The Influence of AQP5 on the Response to Hydrogen Peroxide in Breast Cancer Cell Lines
Abstract
1. Introduction
2. Results
2.1. Overexpression of AQP5 Decreases Cell Viability in MCF7 and SkBr-3 Cells Following Treatment with 40 µM Hydrogen Peroxide
2.2. Overexprexpression of AQP5 Increases Intracellular Levels of ROS in MCF7 Cells While Decreasing ROS Levels in SUM 159 Cells Following Hydrogen Peroxide Treatment
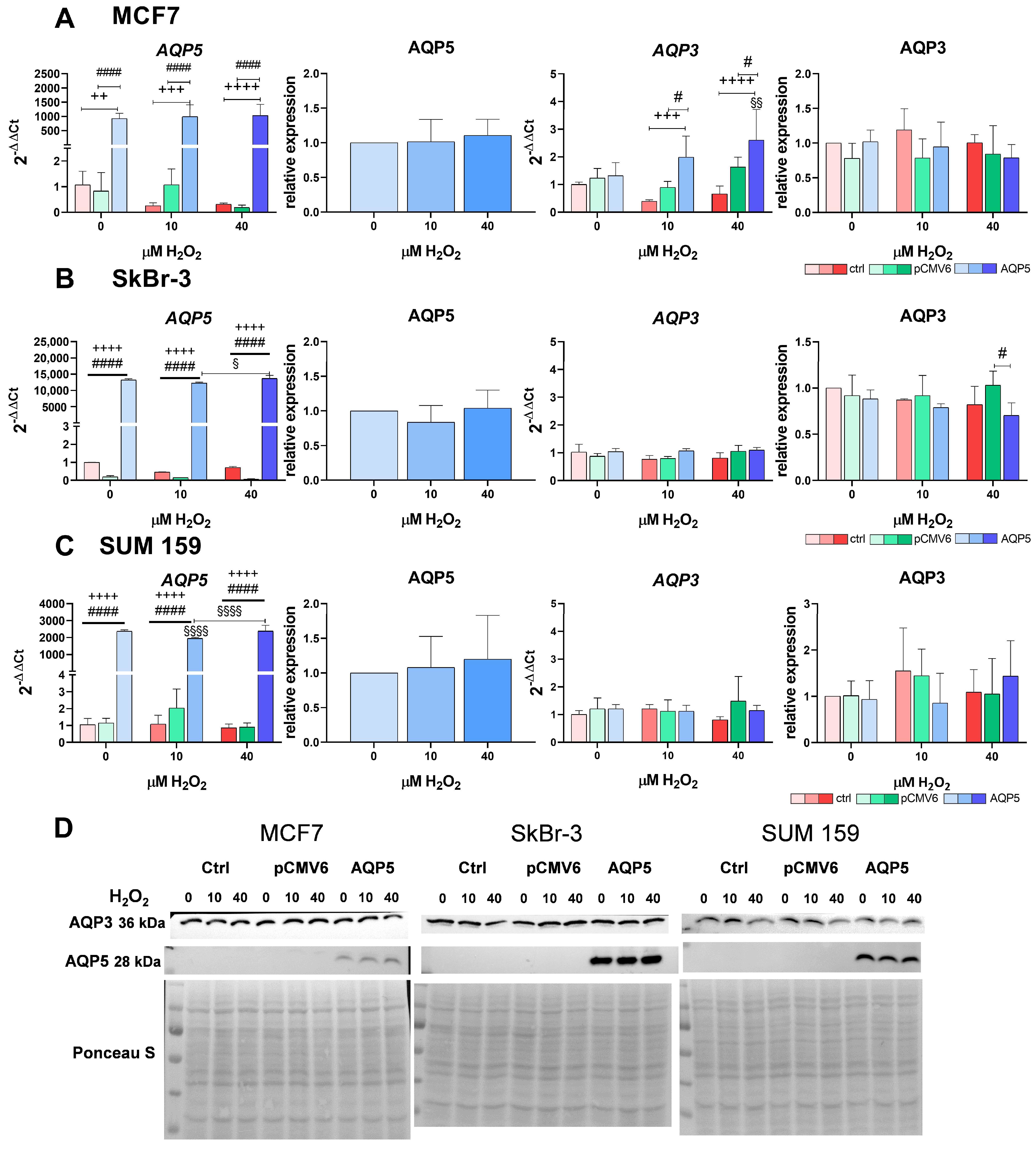
2.3. Overexpression of AQP5 Increases the RNA Expression of AQP3 in MCF7 Cells in Response to Hydrogen Peroxide
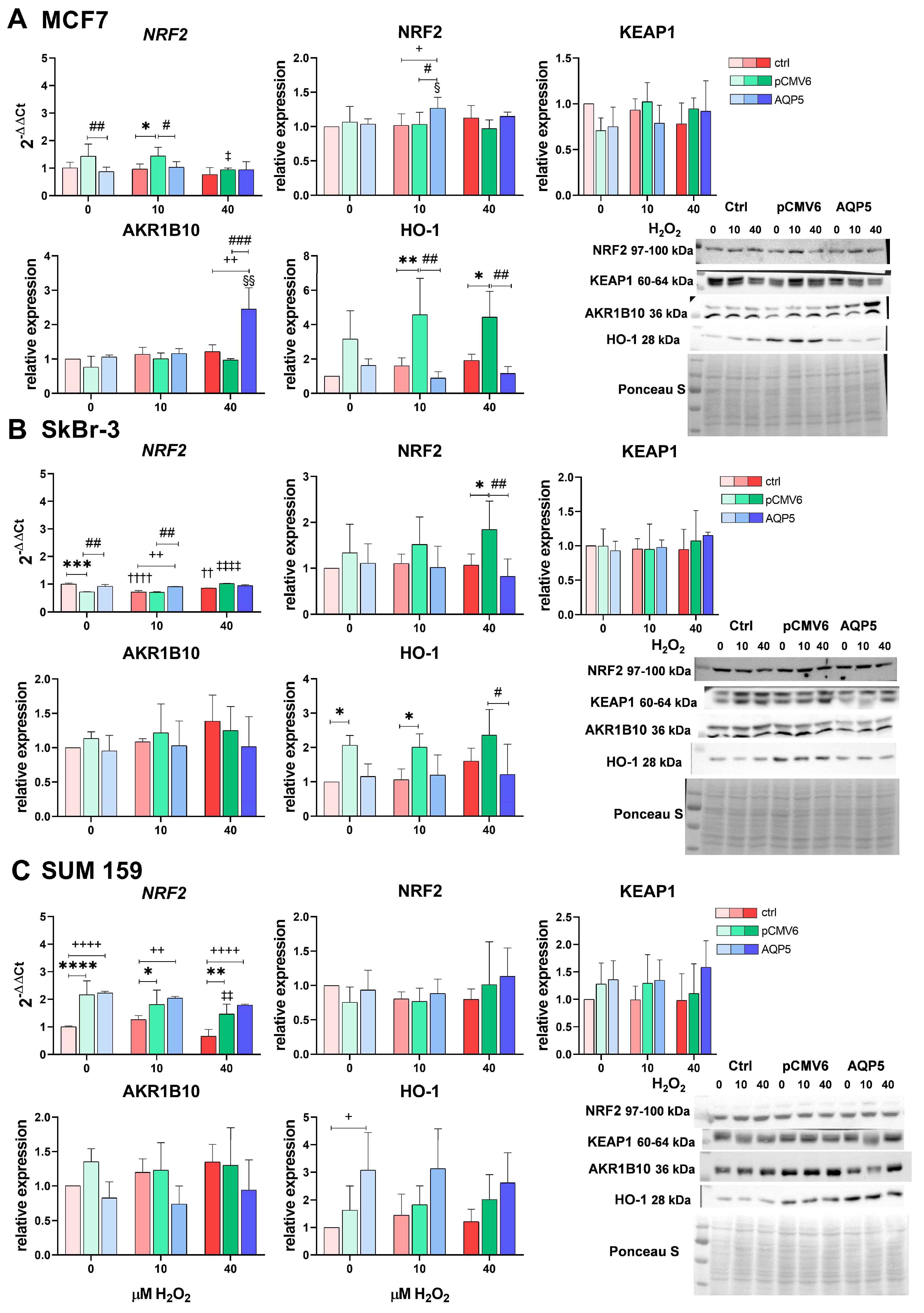
2.4. Overexpression of AQP5 Slightly Affects the NRF2 Signaling Pathway—Mainly the NRF2/AKR1B10 Axis in MCF7 Cells upon H2O2 Treatment
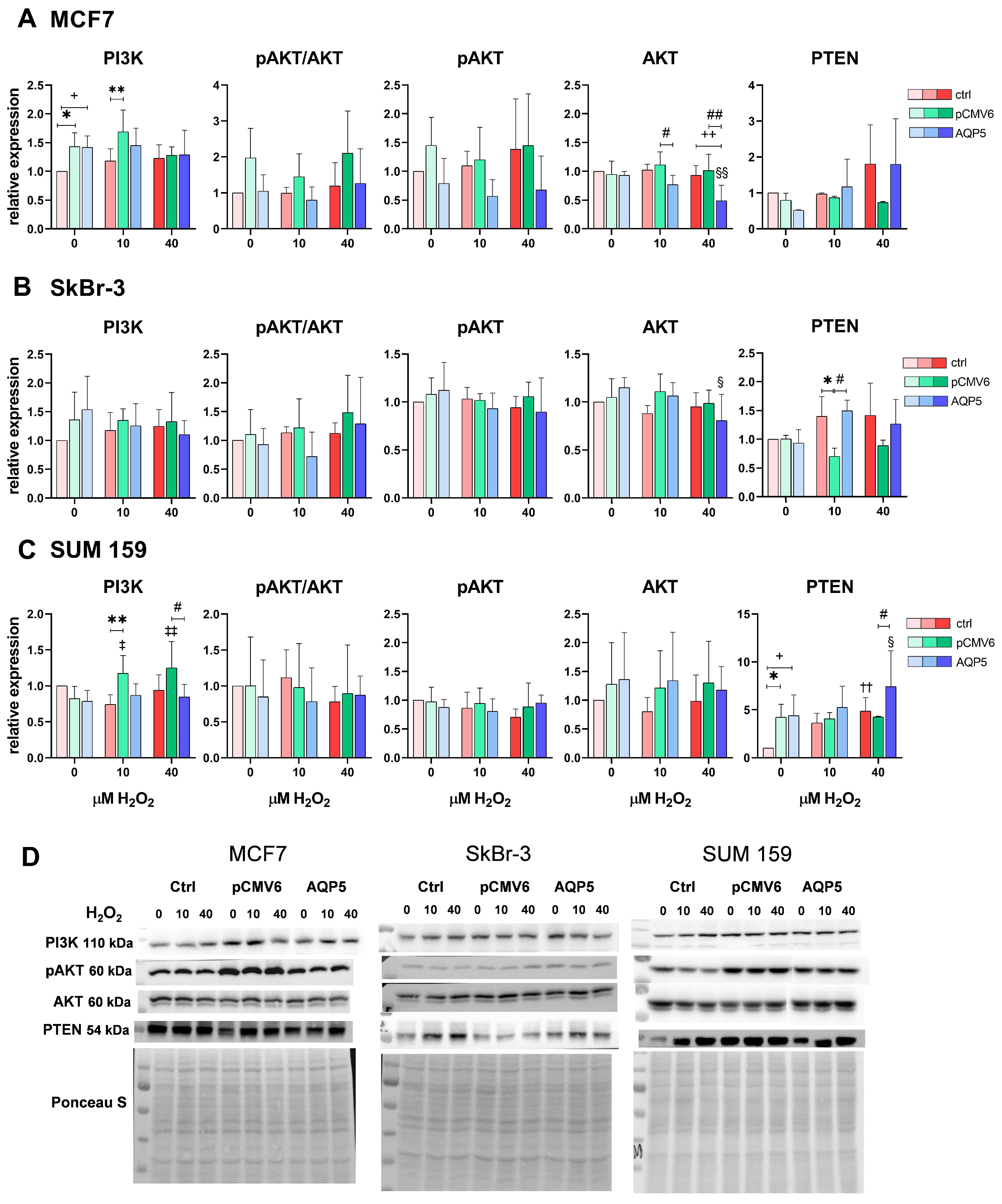
2.5. AQP5 Overexpression Decreases Total AKT Levels in MCF7-AQP5 and SkBr-3-AQP5 Cells and Increases PTEN Levels in SUM 159-AQP5 Cells Following Treatment with 40 µM H2O2
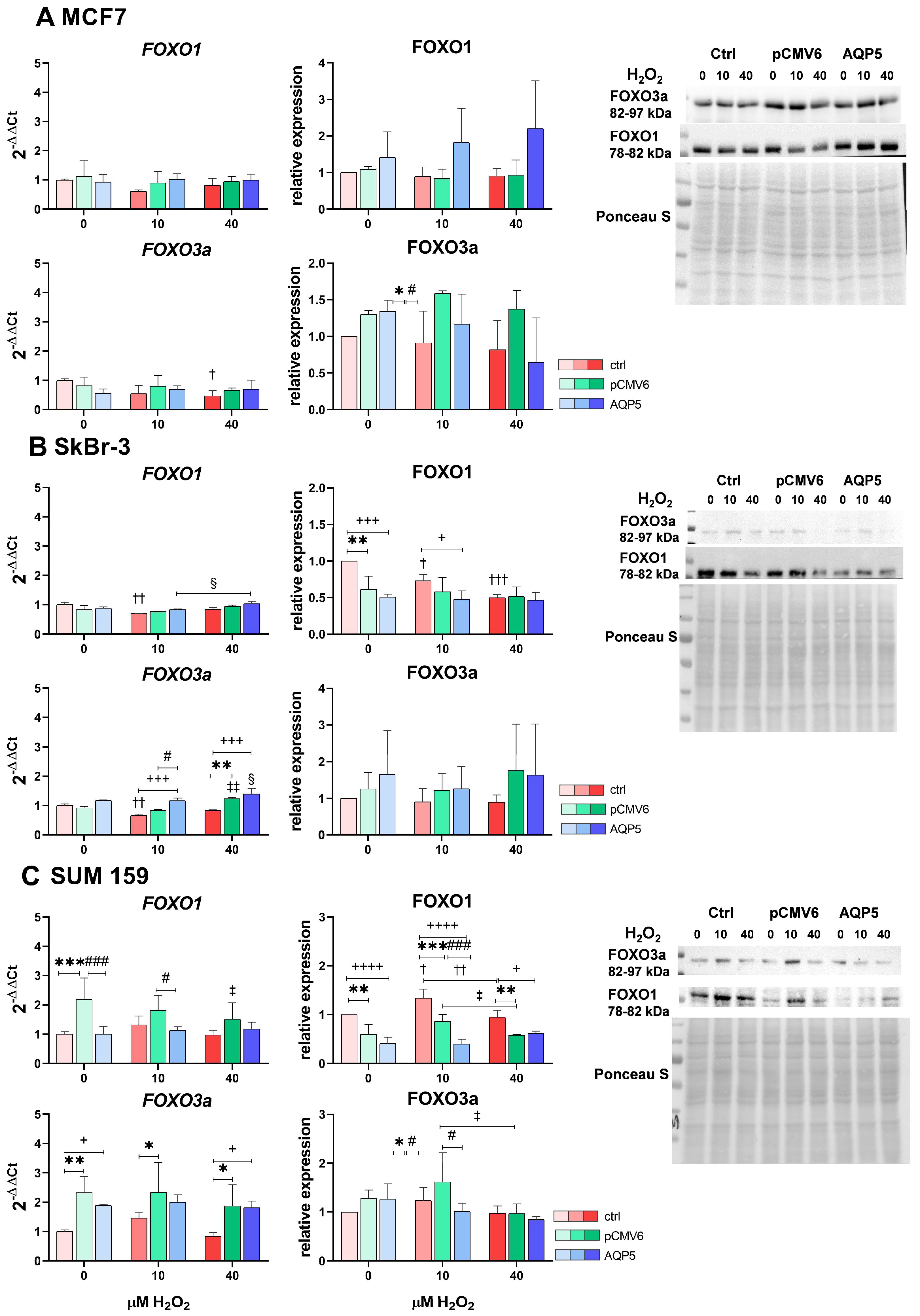
2.6. Overexpression of AQP5 and Hydrogen Peroxide Treatment Slightly Affect FOXO1 and FOXO3a, Primarily Decreasing FOXO1 Protein Levels in SUM 159 Cells and Increasing FOXO3a mRNA Expression in SkBr-3 Cells
3. Discussion
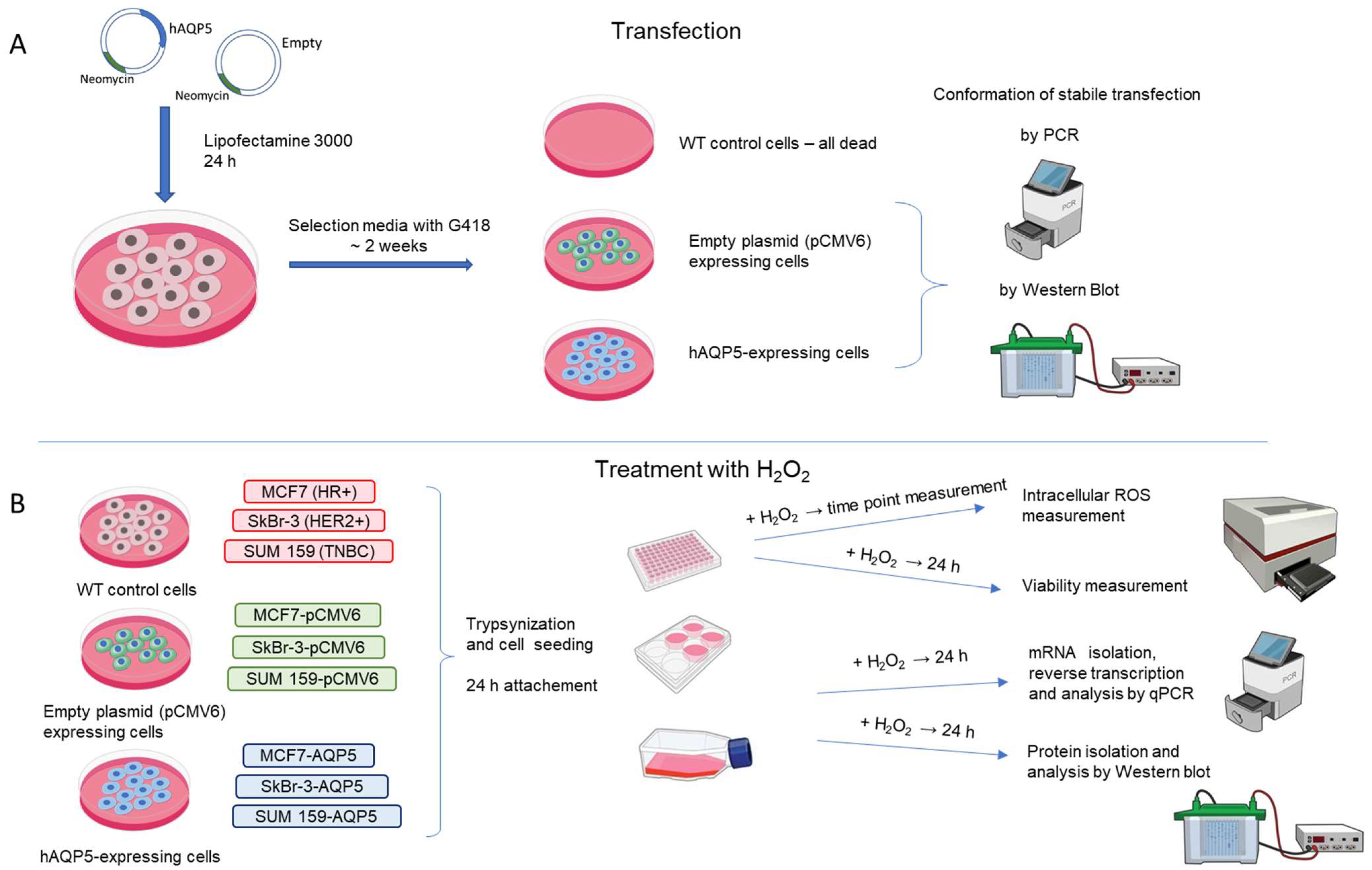
4. Materials and Methods
4.1. Cell Culture
4.2. Establishing Stable AQP5-Overexpressing Cell Lines
4.3. Cell Viability
4.4. ROS Measurement
4.5. mRNA Extraction and qPCR Measurement
4.6. Protein Extraction and WB Analyses
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agre, P.; Bonhivers, M.; Borgnia, M.J. The aquaporins, blueprints for cellular plumbing systems. J. Biol. Chem. 1998, 273, 14659–14662. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Raihan, T.; Ahmed, J.; Hakim, A.; Emon, T.H.; Chowdhury, P.A. Human Aquaporins: Functional Diversity and Potential Roles in Infectious and Non-infectious Diseases. Front. Genet. 2021, 12, 654865. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Méndez-Giménez, L.; Frühbeck, G. Aquaporins in Health. In Aquaporins in Health and Disease, 1st ed.; Soveral, G., Nielsen, S., Casini, A., Eds.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351229159. [Google Scholar]
- Bienert, G.P.; Møller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Milkovic, L.; Cipak Gasparovic, A.; Cindric, M.; Mouthuy, P.-A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef]
- Jaganjac, M.; Milkovic, L.; Sunjic, S.B.; Zarkovic, N. The NRF2, Thioredoxin, and Glutathione System in Tumorigenesis and Anticancer Therapies. Antioxidants 2020, 9, 1151. [Google Scholar] [CrossRef]
- Krafczyk, N.; Klotz, L.-O. FOXO transcription factors in antioxidant defense. IUBMB Life 2022, 74, 53–61. [Google Scholar] [CrossRef]
- Milkovic, L.; Zarkovic, N.; Saso, L. Controversy about pharmacological modulation of Nrf2 for cancer therapy. Redox Biol. 2017, 12, 727–732. [Google Scholar] [CrossRef]
- Farhan, M.; Silva, M.; Li, S.; Yan, F.; Fang, J.; Peng, T.; Hu, J.; Tsao, M.-S.; Little, P.; Zheng, W. The role of FOXOs and autophagy in cancer and metastasis-Implications in therapeutic development. Med. Res. Rev. 2020, 40, 2089–2113. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Schiavoni, V.; Emanuelli, M.; Milanese, G.; Galosi, A.B.; Pompei, V.; Salvolini, E.; Campagna, R. Nrf2 Signaling in Renal Cell Carcinoma: A Potential Candidate for the Development of Novel Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 13239. [Google Scholar] [CrossRef] [PubMed]
- Coomans de Brachène, A.; Demoulin, J.-B. FOXO transcription factors in cancer development and therapy. Cell. Mol. Life Sci. 2016, 73, 1159–1172. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer; Exon Publications: Brisbane, Australia, 2022; pp. 31–42. [Google Scholar]
- Heiser, L.M.; Sadanandam, A.; Kuo, W.-L.; Benz, S.C.; Goldstein, T.C.; Ng, S.; Gibb, W.J.; Wang, N.J.; Ziyad, S.; Tong, F.; et al. Subtype and pathway specific responses to anticancer compounds in breast cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 2724–2729. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-K.; Yang, H.-J.; Lee, M.-S.; Liu, D.-W.; Chen, L.-C.; Chew, C.-H.; Lin, C.-H.; Lee, C.-H.; Li, S.-C.; Hong, C.-L.; et al. Molecular subtypes of breast cancer predicting clinical benefits of radiotherapy after breast-conserving surgery: A propensity-score-matched cohort study. Breast Cancer Res. 2023, 25, 149. [Google Scholar] [CrossRef]
- Rouzier, R.; Perou, C.M.; Symmans, W.F.; Ibrahim, N.; Cristofanilli, M.; Anderson, K.; Hess, K.R.; Stec, J.; Ayers, M.; Wagner, P.; et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 2005, 11, 5678–5685. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, T.; He, F.; Rong, Y.; Lee, S.H.; Wu, S.; Zuo, L. Reactive oxygen species formation and bystander effects in gradient irradiation on human breast cancer cells. Oncotarget 2016, 7, 41622–41636. [Google Scholar] [CrossRef]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef]
- Jang, S.J.; Moon, C. Aquaporin 5 (AQP5) expression in breast cancer and its clinicopathological characteristics. PLoS ONE 2023, 18, e0270752. [Google Scholar] [CrossRef]
- Bijelić, A.; Silovski, T.; Mlinarić, M.; Čipak Gašparović, A. Peroxiporins in Triple-Negative Breast Cancer: Biomarker Potential and Therapeutic Perspectives. Int. J. Mol. Sci. 2024, 25, 6658. [Google Scholar] [CrossRef]
- Edamana, S.; Login, F.H.; Riishede, A.; Dam, V.S.; Tramm, T.; Nejsum, L.N. The cell polarity protein Scribble is downregulated by the water channel aquaporin-5 in breast cancer cells. Am. J. Physiol. Cell Physiol. 2023, 324, C307–C319. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, T.; Wang, H.; Jiao, L. AQP5 promotes epithelial-mesenchymal transition and tumor growth through activating the Wnt/β-catenin pathway in triple-negative breast cancer. Mutat. Res. 2024, 829, 111868. [Google Scholar] [CrossRef]
- Wang, L.; Huo, D.; Zhu, H.; Xu, Q.; Gao, C.; Chen, W.; Zhang, Y. Deciphering the structure, function, expression and regulation of aquaporin-5 in cancer evolution. Oncol. Lett. 2021, 21, 309. [Google Scholar] [CrossRef] [PubMed]
- Pimpão, C.; da Silva, I.V.; Soveral, G. The Expanding Role of Aquaporin-1, Aquaporin-3 and Aquaporin-5 as Transceptors: Involvement in Cancer Development and Potential Druggability. Int. J. Mol. Sci. 2025, 26, 1330. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wu, S.; Yang, Y.; Tang, L.; Wang, Y.; Dong, J.; Lü, B.; Jiang, G.; Zhao, W. AQP5 silencing suppresses p38 MAPK signaling and improves drug resistance in colon cancer cells. Tumour Biol. 2014, 35, 7035–7045. [Google Scholar] [CrossRef]
- Li, X.; Pei, B.; Wang, H.; Tang, C.; Zhu, W.; Jin, F. Effect of AQP-5 silencing by siRNA interference on chemosensitivity of breast cancer cells. OncoTargets Ther. 2018, 11, 3359–3368. [Google Scholar] [CrossRef]
- Edamana, S.; Pedersen, S.F.; Nejsum, L.N. Aquaporin water channels affect the response of conventional anticancer therapies of 3D grown breast cancer cells. Biochem. Biophys. Res. Commun. 2023, 639, 126–133. [Google Scholar] [CrossRef]
- Rodrigues, C.; Mósca, A.F.; Martins, A.P.; Nobre, T.; Prista, C.; Antunes, F.; Cipak Gasparovic, A.; Soveral, G. Rat Aquaporin-5 Is pH-Gated Induced by Phosphorylation and Is Implicated in Oxidative Stress. Int. J. Mol. Sci. 2016, 17, 2090. [Google Scholar] [CrossRef]
- Rodrigues, C.; Milkovic, L.; Bujak, I.T.; Tomljanovic, M.; Soveral, G.; Gasparovic, A.C.; Cipak Gasparovic, A. Lipid Profile and Aquaporin Expression under Oxidative Stress in Breast Cancer Cells of Different Malignancies. Oxid. Med. Cell. Longev. 2019, 2019, 2061830. [Google Scholar] [CrossRef]
- Mlinarić, M.; Lučić, I.; Milković, L.; da Silva, I.V.; Tartaro Bujak, I.; Musani, V.; Soveral, G.; Čipak Gašparović, A. AQP3-Dependent PI3K/Akt Modulation in Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 8133. [Google Scholar] [CrossRef]
- Langlands, F.E.; Horgan, K.; Dodwell, D.D.; Smith, L. Breast cancer subtypes: Response to radiotherapy and potential radiosensitisation. Br. J. Radiol. 2013, 86, 20120601. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Li, D.; Ponnamperumage, T.N.F.; Peterson, A.K.; Pandey, J.; Fatima, K.; Brzezinski, J.; Jakusz, J.A.R.; Gao, H.; Koelsch, G.E.; et al. Generation of Hydrogen Peroxide in Cancer Cells: Advancing Therapeutic Approaches for Cancer Treatment. Cancers 2024, 16, 2171. [Google Scholar] [CrossRef] [PubMed]
- Vilchis-Landeros, M.M.; Vázquez-Meza, H.; Vázquez-Carrada, M.; Uribe-Ramírez, D.; Matuz-Mares, D. Antioxidant Enzymes and Their Potential Use in Breast Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 5675. [Google Scholar] [CrossRef]
- Campagna, R.; Pozzi, V.; Giorgini, S.; Morichetti, D.; Goteri, G.; Sartini, D.; Serritelli, E.N.; Emanuelli, M. Paraoxonase-2 is upregulated in triple negative breast cancer and contributes to tumor progression and chemoresistance. Hum. Cell 2023, 36, 1108–1119. [Google Scholar] [CrossRef]
- Login, F.H.; Palmfeldt, J.; Cheah, J.S.; Yamada, S.; Nejsum, L.N. Aquaporin-5 regulation of cell-cell adhesion proteins: An elusive “tail” story. Am. J. Physiol. Cell Physiol. 2021, 320, C282–C292. [Google Scholar] [CrossRef]
- Chae, Y.K.; Woo, J.; Kim, M.-J.; Kang, S.K.; Kim, M.S.; Lee, J.; Lee, S.K.; Gong, G.; Kim, Y.H.; Soria, J.C.; et al. Expression of aquaporin 5 (AQP5) promotes tumor invasion in human non small cell lung cancer. PLoS ONE 2008, 3, e2162. [Google Scholar] [CrossRef]
- Bystrup, M.; Login, F.H.; Edamana, S.; Borgquist, S.; Tramm, T.; Kwon, T.-H.; Nejsum, L.N. Aquaporin-5 in breast cancer. APMIS 2022, 130, 253–260. [Google Scholar] [CrossRef]
- Lennicke, C.; Rahn, J.; Lichtenfels, R.; Wessjohann, L.A.; Seliger, B. Hydrogen peroxide—Production, fate and role in redox signaling of tumor cells. Cell Commun. Signal. 2015, 13, 39. [Google Scholar] [CrossRef]
- Janosi, L.; Ceccarelli, M. The gating mechanism of the human aquaporin 5 revealed by molecular dynamics simulations. PLoS ONE 2013, 8, e59897. [Google Scholar] [CrossRef]
- Chevriau, J.; De Palma, G.Z.; Jozefkowicz, C.; Vitali, V.; Canessa Fortuna, A.; Ayub, N.; Soto, G.; Bienert, G.P.; Zeida, A.; Alleva, K. Permeation mechanisms of hydrogen peroxide and water through Plasma Membrane Intrinsic Protein aquaporins. Biochem. J. 2024, 481, 1329–1347. [Google Scholar] [CrossRef]
- Rodrigues, C.; Pimpão, C.; Mósca, A.F.; Coxixo, A.S.; Lopes, D.; da Silva, I.V.; Pedersen, P.A.; Antunes, F.; Soveral, G. Human Aquaporin-5 Facilitates Hydrogen Peroxide Permeation Affecting Adaption to Oxidative Stress and Cancer Cell Migration. Cancers 2019, 11, 932. [Google Scholar] [CrossRef] [PubMed]
- Wegler, C.; Ölander, M.; Wiśniewski, J.R.; Lundquist, P.; Zettl, K.; Åsberg, A.; Hjelmesæth, J.; Andersson, T.B.; Artursson, P. Global variability analysis of mRNA and protein concentrations across and within human tissues. NAR Genom. Bioinform. 2020, 2, lqz010. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; da Silva, I.V.; Rodrigues, C.M.P.; Castro, R.E.; Soveral, G. The Emerging Role of microRNAs in Aquaporin Regulation. Front. Chem. 2018, 6, 238. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lu, H.; Bai, Y. Nrf2 in cancers: A double-edged sword. Cancer Med. 2019, 8, 2252–2267. [Google Scholar] [CrossRef]
- Huang, C.; Verhulst, S.; Shen, Y.; Bu, Y.; Cao, Y.; He, Y.; Wang, Y.; Huang, D.; Cai, C.; Rao, K.; et al. AKR1B10 promotes breast cancer metastasis through integrin α5/δ-catenin mediated FAK/Src/Rac1 signaling pathway. Oncotarget 2016, 7, 43779–43791. [Google Scholar] [CrossRef]
- Jensen, H.H.; Login, F.H.; Park, J.-Y.; Kwon, T.-H.; Nejsum, L.N. Immunohistochemical evalulation of activated Ras and Rac1 as potential downstream effectors of aquaporin-5 in breast cancer in vivo. Biochem. Biophys. Res. Commun. 2017, 493, 1210–1216. [Google Scholar] [CrossRef]
- Klotz, L.-O.; Sánchez-Ramos, C.; Prieto-Arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox regulation of FoxO transcription factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Graves, D.T. FOXO transcription factors: Their clinical significance and regulation. BioMed Res. Int. 2014, 2014, 925350. [Google Scholar] [CrossRef]
- Mukherjee, R.; Vanaja, K.G.; Boyer, J.A.; Gadal, S.; Solomon, H.; Chandarlapaty, S.; Levchenko, A.; Rosen, N. Regulation of PTEN translation by PI3K signaling maintains pathway homeostasis. Mol. Cell 2021, 81, 708–723.e5. [Google Scholar] [CrossRef]
- Mahalingaiah, P.K.S.; Singh, K.P. Chronic oxidative stress increases growth and tumorigenic potential of MCF-7 breast cancer cells. PLoS ONE 2014, 9, e87371. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]


| Gene of Interest | Forward | Reverse |
|---|---|---|
| NRF2 | GCTATGGAGACACACTACTTGG | CCAGGACTTACAGGCAATTCT |
| AQP3 | GGGCTGTATTATGATGCAATCTGG | GTCCAGAGGGGTAGGTAGCA |
| AQP5 | CCCGCTCACTGGGTTTTCT | GTCCTCGTCAGGCTCATACG |
| FOXO1 | GTCCTACGCCGACCTCAT | ACTTGCTGTGTAGGGACAGAT |
| FOXO3a | GGGGAACTTCACTGGTGCTA | GTTTGAGGGTCTGCTTTGCC |
| B2M | TGTCTTTCAGCAAGGACTGGT | ACATGTCTCGATCCCACTTAAC |
| Antibody | Catalog Number | Supplier | Dilution |
|---|---|---|---|
| Anti-NRF2 (Rabbit) | CST-12721 | Cell Signaling Technology (CST) | 1:1000 |
| Anti-PI3K (Rabbit) | CST-4249 | Cell Signaling Technology (CST) | 1:1000 |
| Anti-p-AKT (Rabbit) | CST-4060 | Cell Signaling Technology (CST) | 1:1000 |
| Anti-AKT (Rabbit) | CST-4691 | Cell Signaling Technology (CST) | 1:1000 |
| Anti-KEAP1 (Rabbit) | CST-8047 | Cell Signaling Technology (CST) | 1:1000 |
| Anti-HO-1 (Rabbit) | CST-26416 | Cell Signaling Technology (CST) | 1:1000 |
| Anti-PTEN (Rabbit) | CST-9188 | Cell Signaling Technology (CST) | 1:1000 |
| Anti-FOXO3a (Rabbit) | CST-2497 | Cell Signaling Technology (CST) | 1:1000 |
| Anti-FOXO1 (Mouse) | CST-2880 | Cell Signaling Technology (CST) | 1:1000 |
| Anti-AQP3 (Mouse) | sc-518001 | Santa Cruz Biotechnology | 1:200 |
| Anti-AQP5 (Mouse) | sc-514022 | Santa Cruz Biotechnology | 1:200 |
| Anti-AKR1B10 (Rabbit) | ab96417 | Abcam | 1:10,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lučić, I.; Mlinarić, M.; Čipak Gašparović, A.; Milković, L. The Influence of AQP5 on the Response to Hydrogen Peroxide in Breast Cancer Cell Lines. Int. J. Mol. Sci. 2025, 26, 3243. https://doi.org/10.3390/ijms26073243
Lučić I, Mlinarić M, Čipak Gašparović A, Milković L. The Influence of AQP5 on the Response to Hydrogen Peroxide in Breast Cancer Cell Lines. International Journal of Molecular Sciences. 2025; 26(7):3243. https://doi.org/10.3390/ijms26073243
Chicago/Turabian StyleLučić, Ivan, Monika Mlinarić, Ana Čipak Gašparović, and Lidija Milković. 2025. "The Influence of AQP5 on the Response to Hydrogen Peroxide in Breast Cancer Cell Lines" International Journal of Molecular Sciences 26, no. 7: 3243. https://doi.org/10.3390/ijms26073243
APA StyleLučić, I., Mlinarić, M., Čipak Gašparović, A., & Milković, L. (2025). The Influence of AQP5 on the Response to Hydrogen Peroxide in Breast Cancer Cell Lines. International Journal of Molecular Sciences, 26(7), 3243. https://doi.org/10.3390/ijms26073243









