Lentinula edodes as a Source of Bioactive Compounds with Therapeutical Potential in Intestinal Inflammation and Colorectal Cancer
Abstract
1. Introduction
2. Lentinula edodes-Derived Bioactive Compounds
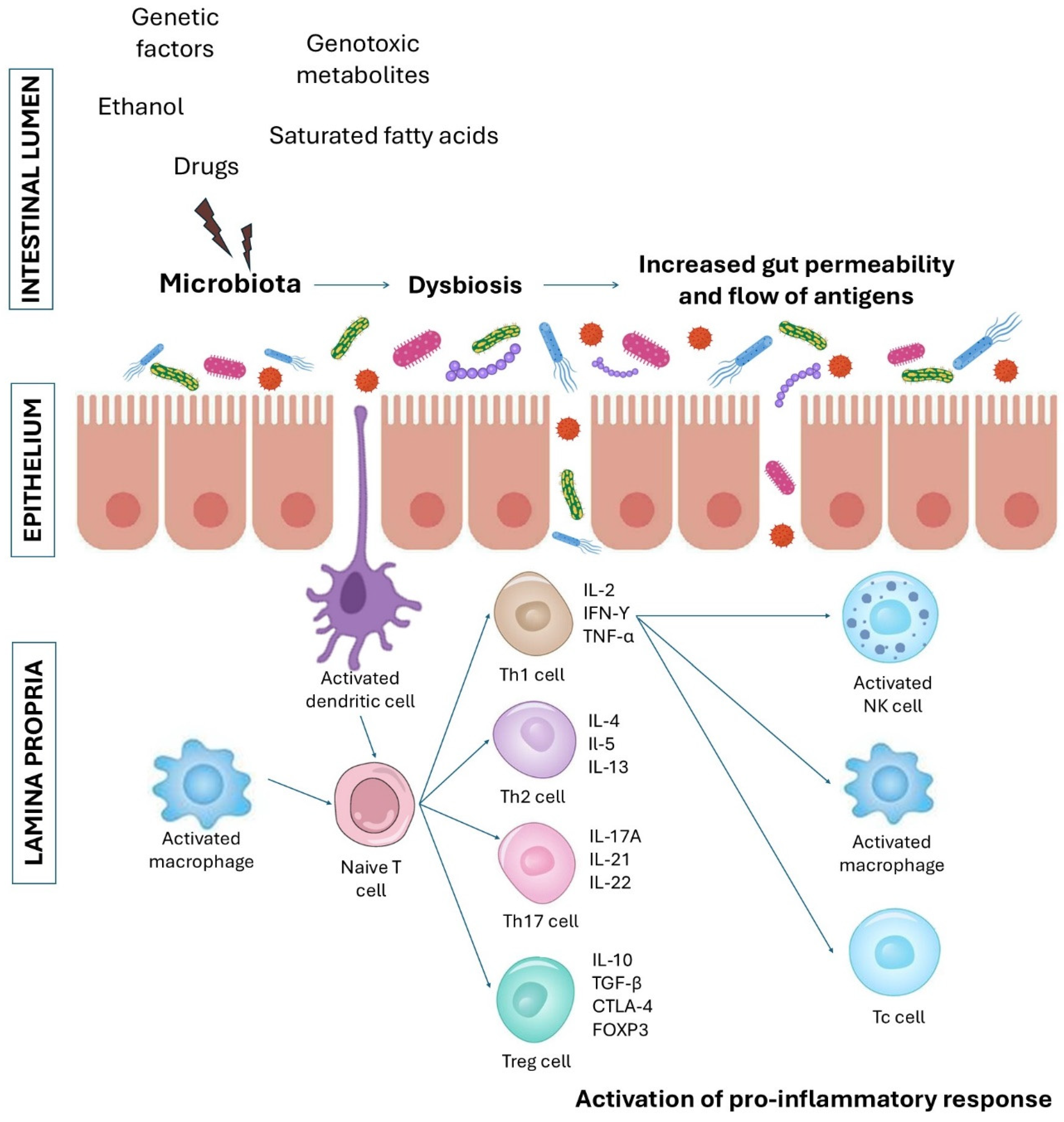
3. The Effects of L. edodes-Derived Bioactive Compounds on Intestinal Barrier and Gastrointestinal Diseases
3.1. Studies Performed on Cell Culture and Animal Models
3.2. Studies Performed in Humans
4. Discussion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AHCC | Active Hexose Correlated Compound |
| CAC | Colitis-associated cancer |
| CaSR | Calcium-sensing receptor |
| CD | Crohn’s disease |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| CRC | Colorectal cancer |
| DCs | Dendritic cells |
| DSS | Dextran sulfate sodium |
| EGFR | Epidermal growth factor receptor |
| FOXP3 | Forkhead box P3 |
| GIT | Gastrointestinal tract |
| IBD | Inflammatory bowel disease |
| ILCs | Innate lymphoid cells |
| IFN | Interferon |
| IL | Interleukin |
| LEM | Lentinula edodes mycelia extract |
| LNT | Lentinan |
| MPO | Myeloperoxidase |
| NFκB | Nuclear factor κB |
| NK | Natural killer |
| NO | Nitric oxide |
| ROS | Reactive oxygen species |
| SCID | Severe combined immunodeficiency |
| SeNPs | Selenonanoparticles |
| Tc | T cytotoxic cells |
| Th | T helper cells |
| Tm | T memory cells |
| TNF | Tumor necrosis factor |
| TGF | Transforming growth factor |
| UC | Ulcerative colitis |
| WT | Wild type |
| ZO | Zonula occludens |
References
- Matar, A.; Damianos, J.A.; Jencks, K.J.; Camilleri, M. Intestinal barrier impairment, preservation, and repair: An update. Nutrients 2024, 16, 3494. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal barrier in human health and disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- De Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut 2014, 63, 776–784. [Google Scholar] [CrossRef]
- Khalili, H.; Higuchi, L.M.; Ananthakrishnan, A.N.; Richter, J.M.; Feskanich, D.; Fuchs, C.S.; Chan, A.T. Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut 2013, 62, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Kvasnovsky, C.L.; Aujla, U.; Bjarnason, I. Nonsteroidal anti-inflammatory drugs and exacerbations of inflammatory bowel disease. Scand. J. Gastroenterol. 2015, 50, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B.; Wu, G.D. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology 2017, 152, 327–339.e4. [Google Scholar] [CrossRef] [PubMed]
- Pott, J.; Hornef, M. Innate immune signaling at the intestinal epithelium in homeostasis and disease. EMBO Rep. 2012, 13, 684–698. [Google Scholar] [CrossRef]
- Wallace, K.L.; Zheng, L.B.; Kanazawa, Y.; Shih, D.Q. Immunopathology of inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 6–21. [Google Scholar] [CrossRef]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- Holleran, G.; Lopetuso, L.; Petito, V.; Graziani, C.; Ianiro, G.; McNamara, D.; Gasbarrini, A.; Scaldaferri, F. The innate and adaptive immune system as targets for biologic therapies in inflammatory bowel disease. Int. J. Mol. Sci. 2017, 18, 2020. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, M.F.; Liang, Y.J.; Xu, J.; Xu, H.M.; Nie, Y.Q.; Wang, L.S.; Yao, J.; Li, D.F. Immunology of inflammatory bowel disease: Molecular mechanisms and therapeutics. J. Inflamm. Res. 2022, 15, 1825–1844. [Google Scholar] [CrossRef]
- Chen, L.; Ruan, G.; Cheng, Y.; Yi, A.; Chen, D.; Wei, Y. The role of Th17 cells in inflammatory bowel disease and the research progress. Front. Immunol. 2023, 13, 1055914. [Google Scholar] [CrossRef]
- Fanizza, J.; Bencardino, S.; Allocca, M.; Furfaro, F.; Zilli, A.; Parigi, T.L.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S.; D’Amico, F. Inflammatory bowel disease and colorectal cancer. Cancers 2024, 16, 2943. [Google Scholar] [CrossRef]
- Clarke, W.T.; Feuerstein, J.D. Colorectal cancer surveillance in inflammatory bowel disease: Practice guidelines and recent developments. World J. Gastroenterol. 2019, 25, 4148–4157. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Smith, S.C.; Gobalakrishnan, S.; McGinn, M.; Yakovlev, V.A.; Rabender, C.S. Uncoupled nitric oxide synthase activity promotes colorectal cancer progression. Front. Oncol. 2023, 13, 1165326. [Google Scholar] [CrossRef] [PubMed]
- Stidham, R.W.; Higgins, P.D.R. Colorectal cancer in inflammatory bowel disease. Clin. Colon Rectal Surg. 2018, 31, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Domínguez, A.; Pastor, N.; Martínez-López, L.; Colón-Pérez, J.; Bermúdez, B.; Orta, M.L. The role of DNA damage response in dysbiosis-induced colorectal cancer. Cells 2021, 10, 1934. [Google Scholar] [CrossRef]
- Muzammil, M.A.; Fariha, F.; Patel, T.; Sohail, R.; Kumar, M.; Khan, E.; Khanam, B.; Kumar, S.; Khatri, M.; Varrassi, G.; et al. Advancements in inflammatory bowel disease: A narrative review of diagnostics, management, epidemiology, prevalence, patient outcomes, quality of life, and clinical presentation. Cureus 2023, 15, e41120. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of inflammatory bowel disease: A comprehensive review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Ashton, J.J.; Beattie, R.M. Inflammatory bowel disease: Recent developments. Arch. Dis. Child. 2024, 109, 370–376. [Google Scholar] [CrossRef]
- Xu, Y.H.; Zhu, W.M.; Guo, Z. Current status of novel biologics and small molecule drugs in the individualized treatment of inflammatory bowel disease. World J. Gastroenterol. 2022, 28, 6888–6899. [Google Scholar] [CrossRef]
- Marmol, I.; Sanchez-De-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M. Colorectal carcinoma: A general overview and future perspectives in colorectal cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef]
- Saha, S.; Ghosh, S.; Ghosh, S.; Nandi, S.; Nayak, A. Unraveling the complexities of colorectal cancer and its promising therapies—An updated review. Int. Immunopharmacol. 2024, 143, 113325. [Google Scholar] [CrossRef]
- Kumar, A.; Gautam, V.; Sandhu, A.; Rawat, K.; Sharma, A.; Saha, L. Current and emerging therapeutic approaches for colorectal cancer: A comprehensive review. World J. Gastrointest. Surg. 2023, 15, 495–519. [Google Scholar] [CrossRef] [PubMed]
- Cornista, A.M.; Giolito, M.V.; Baker, K.; Hazime, H.; Dufait, I.; Datta, J.; Khumukcham, S.S.; De Ridder, M.; Roper, J.; Abreu, M.T.; et al. Colorectal cancer immunotherapy: State of the art and future directions. Gastro Hep Adv. 2023, 2, 1103–1119. [Google Scholar] [CrossRef] [PubMed]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal mushrooms: Their bioactive components, nutritional value and application in functional food production—A review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef]
- Spim, S.R.V.; Castanho, N.R.C.M.; Pistila, A.M.H.; Jozala, A.F.; Oliveira Júnior, J.M.; Grotto, D. Lentinula edodes mushroom as an ingredient to enhance the nutritional and functional properties of cereal bars. J. Food Sci. Technol. 2021, 58, 1349–1357. [Google Scholar] [CrossRef]
- Kaleta, B.; Górski, A.; Zagożdżon, R.; Cieślak, M.; Kaźmierczak-Barańska, J.; Nawrot, B.; Klimaszewska, M.; Malinowska, E.; Górska, S.; Turło, J. Selenium-containing polysaccharides from Lentinula edodes—Biological activity. Carbohydr. Polym. 2019, 223, 115078. [Google Scholar] [CrossRef]
- Kaleta, B.; Roszczyk, A.; Zych, M.; Kniotek, M.; Zagożdżon, R.; Klimaszewska, M.; Malinowska, E.; Pac, M.; Turło, J. Selective biological effects of selenium-enriched polysaccharide (Se-Le-30) isolated from Lentinula edodes mycelium on human immune cells. Biomolecules 2021, 11, 1777. [Google Scholar] [CrossRef]
- Roszczyk, A.; Zych, M.; Zielniok, K.; Krata, N.; Turło, J.; Klimaszewska, M.; Zagożdżon, R.; Kaleta, B. The effect of novel selenopolysaccharide isolated from Lentinula edodes mycelium on human T lymphocytes activation, proliferation, and cytokines synthesis. Biomolecules 2022, 12, 1900. [Google Scholar] [CrossRef]
- Kaleta, B.; Zielniok, K.; Roszczyk, A.; Turło, J.; Zagożdżon, R. Selenopolysaccharide isolated from Lentinula edodes mycelium affects human T-cell function. Int. J. Mol. Sci. 2024, 25, 11576. [Google Scholar] [CrossRef]
- Xu, X.; Yu, C.; Liu, Z.; Cui, X.; Guo, X.; Wang, H. Chemical composition, antioxidant and anti-inflammatory activity of Shiitake mushrooms (Lentinus edodes). J. Fungi 2024, 10, 552. [Google Scholar] [CrossRef]
- Rao, J.R.; Smyth, T.J.; Millar, B.C.; Moore, J.E. Antimicrobial properties of shiitake mushrooms (Lentinula edodes). Int. J. Antimicrob. Agents 2009, 33, 591–592. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Yan, W. Treatment effects of natural products on inflammatory bowel disease in vivo and their mechanisms: Based on animal experiments. Nutrients 2023, 15, 1031. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Fujii, G.; Hamoya, T.; Kurokawa, Y.; Matsuzawa, Y.; Miki, K.; Komiya, M.; Narita, T.; Mutoh, M. Activation of NF-E2 p45-related factor-2 transcription and inhibition of intestinal tumor development by AHCC, a standardized extract of cultured Lentinula edodes mycelia. J. Clin. Biochem. Nutr. 2019, 65, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Boo, S.; Park, I.; Shin, M.S.; Takahashi, T.; Takanari, J.; Homma, K.; Kang, I. AHCC®, a standardized extract of cultured Lentinula edodes mycelia, promotes the anti-tumor effect of dual immune checkpoint blockade effect in murine colon cancer. Front. Immunol. 2022, 13, 875872. [Google Scholar] [CrossRef]
- Doursout, M.F.; Liang, Y.; Sundaresan, A.; Wakame, K.; Fujii, H.; Takanari, J.; Devakottai, S.; Kulkarni, A. Active hexose correlated compound modulates LPS-induced hypotension and gut injury in rats. Int. Immunopharmacol. 2016, 39, 280–286. [Google Scholar] [CrossRef]
- You, C.; Xing, J.; Sun, J.; Zhang, D.; Yan, Y.; Dong, Y. Anti-inflammatory and anti-oxidant impacts of lentinan combined with probiotics in ulcerative colitis. Mol. Biotechnol. 2024, 66, 2778–2791. [Google Scholar] [CrossRef]
- Nishitani, Y.; Zhang, L.; Yoshida, M.; Azuma, T.; Kanazawa, K.; Hashimoto, T.; Mizuno, M. Intestinal anti-inflammatory activity of lentinan: Influence on IL-8 and TNFR1 expression in intestinal epithelial cells. PLoS ONE 2013, 8, e62441. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Shirai, Y.; Toshiki, I.; Mizuno, M. Lentinan exerts its anti-inflammatory activity by suppressing TNFR1 transfer to the surface of intestinal epithelial cells through Dectin-1 in an in vitro and mice model. Immunome Res. 2018, 14, 1000165. [Google Scholar] [CrossRef]
- Minato, K.I.; Oura, K.; Mizuno, M. The inhibitory effect of oral administration of lentinan on DSS-induced inflammation is exerted by the migration of T cells activated in the ileum to the colon. Eur. J. Pharmacol. 2023, 946, 175631. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, W.; Huang, X.; Liu, Y.; Li, Q.; Zheng, Z.; Wang, K. A polysaccharide from Lentinus edodes inhibits human colon cancer cell proliferation and suppresses tumor growth in athymic nude mice. Oncotarget 2017, 8, 610–623. [Google Scholar] [CrossRef]
- Aral, K.; Aral, C.A.; Kapila, Y. The role of caspase-8, caspase-9, and apoptosis inducing factor in periodontal disease. J. Periodontol. 2019, 90, 288–294. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhou, Y.; Zheng, Z.; Tang, W.; Song, M.; Wang, J.; Wang, K. Lentinan inhibited colon cancer growth by inducing endoplasmic reticulum stress-mediated autophagic cell death and apoptosis. Carbohydr. Polym. 2021, 267, 118154. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Su, L.; Zhang, P.; Yue, Q.; Zhao, C.; Sun, X.; Li, K.; Liu, X.; Zhang, S.; Zhao, L. Lentinan improves intestinal inflammation and gut dysbiosis in antibiotics-induced mice. Sci. Rep. 2022, 12, 19609. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Zhao, Y.; Zong, S.; Tian, Y.; Chen, S.; Li, M.; Liu, H.; Zhang, Q.; Jing, X.; et al. Therapeutic effects of lentinan on inflammatory bowel disease colitis-associated cancer. J. Cell. Mol. Med. 2019, 23, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Mody, K.; Baldeo, C.; Bekaii-Saab, T. Antiangiogenic therapy in colorectal cancer. Cancer J. 2018, 24, 165–170. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, G.; Kuai, J.; Fan, P.; Wang, X.; Zhou, P.; Yang, D.; Zheng, X.; Liu, X.; Wu, Q.; et al. Lentinan inhibits tumor angiogenesis via interferon γ and in a T cell independent manner. J. Exp. Clin. Cancer Res. 2018, 37, 260. [Google Scholar] [CrossRef]
- Connelly-Frost, A.; Poole, C.; Satia, J.A.; Kupper, L.L.; Millikan, R.C.; Sandler, R.S. Selenium, folate, and colon cancer. Nutr. Cancer 2009, 61, 165–178. [Google Scholar] [CrossRef]
- Zhang, J.; Teng, Z.; Yuan, Y.; Zeng, Q.Z.; Lou, Z.; Lee, S.H.; Wang, Q. Development, physicochemical characterization and cytotoxicity of selenium nanoparticles stabilized by beta-lactoglobulin. Int. J. Biol. Macromol. 2018, 107, 1406–1413. [Google Scholar] [CrossRef]
- Blinov, A.V.; Kostenko, K.V.; Gvozdenko, A.A.; Maglakelidze, D.G.; Golik, A.B.; Nagdalian, A.A.; Statsenko, E.N.; Nikulnikova, N.N.; Remizov, D.M.; Verevkina, M.N.; et al. Study of stabilization of selenium nanoparticles by polysaccharides. J. Hyg. Eng. Des. 2021, 34, 209–216. [Google Scholar]
- Gao, X.; Yao, Y.; Chen, X.; Lin, X.; Yang, X.; Ho, C.T.; Li, B.; Chen, Z. Lentinan-functionalized selenium nanoparticles induce apoptosis and cell cycle arrest in human colon carcinoma HCT-116 cells. Front. Nutr. 2022, 9, 987807. [Google Scholar] [CrossRef]
- Liu, G.; Ling, J.; He, L.; Xu, Y.; Chen, T.; Shi, C.; Luo, L. Theranostic cancer treatment using lentinan-coated selenium nanoparticles and label-free CEST MRI. Pharmaceutics 2022, 15, 120. [Google Scholar] [CrossRef]
- Lin, M.; Dong, L.; Chen, Q.; Xu, H.; Han, X.; Luo, R.; Pu, X.; Qi, S.; Nie, W.; Ma, M.; et al. Lentinan-based oral nanoparticle loaded budesonide with macrophage-targeting ability for treatment of ulcerative colitis. Front. Bioeng. Biotechnol. 2021, 9, 702173. [Google Scholar] [CrossRef]
- Liu, Y.R.; Sun, B.; Zhu, G.H.; Li, W.W.; Tian, Y.X.; Wang, L.M.; Zong, S.M.; Sheng, P.Z.; Li, M.; Chen, S.; et al. Selenium-lentinan inhibits tumor progression by regulating epithelial-mesenchymal transition. Toxicol. Appl. Pharmacol. 2018, 360, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Kornmann, M.; Traub, B. Role of epithelial to mesenchymal transition in colorectal cancer. Int. J. Mol. Sci. 2023, 24, 14815. [Google Scholar] [CrossRef]
- Ng, M.L.; Yap, A.T. Inhibition of human colon carcinoma development by lentinan from shiitake mushrooms (Lentinus edodes). J. Altern. Complement. Med. 2002, 8, 581–589. [Google Scholar] [CrossRef]
- Jeannin, J.F.; Lagadec, P.; Pelletier, H.; Reisser, D.; Olsson, N.O.; Chihara, G.; Martin, F. Regression induced by lentinan, of peritoneal carcinomatoses in a model of colon cancer in rat. Int. J. Immunopharmacol. 1988, 10, 855–861. [Google Scholar] [CrossRef]
- Iamartino, L.; Elajnaf, T.; Kallay, E.; Schepelmann, M. Calcium-sensing receptor in colorectal inflammation and cancer: Current insights and future perspectives. World J. Gastroenterol. 2018, 24, 4119–4131. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.; Singh, N.; Promkan, M.; Liu, G. Effects of potential calcium sensing receptor inducers on promoting chemosensitivity of human colon carcinoma cells. Int. J. Oncol. 2010, 36, 1573–1580. [Google Scholar] [CrossRef]
- Suga, Y.; Takehana, K. Lentinan diminishes apoptotic bodies in the ileal crypts associated with S-1 administration. Int. Immunopharmacol. 2017, 50, 55–60. [Google Scholar] [CrossRef]
- Mushiake, H.; Tsunoda, T.; Nukatsuka, M.; Shimao, K.; Fukushima, M.; Tahara, H. Dendritic cells might be one of key factors for eliciting antitumor effect by chemoimmunotherapy in vivo. Cancer Immunol. Immunother. 2005, 54, 120–128. [Google Scholar] [CrossRef]
- Mitamura, T.; Sakamoto, S.; Suzuki, S.; Yoshimura, S.; Maemura, M.; Kudo, H. Effects of lentinan on colorectal carcinogenesis in mice with ulcerative colitis. Oncol. Rep. 2000, 7, 599–601. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Khan, A.I.; Rehman, A.U.; Khinsar, K.H.; Xin, Y. Lentinan’s effect on gut microbiota and inflammatory cytokines in 5-FU-induced mucositis mice. AMB Express 2025, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zou, S.; Xie, C.; Meng, Y.; Xu, X. Effect of the β-glucan from Lentinus edodes on colitis-associated colorectal cancer and gut microbiota. Carbohydr. Polym. 2023, 316, 121069. [Google Scholar] [CrossRef]
- Mao, X.; Hu, H.; Xiao, X.; Chen, D.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, J.; Luo, Y.; et al. Lentinan administration relieves gut barrier dysfunction induced by rotavirus in a weaned piglet model. Food Funct. 2019, 10, 2094–2101. [Google Scholar] [CrossRef]
- Alagbaoso, C.A.; Mizuno, M. Lentinula edodes polysaccharides suppressed pro-inflammatory cytokines expression and colitis in mice. Arq. Gastroenterol. 2022, 59, 288–295. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushrooms in human clinical studies. part i. anticancer, oncoimmunological, and immunomodulatory activities: A review. Int. J. Med. Mushrooms 2017, 19, 279–317. [Google Scholar] [CrossRef]
- Tanaka, K.; Matsui, Y.; Ishikawa, S.; Kawanishi, T.; Harada, M. Oral ingestion of Lentinula edodes mycelia extract can restore the antitumor T cell response of mice inoculated with colon-26 cells into the subserosal space of the cecum. Oncol. Rep. 2012, 27, 325–332. [Google Scholar] [CrossRef][Green Version]
- Matsuhisa, K.; Yamane, S.; Okamoto, T.; Watari, A.; Kondoh, M.; Matsuura, Y.; Yagi, K. Anti-HCV effect of Lentinula edodes mycelia solid culture extracts and low-molecular-weight lignin. Biochem. Biophys. Res. Commun. 2015, 462, 52–57. [Google Scholar] [CrossRef]
- Matsuhisa, K.; Watari, A.; Iwamoto, K.; Kondoh, M.; Yagi, K. Lignosulfonic acid attenuates NF-κB activation and intestinal epithelial barrier dysfunction induced by TNF-α/IFN-γ in Caco-2 cells. J. Nat. Med. 2018, 72, 448–455. [Google Scholar] [CrossRef]
- Currò, D.; Ianiro, G.; Pecere, S.; Bibbò, S.; Cammarota, G. Probiotics, fiber and herbal medicinal products for functional and inflammatory bowel disorders. Br. J. Pharmacol. 2017, 174, 1426–1449. [Google Scholar] [CrossRef]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, probiotics, synbiotics, paraprobiotics and postbiotic compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef]
- Darb Emamie, A.; Rajabpour, M.; Ghanavati, R.; Asadolahi, P.; Farzi, S.; Sobouti, B.; Darbandi, A. The effects of probiotics, prebiotics and synbiotics on the reduction of IBD complications, a periodic review during 2009–2020. J. Appl. Microbiol. 2021, 130, 1823–1838. [Google Scholar] [CrossRef]
- Xue, Z.; Li, R.; Liu, J.; Zhou, J.; Zhang, X.; Zhang, T.; Zhang, M.; Yang, Y.; Chen, H. Preventive and synbiotic effects of the soluble dietary fiber obtained from Lentinula edodes byproducts and Lactobacillus plantarum LP90 against dextran sulfate sodium-induced colitis in mice. J. Sci. Food Agric. 2023, 103, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Gu, F.T.; Yang, Y.; Zhao, Z.C.; Huang, L.X.; Zhu, Y.Y.; Chen, S.; Wu, J.Y. Simulated human digestion and fermentation of a high-molecular weight polysaccharide from Lentinula edodes mushroom and protective effects on intestinal barrier. Carbohydr. Polym. 2024, 343, 122478. [Google Scholar] [CrossRef] [PubMed]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by β-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef] [PubMed]
- De Marco Castro, E.; Calder, P.C.; Roche, H.M. β-1,3/1,6-glucans and immunity: State of the art and future directions. Mol. Nutr. Food Res. 2021, 65, e1901071. [Google Scholar] [CrossRef]
- Morales, D.; Shetty, S.A.; López-Plaza, B.; Gómez-Candela, C.; Smidt, H.; Marín, F.R.; Soler-Rivas, C. Modulation of human intestinal microbiota in a clinical trial by consumption of a β-D-glucan-enriched extract obtained from Lentinula edodes. Eur. J. Nutr. 2021, 60, 3249–3265. [Google Scholar] [CrossRef]
- Gong, D.; Gong, X.; Wang, L.; Yu, X.; Dong, Q. Involvement of reduced microbial diversity in inflammatory bowel disease. Gastroenterol. Res. Pract. 2016, 2016, 6951091. [Google Scholar] [CrossRef]
- Wakui, A.; Kasai, M.; Konno, K.; Abe, R.; Kanamaru, R.; Takahashi, K.; Nakai, Y.; Yoshida, Y.; Koie, H.; Masuda, H.; et al. Randomized study of lentinan on patients with advanced gastric and colorectal cancer. Tohoku Lentinan Study Group. Gan Kagaku Ryoho 1986, 13, 1050–1059. (In Japanese) [Google Scholar]
- Satake, H.; Boku, S.; Mitani, S.; Maeda, K.; Kudo, T.; Gotoh, M.; Takagi, T.; Kawakami, H. A placebo-controlled study comparing doses and efficacy of Lentinula edodes mycelia for chemotherapy-induced peripheral neuropathy in colorectal cancer: The LEMON trial. Ann. Oncol. 2024, 35, S6–S7. [Google Scholar] [CrossRef]
- Okuno, K.; Uno, K. Efficacy of orally administered Lentinula edodes mycelia extract for advanced gastrointestinal cancer patients undergoing cancer chemotherapy: A pilot study. Asian Pac. J. Cancer Prev. 2011, 12, 1671–1674. [Google Scholar]
- Zi, Y.; Zhang, B.; Jiang, B.; Yang, X.; Liang, Z.; Liu, W.; He, C.; Liu, L. Antioxidant action and protective and reparative effects of lentinan on oxidative damage in HaCaT cells. J. Cosmet. Dermatol. 2018, 17, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Urushima, H.; Matsubara, T.; Qiongya, G.; Daikoku, A.; Takayama, M.; Kadono, C.; Nakai, H.; Ikeya, Y.; Yuasa, H.; Ikeda, K. AHCC inhibited hepatic stellate cells activation by regulation of cytoglobin induction via TLR2-SAPK/JNK pathway collagen production via TLR4-NF-κβ pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2024, 327, G741–G753. [Google Scholar] [CrossRef] [PubMed]
- Roszczyk, A.; Turło, J.; Zagożdżon, R.; Kaleta, B. Immunomodulatory properties of polysaccharides from Lentinula edodes. Int. J. Mol. Sci. 2022, 23, 8980. [Google Scholar] [CrossRef] [PubMed]

| L. edodes Compound | Results | References |
|---|---|---|
AHCC 1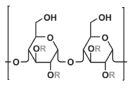 The active compound of AHCC: α-1,4-glucan (R:H or CH3CO-) |
| [42,43] |
| [44] | |
Lentinan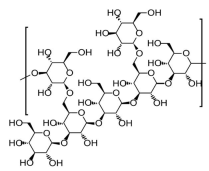 1-3;1-6-β-D-glucan |
| [45] |
| [46] | |
| [48] | |
| [49,51] | |
| [52] | |
| [53] | |
| [55] | |
| [59] | |
| [60] | |
| [61] | |
| [62] | |
| [64] | |
| [65] | |
| [67] | |
| [68] | |
| [69] | |
| [70] | |
| [71] | |
| [73] | |
| β-glucan from L. edodes degraded into three fractions |
| [72] |
| LeAP1 and LeAP2 (crude polysaccharide fractions extracted from L. edodes) |
| [74] |
| LEM (dried powder hot water extract from L. edodes mycelia) |
| [76] |
| MSCE (L. edodes mycelia solid culture extract) |
| [78] |
| Soluble dietary fiber (SDF) from L. edodes |
| [82] |
LePS40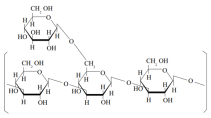 β-1,3-D-glucan with 1,6-linked glucose side chains |
| [83] |
| Treatment | Number of Participants and Condition | Results | Reference |
|---|---|---|---|
| β-D-glucan-enriched (BGE) mixture (containing 3.5 g of β-D-glucans) isolated from L. edodes consumed 10.4 g/day for 8 weeks | 52 patients with untreated mild hypercholesterolemia and dysbiosis | BGE administration increased microbial diversity | [86] |
| Lentinan (2 mg/week administered intravenously) in combination with mitomycin and 5-fluorouracil | 115 gastric cancer and 51 CRC 1 patients | Lentinan, in combination with chemotherapy, prolonged the survival time of gastric cancer and CRC patients | [87] |
| L. edodes mycelia extract (LEM) administered orally 300 mg twice a day (low dose) or 900 mg twice a day (high dose) for 12 weeks | 29 CRC patients with oxaliplatin-induced peripheral neuropathy | High-dose LEM administration reduced VAS numbness and pain and improved walking problems | [88] |
| LEM orally ingested during the second course of chemotherapy (5-fluorouracil), irinotecan, uracil and tegafur, levofolinate, mitomycin or taxol) at a dose of 1800 mg/day for 4 weeks | 7 CRC patients and one gastric cancer patient | LEM ingestion reduced adverse effects of chemotherapy and increased IFN-γ production by T CD4+, T CD8+ and NK/NKT CD56+ cells | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugajewski, M.; Angerhoefer, N.; Pączek, L.; Kaleta, B. Lentinula edodes as a Source of Bioactive Compounds with Therapeutical Potential in Intestinal Inflammation and Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 3320. https://doi.org/10.3390/ijms26073320
Bugajewski M, Angerhoefer N, Pączek L, Kaleta B. Lentinula edodes as a Source of Bioactive Compounds with Therapeutical Potential in Intestinal Inflammation and Colorectal Cancer. International Journal of Molecular Sciences. 2025; 26(7):3320. https://doi.org/10.3390/ijms26073320
Chicago/Turabian StyleBugajewski, Mikołaj, Norbert Angerhoefer, Leszek Pączek, and Beata Kaleta. 2025. "Lentinula edodes as a Source of Bioactive Compounds with Therapeutical Potential in Intestinal Inflammation and Colorectal Cancer" International Journal of Molecular Sciences 26, no. 7: 3320. https://doi.org/10.3390/ijms26073320
APA StyleBugajewski, M., Angerhoefer, N., Pączek, L., & Kaleta, B. (2025). Lentinula edodes as a Source of Bioactive Compounds with Therapeutical Potential in Intestinal Inflammation and Colorectal Cancer. International Journal of Molecular Sciences, 26(7), 3320. https://doi.org/10.3390/ijms26073320






