficial dorsal horn neurons was significantly elevated in PTX-treated mice but not in those treated with the vehicle only on day 14 (Figure 4B, day 14, 1.76 ± 0.20, PTX-vehicle group; 0.08 ± 0.04, sham-vehicle group, p < 0.0001). The repeated administration of K/Na citrate (2 g/kg/day, p.o.) significantly attenuated this effect by 80% (Figure 4B, day 14, 0.36 ± 0.07, PTX-K/Na citrate group, p < 0.0001). Mechanical stimuli-induced firing with the vFF elicited transient firing in the superficial dorsal horn neurons of vehicle-treated mice after filament detachment (Figure 4C, day 14, 2.80 ± 0.23). PTX-treated mice exhibited sustained and intense firing in their superficial dorsal horn neurons upon stimulation with mechanical stimuli (Figure 4C, day 14, 11.18 ± 0.58). Repeated administration of K/Na citrate (2 g/kg/day, p.o.) also suppressed the elevated mechanical stimuli-induced firing in PTX-treated mice by 57% (Figure 4C, day 14, 4.76 ± 0.40, p < 0.0001).
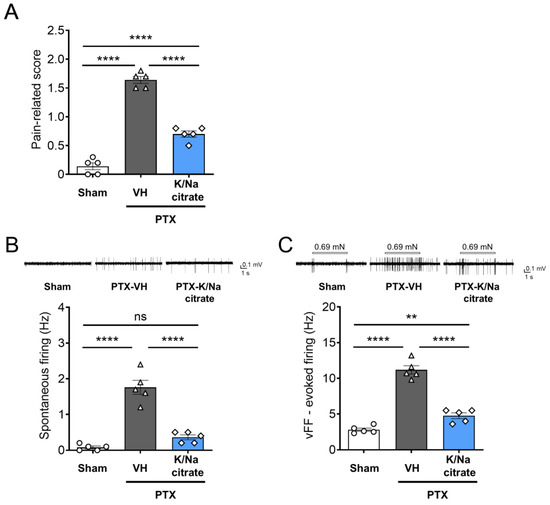
Figure 4.
Effect of repeated oral administration of K/Na citrate on spontaneous firing and vFF-evoked firing in superficial dorsal horn neurons in PTX-treated mice. (A) Pain-related scores in PTX-induced CIPN model mice with or without K/Na citrate treatment on day 13. (B,C) Representative traces and quantitative evaluation of spontaneous firing (B) and vFF-evoked firing (C) in superficial dorsal horn neurons in mice receiving each treatment. Data are presented as mean ± SEM (n = 5) (the total number of neurons was 25). ** p < 0.01, **** p < 0.0001 (one-way ANOVA with post hoc Holm–Sidak multiple comparison test).
2.5. The Preventive Effect of Repeated Oral Administration of K/Na Citrate on PTX-Induced Mechanical Allodynia and Plasma Citric Acid Levels in Rats
We investigated whether K/Na citrate could alleviate the allodynia induced by repeated PTX injection in rats. Mechanical allodynia was induced by administering PTX (2 mg/kg, i.p.) on four alternate days (days 0, 2, 4, and 6) in rats, resulting in a gradual decrease in the withdrawal threshold of the hind paw to mechanical stimulation on day 14 (5.18 ± 0.33 g, PTX-vehicle group; 11.71 ± 0.83 g, sham-vehicle group, p < 0.0001) and day 21 (4.18 ± 0.34 g, PTX-vehicle; 11.53 ± 0.81 g, sham-vehicle group, p < 0.0001) (Figure 5A). K/Na citrate (2 g/kg/day, p.o.) was administered twice daily from days −2 to 22, leading to a significant suppression of mechanical allodynia on days 14 (9.55 ± 0.98 g, PTX-K/Na citrate group, p < 0.05) and 21 (10.77 ± 0.86 g, PTX-K/Na citrate group, p < 0.0001) (Figure 5A). In the PTX-induced CIPN rats, the repeated administration of K/Na citrate significantly increased blood HCO3− concentrations (Figure 5B, 38.8 ± 0.7, PTX- K/Na citrate; 32.0 ± 0.5, PTX-vehicle group, p < 0.0001) and blood pH (Figure 5C, 7.48 ± 0.01, PTX- K/Na citrate group; 7.39 ± 0.01, PTX-vehicle group, p < 0.0001) on day 21 at 3 h post administration; this is consistent with the effects observed in PTX-induced CIPN mice.
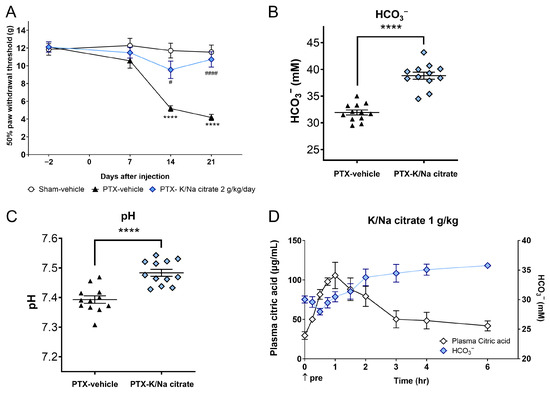
Figure 5.
Preventive effect of repeated oral administration of K/Na citrate on PTX-induced mechanical allodynia and plasma citric acid levels in rats. (A) Time course of paw withdrawal threshold in the PTX-induced CIPN rat model with or without K/Na citrate treatment (2 g/kg/day, p.o.). Data are presented as the mean ± SEM (n = 12). **** p < 0.0001 vs. sham-vehicle. # p < 0.05, #### p < 0.0001 vs. PTX-vehicle (two-way repeated measures ANOVA with post hoc Tukey–Kramer multiple comparison tests). (B,C) Blood HCO3− concentrations (B) and pH (C) in rats from each group in (A), measured on day 21 at 3 h after the administration of K/Na citrate. Data are presented as mean ± SEM (n = 12). **** p < 0.0001 vs. PTX-vehicle (Student’s t-test). (D) Changes in the plasma concentrations of citric acid and HCO3− in normal Wistar rats after administration of K/Na citrate (1 g/kg, p.o.) only. Data are presented as mean ± SEM (n = 3).
Furthermore, we assessed the impact of a single dose of K/Na citrate on plasma citrate and HCO3− levels. The plasma citrate level peaked 1 h after K/Na citrate administration and returned to baseline levels approximately 3 h after K/Na citrate administration. In contrast, the plasma HCO3− level started to increase 2 h after the administration and remained elevated up to 6 h later (Figure 5B).
2.6. Effect of Repeated Oral Administration of K/Na Citrate on Plasma Complement Levels
The activation of complement components C3a and C5a, which have been reported to be associated with PTX-induced peripheral neuropathy [27,28,29], was assessed in PTX-treated rats. The rats were given repeated injections of PTX (2 mg/kg, i.p.) or vehicle on four alternate days (days 0, 2, 4, and 6). K/Na citrate (2 g/kg/day, p.o.) or vehicle was administered twice daily from days −2 to 22. On day 22, the PTX injection led to a nonsignificant increase in plasma C3a levels (Figure 6A, 23.2 ± 3.2 ng/mL, sham-vehicle group; 31.7 ± 4.7 ng/mL, PTX-vehicle group, p = 0.0601). The prophylactic administration of K/Na citrate significantly decreased plasma C3a levels (Figure 6A, 21.9 ± 2.9 ng/mL, PTX-K/Na citrate group, p < 0.05). Plasma C5a levels were not affected by either the PTX or K/Na citrate treatment (Figure 6B, 491.5 ± 54.4 pg/mL, sham-vehicle group; 486.1 ± 23.1 pg/mL, PTX-vehicle group; 584.4 ± 54.6 pg/mL, PTX-K/Na citrate group).
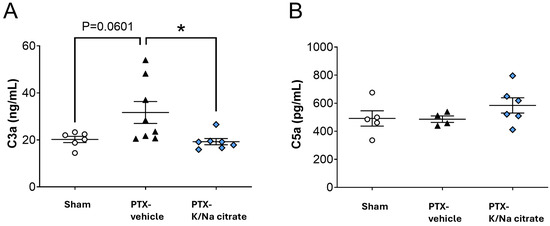
Figure 6.
Effect of repeated oral administration of K/Na citrate on the concentrations of complement component anaphylatoxins C3a and C5a in rat plasma. (A) C3a and (B) C5a rat plasma complement component levels on day 22 in rat PTX-treated model measured using an enzyme-linked immunosorbent assay (ELISA) kit. Data are presented as mean ± SEM (n = 4–8). * p < 0.05 (one-way ANOVA with post hoc Tukey–Kramer multiple comparison tests).
2.7. Na Citrate Prevented the Reduction in Neurite Outgrowth in PTX-Exposed Primary Dorsal Root Ganglion (DRG) Neurons
We investigated the direct effect of Na citrate on axonal integrity in primary cultured adult rat DRG neurons exposed to PTX; the 0.1 μM PTX treatment caused a 48% decrease in neurite outgrowth from DRG neurons compared with untreated neurons (Figure 7A,B, 804.7 ± 30.9 µm, PTX-treated group; 1534.3 ± 44.3 µm, untreated group, p < 0.0001). Pretreatment with Na citrate at different concentrations significantly prevented the reduction in the neurite outgrowth of PTX-treated DRG neurons (Figure 7A,B, 1022.2 ± 24.3, 1077.4 ± 28.0, and 1122.5 ± 24.6 µm for 0.25, 0.5, and 1 mM of Na citrate, respectively, p < 0.0001). This result indicates that Na citrate exerts a direct protective effect on DRG neurons stressed by PTX exposure.
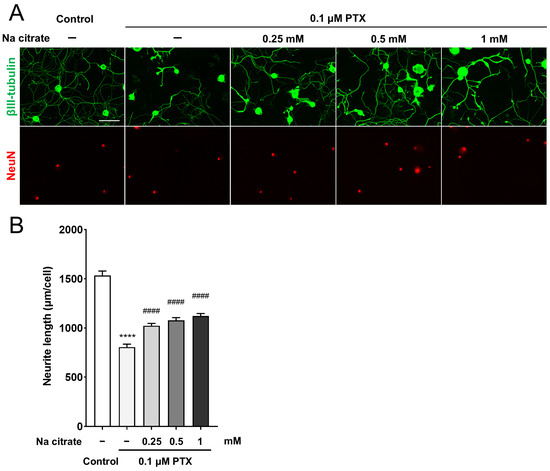
Figure 7.
Effect of Na citrate on neurite outgrowth of primary adult rat DRG neurons. (A) Representative images of βIII-tubulin (green) and NeuN (red) immunostaining of adult rat DRG neuron cultures exposed to 0.1 µM PTX for 24 h in the presence or absence of Na citrate. Scale bar: 100 µm. (B) Total neurite outgrowth in cultures with each treatment. Data are presented as mean ± SEM (n = 14 replicate cultures). **** p < 0.0001 vs. control, #### p < 0.0001 vs. 0.1 μM PTX (one-way ANOVA with post hoc Tukey–Kramer multiple comparison tests).
2.8. Therapeutic Effect of Repeated Oral Administration of K/Na Citrate on PTX-Induced Mechanical Allodynia
We also investigated the potential therapeutic effects of K/Na citrate on mechanical allodynia by administering K/Na citrate after the development of PTX-induced mechanical allodynia in mice. Mice were given a single injection of PTX (5 mg/kg, i.p.) or vehicle on day 0. K/Na citrate (2 g/kg/day, p.o.) or vehicle was administered twice daily from days 5 to 18. Consistent with previous findings, the PTX treatment led to an increase in the pain-related scores on day 5 (Figure 8, 0.9 ± 0.1, PTX-vehicle group; 0.0 ± 0.0, sham-vehicle group, p < 0.0001). When K/Na citrate administration commenced on day 5, the PTX-vehicle group showed a continued increase in their pain-related scores until day 14, whereas the PTX-K/Na citrate group maintained pain-related scores similar to those on day 5 (Figure 8, day 14, 1.8 ± 0.1, PTX-vehicle group; 0.7 ± 0.1, PTX-K/Na citrate group, p < 0.05).
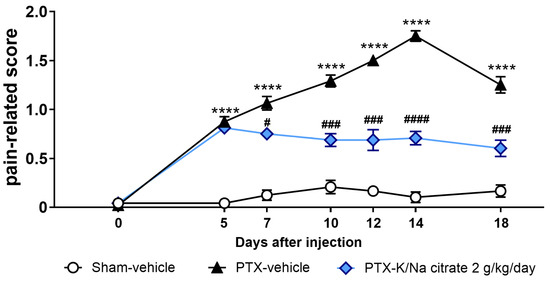
Figure 8.
Therapeutic effect of repeated oral administration of K/Na citrate on PTX-induced mechanical allodynia in mice. Time course of the pain-related scores in response to mechanical stimulus in PTX-treated mice. The mice were administered K/Na citrate from days 5 to 18. Data are presented as mean ± SEM (n = 8). **** p < 0.0001 vs. sham-vehicle. # p < 0.05, ### p < 0.001, #### p < 0.0001 vs. PTX-vehicle (two-way repeated measures ANOVA with post hoc Holm–Sidak multiple comparison test).
3. Discussion
In this study, the alkalization of blood or extracellular fluid was demonstrated to be effective in inhibiting PTX-induced peripheral neuropathy. Our study using murine and rat models revealed that the repeated prophylactic administration of K/Na citrate can prevent the development of PTX-induced mechanical allodynia (Figure 1 and Figure 5). Additionally, K/Na citrate was able to prevent the PTX-induced exacerbation of mechanical allodynia, even when treatment was started immediately after the onset of allodynia (Figure 8).
K/Na citrate, which contains Na and K citrate as its active ingredients, has been used in the treatment of metabolic acidosis [30]. Citrate is metabolized to HCO3− mainly in the liver after oral administration [31,32,33,34,35]. NaHCO3 provides HCO3− by dissociating into Na+ and HCO3− in the body. The HCO3− buffering system plays a crucial role in maintaining pH homeostasis in the bloodstream by regulating the balance of carbonic acid (H2CO3), HCO3−, and carbon dioxide (CO2), thereby contributing to the overall regulation of pH in the body.
In this study, K/Na citrate and NaHCO3 were used to alkalinize blood pH. Both compounds attenuated PTX-induced mechanical allodynia, with K/Na citrate exhibiting a stronger effect against mechanical allodynia (Figure 3). This suggests that mechanisms other than alkalinization may contribute to the observed effects. The distinct modes of action in the alkalinizing achieved by K/Na citrate and NaHCO3 imply that citrate, before metabolizing into HCO3−, may possess inherent properties that allow it to attenuate mechanical allodynia.
To better understand the mechanisms of CIPN improvement involved in this alkalinizing effect, we investigated its impact on the immune system. Studies have shown that the immune system and immune-mediated neuroinflammation play a critical role in the development of CIPN, including that induced by PTX [36,37,38,39,40]. Additionally, studies suggest that a complement, a key component of the innate immune system, is involved in the pathogenesis of PTX-induced CIPN [27,28,29,41].
The activation of complements through the classical, lectin, or alternative pathways leads to the activation of the complement components C3 and C5, which are central components of the complement system, resulting in the formation of a membrane attack complex that can cause direct cell damage or lysis.
K/Na citrate reduced plasma C3a levels in a PTX-induced CIPN rat model (Figure 6). It has been reported that C3a and C5a, produced through complement activation, can promote inflammation and potentially mediate neuropathic pain [42]. In our study, the plasma C3a levels in the PTX-induced CIPN rat model exhibited a tendency to increase, indicating an inflammatory response. However, treatment with K/Na citrate appeared to suppress this increase in C3a levels, indicating a potential mechanism for inhibiting the development of mechanical allodynia.
Complement activation is known to be influenced by pH, with both animal and clinical studies showing that hypoxia and reperfusion can lead to complement activation [43,44,45,46]. Furthermore, a low pH has been found to activate alternative pathways [47], and in acidotic and hypoxic conditions, the complement system is activated, resulting in the increased production of C3a and C5a [43,48]. The reduced peripheral blood flow velocity caused by PTX and neuroinflammation involving immune cells like microglia, astrocytes, and macrophages may cause a local pH reduction in peripheral nerves [14,15,16,18]. Therefore, we hypothesize that alkalization plays a role in inhibiting PTX-induced mechanical allodynia through K/Na citrate.
K/Na citrate is presumed to involve citrate’s alkalizing properties; hence, we focused on its potential neuroprotective effects. Previous studies have shown that PTX can induce neurotoxicity, resulting in demyelination, axonal degeneration, impaired axonal trafficking, direct injury to peripheral nerves, and a loss of nerve fibers [49,50,51,52]. Among these neurotoxic effects, a decrease in intraepidermal nerve fibers has been linked to the development of peripheral neuropathy [53]. In this study, we investigated the impact of Na citrate on neurite outgrowth in primary DRG cells as a measure of neurotoxicity. Our results revealed that pretreatment with Na citrate effectively prevented PTX-induced reductions in neurite outgrowth in a dose-dependent manner (Figure 7). These findings suggest that Na citrate helps mitigate the neurotoxic effects of PTX.
In this study, we recorded the activity of superficial dorsal horn neurons using extracellular electrophysiological recording methods. Previous findings indicated that mechanical stimulation increased the firing and spontaneous firing of superficial dorsal horn neurons in PTX-treated mice, resembling mechanical allodynia [54]. Interestingly, the prophylactic administration of K/Na citrate suppressed both mechanically stimulated firing and spontaneous firing (Figure 4). This indicates that inhibiting the input of abnormal stimuli into the dorsal horn of the spinal cord, which is the input site for the peripheral communication of information, may preserve the normal ascending transmission of pain sensory signals from the periphery through the inhibition of complement activity and the neuroprotective action of K/Na citrate.
Furthermore, we confirmed that the repeated administration of K/Na citrate does not inhibit the anticancer effects of PTX in pancreatic tumor-bearing mice (unpublished data). In addition, K/Na citrate was found to inhibit the exacerbation of CIPN even when administered at the early stage of pain onset (Figure 8). Therefore, the clinical application of K/Na citrate is expected to offer a valuable therapeutic approach that can manage the onset of CIPN without compromising the anticancer effects of PTX.
This study suggests that K/Na citrate is beneficial in preventing and alleviating PTX-induced peripheral neuropathy, potentially through its alkalinizing effect and citrate properties. However, the specific contributions of these mechanisms to CIPN improvement and their underlying mechanisms require further investigation.
In this study, we demonstrated that K/Na citrate can suppress the increase in plasma C3a concentration. However, the specific effects of Na/K citrate on the complement cascade and ion channels (such as transient receptor potential channels and Na+ channels) associated with the development of CIPN, which have been shown to be upregulated or enhanced by complement activation [28,55], are not fully understood. Further investigations are needed to elucidate these mechanisms. Additionally, our study revealed the protective effect of citrate against PTX neurotoxicity in vitro using primary DRG cells. Future studies are warranted to assess whether K/Na citrate can improve histopathological changes, such as demyelination and axonal degeneration, in animal models of PTX-induced neuropathy.
4. Materials and Methods
4.1. Animals
4.1.1. Mice
Male C57BL/6 mice weighing 20–26 g at 6 weeks of age were obtained from Japan SLC, Inc. (Shizuoka, Japan) and The Jackson Laboratory Japan, Inc. (Kanagawa, Japan). The mice were housed in a controlled environment with a temperature of 21–23 °C and humidity of 30–62%. The room was lit from 07:00 to 19:00, and food and water were provided ad libitum. In total, 146 mice were used: 112 for 4 behavioral studies (n = 8 per group), 15 for the electrophysiological study (n = 5 per group), and 19 for assessing blood HCO3− concentrations and pH (n = 3 or 4 per treatment). One mouse was excluded from the behavioral study because it died due to excessive fighting (Figure 1, PTX-Na/K citrate-2 g/kg/day group). All animal procedures were approved by the Committee for Animal Experiments at the University of Toyama.
4.1.2. Rats
Male Sprague–Dawley rats weighing 250–390 g at 7 weeks of age were obtained from The Jackson Laboratory Japan, Inc. (Kanagawa, Japan). The rats were housed in a controlled environment with a temperature of 22.7–24.1 °C and humidity of 45–65%. The room was lit from 07:00 to 19:00, and food and water were provided ad libitum. A total of 36 rats were used for the behavioral study followed by plasma sampling for analyzing complement components (n = 12 per group). All animal procedures were approved by the Committee for Animal Experiments at the Biological Science Center, KAC Co., Ltd. (Shiga, Japan).
Male Sprague–Dawley rats weighing 180–250 g at 5–7 weeks of age and male Wistar rats weighing 180–210 g at 6–7 weeks of age were obtained from The Jackson Laboratory Japan, Inc. (Kanagawa, Japan). The animals were housed in a controlled environment with a temperature of 21–25 °C and humidity of 40–70%. The room was lit from 07:00 to 19:00, and food and water were provided ad libitum. Two Sprague–Dawley rats were used for the primary culture of DRG neurons, and three Wistar rats were used for assessing plasma concentrations of citric acid and HCO3−. All animal procedures were approved by the Committee for Animal Experiments at Discovery Research Laboratories, Nippon Chemiphar Co., Ltd. (Saitama, Japan).
4.2. Test Compound
K/Na citrate (Uralyt-U Combination Powder, Nippon Chemiphar Co., Ltd., Tokyo, Japan), a mixture composed of 2 mol potassium citrate and 2 mol sodium citrate or NaHCO3 (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan), was dissolved in distilled water (Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan).
4.3. Animal Models
4.3.1. Mouse Study
PTX (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) was dissolved in a vehicle that consisted of saline (Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan) containing 10% (v/v) Kolliphor (C5135, Sigma-Aldrich, Inc., St. Louis, MO, USA) and 10% (v/v) ethanol. PTX was administered i.p. at a dose of 5 mg/kg [18] on day 0. K/Na citrate or NaHCO3 was administered p.o. twice daily at doses of 2 g/kg/day or 1.5 g/kg/day, respectively, starting from 2 days before PTX administration until the end of the study. However, in testing for the therapeutic effect of K/Na citrate on PTX-induced mechanical allodynia, K/Na citrate was administered p.o. at a dose of 2 g/kg/day twice daily from days 5 to 18.
4.3.2. Rat Study
Taxol (PTX, Bristol-Myers Squibb Company, Princeton, NJ, USA) was reconstituted in a saline vehicle. Taxol was administered i.p. at a dose of 2 mg/kg every other day (4 times), starting from day 0. K/Na citrate, at 2 g/kg/day, or vehicle was administered p.o. twice daily from days −2 to 22.
4.4. Behavioral Experiments
4.4.1. Mouse Study
Mice were individually placed in plastic cages (11 cm × 18 cm × 15 cm) with a wire mesh bottom and allowed to acclimatize for at least 30 min. Mechanical allodynia in the hind paw was assessed using a fine vFF with a bending force of 0.69 mN (North Coast Medical Inc., Morgan Hill, CA, USA) [54,56]. The vFF was pressed perpendicularly against the central part of the plantar hind paw and held for 1–3 s with slight pressure. Responses to the stimulus were scored as follows: no reaction (0), lifting of the hind paw (1), and licking and flinching of the hind paw (2). The stimulation was repeated three times on each hind paw with intervals of several seconds and at the same intensity, and the average value of six trials was used as the response score (maximum score of 2).
4.4.2. Rat Study
Rats were placed in individual cages. Mechanical allodynia in the hind paw was assessed using a fine vFF and bending forces of 0.4, 0.6, 1, 2, 4, 6, 8, and 15 g. The vFF was pressed perpendicularly against the central part of the plantar hind paw for 3–4 s, and the avoidance response (e.g., foot retraction) was recorded. The 50% paw withdrawal threshold was calculated using the up–down method [57].
4.5. Electrophysiological Recording
The mice were anesthetized with urethane (1.5 g/kg, i.p.), which provides long-lasting and steady anesthesia without the need for additional doses in most cases. A thoracolumbar laminectomy was performed to expose the L1–L6 vertebrae, after which the animal was placed in a stereotaxic apparatus. Subsequently, the dura mater was removed, and the arachnoid membrane was incised to create a large window for a tungsten microelectrode. The spinal cord surface was irrigated with Krebs solution equilibrated with 95% O2 and 5% CO2 at a flow rate of 10–15 mL/min, containing 117 mM NaCl, 3.6 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 1.2 mM NaH2PO4, 11 mM glucose, and 25 mM NaHCO3 at 37 ± 1 °C. Extracellular single-unit recordings of superficial dorsal horn (lamina I and II) neurons were conducted as previously described [58,59,60,61] at a depth of 20–150 μm from the surface. The recordings were obtained from the superficial dorsal horn neurons in slices from the same spinal level of same-age mice. Unit signals were amplified using an EX1 amplifier (Dagan Corporation, Minneapolis, MN, USA), digitized with an analog-to-digital converter (Digidata 1400A, Molecular Devices, LLC., San Jose, CA, USA), and stored on a personal computer using a data acquisition program (Clampex software, version 10.2, Molecular Devices, LLC., San Jose, CA, USA). The skin areas where touch (with a cotton wisp) or noxious pinch (with forceps) stimuli elicited a neural response were identified. Mechanical stimuli were applied to the skin folding of the ipsilateral hind limb using a fine vFF with a bending force of 0.69 mN for 5 s at the maximal response point of each mouse’s respective receptive area.
4.6. Measurement of Plasma Citric Acid, Blood HCO3−, and pH
C57BL/6 mice were orally administered K/Na citrate (1 g/kg) under non-fasting conditions. Blood samples were collected from the tail vein of the mice at predose, 1.5, 3, 5.5, and 8 h after oral administration. In PTX-treated mice, blood samples were collected from the tail vein of the mice 2 h after administering K/Na citrate on day 14 after PTX treatment.
Wistar rats were orally administered K/Na citrate (1 g/kg) under fasting conditions. Blood samples were collected from the tail vein of the rats at predose, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, and 6 h after oral administration. In the PTX-induced CIPN rat models, blood samples were collected from the tail vein of the rat 3 h after administering K/Na citrate on day 21 after PTX treatment.
In the above experiment, blood samples were drawn using lithium heparin as an anticoagulant. Blood pH and HCO3− concentrations were measured using the i-STAT 1 analyzer (Abbott Laboratories, Abbott Park, IL, USA). Plasma samples were obtained by centrifugation of the blood (1500× g, 4 °C, 15 min) and were used for citric acid measurements using a Citrate Assay Kit (Sigma-Aldrich, Inc., St. Louis, MO, USA).
4.7. Preventive and Dose-Dependent Effect of K/Na Citrate on Mechanical Allodynia
In the mouse study, mechanical allodynia was induced by a single injection of PTX at 5 mg/kg. K/Na citrate was administered p.o. at doses of 0.25, 0.5, and 1 g/kg twice daily, starting 2 days before PTX injection until the end of the study. Blood HCO3− levels were measured 2 h after K/Na citrate administration on day 14 after PTX treatment.
In the rat study, mechanical allodynia was induced by repeated injections of PTX (2 mg/kg, i.p.) on four alternate days (days 0, 2, 4, and 6). K/Na citrate was administered p.o. at a dose of 1 g/kg twice daily, starting 2 days before the first PTX injection until the end of the study.
4.8. Therapeutic Effect of K/Na Citrate on Mechanical Allodynia in PTX-Treated Mice
PTX-induced mechanical allodynia was established using the abovementioned method (see Section 4.4.1). K/Na citrate was administered p.o. at a dose of 1 g/kg twice daily starting from 5 days after the PTX injection until the end of the study.
4.9. Effect of Repeated Oral Administration of K/Na Citrate on the Concentrations of Complement Components, Anaphylatoxins C3a and C5a, in Rat Plasma
Rats were given four injections of PTX (2 mg/kg, i.p., every other day) or vehicle on day 0. K/Na citrate (2 g/kg/day, p.o.) or vehicle was administered twice daily from days −2 to 22. Plasma samples were collected from the rats’ jugular veins using lithium heparin as an anticoagulant on day 22. Plasma C3a and C5a levels were measured using the Rat Complement Fragment 3a (C3a) ELISA Kit (CSB-E08510r, Cusabio Technology LLC, Houston, TX, USA) and the Rat Complement C5a ELISA Kit (LS-F34383-1, Lifespan Biosciences, Inc., Seattle, WA, USA), respectively, following the manufacturer’s instructions.
4.10. Assessment of Neurite Outgrowth in Primary Adult Rat DRG Cultures
Based on previous studies [62,63,64], DRG neurons from 5–7-week-old Sprague–Dawley rats were dissociated. Briefly, bilateral thoracic and lumbar DRG were isolated and treated with 1.25 mg/mL of collagenase A (10103578001, Roche, Mannheim, Germany) and 2.5 mg/mL of dispase II (D4693, Sigma-Aldrich, Inc., St. Louis, MO, USA) in Hank’s Balanced Salt Solution without calcium and magnesium (14175095, Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 90 min. Then, they were treated with 0.25% trypsin-ethylenediaminetetraacetic acid (25200056, Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 3 min. The digest was neutralized using Dulbecco’s Modified Eagle Medium (11885084, Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (S1600, Biowest, Nuaillé, France) and 0.1% penicillin–streptomycin (15140122, Thermo Fisher Scientific, Inc., Waltham, MA, USA) and mechanically dissociated using a pipette and 23- and 28-gauge needles. The resulting cell suspension was layered on a 30%/60% Percoll (17089102, Global Life Sciences Technologies Japan K.K., Tokyo, Japan) gradient and centrifuged for 15 min at 1000× g at 4 °C, and the cell fraction between the 30% and 60% interface was collected. The pellet was resuspended into Neurobasal A medium (10888022, Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 2% B-27 supplement without antioxidants (10889038, Thermo Fisher Scientific, Inc., Waltham, MA, USA), GlutaMAX supplement (35050061, Thermo Fisher Scientific, Inc., Waltham, MA, USA), 0.1% penicillin–streptomycin, 0.1 ng/mL nerve growth factor (13257-019, Thermo Fisher Scientific, Inc.), 1 ng/mL glial cell line-derived neurotrophic factor, and 2’-deoxy-5-fluorouridine (D2235, Tokyo Chemical Industry Co., Ltd., Tokyo, Japan). The cells were plated on 96-well glass-bottom plates which were previously coated with 0.1 mg/mL poly-D-lysine (P6407, Sigma-Aldrich, Inc., St. Louis, MO, USA) and 10 µg/mL laminin (L2020, Sigma-Aldrich, Inc., St. Louis, MO, USA) at a density of 1000 neurons per well. The following day, the cells were pretreated with Na citrate for 4 h and exposed to PTX (0.1 μM) for 24 h. After treatment, the cells were fixed with ice-cold methanol for 15 min at −30 °C, washed thrice with phosphate-buffered saline (05913, Shimazu Corporation, Tokyo, Japan), blocked with 1% bovine serum albumin (B4287, Sigma-Aldrich, Inc., St. Louis, MO, USA) for 1 h at room temperature, immunostained with anti-βIII-tubulin antibody (1:2000, G712A, Promega Corporation, Madison, WI, USA) and anti-NeuN antibody (1:800, 24307, Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at 4 °C, and exposed to Alexa Fluor 488-conjugated antimouse IgG (1:1000, A-11001, Thermo Fisher Scientific, Inc., Waltham, MA, USA) and Alexa Fluor 594-conjugated anti-rabbit IgG (1:1000, A-11012, Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 1 h at room temperature. Images were captured using a BZ-X810 microscope (Keyence Corporation, Osaka, Japan) with an original image size of 960 × 720 pixels and cropped to 672 × 503 pixels using a BZ-X800 Image Converter 1.1.2.4 software (Keyence Corporation, Osaka, Japan) to trim overlapping parts. Images for βIII-tubulin were subjected to haze reduction using the BZ-X800 analyzer 1.1.2.4 software (Keyence Corporation, Osaka, Japan). The total neurite length, in pixels, was quantified using ImageJ 1.54f software (National Institute of Health, Bethesda, MD, USA) with the NeurphologyJ plugin [65]. Soma detection was performed using ImageJ 1.54f software on NeuN images. The total neurite length in pixels was converted to that in µm and adjusted for the total soma count.
4.11. Statistical Analysis
Statistical analyses were conducted using GraphPad Prism 10.3.0 software (GraphPad Software, Inc., San Diego, CA, USA). Data were presented as the mean and SEM. Statistical significance was assessed using Student’s t-test (Figure 2C,D and Figure 5B,D) or a one-way ANOVA followed by a post hoc Holm–Sidak test (Figure 4A–C) or Tukey–Kramer test (Figure 6A,B, and Figure 7) or two-way repeated measures ANOVA followed by a post hoc Holm–Sidak test (Figure 1A–C, Figure 2A,B, Figure 3 and Figure 8) or Tukey–Kramer test (Figure 5A). A p-value of less than 0.05 was considered statistically significant. Outliers were identified and eliminated using the Smirnov–Grubbs test (Figure 6A,B), a method for outlier detection that assumes a normal distribution of the data.
5. Conclusions
Our findings suggest that K/Na citrate may be useful in the prevention and treatment of PTX-induced peripheral neuropathy.
Author Contributions
Conceptualization, D.U. and H.N.; methodology, D.U. and H.N.; software, D.U. and H.N.; validation, D.U. and H.N.; formal analysis, D.U. and H.N.; investigation, D.U., H.N., K.M. (Kengo Maruo), K.M. (Kanoko Matsumura) and Y.U.; resources, D.U. and H.N.; data curation, D.U., H.N., K.M. (Kengo Maruo) and K.M. (Kanoko Matsumura); writing—original draft preparation, D.U. and H.N.; writing—review and editing, D.U., H.N. and T.K.; visualization, D.U. and H.N.; supervision, D.U.; project administration, D.U.; funding acquisition, D.U. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI, who provided grants to Daisuke Uta, grant numbers 19K09323 and 22K09020, and in part by Nippon Chemiphar Co., Ltd., Tokyo, Japan (Daisuke Uta).
Institutional Review Board Statement
This study did not use human-derived experimental materials. The animal study was approved by the Institutional Animal Care and Use Committee of the University of Toyama (Approval nos. A2020PHA-13, approved on 20 April 2020; A2020PHA-18, approved on 2 Jun 2020; and A2023PHA-13, approved on 31 January 2023) and Nippon Chemiphar Co., Ltd. (Approval nos. 21-07, approved on 28 Jun 2021; 21-20, approved on 2 December 2021; 22-24, approved on 6 October 2022; 22-31, approved on 10 March 2023; and 23-02, approved on 21 April 2023) and conducted in strict accordance with the guidelines of each institution. All efforts were made to minimize animal suffering and the number of animals used in the studies.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are grateful to Karin Yamada for her help with animal care.
Conflicts of Interest
Daisuke Uta was funded by Nippon Chemiphar Co., Ltd. Hideki Nakamura, Kengo Maruo, Kanoko Matsumura, and Yohei Usami are employees of Nippon Chemiphar Co., Ltd. Toshiaki Kume has no conflicts of interest to declare.
Abbreviations
The following abbreviations are used in this manuscript:
| ANOVA | Analysis of variance |
| CIPN | Chemotherapy-induced peripheral neuropathy |
| DRG | Dorsal root ganglion |
| ELISA | Enzyme-linked immunosorbent assay |
| i.p. | Intraperitoneal |
| p.o. | Per os |
| SEM | Standard error of the mean |
| vFF | von Frey filament |
References
- Badros, A.; Goloubeva, O.; Dalal, J.S.; Can, I.; Thompson, J.; Rapoport, A.P.; Heyman, M.; Akpek, G.; Fenton, R.G. Neurotoxicity of bortezomib therapy in multiple myeloma: A single-center experience and review of the literature. Cancer 2007, 110, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Quasthoff, S.; Hartung, H. Chemotherapy-induced peripheral neuropathy. J. Neurol. 2002, 249, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Barton, D.; Kottschade, L.; Grothey, A.; Loprinzi, C. Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur. J. Cancer 2008, 44, 1507–1515. [Google Scholar] [CrossRef]
- Kanbayashi, Y.; Hosokawa, T.; Okamoto, K.; Konishi, H.; Otsuji, E.; Yoshikawa, T.; Takagi, T.; Taniwaki, M. Statistical identification of predictors for peripheral neuropathy associated with administration of bortezomib, taxanes, oxaliplatin or vincristine using ordered logistic regression analysis. Anticancer Drugs 2010, 21, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Alberti, P.; Frigeni, B.; Piatti, M.; Susani, E. Chemotherapy-induced neuropathy. Curr. Treat. Options Neurol. 2011, 13, 180–190. [Google Scholar] [CrossRef]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Qin, R.; Dakhil, S.R.; Fehrenbacher, L.; Flynn, K.A.; Atherton, P.; Seisler, D.; Qamar, R.; Lewis, G.C.; Grothey, A. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). J. Clin. Oncol. 2014, 32, 997–1005. [Google Scholar] [CrossRef]
- Oki, E.; Emi, Y.; Kojima, H.; Higashijima, J.; Kato, T.; Miyake, Y.; Kon, M.; Ogata, Y.; Takahashi, K.; Ishida, H.; et al. Preventive effect of Goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): A placebo-controlled, double-blind, randomized phase III study. Int. J. Clin. Oncol. 2015, 20, 767–775. [Google Scholar] [CrossRef]
- Hirayama, Y.; Ishitani, K.; Sato, Y.; Iyama, S.; Takada, K.; Murase, K.; Kuroda, H.; Nagamachi, Y.; Konuma, Y.; Fujimi, A.; et al. Effect of duloxetine in Japanese patients with chemotherapy-induced peripheral neuropathy: A pilot randomized trial. Int. J. Clin. Oncol. 2015, 20, 866–871. [Google Scholar] [CrossRef]
- Vo, T.; Rice, A.S.C.; Dworkin, R.H. Nonsteroidal anti-inflammatory drugs for neuropathic pain: How do we explain continued widespread use? Pain 2009, 143, 169–171. [Google Scholar] [CrossRef]
- Takenaka, M.; Iida, H.; Matsumoto, S.; Yamaguchi, S.; Yoshimura, N.; Miyamoto, M. Successful treatment by adding duloxetine to pregabalin for peripheral neuropathy induced by paclitaxel. Am. J. Hosp. Palliat. Care 2013, 30, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.D.; Michalak, J.C.; Sloan, J.A.; Loprinzi, C.L.; Soori, G.S.; Nikcevich, D.A.; Warner, D.O.; Novotny, P.; Kutteh, L.A.; Wong, G.Y.; et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: A phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer 2007, 110, 2110–2118. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, M.; Ooshiro, M.; Moriyama, A.; Sugishita, Y.; Kadoya, K.; Sato, A.; Kitahara, T.; Takagi, R.; Urita, T.; Yoshida, Y.; et al. Efficacy and tolerability of controlled-release oxycodone for oxaliplatin-induced peripheral neuropathy and the extension of FOLFOX therapy in advanced colorectal cancer patients. Support. Care Cancer 2014, 22, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yoon, S.Y.; Zhang, H.; Dougherty, P.M. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J. Pain 2012, 13, 293–303. [Google Scholar] [CrossRef]
- Ruiz-Medina, J.; Baulies, A.; Bura, S.A.; Valverde, O. Paclitaxel-induced neuropathic pain is age dependent and devolves on glial response. Eur. J. Pain 2013, 17, 75–85. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; de Carvalho-Barbosa, M.; Kavelaars, A.; Heijnen, C.J.; Albrecht, P.J.; Dougherty, P.M. Dorsal root ganglion infiltration by macrophages contributes to paclitaxel chemotherapy-induced peripheral neuropathy. J. Pain 2016, 17, 775–786. [Google Scholar] [CrossRef]
- Miyamoto, K.; Ishikura, K.I.; Kume, K.; Ohsawa, M. Astrocyte-neuron lactate shuttle sensitizes nociceptive transmission in the spinal cord. Glia 2019, 67, 27–36. [Google Scholar] [CrossRef]
- Gauchan, P.; Andoh, T.; Kato, A.; Sasaki, A.; Kuraishi, Y. Effects of the prostaglandin E1 analog limaprost on mechanical allodynia caused by chemotherapeutic agents in mice. J. Pharmacol. Sci. 2009, 109, 469–472. [Google Scholar] [CrossRef]
- Reeh, P.W.; Steen, K.H. Tissue acidosis in nociception and pain. Prog. Brain Res. 1996, 113, 143–151. [Google Scholar] [CrossRef]
- Heming, T.A.; Davé, S.K.; Tuazon, D.M.; Chopra, A.K.; Peterson, J.W.; Bidani, A. Effects of extracellular pH on tumour necrosis factor-α production by resident alveolar macrophages. Clin. Sci. 2001, 101, 267–274. [Google Scholar] [CrossRef]
- Opie, L.H.; Owen, P.; Riemersma, R.A. Relative rates of oxidation of glucose and free fatty acids by ischaemic and nonischaemic myocardium after coronary artery ligation in the dog. Eur. J. Clin. Investig. 1973, 3, 419–435. [Google Scholar] [CrossRef]
- Schwaiger, M.; Neese, R.A.; Araujo, L.; Wyns, W.; Wisneski, J.A.; Sochor, H.; Swank, S.; Kulber, D.; Selin, C.; Phelps, M.; et al. Sustained nonoxidative glucose utilization and depletion of glycogen in reperfused canine myocardium. J. Am. Coll. Cardiol. 1989, 13, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Eshima, K.; Ishida, T. Neutralization of acidic tumor microenvironment (tme) with daily oral dosing of sodium potassium citrate (K/Na citrate) increases therapeutic effect of anti-cancer agent in pancreatic cancer xenograft mice model. Biol. Pharm. Bull. 2021, 44, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Renschler, M.F.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Ueno, H.; Ikeda, M.; Ueno, M.; Mizuno, N.; Ioka, T.; Omuro, Y.; Nakajima, E.T.; Furuse, J. Phase I/II study of nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother. Pharmacol. 2016, 77, 595–603. [Google Scholar] [CrossRef]
- Mizuta, T.; Miyake, N.; Shika, K. General pharmacological study of citrate citric acid combination (Uralyt-U®). Oyo Yakuri Pharmacometr. 1981, 21, 715–730. [Google Scholar]
- Cristiano, C.; Giorgio, C.; Cocchiaro, P.; Boccella, S.; Cesta, M.C.; Castelli, V.; Liguori, F.M.; Cuozzo, M.R.; Brandolini, L.; Russo, R.; et al. Inhibition of C5aR1 as a promising approach to treat taxane-induced neuropathy. Cytokine 2023, 171, 156370. [Google Scholar] [CrossRef]
- Xu, J.; Huang, P.; Bie, B.; Dai, Y.; Ben-Salem, S.; Borjini, N.; Zhang, L.; Chen, J.; Olman, M.; Cheng, J.; et al. Complement receptor C3aR1 contributes to paclitaxel-induced peripheral neuropathic pain in mice and rats. J. Immunol. 2023, 211, 1736–1746. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Xie, M.; Li, Y.; Huang, P.; Saunders, T.L.; Fox, D.A.; Rosenquist, R.; Lin, F. Role of complement in a rat model of paclitaxel-induced peripheral neuropathy. J. Immunol. 2018, 200, 4094–4101. [Google Scholar] [CrossRef]
- Starke, A.; Corsenca, A.; Kohler, T.; Knubben, J.; Kraenzlin, M.; Uebelhart, D.; Wüthrich, R.P.; von Rechenberg, B.; Müller, R.; Ambühl, P.M. Correction of metabolic acidosis with potassium citrate in renal transplant patients and its effect on bone quality. Clin. J. Am. Soc. Nephrol. 2012, 7, 1461–1472. [Google Scholar] [CrossRef]
- McNaughton, L.; Cedaro, R. Sodium citrate ingestion and its effects on maximal anaerobic exercise of different durations. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 64, 36–41. [Google Scholar] [CrossRef]
- Remer, T. ACID-BASE IN RENAL FAILURE: Influence of diet on acid-base balance. Semin. Dial. 2000, 13, 221–226. [Google Scholar] [CrossRef]
- Sabboh, H.; Besson, C.; Tressol, J.C.; Rémésy, C.; Demigné, C. Excess casein in the diet is not the unique cause of low-grade metabolic acidosis: Role of a deficit in potassium citrate in a rat model. Ann. Nutr. Metab. 2006, 50, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kopel, J.J.; Bhutia, Y.D.; Sivaprakasam, S.; Ganapathy, V. Consequences of NaCT/SLC13A5/mINDY deficiency: Good versus evil, separated only by the blood-brain barrier. Biochem. J. 2021, 478, 463–486. [Google Scholar] [CrossRef] [PubMed]
- Mycielska, M.E.; Patel, A.; Rizaner, N.; Mazurek, M.P.; Keun, H.; Patel, A.; Ganapathy, V.; Djamgoz, M.B.A. Citrate transport and metabolism in mammalian cells: Prostate epithelial cells and prostate cancer. Bioessays 2009, 31, 10–20. [Google Scholar] [CrossRef]
- Krukowski, K.; Eijkelkamp, N.; Laumet, G.; Hack, C.E.; Li, Y.; Dougherty, P.M.; Heijnen, C.J.; Kavelaars, A. CD8+ T cells and endogenous IL-10 are required for resolution of chemotherapy-induced neuropathic pain. J. Neurosci. 2016, 36, 11074–11083. [Google Scholar] [CrossRef] [PubMed]
- Ledeboer, A.; Jekich, B.M.; Sloane, E.M.; Mahoney, J.H.; Langer, S.J.; Milligan, E.D.; Martin, D.; Maier, S.F.; Johnson, K.W.; Leinwand, L.A.; et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav. Immun. 2007, 21, 686–698. [Google Scholar] [CrossRef]
- Janes, K.; Wahlman, C.; Little, J.W.; Doyle, T.; Tosh, D.K.; Jacobson, K.A.; Salvemini, D. Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy. Brain Behav. Immun. 2015, 44, 91–99. [Google Scholar] [CrossRef]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008, 8, 59–73. [Google Scholar] [CrossRef]
- Makker, P.G.; Duffy, S.S.; Lees, J.G.; Perera, C.J.; Tonkin, R.S.; Butovsky, O.; Park, S.B.; Goldstein, D.; Moalem-Taylor, G. Characterisation of immune and neuroinflammatory changes associated with chemotherapy-induced peripheral neuropathy. PLOS ONE 2017, 12, e0170814. [Google Scholar] [CrossRef]
- Song, W.C.; Sarrias, M.R.; Lambris, J.D. Complement and innate immunity. Immunopharmacology 2000, 49, 187–198. [Google Scholar] [CrossRef]
- Jang, J.H.; Clark, D.J.; Li, X.; Yorek, M.S.; Usachev, Y.M.; Brennan, T.J. Nociceptive sensitization by complement C5a and C3a in mouse. Pain 2010, 148, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Lindsay, T.F.; Ortiz, F.; Yeh, C.G.; Hechtman, H.B.; Moore, F.D., Jr. Soluble complement receptor type 1 ameliorates the local and remote organ injury after intestinal ischemia-reperfusion in the rat. J. Immunol. 1992, 149, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Brus, F.; van Oeveren, W.; Okken, A.; Bambang, S.O. Activation of circulating polymorphonuclear leukocytes in preterm infants with severe idiopathic respiratory distress syndrome. Pediatr. Res. 1996, 39, 456–463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sonntag, J.; Wagner, M.H.; Strauss, E.; Obladen, M. Complement and contact activation in term neonates after fetal acidosis. Arch. Dis. Child. Fetal Neonatal Ed. 1998, 78, F125–F128. [Google Scholar] [CrossRef][Green Version]
- Horstick, G.; Heimann, A.; Götze, O.; Hafner, G.; Berg, O.; Böhmer, P.; Becker, P.; Darius, H.; Rupprecht, H.J.; Loos, M.; et al. Intracoronary application of C1 esterase inhibitor improves cardiac function and reduces myocardial necrosis in an experimental model of ischemia and reperfusion. Circulation 1997, 95, 701–708. [Google Scholar] [CrossRef]
- Morita, Y.; Ikeguchi, H.; Nakamura, J.; Hotta, N.; Yuzawa, Y.; Matsuo, S. Complement activation products in the urine from proteinuric patients. J. Am. Soc. Nephrol. 2000, 11, 700–707. [Google Scholar] [CrossRef]
- Emeis, M.; Sonntag, J.; Willam, C.; Strauss, E.; Walka, M.M.; Obladen, M. Acidosis activates complement system in vitro. Mediat. Inflamm. 1998, 7, 417–420. [Google Scholar] [CrossRef]
- Sahenk, Z.; Barohn, R.; New, P.; Mendell, J.R. Taxol neuropathy. Electrodiagnostic and sural nerve biopsy findings. Arch. Neurol. 1994, 51, 726–729. [Google Scholar] [CrossRef]
- Boehmerle, W.; Huehnchen, P.; Peruzzaro, S.; Balkaya, M.; Endres, M. Electrophysiological, behavioral and histological characterization of paclitaxel, cisplatin, vincristine and bortezomib-induced neuropathy in C57BL/6 mice. Sci. Rep. 2014, 4, 6370. [Google Scholar] [CrossRef]
- Siau, C.; Xiao, W.; Bennett, G.J. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: Loss of epidermal innervation and activation of Langerhans cells. Exp. Neurol. 2006, 201, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Gornstein, E.; Schwarz, T.L. The paradox of paclitaxel neurotoxicity: Mechanisms and unanswered questions. Neuropharmacology 2014, 76, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Boyette-Davis, J.; Xin, W.; Zhang, H.; Dougherty, P.M. Intraepidermal nerve fiber loss corresponds to the development of Taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain 2011, 152, 308–313. [Google Scholar] [CrossRef]
- Andoh, T.; Uta, D.; Kato, M.; Toume, K.; Komatsu, K.; Kuraishi, Y. Prophylactic administration of aucubin inhibits paclitaxel-induced mechanical allodynia via the inhibition of endoplasmic reticulum stress in peripheral Schwann cells. Biol. Pharm. Bull. 2017, 40, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Warwick, C.A.; Keyes, A.L.; Woodruff, T.M.; Usachev, Y.M. The complement cascade in the regulation of neuroinflammation, nociceptive sensitization, and pain. J. Biol. Chem. 2021, 297, 101085. [Google Scholar] [CrossRef]
- Andoh, T.; Fukutomi, D.; Uta, D.; Kuraishi, Y. Prophylactic repetitive treatment with the herbal medicine Kei-kyoh-zoh-soh-oh-shin-bu-toh attenuates oxaliplatin-induced mechanical allodynia by decreasing spinal astrocytes. Evid. Based Complement. Altern. Med. 2019, 2019, 4029694. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Ishikawa, T.; Uta, D.; Okuda, H.; Potapenko, I.; Hori, K.; Kume, T.; Ozaki, N. Combined experiments with in vivo fiber photometry and behavior tests can facilitate the measurement of neuronal activity in the primary somatosensory cortex and hyperalgesia in an inflammatory pain mice model. Biol. Pharm. Bull. 2024, 47, 591–599. [Google Scholar] [CrossRef]
- Uta, D.; Inami, Y.; Fukushima, M.; Kume, T. Light-touch-induced after discharge firing in the superficial spinal dorsal horn neurons in hairless mice with irritant contact dermatitis. Biol. Pharm. Bull. 2022, 45, 1678–1683. [Google Scholar] [CrossRef]
- Uta, D.; Tsuboshima, K.; Nishijo, H.; Mizumura, K.; Taguchi, T. Neuronal sensitization and synaptic facilitation in the superficial dorsal horn of a rat reserpine-induced pain model. Neuroscience 2021, 479, 125–139. [Google Scholar] [CrossRef]
- Uta, D.; Koga, K.; Furue, H.; Imoto, K.; Yoshimura, M. L-bupivacaine inhibition of nociceptive transmission in rat peripheral and dorsal horn neurons. Anesthesiology 2021, 134, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Perner, C.; Sokol, C.L. Protocol for dissection and culture of murine dorsal root ganglia neurons to study neuropeptide release. STAR Protoc. 2021, 2, 100333. [Google Scholar] [CrossRef] [PubMed]
- Mecklenburg, J.; Zou, Y.; Wangzhou, A.; Garcia, D.; Lai, Z.; Tumanov, A.V.; Dussor, G.; Price, T.J.; Akopian, A.N. Transcriptomic sex differences in sensory neuronal populations of mice. Sci. Rep. 2020, 10, 15278. [Google Scholar] [CrossRef]
- Calcutt, N.A.; Smith, D.R.; Frizzi, K.; Sabbir, M.G.; Chowdhury, S.K.; Mixcoatl-Zecuatl, T.; Saleh, A.; Muttalib, N.; Van der Ploeg, R.; Ochoa, J.; et al. Selective antagonism of muscarinic receptors is neuroprotective in peripheral neuropathy. J. Clin. Investig. 2017, 127, 608–622. [Google Scholar] [CrossRef]
- Ho, S.Y.; Chao, C.Y.; Huang, H.L.; Chiu, T.W.; Charoenkwan, P.; Hwang, E. NeurphologyJ: An automatic neuronal morphology quantification method and its application in pharmacological discovery. BMC Bioinform. 2011, 12, 230. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).