Endometrial Cancer Is Associated with Altered Metabolism and Composition of Fatty Acids
Abstract
1. Introduction
2. Results
2.1. Anthropometric and Biochemical Characteristics of the Study Population
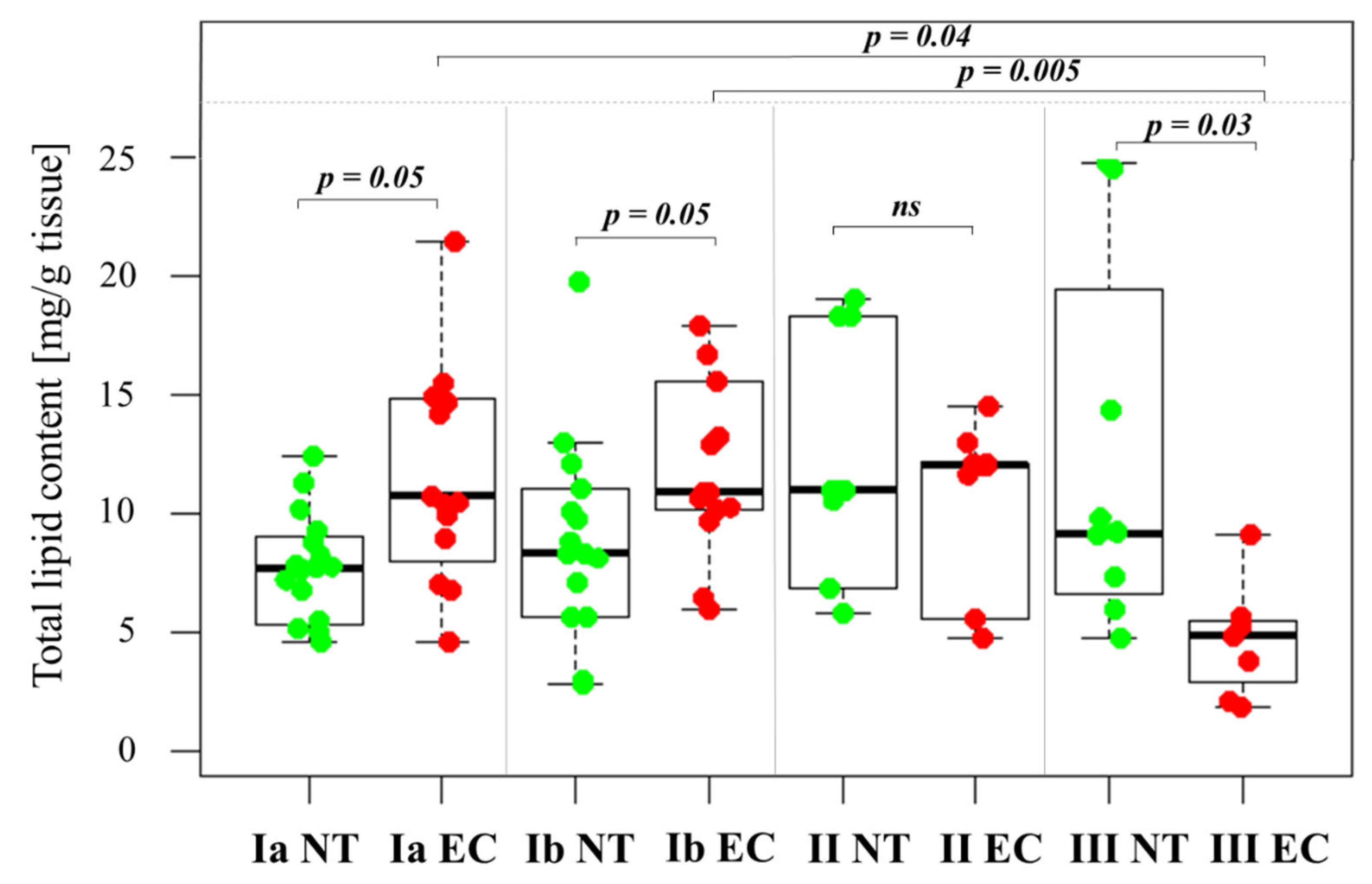
2.2. Changes in Total Lipid Content in the Tissue Samples
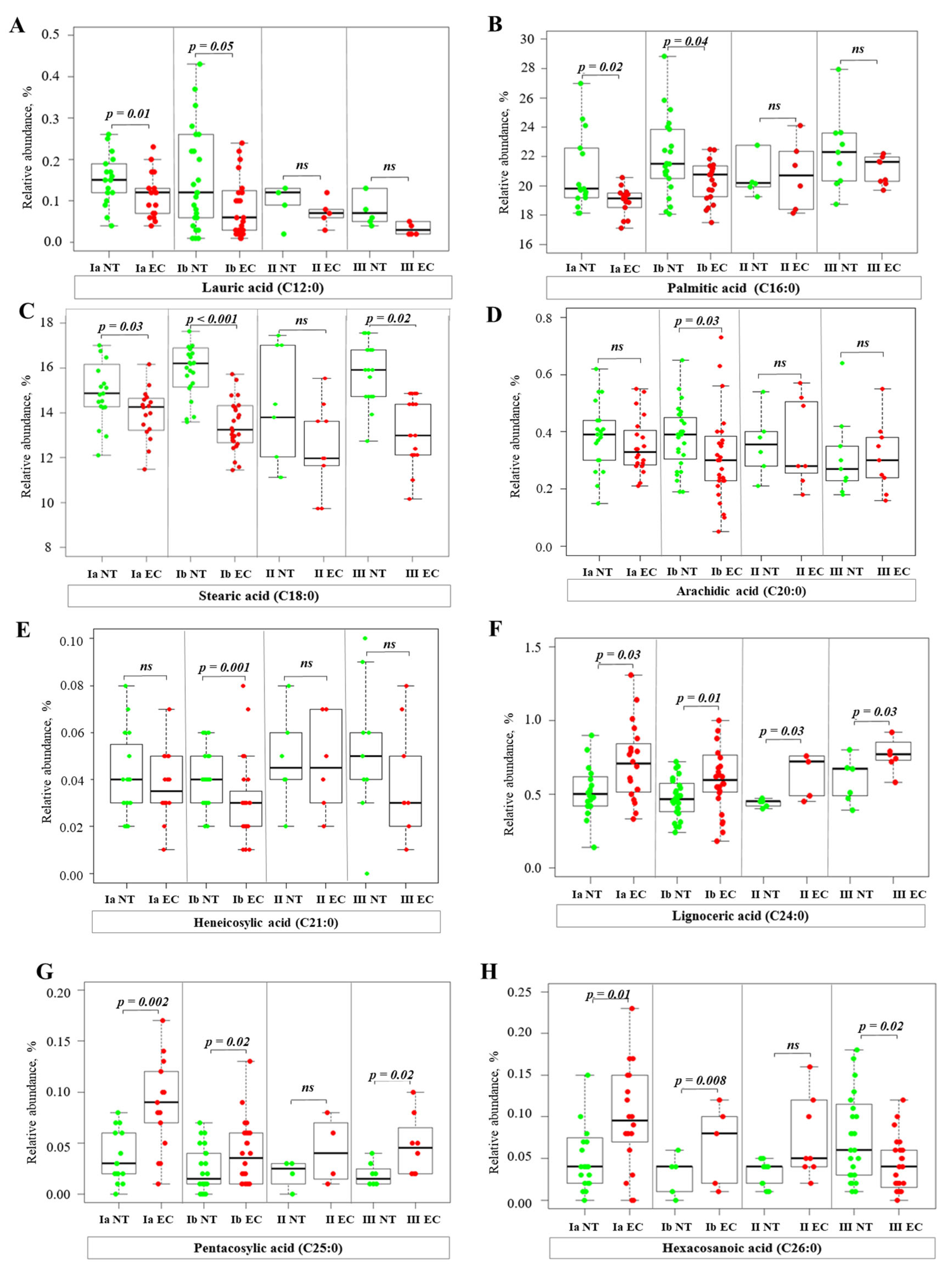
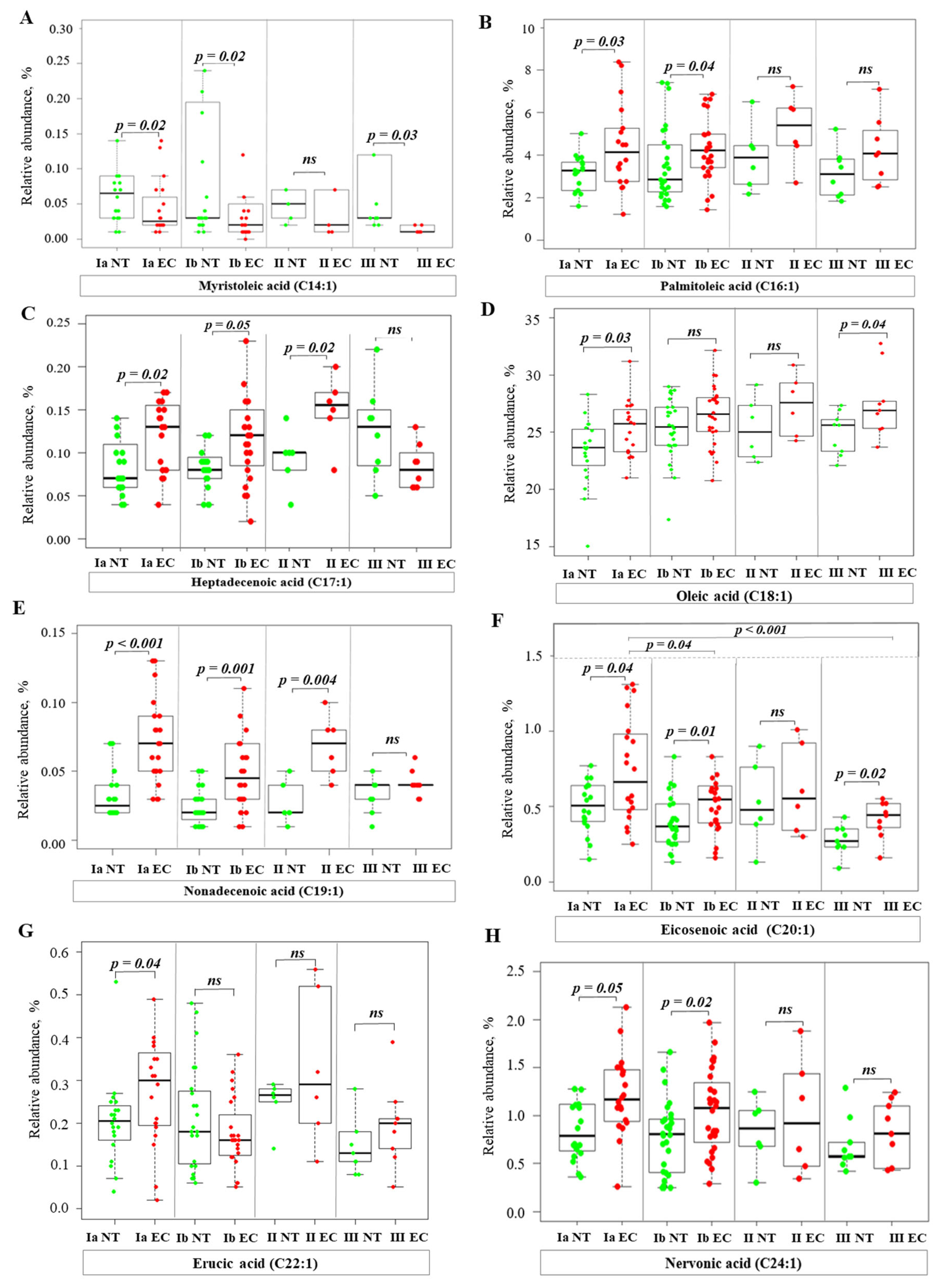
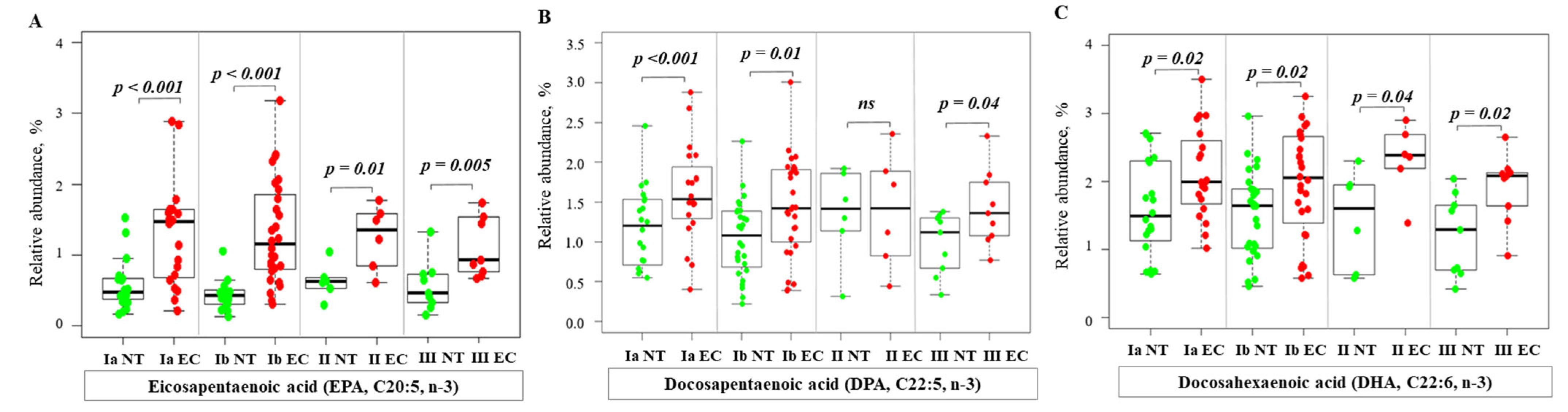
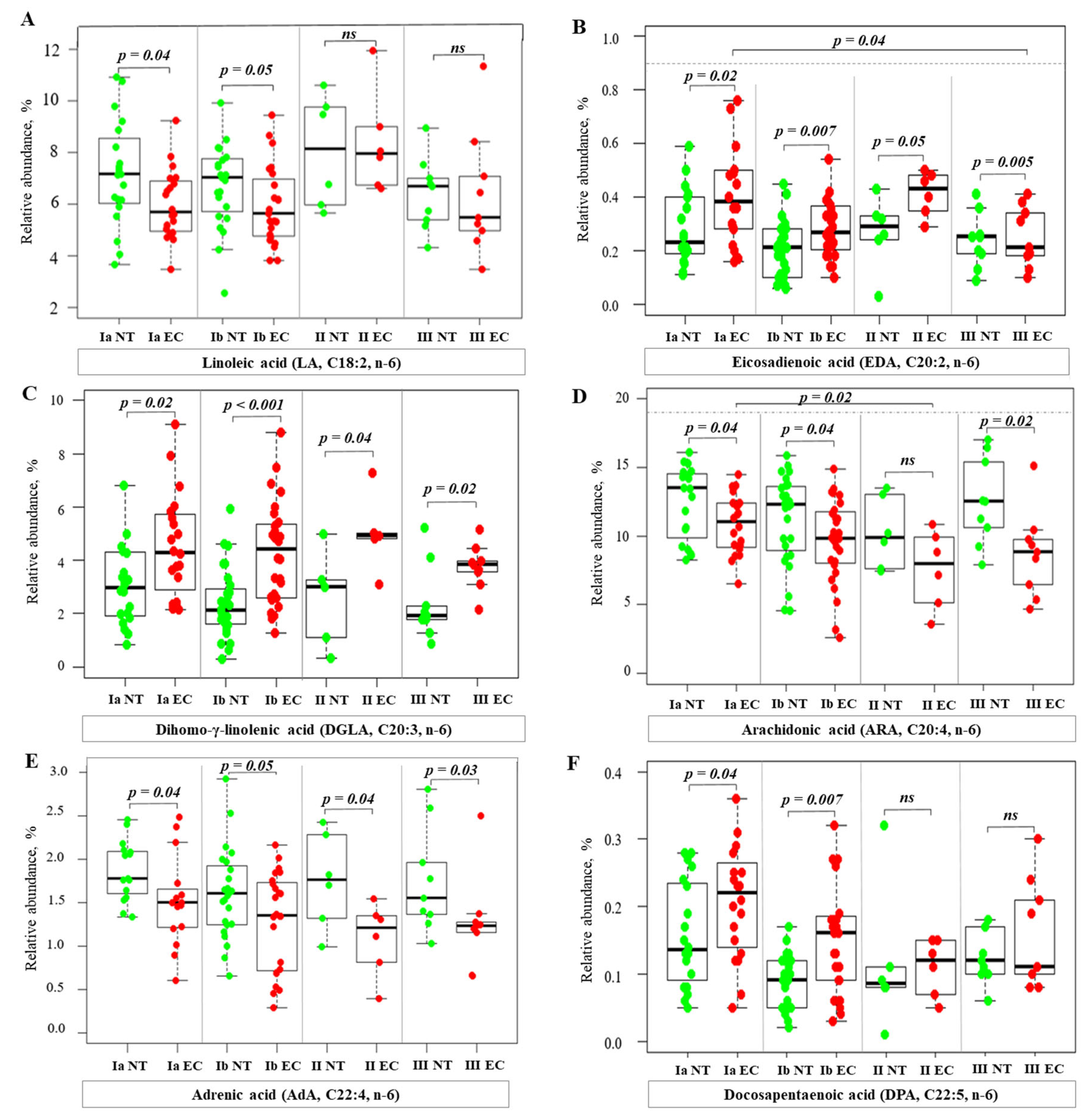
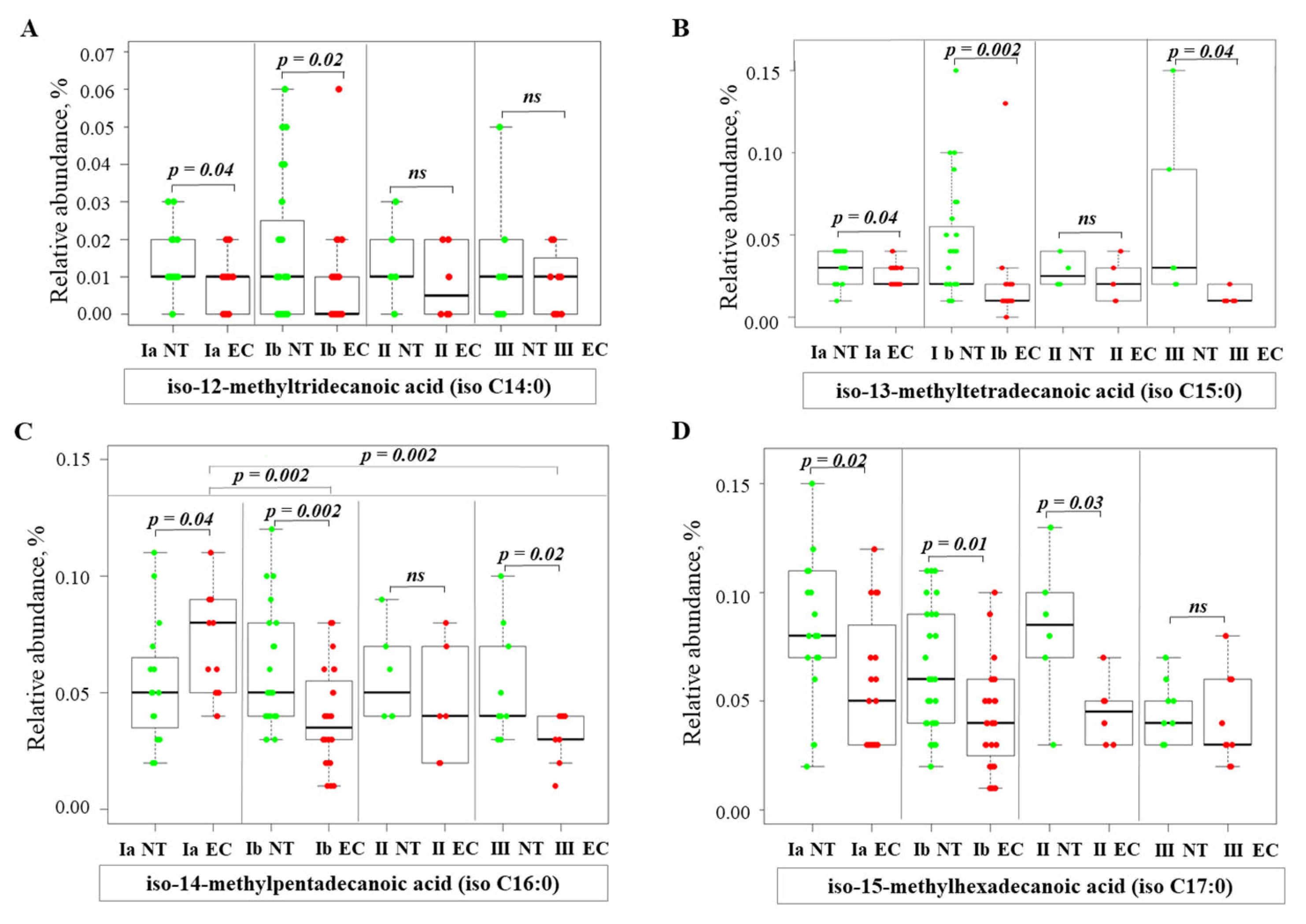
2.3. Alterations of Fatty Acid Profile in Endometrial Cancer Tissue
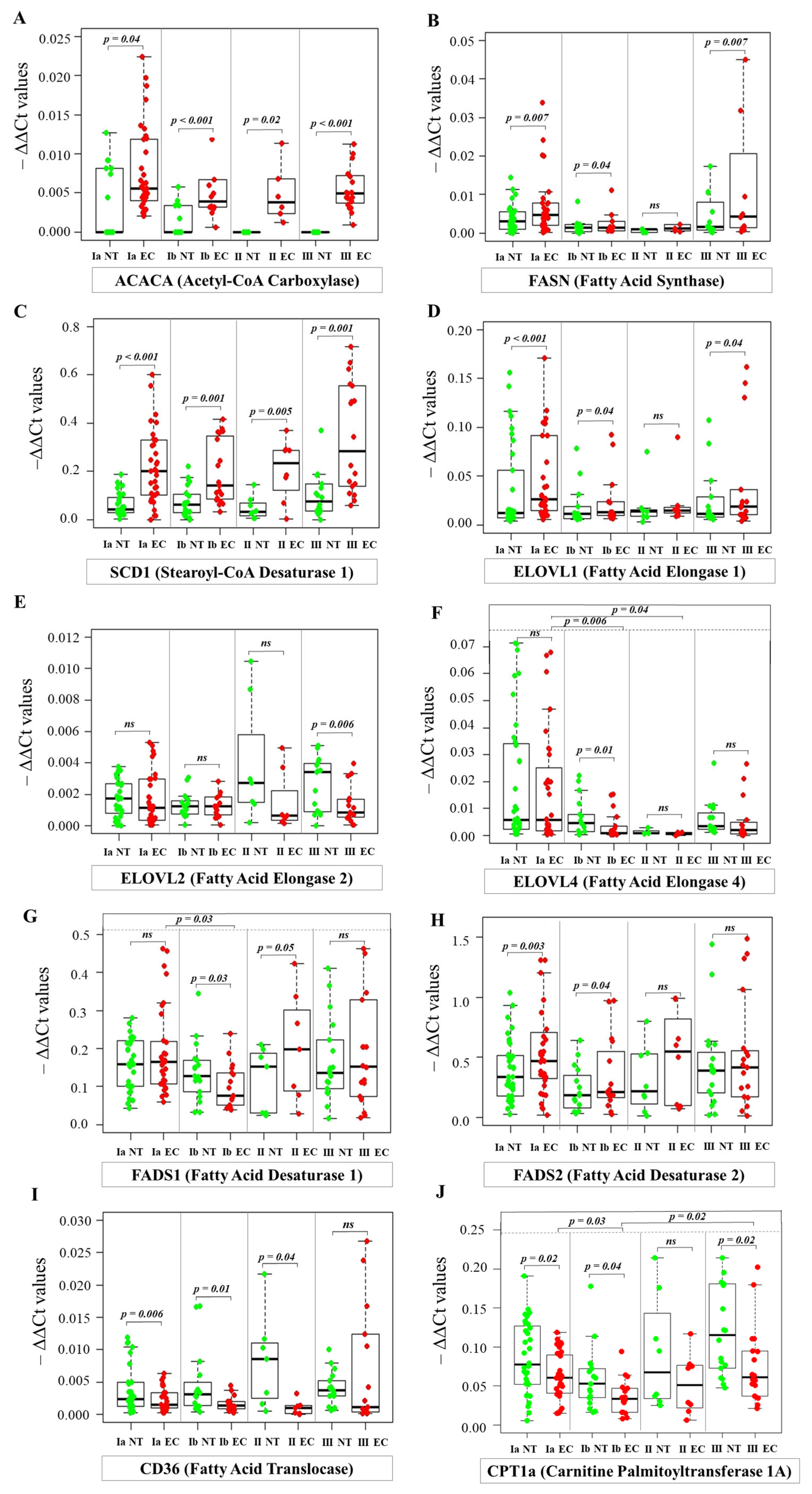
2.4. Analysis of mRNA Levels by Real-Time PCR
2.5. Comparison of RT-PCR and RNA-Seq Expression Profiles of Fatty Acid Metabolism Genes in Endometrial Cancer
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Tissue Collection
4.3. Fatty Acids Analysis
4.4. Real-Time PCR Analysis of mRNA Levels
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| B | BCFA | monomethyl branched-chain fatty acids |
| D | DSS | disease-specific survival |
| E | EC | endometrial cancer |
| F | FA | fatty acid |
| FAME | fatty acid methyl esters | |
| FIGO | The International Federation of Gynecology and Obstetrics | |
| G | GSCA | Gene Set Cancer Analysis |
| H | HC | healthy controls |
| HDL | high-density lipoprotein cholesterol | |
| HOMA-IR | homeostatic model assessment for insulin resistance | |
| HR | hazard ratios | |
| L | LCFA | long-chain fatty acids |
| LDL | low density lipoprotein cholesterol | |
| LVSI | lymphovascular space invasion | |
| M | MUFA | monounsaturated fatty acid |
| MUG | Medical University of Gdansk | |
| N | NT | normal endometrial tissue |
| O | OA | oleic acid |
| OS | overall survival | |
| P | PFS | progression-free survival |
| PUFA | polyunsaturated fatty acid | |
| R | RA | relative abundance |
| S | SFA | saturated fatty acids |
| T | TCGA | the cancer genome atlas |
| TG | triglyceride | |
| U | UALCAN | The University of Alabama at Birmingham Cancer Data Analysis Portal |
| UCEC | uterine corpus endometrial carcinoma | |
| V | VLCFA | very long chain fatty acids |
References
- Global Cancer Observatory: Cancer Tomorrow. International Agency for Research on Cancer: Lyon, France. Available online: https://gco.iarc.who.int/tomorrow/en/dataviz/trends (accessed on 30 May 2024).
- Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer: Lyon, France. Available online: https://gco.iarc.fr/today/en/fact-sheets-cancers (accessed on 18 June 2024).
- World Cancer Research Fund International: Worldwide Cancer Data London, UK. Available online: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/ (accessed on 15 June 2024).
- Terzic, M.; Aimagambetova, G.; Kunz, J.; Bapayeva, G.; Aitbayeva, B.; Terzic, S.; Laganà, A.S. Molecular Basis of Endometriosis and Endometrial Cancer: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 9274. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, H.; Zhang, L.; Zhang, S.; Dai, Y. Metabolic reprogramming and interventions in endometrial carcinoma. Biomed. Pharmacother. 2023, 161, 11452. [Google Scholar] [CrossRef]
- Zhai, L.; Yang, X.; Cheng, Y.; Wang, J. Glutamine and amino acid metabolism as a prognostic signature and therapeutic target in endometrial cancer. Cancer Med. 2023, 12, 16337–16358. [Google Scholar] [CrossRef]
- Huang, P.; Fan, X.; Yu, H.; Zhang, K.; Li, H.; Wang, Y.; Xue, F. Glucose metabolic reprogramming and its therapeutic potential in obesity-associated endometrial cancer. J. Transl. Med. 2023, 21, 94. [Google Scholar] [CrossRef]
- Mika, A.; Sledzinski, T.; Stepnowski, P. Current Progress of Lipid Analysis in Metabolic Diseases by Mass Spectrometry Methods. Curr. Med. Chem. 2019, 26, 60–103. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, M.; Su, J.; Li, Y.; Long, J.; Chu, J.; Wan, X.; Cao, Y.; Li, Q. Lipid Metabolism and Cancer. Life 2022, 12, 784. [Google Scholar] [CrossRef]
- Li, W.; Xu, Y.; Zeng, X.; Tan, J.; Wang, Y.; Wu, H.; Li, M.; Yi, C. Etiological relationship between lipid metabolism and endometrial carcinoma. Lipids Health Dis. 2023, 22, 116. [Google Scholar] [CrossRef]
- Deng, B.; Kong, W.; Suo, H.; Shen, X.; Newton, M.A.; Burkett, W.C.; Zhao, Z.; John, C.; Sun, W.; Zhang, X.; et al. Oleic Acid Exhibits Anti-Proliferative and Anti-Invasive Activities via the PTEN/AKT/mTOR Pathway in Endometrial Cancer. Cancers 2023, 15, 5407. [Google Scholar] [CrossRef]
- Wu, Z.S.; Huang, S.M.; Wang, Y.C. Palmitate Enhances the Efficacy of Cisplatin and Doxorubicin against Human Endometrial Carcinoma Cells. Int. J. Mol. Sci. 2022, 23, 80. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]
- Liu, C.J.; Hu, F.F.; Xie, G.Y.; Miao, Y.R.; Li, X.W.; Zeng, Y.; Guo, A.-Y. GSCA: An integrated platform for gene set cancer analysis at genomic, pharmacogenomic and immunogenomic levels. Brief. Bioinform. 2023, 24, bbac558. [Google Scholar] [CrossRef]
- Liu, C.J.; Hu, F.F.; Xia, M.X.; Han, L.; Zhang, Q.; Guo, A.Y. GSCALite: A web server for gene set cancer analysis. Bioinformatics 2018, 34, 3771–3772. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, S.; Guillaumond, F. Lipids in cancer: A global view of the contribution of lipid pathways to metastatic formation and treatment resistance. Oncogenesis 2022, 11, 46. [Google Scholar] [PubMed]
- Mika, A.; Pakiet, A.; Czumaj, A.; Kaczynski, Z.; Liakh, I.; Kobiela, J.; Perdyan, A.; Adrych, K.; Makarewicz, W.; Sledzinski, T. Decreased triacylglycerol content and elevated contents of cell membrane lipids in colorectal cancer tissue: A lipidomic study. J. Clin. Med. 2020, 9, 1095. [Google Scholar] [CrossRef]
- Nagarajan, S.R.; Butler, L.M.; Hoy, A.J. The diversity and breadth of cancer cell fatty acid metabolism. Cancer Metab. 2021, 9, 2. [Google Scholar] [CrossRef]
- Zierfuss, B.; Buda, A.; Villoria-González, A.; Logist, M.; Fabjan, J.; Parzer, P.; Battin, C.; Vandersteene, S.; Dijkstra, I.M.E.; Waidhofer-Söllner, P.; et al. Saturated very long-chain fatty acids regulate macrophage plasticity and invasiveness. J. Neuroinflamm. 2022, 19, 305. [Google Scholar] [CrossRef]
- Westheim, A.J.F.; Stoffels, L.M.; Dubois, L.J.; van Bergenhenegouwen, J.; van Helvoort, A.; Langen, R.C.J.; Shiri-Sverdlov, R.; Theys, J. The modulatory effects of fatty acids on cancer progression. Biomedicines 2023, 11, 280. [Google Scholar] [CrossRef]
- Guo, Z.; Bergeron, K.F.; Lingrand, M.; Mounier, C. Unveiling the MUFA–Cancer Connection: Insights from endogenous and exogenous perspectives. Int. J. Mol. Sci. 2023, 24, 9921. [Google Scholar] [CrossRef]
- Iguchi, K.; Okumura, N.; Usui, S.; Sajiki, H.; Hirota, K.; Hirano, K. Myristoleic acid, a cytotoxic component in the extract from Serenoa repens, induces apoptosis and necrosis in human prostatic LNCaP cells. Prostate 2001, 47, 59–65. [Google Scholar] [CrossRef]
- Karayama, M.; Inui, N.; Inoue, Y.; Yoshimura, K.; Mori, K.; Hozumi, H.; Suzuki, Y.; Furuhashi, K.; Fujisawa, T.; Enomoto, N.; et al. Increased serum cholesterol and long-chain fatty acid levels are associated with the efficacy of nivolumab in patients with non-small cell lung cancer. Cancer Immunol. Immunother. 2022, 71, 203–217. [Google Scholar] [CrossRef]
- Pan, J.; Cheng, L.; Bi, X.; Zhang, X.; Liu, S.; Bai, X.; Li, F.; Zhao, A.Z. Elevation of ω-3 polyunsaturated fatty acids attenuates PTEN-deficiency induced endometrial cancer development through regulation of COX-2 and PGE2 production. Sci. Rep. 2015, 5, 14958. [Google Scholar] [CrossRef] [PubMed]
- Berquin, I.M.; Edwards, I.J.; Chen, Y.Q. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008, 269, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Mansara, P.P.; Deshpande, R.A.; Vaidya, M.M.; Kaul-Ghanekar, R. Differential ratios of omega fatty acids (AA/EPA+DHA) modulate growth, lipid peroxidation and expression of tumor regulatory MARBPs in breast cancer cell lines MCF7 and MDA-MB-231. PLoS ONE 2015, 10, e0136542. [Google Scholar] [CrossRef] [PubMed]
- Gozdzik, P.; Magkos, F.; Sledzinski, T.; Mika, A. Monomethyl branched-chain fatty acids: Health effects and biological mechanisms. Prog. Lipid Res. 2023, 90, 101226. [Google Scholar] [CrossRef]
- Wongtangtintharn, S.; Oku, H.; Iwasaki, H.; Toda, T. Effect of branched-chain fatty acids on fatty acid biosynthesis of human breast cancer cells. J. Nutr. Sci. Vitaminol. 2004, 50, 137–143. [Google Scholar] [CrossRef]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Mills Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- GDC Data Portal Homepage. Available online: https://portal.gdc.cancer.gov/ (accessed on 9 September 2024).

| Characteristic | Control (HC) | Stage IA | Stage IB | Stage II | Stage III | p-Value | |
|---|---|---|---|---|---|---|---|
| Sample size | n | 58 | 36 | 19 | 9 | 19 | - |
| Demographic characteristics | Age (years) | 58.3 ± 1.2 | 59.0 ± 2.1 | 64.6 ± 1.8 | 69.7 ± 4.0 | 65.7 ± 1.4 | 1a vs. II p = 0.04 |
| Body composition | BMI (kg/m2) | 26.7 ± 0.6 | 33.4 ± 1.4 | 31.6 ± 1.7 | 29.5 ± 2.5 | 33.1 ± 1.9 | HC vs. III p = 0.02 HC vs. IA p = 0.002 |
| Glycemic analysis | HbA1c (%) | 5.7 ± 0.09 | 5.9 ± 0.2 | 5.7 ± 0.3 | 6.6 ± 0.5 | 6.5 ± 0.3 | ns |
| Glucose (mg/dL) | 89.6 ± 2.7 | 98.8 ± 4.3 | 101.6 ± 4.7 | 130.2 ± 17.7 | 128.0 ± 11.3 | 1a vs. III p = 0.02 HC vs. III p < 0.001 | |
| Insulin (µU/mL) | 6.5 ± 0.7 | 11.1 ± 1.6 | 9.7 ± 1.9 | 16.6 ± 3.6 | 10.1 ± 1.8 | HC vs. II p = 0.02 | |
| HOMA-IR | 1.2 ± 0.09 | 3.1 ± 0.6 | 2.6 ± 0.6 | 5.4 ±1.8 | 3.1 ± 0.6 | HC vs. II p = 0.03 HC vs. III p = 0.01 | |
| Inflammatory marker | CRP (mg/dL) | 2.2 ± 0.3 | 7.5 ± 3.2 | 3.3 ± 0.8 | 3.3 ± 0.6 | 8.0 ± 2.4 | HC vs. III p = 0.04 |
| Tumor markers | AFP (ng/mL) | 2.5 ± 0.2 | 4.2 ± 1.0 | 3.9 ± 0.7 | 3.0 ± 1.1 | 2.4 ± 0.4 | HC vs. IA p = 0.009 HC vs. IB p = 0.050 |
| β-HCG (IU/L) | 2.0 ± 0.2 | 1.7 ± 0.3 | 2.9 ± 0.4 | 2.7 ± 0.9 | 3.1 ± 0.6 | HC vs. IB p = 0.028 HC vs. III p = 0.034 1a vs. IB p= 0.04 1a vs. III p = 0.012 | |
| CEA (ng/mL) | 1.6 ± 0.1 | 1.4 ± 0.2 | 2.3 ± 0.6 | 1.9 ± 0.3 | 2.8 ± 0.5 | HC vs. III p = 0.009 1a vs. III p = 0.046 | |
| Ca19 -9 (U/mL) | 5.9 ± 0.8 | 17.8 ± 12.6 | 102.3 ± 49.9 | 169.5 ± 146.7 | 113.6 ± 70.7 | HC vs. IB p = 0.007 HC vs. II p = 0.012 HC vs. III p = 0.002 | |
| Ca125 (U/mL) | 14.2 ± 0.8 | 44.8 ± 27.2 | 47.3 ± 17.6 | 30.9 ± 11.8 | 45.9 ± 14.3 | HC vs. IA p = 0.050 HC vs. IB p = 0.015 HC vs. II p = 0.014 HC vs. III p = 0.004 | |
| Lipid profiles | TC (mg/dL) | 192.1 ± 6.7 | 196.5 ± 7.8 | 213.6 ± 14.8 | 199.5 ± 18.2 | 182.5 ± 10.6 | ns |
| HDL (mg/dL) | 55.1 ± 1.7 | 49.0 ± 2.7 | 56.8 ± 3.9 | 47.5 ± 4.5 | 49.9 ± 6.9 | ns | |
| LDL (mg/dL) | 118.9 ± 6.1 | 132.6 ± 8.2 | 138.4 ± 14.0 | 132.2 ± 18.6 | 114.1 ± 10.7 | ns | |
| TG (mg/dL) | 119.0 ± 9.7 | 140.8 ± 12.2 | 130.9 ± 11.4 | 150.0 ± 27.9 | 167.8 ± 23.7 | ns | |
| TG/HDL ratio | 2.4 ± 0.2 | 3.4 ± 0.5 | 2.5 ± 0.3 | 3.4 ± 2.1 | 4.2 ± 0.9 | HC vs. III p = 0.05 | |
| TC/HDL ratio | 3.6 ± 0.1 | 4.3 ± 0.3 | 3.9 ± 0.3 | 4.3 ± 0.4 | 4.0 ± 0.3 | ns | |
| LDL/HDL ratio | 2.2 ± 0.1 | 3.0 ± 0.2 | 2.5 ± 0.3 | 2.8 ± 0.4 | 2.5 ± 0.3 | ns | |
| Medication use (yes; %) | diabetes medications | 25% | 22% | 21% | 33% | 53% | - |
| cholesterol-lowering medication | 36% | 25% | 16% | 33% | 37% | - | |
| Hormonal status (yes; %) | Postmenopausal | 91% | 75% | 84% | 89% | 100% | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razghonova, Y.; Mika, A.; Czapiewska, M.; Stanczak, A.; Zygowska, P.; Wydra, D.G.; Sledzinski, T.; Abacjew-Chmylko, A. Endometrial Cancer Is Associated with Altered Metabolism and Composition of Fatty Acids. Int. J. Mol. Sci. 2025, 26, 3322. https://doi.org/10.3390/ijms26073322
Razghonova Y, Mika A, Czapiewska M, Stanczak A, Zygowska P, Wydra DG, Sledzinski T, Abacjew-Chmylko A. Endometrial Cancer Is Associated with Altered Metabolism and Composition of Fatty Acids. International Journal of Molecular Sciences. 2025; 26(7):3322. https://doi.org/10.3390/ijms26073322
Chicago/Turabian StyleRazghonova, Yelyzaveta, Adriana Mika, Monika Czapiewska, Agata Stanczak, Paulina Zygowska, Dariusz Grzegorz Wydra, Tomasz Sledzinski, and Anna Abacjew-Chmylko. 2025. "Endometrial Cancer Is Associated with Altered Metabolism and Composition of Fatty Acids" International Journal of Molecular Sciences 26, no. 7: 3322. https://doi.org/10.3390/ijms26073322
APA StyleRazghonova, Y., Mika, A., Czapiewska, M., Stanczak, A., Zygowska, P., Wydra, D. G., Sledzinski, T., & Abacjew-Chmylko, A. (2025). Endometrial Cancer Is Associated with Altered Metabolism and Composition of Fatty Acids. International Journal of Molecular Sciences, 26(7), 3322. https://doi.org/10.3390/ijms26073322






