Caveolae: Metabolic Platforms at the Crossroads of Health and Disease
Abstract
1. Introduction
2. Lipid Rafts, Caveolae, and Caveolins
2.1. Structural Features of the Caveolin Family of Proteins
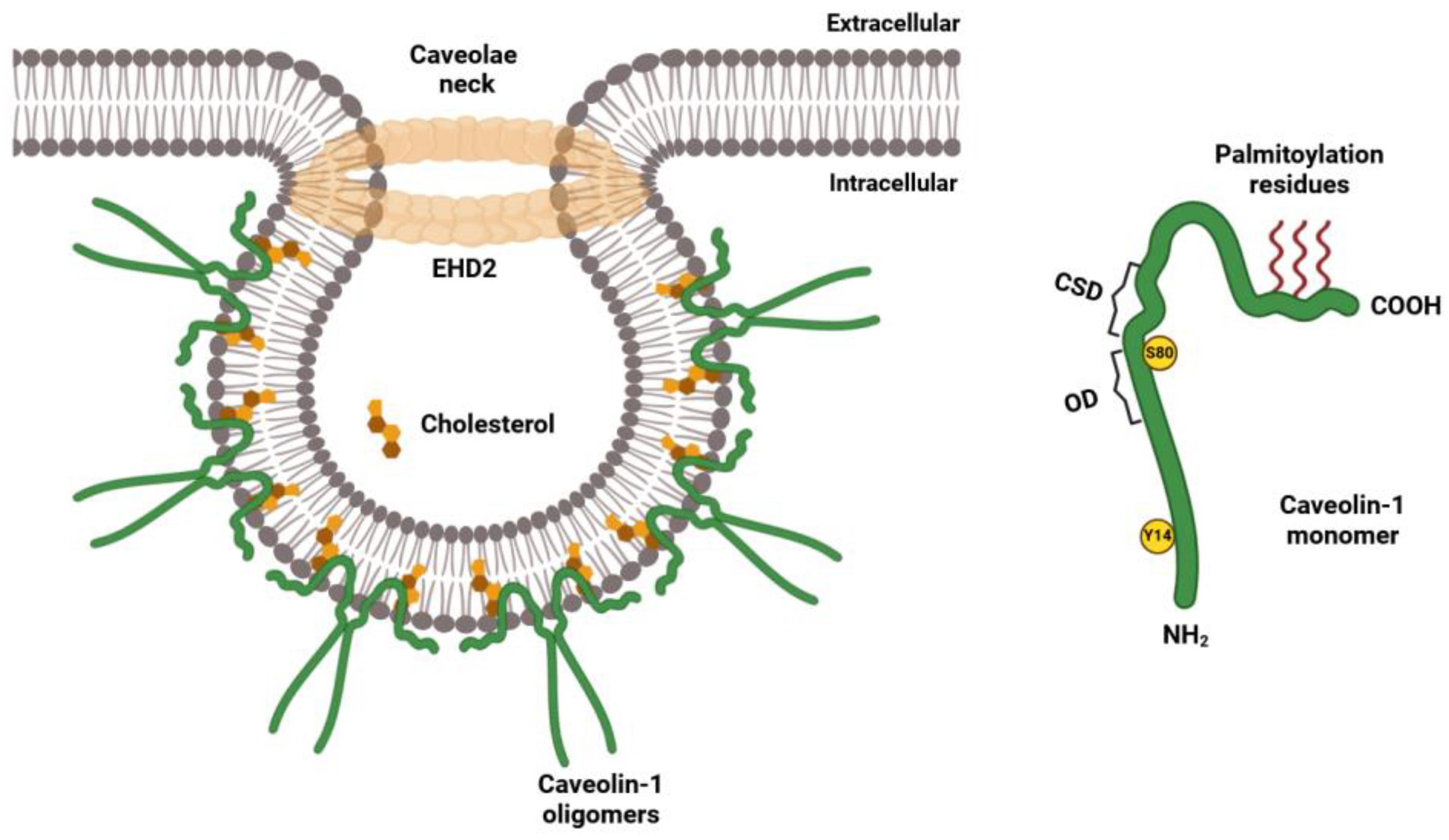
2.2. Biogenesis of Lipid Rafts and Caveolae
3. The Caveolar Network in Cell Metabolism and Metabolic Disorders
3.1. Caveolin Proteins in Glucose Metabolism and Glycolysis
3.2. Caveolae and Caveolin Proteins in Insulin Signaling and Diabetes
3.3. Caveolae and Caveolin Proteins in Obesity
4. Caveolae and Caveolin Proteins in Endothelial Dysfunction and Cardiovascular Disease
4.1. Caveolae and Caveolins in the Regulation of NO Synthesis in ECs
4.2. The Role of Caveolae and Caveolins in Atherosclerosis
5. The Involvement of the Caveolar Network in Autoimmune Diseases
6. The Caveolar Network in Ocular Diseases
7. Caveolae and Caveolins in the Central Nervous System (CNS)
7.1. Caveolae and Caveolins in Alzheimer’s Disease (AD)
7.2. Involvement of Caveolins in Parkinson’s Disease (PD)
7.3. The Role of Caveolins in the Nervous System Tumors
8. Conclusions and Prospective Therapeutic Strategies Targeting Caveolae/Caveolins in Human Disease
Author Contributions
Funding
Conflicts of Interest
References
- Robertson, J.D. The ultrastructure of cell membranes and their derivatives. Biochem. Soc. Symp. 1959, 16, 3–43. [Google Scholar] [PubMed]
- Singer, S.J.; Nicolson, G.L. The fluid mosaic model of the structure of cell membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos-Cardoso, M.; Batista-Almeida, D.; Rios-Barros, L.V.; Castro-Gomes, T.; Girao, H. Cellular and molecular mechanisms underlying plasma membrane functionality and integrity. J. Cell Sci. 2022, 135, jcs259806. [Google Scholar] [CrossRef]
- Regen, S.L. The origin of lipid rafts. Biochemistry 2020, 59, 4617–4621. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Brown, D.A.; London, E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 2000, 275, 17221–17224. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.F.; Peters, F.S.; Camerini, E.; Cretenet, G.; Rietveld, J.; Schomakers, B.V.; van Weeghel, M.; Hahn, N.; Verberk, S.G.S.; Van den Bossche, J.; et al. Cholesterol homeostasis and lipid raft dynamics at the basis of tumor-induced immune dysfunction in chronic lymphocytic leukemia. Cell Mol. Immunol. 2025, 1–16. [Google Scholar] [CrossRef]
- Roy, A.; Patra, S.K. Lipid raft facilitated receptor organization and signaling: A functional rheostat in embryonic development, stem cell biology and cancer. Stem. Cell Rev. Rep. 2023, 19, 2–25. [Google Scholar] [CrossRef]
- Isik, O.A.; Cizmecioglu, O. Rafting on the plasma membrane: Lipid rafts in signaling and disease. Adv. Exp. Med. Biol. 2023, 1436, 87–108. [Google Scholar]
- Bakillah, A.; Hejji, F.A.; Almasaud, A.; Jami, H.A.; Hawwari, A.; Qarni, A.A.; Iqbal, J.; Alharbi, N.K. Lipid raft integrity and cellular cholesterol homeostasis are critical for SARS-COV-2 entry into cells. Nutrients 2022, 14, 3417. [Google Scholar] [CrossRef]
- Mlinac-Jerkovic, K.; Kalanj-Bognar, S.; Heffer, M.; Blazetic, S. Methodological pitfalls of investigating lipid rafts in the brain: What are we still missing? Biomolecules 2024, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Ouweneel, A.B.; Thomas, M.J.; Sorci-Thomas, M.G. The ins and outs of lipid rafts: Functions in intracellular cholesterol homeostasis, microparticles, and cell membranes: Thematic review series: Biology of lipid rafts. J. Lipid Res. 2020, 61, 676–686. [Google Scholar]
- Levental, I.; Levental, K.R.; Heberle, F.A. Lipid rafts: Controversies resolved, mysteries remain. Trends Cell Biol. 2020, 30, 341–353. [Google Scholar] [PubMed]
- D’Alessio, A. Unraveling the cave: A seventy-year journey into the caveolar network, cellular signaling, and human disease. Cells 2023, 12, 2680. [Google Scholar] [CrossRef]
- Parton, R.G.; del Pozo, M.A. Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol. 2013, 14, 98–112. [Google Scholar] [PubMed]
- Stan, R.V. Structure of caveolae. Biochim. Biophys. Acta 2005, 1746, 334–348. [Google Scholar]
- Popov, L.D. Deciphering the relationship between caveolae-mediated intracellular transport and signalling events. Cell. Signal. 2022, 97, 110399. [Google Scholar]
- Pelkmans, L.; Kartenbeck, J.; Helenius, A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the er. Nat. Cell Biol. 2001, 3, 473–483. [Google Scholar]
- Xing, Y.; Wen, Z.; Gao, W.; Lin, Z.; Zhong, J.; Jiu, Y. Multifaceted functions of host cell caveolae/caveolin-1 in virus infections. Viruses 2020, 12, 487. [Google Scholar] [CrossRef]
- Del Pozo, M.A.; Lolo, F.N.; Echarri, A. Caveolae: Mechanosensing and mechanotransduction devices linking membrane trafficking to mechanoadaptation. Curr. Opin. Cell Biol. 2021, 68, 113–123. [Google Scholar]
- Sinha, B.; Koster, D.; Ruez, R.; Gonnord, P.; Bastiani, M.; Abankwa, D.; Stan, R.V.; Butler-Browne, G.; Vedie, B.; Johannes, L.; et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 2010, 144, 402–413. [Google Scholar]
- Stefl, M.; Takamiya, M.; Middel, V.; Tekpinar, M.; Nienhaus, K.; Beil, T.; Rastegar, S.; Strahle, U.; Nienhaus, G.U. Caveolae disassemble upon membrane lesioning and foster cell survival. iScience 2024, 27, 108849. [Google Scholar] [CrossRef]
- Madaro, L.; Antonangeli, F.; Favia, A.; Esposito, B.; Biamonte, F.; Bouche, M.; Ziparo, E.; Sica, G.; Filippini, A.; D’Alessio, A. Knock down of caveolin-1 affects morphological and functional hallmarks of human endothelial cells. J. Cell. Biochem. 2013, 114, 1843–1851. [Google Scholar]
- Parton, R.G. Caveolae: Structure, function, and relationship to disease. Annu. Rev. Cell Dev. Biol. 2018, 34, 111–136. [Google Scholar] [PubMed]
- Drab, M.; Verkade, P.; Elger, M.; Kasper, M.; Lohn, M.; Lauterbach, B.; Menne, J.; Lindschau, C.; Mende, F.; Luft, F.C.; et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 2001, 293, 2449–2452. [Google Scholar] [PubMed]
- Pol, A.; Morales-Paytuvi, F.; Bosch, M.; Parton, R.G. Non-caveolar caveolins—Duties outside the caves. J. Cell Sci. 2020, 133, jcs241562. [Google Scholar] [PubMed]
- Head, B.P.; Insel, P.A. Do caveolins regulate cells by actions outside of caveolae? Trends Cell Biol. 2007, 17, 51–57. [Google Scholar]
- Goutas, A.; Outskouni, Z.; Papathanasiou, I.; Satra, M.; Koliakos, G.; Trachana, V. Dysregulation of caveolin-1 phosphorylation and nuclear translocation is associated with senescence onset. Cells 2021, 10, 2939. [Google Scholar] [CrossRef]
- Hau, A.M.; Gupta, S.; Leivo, M.Z.; Nakashima, K.; Macias, J.; Zhou, W.; Hodge, A.; Wulfkuhle, J.; Conkright, B.; Bhuvaneshwar, K.; et al. Dynamic regulation of caveolin-1 phosphorylation and caveolae formation by mammalian target of rapamycin complex 2 in bladder cancer cells. Am. J. Pathol. 2019, 189, 1846–1862. [Google Scholar]
- Meng, F.; Saxena, S.; Liu, Y.; Joshi, B.; Wong, T.H.; Shankar, J.; Foster, L.J.; Bernatchez, P.; Nabi, I.R. The phospho-caveolin-1 scaffolding domain dampens force fluctuations in focal adhesions and promotes cancer cell migration. Mol. Biol. Cell 2017, 28, 2190–2201. [Google Scholar]
- Wong, T.H.; Dickson, F.H.; Timmins, L.R.; Nabi, I.R. Tyrosine phosphorylation of tumor cell caveolin-1: Impact on cancer progression. Cancer Metastasis Rev. 2020, 39, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.; Pawling, J.; Shankar, J.; Pacholczyk, K.; Kim, Y.; Tran, W.; Meng, F.; Rahman, A.M.A.; Foster, L.J.; Leong, H.S.; et al. Caveolin-1 y14 phosphorylation suppresses tumor growth while promoting invasion. Oncotarget 2019, 10, 6668–6677. [Google Scholar] [PubMed]
- Nah, J.; Yoo, S.M.; Jung, S.; Jeong, E.I.; Park, M.; Kaang, B.K.; Jung, Y.K. Phosphorylated cav1 activates autophagy through an interaction with becn1 under oxidative stress. Cell Death Dis. 2017, 8, e2822. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, G.; Zhang, X.; Minshall, R.D. Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary vascular permeability via paracellular and transcellular pathways. Circ. Res. 2009, 105, 676–685. [Google Scholar]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar]
- D’Aprile, C.; Prioni, S.; Mauri, L.; Prinetti, A.; Grassi, S. Lipid rafts as platforms for sphingosine 1-phosphate metabolism and signalling. Cell. Signal. 2021, 80, 109929. [Google Scholar] [PubMed]
- Mollinedo, F.; Gajate, C. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: Implications in tumor progression and therapy: Thematic review series: Biology of lipid rafts. J. Lipid Res. 2020, 61, 611–635. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J. Lipid rafts: Bringing order to chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar]
- Hogue, I.B.; Grover, J.R.; Soheilian, F.; Nagashima, K.; Ono, A. Gag induces the coalescence of clustered lipid rafts and tetraspanin-enriched microdomains at hiv-1 assembly sites on the plasma membrane. J. Virol. 2011, 85, 9749–9766. [Google Scholar]
- Hofman, E.G.; Ruonala, M.O.; Bader, A.N.; van den Heuvel, D.; Voortman, J.; Roovers, R.C.; Verkleij, A.J.; Gerritsen, H.C.; van Bergen En Henegouwen, P.M. Egf induces coalescence of different lipid rafts. J. Cell Sci. 2008, 121, 2519–2528. [Google Scholar]
- Palade, G.E. Fine structure of blood capillaries. J. Appl. Phys. 1953, 24, 1424. [Google Scholar]
- Yamada, E. The fine structure of the gall bladder epithelium of the mouse. J. Biophys. Biochem. Cytol. 1955, 1, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Kurzchalia, T.V.; Dupree, P.; Parton, R.G.; Kellner, R.; Virta, H.; Lehnert, M.; Simons, K. Vip21, a 21-kd membrane protein is an integral component of trans-golgi-network-derived transport vesicles. J. Cell Biol. 1992, 118, 1003–1014. [Google Scholar] [CrossRef]
- Scherer, P.E.; Okamoto, T.; Chun, M.; Nishimoto, I.; Lodish, H.F.; Lisanti, M.P. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc. Natl. Acad. Sci. USA 1996, 93, 131–135. [Google Scholar] [CrossRef]
- Tang, Z.; Scherer, P.E.; Okamoto, T.; Song, K.; Chu, C.; Kohtz, D.S.; Nishimoto, I.; Lodish, H.F.; Lisanti, M.P. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J. Biol. Chem. 1996, 271, 2255–2261. [Google Scholar] [CrossRef]
- Brown, D.A.; Rose, J.K. Sorting of gpi-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 1992, 68, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kabayama, K. Structural analyses of the glycolipids in lipid rafts. Methods Mol. Biol. 2023, 2613, 145–152. [Google Scholar]
- Gajate, C.; Mollinedo, F. Lipid raft isolation by sucrose gradient centrifugation and visualization of raft-located proteins by fluorescence microscopy: The use of combined techniques to assess fas/cd95 location in rafts during apoptosis triggering. Methods Mol. Biol. 2021, 2187, 147–186. [Google Scholar]
- D’Alessio, A.; Esposito, B.; Giampietri, C.; Ziparo, E.; Pober, J.S.; Filippini, A. Plasma membrane micro domains regulate tace-dependent tnfr1 shedding in human endothelial cells. J. Cell. Mol. Med. 2011, 16, 626–635. [Google Scholar] [CrossRef]
- D’Alessio, A.; Al-Lamki, R.S.; Bradley, J.R.; Pober, J.S. Caveolae participate in tumor necrosis factor receptor 1 signaling and internalization in a human endothelial cell line. Am. J. Pathol. 2005, 166, 1273–1282. [Google Scholar] [CrossRef]
- Dupree, P.; Parton, R.G.; Raposo, G.; Kurzchalia, T.V.; Simons, K. Caveolae and sorting in the trans-golgi network of epithelial cells. EMBO J. 1993, 12, 1597–1605. [Google Scholar] [CrossRef]
- Nystrom, F.H.; Chen, H.; Cong, L.N.; Li, Y.; Quon, M.J. Caveolin-1 interacts with the insulin receptor and can differentially modulate insulin signaling in transfected cos-7 cells and rat adipose cells. Mol. Endocrinol. 1999, 13, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cardena, G.; Martasek, P.; Masters, B.S.; Skidd, P.M.; Couet, J.; Li, S.; Lisanti, M.P.; Sessa, W.C. Dissecting the interaction between nitric oxide synthase (nos) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J. Biol. Chem. 1997, 272, 25437–25440. [Google Scholar] [CrossRef]
- Li, S.; Couet, J.; Lisanti, M.P. Src tyrosine kinases, galpha subunits, and h-ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of src tyrosine kinases. J. Biol. Chem. 1996, 271, 29182–29190. [Google Scholar] [CrossRef] [PubMed]
- Vihanto, M.M.; Vindis, C.; Djonov, V.; Cerretti, D.P.; Huynh-Do, U. Caveolin-1 is required for signaling and membrane targeting of ephb1 receptor tyrosine kinase. J. Cell Sci. 2006, 119, 2299–2309. [Google Scholar] [CrossRef]
- Bauer, P.M.; Yu, J.; Chen, Y.; Hickey, R.; Bernatchez, P.N.; Looft-Wilson, R.; Huang, Y.; Giordano, F.; Stan, R.V.; Sessa, W.C. Endothelial-specific expression of caveolin-1 impairs microvascular permeability and angiogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Bucci, M.; Gratton, J.P.; Rudic, R.D.; Acevedo, L.; Roviezzo, F.; Cirino, G.; Sessa, W.C. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat. Med. 2000, 6, 1362–1367. [Google Scholar] [CrossRef]
- Couet, J.; Li, S.; Okamoto, T.; Ikezu, T.; Lisanti, M.P. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J. Biol. Chem. 1997, 272, 6525–6533. [Google Scholar] [CrossRef]
- Collins, B.M.; Davis, M.J.; Hancock, J.F.; Parton, R.G. Structure-based reassessment of the caveolin signaling model: Do caveolae regulate signaling through caveolin-protein interactions? Dev. Cell 2012, 23, 11–20. [Google Scholar] [CrossRef]
- Schlegel, A.; Lisanti, M.P. A molecular dissection of caveolin-1 membrane attachment and oligomerization. Two separate regions of the caveolin-1 c-terminal domain mediate membrane binding and oligomer/oligomer interactions in vivo. J. Biol. Chem. 2000, 275, 21605–21617. [Google Scholar] [CrossRef]
- Dietzen, D.J.; Hastings, W.R.; Lublin, D.M. Caveolin is palmitoylated on multiple cysteine residues. Palmitoylation is not necessary for localization of caveolin to caveolae. J. Biol. Chem. 1995, 270, 6838–6842. [Google Scholar]
- Scherer, P.E.; Tang, Z.; Chun, M.; Sargiacomo, M.; Lodish, H.F.; Lisanti, M.P. Caveolin isoforms differ in their n-terminal protein sequence and subcellular distribution. Identification and epitope mapping of an isoform-specific monoclonal antibody probe. J. Biol. Chem. 1995, 270, 16395–16401. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Courchesne, W.E.; Mastick, C.C. A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: Recruitment of c-terminal src kinase. J. Biol. Chem. 2002, 277, 8771–8774. [Google Scholar] [PubMed]
- Li, S.; Seitz, R.; Lisanti, M.P. Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-src in vivo. J. Biol. Chem. 1996, 271, 3863–3868. [Google Scholar]
- Lu, T.; Zhang, Z.; Pan, X.; Zhang, J.; Wang, X.; Wang, M.; Li, H.; Yan, M.; Chen, W. Caveolin-1 promotes cancer progression via inhibiting ferroptosis in head and neck squamous cell carcinoma. J. Oral Pathol. Med. 2022, 51, 52–62. [Google Scholar]
- Yoon, H.J.; Surh, Y.J. Modulation of cancer cell growth and progression by caveolin-1 in the tumor microenvironment. Adv. Exp. Med. Biol. 2020, 1277, 63–74. [Google Scholar] [PubMed]
- Scherer, P.E.; Lewis, R.Y.; Volonte, D.; Engelman, J.A.; Galbiati, F.; Couet, J.; Kohtz, D.S.; van Donselaar, E.; Peters, P.; Lisanti, M.P. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J. Biol. Chem. 1997, 272, 29337–29346. [Google Scholar] [CrossRef]
- Sowa, G. Novel insights into the role of caveolin-2 in cell- and tissue-specific signaling and function. Biochem. Res. Int. 2011, 2011, 809259. [Google Scholar]
- Liu, Y.; Qi, X.; Li, G.; Sowa, G. Caveolin-2 deficiency induces a rapid anti-tumor immune response prior to regression of implanted murine lung carcinoma tumors. Sci. Rep. 2019, 9, 18970. [Google Scholar]
- Razani, B.; Wang, X.B.; Engelman, J.A.; Battista, M.; Lagaud, G.; Zhang, X.L.; Kneitz, B.; Hou, H., Jr.; Christ, G.J.; Edelmann, W.; et al. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol. Cell. Biol. 2002, 22, 2329–2344. [Google Scholar]
- Matsunobe, M.; Motohashi, N.; Aoki, E.; Tominari, T.; Inada, M.; Aoki, Y. Caveolin-3 regulates the activity of Ca2+/calmodulin-dependent protein kinase ii in c2c12 cells. Am. J. Physiol. Cell Physiol. 2022, 323, C1137–C1148. [Google Scholar] [PubMed]
- Madaro, L.; Marrocco, V.; Fiore, P.; Aulino, P.; Smeriglio, P.; Adamo, S.; Molinaro, M.; Bouche, M. Pkctheta signaling is required for myoblast fusion by regulating the expression of caveolin-3 and beta1d integrin upstream focal adhesion kinase. Mol. Biol. Cell 2011, 22, 1409–1419. [Google Scholar] [PubMed]
- Volonte, D.; Peoples, A.J.; Galbiati, F. Modulation of myoblast fusion by caveolin-3 in dystrophic skeletal muscle cells: Implications for duchenne muscular dystrophy and limb-girdle muscular dystrophy-1c. Mol. Biol. Cell 2003, 14, 4075–4088. [Google Scholar] [CrossRef]
- Whiteley, G.; Collins, R.F.; Kitmitto, A. Characterization of the molecular architecture of human caveolin-3 and interaction with the skeletal muscle ryanodine receptor. J. Biol. Chem. 2012, 287, 40302–40316. [Google Scholar] [PubMed]
- Nakashima, M.; Suga, N.; Yoshikawa, S.; Matsuda, S. Caveolin and NOS in the development of muscular dystrophy. Int. J. Mol. Sci. 2024, 25, 8771. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, Z.; Zhao, J.; Liu, C.; Yao, P.; Zhang, L.; Xie, D.; Lau, W.B.; Tsukuda, J.; Christopher, T.A.; et al. Nitrative modification of caveolin-3: A novel mechanism of cardiac insulin resistance and a potential therapeutic target against ischemic heart failure in prediabetic animals. Circulation 2023, 147, 1162–1179. [Google Scholar]
- Shang, L.; Chen, T.; Deng, Y.; Huang, Y.; Huang, Y.; Xian, J.; Lu, W.; Yang, L.; Huang, Q. Caveolin-3 promotes glycometabolism, growth and proliferation in muscle cells. PLoS ONE 2017, 12, e0189004. [Google Scholar]
- Chaudhry, R.; Varacallo, M.A. Biochemistry, glycolysis. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Galbiati, F.; Volonte, D.; Minetti, C.; Chu, J.B.; Lisanti, M.P. Phenotypic behavior of caveolin-3 mutations that cause autosomal dominant limb girdle muscular dystrophy (lgmd-1c). Retention of lgmd-1c caveolin-3 mutants within the golgi complex. J. Biol. Chem. 1999, 274, 25632–25641. [Google Scholar]
- Minetti, C.; Sotgia, F.; Bruno, C.; Scartezzini, P.; Broda, P.; Bado, M.; Masetti, E.; Mazzocco, M.; Egeo, A.; Donati, M.A.; et al. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat. Genet. 1998, 18, 365–368. [Google Scholar]
- Hayer, A.; Stoeber, M.; Bissig, C.; Helenius, A. Biogenesis of caveolae: Stepwise assembly of large caveolin and cavin complexes. Traffic 2010, 11, 361–382. [Google Scholar] [CrossRef]
- Monier, S.; Parton, R.G.; Vogel, F.; Behlke, J.; Henske, A.; Kurzchalia, T.V. Vip21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol. Biol. Cell 1995, 6, 911–927. [Google Scholar] [PubMed]
- Porta, J.C.; Han, B.; Gulsevin, A.; Chung, J.M.; Peskova, Y.; Connolly, S.; McHaourab, H.S.; Meiler, J.; Karakas, E.; Kenworthy, A.K.; et al. Molecular architecture of the human caveolin-1 complex. Sci. Adv. 2022, 8, eabn7232. [Google Scholar]
- Hill, M.M.; Bastiani, M.; Luetterforst, R.; Kirkham, M.; Kirkham, A.; Nixon, S.J.; Walser, P.; Abankwa, D.; Oorschot, V.M.; Martin, S.; et al. Ptrf-cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell 2008, 132, 113–124. [Google Scholar] [PubMed]
- Anderson, R.G. The caveolae membrane system. Annu. Rev. Biochem. 1998, 67, 199–225. [Google Scholar] [PubMed]
- Parton, R.G.; Richards, A.A. Lipid rafts and caveolae as portals for endocytosis: New insights and common mechanisms. Traffic 2003, 4, 724–738. [Google Scholar]
- Head, B.P.; Patel, H.H.; Insel, P.A. Interaction of membrane/lipid rafts with the cytoskeleton: Impact on signaling and function: Membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim. Biophys. Acta 2014, 1838, 532–545. [Google Scholar]
- Razani, B.; Woodman, S.E.; Lisanti, M.P. Caveolae: From cell biology to animal physiology. Pharmacol. Rev. 2002, 54, 431–467. [Google Scholar]
- Parton, R.G.; Hanzal-Bayer, M.; Hancock, J.F. Biogenesis of caveolae: A structural model for caveolin-induced domain formation. J. Cell Sci. 2006, 119, 787–796. [Google Scholar]
- Parton, R.G.; Simons, K. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 2007, 8, 185–194. [Google Scholar]
- Anderson, R.G.; Jacobson, K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 2002, 296, 1821–1825. [Google Scholar]
- Norkin, L.C.; Anderson, H.A.; Wolfrom, S.A.; Oppenheim, A. Caveolar endocytosis of simian virus 40 is followed by brefeldin a-sensitive transport to the endoplasmic reticulum, where the virus disassembles. J. Virol. 2002, 76, 5156–5166. [Google Scholar]
- Bernatchez, P.N.; Tao, B.; Bradshaw, R.A.; Eveleth, D.; Sessa, W.C. Characterization of a novel caveolin modulator that reduces vascular permeability and ocular inflammation. Transl. Vis. Sci. Technol. 2021, 10, 21. [Google Scholar] [PubMed]
- Hashimoto, T.; Isaji, T.; Hu, H.; Yamamoto, K.; Bai, H.; Santana, J.M.; Kuo, A.; Kuwahara, G.; Foster, T.R.; Hanisch, J.J.; et al. Stimulation of caveolin-1 signaling improves arteriovenous fistula patency. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 754–764. [Google Scholar]
- Yan, S.; Xue, P.; Sun, Y.; Bai, T.; Shao, S.; Zeng, X. Cupric doping hollow prussian blue nanoplatform for enhanced cholesterol depletion: A promising strategy for breast cancer therapy and metastasis inhibition. Adv. Sci. 2025, 12, e2409967. [Google Scholar]
- Liu, Y.; Niu, R.; Deng, R.; Wang, Y.; Song, S.; Zhang, H. Multi-enzyme co-expressed nanomedicine for anti-metastasis tumor therapy by up-regulating cellular oxidative stress and depleting cholesterol. Adv. Mater. 2024, 36, e2307752. [Google Scholar]
- Terao, J. Caveolae and caveolin-1 as targets of dietary polyphenols for protection against vascular endothelial dysfunction. J. Clin. Biochem. Nutr. 2024, 75, 7–16. [Google Scholar]
- Huang, F.; Mao, F.; Nong, W.; Gong, Z.; Lao, D.; Huang, W. Inhibiting caveolin-1-related akt/mtor signaling pathway protects against n-methyl-d-aspartate receptor activation-mediated dysfunction of blood-brain barrier in vitro. Mol. Neurobiol. 2024, 61, 4166–4177. [Google Scholar] [PubMed]
- Arya, A.; Yadav, H.N.; Sharma, P.L. Involvement of vascular endothelial nitric oxide synthase in development of experimental diabetic nephropathy in rats. Mol. Cell. Biochem. 2011, 354, 57–66. [Google Scholar]
- Larsson, E.; Moren, B.; McMahon, K.A.; Parton, R.G.; Lundmark, R. Dynamin2 functions as an accessory protein to reduce the rate of caveola internalization. J. Cell Biol. 2023, 222, e202205122. [Google Scholar]
- Wang, Y.; Zhang, Y.; Zuo, W.; Bo, Z.; Zhang, C.; Zhang, X.; Wu, Y. Avian reovirus sigmab interacts with caveolin-1 in lipid rafts during dynamin-dependent caveolae-mediated endocytosis. Viruses 2022, 14, 2201. [Google Scholar]
- Tachikawa, M.; Morone, N.; Senju, Y.; Sugiura, T.; Hanawa-Suetsugu, K.; Mochizuki, A.; Suetsugu, S. Measurement of caveolin-1 densities in the cell membrane for quantification of caveolar deformation after exposure to hypotonic membrane tension. Sci. Rep. 2017, 7, 7794. [Google Scholar]
- Maddila, S.C.; Voshavar, C.; Arjunan, P.; Chowath, R.P.; Rachamalla, H.K.R.; Balakrishnan, B.; Balasubramanian, P.; Banerjee, R.; Marepally, S. Cholesterol sequestration from caveolae/lipid rafts enhances cationic liposome-mediated nucleic acid delivery into endothelial cells. Molecules 2021, 26, 4626. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, J.E.; Oh, P.; Pinney, E.; Allard, J. Filipin-sensitive caveolae-mediated transport in endothelium: Reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell Biol. 1994, 127, 1217–1232. [Google Scholar] [PubMed]
- Gyoten, M.; Luo, Y.; Fujiwara-Tani, R.; Mori, S.; Ogata, R.; Kishi, S.; Kuniyasu, H. Lovastatin treatment inducing apoptosis in human pancreatic cancer cells by inhibiting cholesterol rafts in plasma membrane and mitochondria. Int. J. Mol. Sci. 2023, 24, 16814. [Google Scholar] [CrossRef]
- Pereira, P.M.R.; Mandleywala, K.; Monette, S.; Lumish, M.; Tully, K.M.; Panikar, S.S.; Cornejo, M.; Mauguen, A.; Ragupathi, A.; Keltee, N.C.; et al. Caveolin-1 temporal modulation enhances antibody drug efficacy in heterogeneous gastric cancer. Nat. Commun. 2022, 13, 2526. [Google Scholar]
- Fu, J.; Mansfield, C.; Diakonov, I.; Judina, A.; Delahaye, M.; Bhogal, N.; Sanchez-Alonso, J.L.; Kamp, T.; Gorelik, J. Stretch regulation of beta2-adrenoceptor signalling in cardiomyocytes requires caveolae. Cardiovasc. Res. 2025, cvae265. [Google Scholar] [CrossRef]
- Fadeyibi, O.; Rybalchenko, N.; Mabry, S.; Nguyen, D.H.; Cunningham, R.L. The role of lipid rafts and membrane androgen receptors in androgen’s neurotoxic effects. J. Endocr. Soc. 2022, 6, bvac030. [Google Scholar]
- Tillu, V.A.; Rae, J.; Gao, Y.; Ariotti, N.; Floetenmeyer, M.; Kovtun, O.; McMahon, K.A.; Chaudhary, N.; Parton, R.G.; Collins, B.M. Cavin1 intrinsically disordered domains are essential for fuzzy electrostatic interactions and caveola formation. Nat. Commun. 2021, 12, 931. [Google Scholar]
- Wang, L.; Song, Y.; Shu, Y.; Xue, B.; Yu, F.; Yin, Y.; Feng, Z.; Ma, X.; Yao, Y.; Pan, Y.; et al. Cavin-2 positively correlates with diabetic pad and promotes ldl transcytosis by inhibiting enos activation. Ann. Med. 2025, 57, 2457526. [Google Scholar]
- Hoernke, M.; Mohan, J.; Larsson, E.; Blomberg, J.; Kahra, D.; Westenhoff, S.; Schwieger, C.; Lundmark, R. Ehd2 restrains dynamics of caveolae by an atp-dependent, membrane-bound, open conformation. Proc. Natl. Acad. Sci. USA 2017, 114, E4360–E4369. [Google Scholar]
- Yeow, I.; Howard, G.; Chadwick, J.; Mendoza-Topaz, C.; Hansen, C.G.; Nichols, B.J.; Shvets, E. Ehd proteins cooperate to generate caveolar clusters and to maintain caveolae during repeated mechanical stress. Curr. Biol. 2017, 27, 2951–2962.e2955. [Google Scholar] [CrossRef]
- Hansen, C.G.; Howard, G.; Nichols, B.J. Pacsin 2 is recruited to caveolae and functions in caveolar biogenesis. J. Cell Sci. 2011, 124, 2777–2785. [Google Scholar] [CrossRef]
- Kovtun, O.; Tillu, V.A.; Ariotti, N.; Parton, R.G.; Collins, B.M. Cavin family proteins and the assembly of caveolae. J. Cell Sci. 2015, 128, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic syndrome: Updates on pathophysiology and management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Yang, M.; Zhao, H.; Han, Y.; Jiang, N.; Yang, J.; Chen, W.; Li, C.; Liu, Y.; Zhao, C.; et al. Caveolin-1 regulates cellular metabolism: A potential therapeutic target in kidney disease. Front. Pharmacol. 2021, 12, 768100. [Google Scholar] [CrossRef] [PubMed]
- de Souza, G.M.; de Albuquerque Borborema, M.E.; de Lucena, T.M.C.; da Silva Santos, A.F.; de Lima, B.R.; de Oliveira, D.C.; de Azevedo Silva, J. Caveolin-1 (cav-1) up regulation in metabolic syndrome: All roads leading to the same end. Mol. Biol. Rep. 2020, 47, 9245–9250. [Google Scholar] [CrossRef]
- Shah, D.S.; Nisr, R.B.; Krasteva-Christ, G.; Hundal, H.S. Caveolin-3 loss linked with the p104l lgmd-1c mutation modulates skeletal muscle mtorc1 signalling and cholesterol homeostasis. J. Cachexia Sarcopenia Muscle 2023, 14, 2310–2326. [Google Scholar] [CrossRef]
- Cohen, A.W.; Razani, B.; Wang, X.B.; Combs, T.P.; Williams, T.M.; Scherer, P.E.; Lisanti, M.P. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am. J. Physiol. Cell Physiol. 2003, 285, C222–C235. [Google Scholar] [CrossRef]
- Haddad, D.; Al Madhoun, A.; Nizam, R.; Al-Mulla, F. Role of caveolin-1 in diabetes and its complications. Oxidative Med. Cell. Longev. 2020, 2020, 9761539. [Google Scholar] [CrossRef]
- Frank, P.G.; Cheung, M.W.; Pavlides, S.; Llaverias, G.; Park, D.S.; Lisanti, M.P. Caveolin-1 and regulation of cellular cholesterol homeostasis. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H677–H686. [Google Scholar] [CrossRef]
- Mercier, I.; Jasmin, J.F.; Pavlides, S.; Minetti, C.; Flomenberg, N.; Pestell, R.G.; Frank, P.G.; Sotgia, F.; Lisanti, M.P. Clinical and translational implications of the caveolin gene family: Lessons from mouse models and human genetic disorders. Lab. Investig. 2009, 89, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Lee, Y.H.; Kim, Y.D.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, B.W.; Kang, E.S.; Cha, B.S.; et al. Nonalcoholic fatty liver disease and sarcopenia are independently associated with cardiovascular risk. Am. J. Gastroenterol. 2020, 115, 584–595. [Google Scholar] [CrossRef]
- Al Madhoun, A.; Kochumon, S.; Haddad, D.; Thomas, R.; Nizam, R.; Miranda, L.; Sindhu, S.; Bitar, M.S.; Ahmad, R.; Al-Mulla, F. Adipose tissue caveolin-1 upregulation in obesity involves tnf-alpha/nf-kappab mediated signaling. Cells 2023, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S. Glycolysis. Cold Spring Harb. Perspect. Biol. 2021, 13, a040535. [Google Scholar]
- Wang, Y.; Dang, C.V. The warburg effect revisited through blood and electron flow. Cancer Res. 2024, 84, 2046–2048. [Google Scholar]
- Barba, I.; Carrillo-Bosch, L.; Seoane, J. Targeting the warburg effect in cancer: Where do we stand? Int. J. Mol. Sci. 2024, 25, 3142. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar]
- Weinhouse, S. Studies on the fate of isotopically labeled metabolites in the oxidative metabolism of tumors. Cancer Res. 1951, 11, 585–591. [Google Scholar]
- Hay, N. Reprogramming glucose metabolism in cancer: Can it be exploited for cancer therapy? Nat. Rev. Cancer 2016, 16, 635–649. [Google Scholar] [CrossRef]
- Yang, L.; Li, S.; Yu, L.; Leng, J.; Li, N. Targeting glycolysis: Exploring a new frontier in glioblastoma therapy. Front. Immunol. 2024, 15, 1522392. [Google Scholar]
- Zhao, J.; Jin, D.; Huang, M.; Ji, J.; Xu, X.; Wang, F.; Zhou, L.; Bao, B.; Jiang, F.; Xu, W.; et al. Glycolysis in the tumor microenvironment: A driver of cancer progression and a promising therapeutic target. Front. Cell Dev. Biol. 2024, 12, 1416472. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Tahir, S.A.; Yang, G.; Goltsov, A.; Song, K.D.; Ren, C.; Wang, J.; Chang, W.; Thompson, T.C. Caveolin-1-lrp6 signaling module stimulates aerobic glycolysis in prostate cancer. Cancer Res. 2013, 73, 1900–1911. [Google Scholar] [CrossRef]
- Diaz-Valdivia, N.; Simon, L.; Diaz, J.; Martinez-Meza, S.; Contreras, P.; Burgos-Ravanal, R.; Perez, V.I.; Frei, B.; Leyton, L.; Quest, A.F.G. Mitochondrial dysfunction and the glycolytic switch induced by caveolin-1 phosphorylation promote cancer cell migration, invasion, and metastasis. Cancers 2022, 14, 2862. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.K.; Her, N.G.; Lee, M.G.; Ryu, B.K.; Lee, J.H.; Han, J.; Jeong, S.I.; Kang, M.J.; Kim, N.H.; Kim, H.J.; et al. Caveolin-1 increases aerobic glycolysis in colorectal cancers by stimulating hmga1-mediated glut3 transcription. Cancer Res. 2012, 72, 4097–4109. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xu, X.; Li, Q.; Chen, X.; Yuan, X.; Qiu, S.; Yao, C.; Zhang, D.; Wang, F. Caveolin-1 increases glycolysis in pancreatic cancer cells and triggers cachectic states. FASEB J. 2021, 35, e21826. [Google Scholar] [CrossRef]
- Gazzerro, E.; Sotgia, F.; Bruno, C.; Lisanti, M.P.; Minetti, C. Caveolinopathies: From the biology of caveolin-3 to human diseases. Eur. J. Hum. Genet. 2010, 18, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.E.; Lisanti, M.P. Association of phosphofructokinase-m with caveolin-3 in differentiated skeletal myotubes. Dynamic regulation by extracellular glucose and intracellular metabolites. J. Biol. Chem. 1997, 272, 20698–20705. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]
- Karlsson, M.; Thorn, H.; Parpal, S.; Stralfors, P.; Gustavsson, J. Insulin induces translocation of glucose transporter glut4 to plasma membrane caveolae in adipocytes. FASEB J. 2002, 16, 249–251. [Google Scholar] [CrossRef]
- Scherer, P.E.; Lisanti, M.P.; Baldini, G.; Sargiacomo, M.; Mastick, C.C.; Lodish, H.F. Induction of caveolin during adipogenesis and association of glut4 with caveolin-rich vesicles. J. Cell Biol. 1994, 127, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; Way, M.; Zorzi, N.; Stang, E. Caveolin-3 associates with developing t-tubules during muscle differentiation. J. Cell Biol. 1997, 136, 137–154. [Google Scholar] [CrossRef]
- Watson, R.T.; Shigematsu, S.; Chiang, S.H.; Mora, S.; Kanzaki, M.; Macara, I.G.; Saltiel, A.R.; Pessin, J.E. Lipid raft microdomain compartmentalization of tc10 is required for insulin signaling and glut4 translocation. J. Cell Biol. 2001, 154, 829–840. [Google Scholar] [CrossRef]
- Gustavsson, J.; Parpal, S.; Karlsson, M.; Ramsing, C.; Thorn, H.; Borg, M.; Lindroth, M.; Peterson, K.H.; Magnusson, K.E.; Stralfors, P. Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J. 1999, 13, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Hong, S.; Yao, Y.; Liao, K. Glut-4 is translocated to both caveolae and non-caveolar lipid rafts, but is partially internalized through caveolae in insulin-stimulated adipocytes. Cell Res. 2007, 17, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Couet, J.; Sargiacomo, M.; Lisanti, M.P. Interaction of a receptor tyrosine kinase, egf-r, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J. Biol. Chem. 1997, 272, 30429–30438. [Google Scholar] [CrossRef]
- Kimura, A.; Mora, S.; Shigematsu, S.; Pessin, J.E.; Saltiel, A.R. The insulin receptor catalyzes the tyrosine phosphorylation of caveolin-1. J. Biol. Chem. 2002, 277, 30153–30158. [Google Scholar] [CrossRef]
- Mastick, C.C.; Saltiel, A.R. Insulin-stimulated tyrosine phosphorylation of caveolin is specific for the differentiated adipocyte phenotype in 3t3-l1 cells. J. Biol. Chem. 1997, 272, 20706–20714. [Google Scholar] [CrossRef]
- Mastick, C.C.; Brady, M.J.; Saltiel, A.R. Insulin stimulates the tyrosine phosphorylation of caveolin. J. Cell Biol. 1995, 129, 1523–1531. [Google Scholar] [CrossRef]
- Nevins, A.K.; Thurmond, D.C. Caveolin-1 functions as a novel cdc42 guanine nucleotide dissociation inhibitor in pancreatic beta-cells. J. Biol. Chem. 2006, 281, 18961–18972. [Google Scholar] [CrossRef]
- Otsu, K.; Toya, Y.; Oshikawa, J.; Kurotani, R.; Yazawa, T.; Sato, M.; Yokoyama, U.; Umemura, S.; Minamisawa, S.; Okumura, S.; et al. Caveolin gene transfer improves glucose metabolism in diabetic mice. Am. J. Physiol. Cell Physiol. 2010, 298, C450–C456. [Google Scholar] [PubMed]
- Razani, B.; Combs, T.P.; Wang, X.B.; Frank, P.G.; Park, D.S.; Russell, R.G.; Li, M.; Tang, B.; Jelicks, L.A.; Scherer, P.E.; et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J. Biol. Chem. 2002, 277, 8635–8647. [Google Scholar]
- Bae, G.D.; Park, E.Y.; Kim, K.; Jang, S.E.; Jun, H.S.; Oh, Y.S. Upregulation of caveolin-1 and its colocalization with cytokine receptors contributes to beta cell apoptosis. Sci. Rep. 2019, 9, 16785. [Google Scholar]
- Briand, N.; Prado, C.; Mabilleau, G.; Lasnier, F.; Le Liepvre, X.; Covington, J.D.; Ravussin, E.; Le Lay, S.; Dugail, I. Caveolin-1 expression and cavin stability regulate caveolae dynamics in adipocyte lipid store fluctuation. Diabetes 2014, 63, 4032–4044. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.W.; Wang, J.; Guo, H.; Zhao, Y.Y.; Sun, H.H.; Li, Y.F.; Lai, X.Y.; Zhao, N.; Wang, X.; Xie, C.; et al. Cd36 facilitates fatty acid uptake by dynamic palmitoylation-regulated endocytosis. Nat. Commun. 2020, 11, 4765. [Google Scholar] [PubMed]
- Pilch, P.F.; Meshulam, T.; Ding, S.; Liu, L. Caveolae and lipid trafficking in adipocytes. Clin. Lipidol. 2011, 6, 49–58. [Google Scholar]
- Fernández-Real, J.M.; Catalán, V.; Moreno-Navarrete, J.M.; Gómez-Ambrosi, J.; Ortega, F.J.; I Rodriguez-Hermosa, J.; Ricart, W.; Frühbeck, G. Study of caveolin-1 gene expression in whole adipose tissue and its subfractions and during differentiation of human adipocytes. Nutr. Metab. 2010, 7, 1–9. [Google Scholar] [CrossRef]
- Grayson, T.H.; Chadha, P.S.; Bertrand, P.P.; Chen, H.; Morris, M.J.; Senadheera, S.; Murphy, T.V.; Sandow, S.L. Increased caveolae density and caveolin-1 expression accompany impaired no-mediated vasorelaxation in diet-induced obesity. Histochem. Cell Biol. 2013, 139, 309–321. [Google Scholar]
- Gallo, G.; Savoia, C. New insights into endothelial dysfunction in cardiometabolic diseases: Potential mechanisms and clinical implications. Int. J. Mol. Sci. 2024, 25, 2973. [Google Scholar] [CrossRef]
- Wang, X.; He, B. Endothelial dysfunction: Molecular mechanisms and clinical implications. MedComm 2024, 5, e651. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Ren, J. Endothelial dysfunction: Mechanisms and contribution to diseases. Acta Pharmacol. Sin. 2024, 45, 2023–2031. [Google Scholar] [CrossRef]
- Augustin, H.G.; Koh, G.Y. A systems view of the vascular endothelium in health and disease. Cell 2024, 187, 4833–4858. [Google Scholar] [CrossRef]
- Trimm, E.; Red-Horse, K. Vascular endothelial cell development and diversity. Nat. Rev. Cardiol. 2023, 20, 197–210. [Google Scholar]
- Becker, L.M.; Chen, S.H.; Rodor, J.; de Rooij, L.; Baker, A.H.; Carmeliet, P. Deciphering endothelial heterogeneity in health and disease at single-cell resolution: Progress and perspectives. Cardiovasc. Res. 2023, 119, 6–27. [Google Scholar]
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [PubMed]
- Ignarro, L.J.; Byrns, R.E.; Buga, G.M.; Wood, K.S. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ. Res. 1987, 61, 866–879. [Google Scholar] [PubMed]
- Arnold, W.P.; Mittal, C.K.; Katsuki, S.; Murad, F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc. Natl. Acad. Sci. USA 1977, 74, 3203–3207. [Google Scholar] [PubMed]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric oxide and endothelial dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar]
- Walford, G.; Loscalzo, J. Nitric oxide in vascular biology. J. Thromb. Haemost. 2003, 1, 2112–2118. [Google Scholar] [CrossRef]
- Fleming, I.; Fisslthaler, B.; Dimmeler, S.; Kemp, B.E.; Busse, R. Phosphorylation of thr495 regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ. Res. 2001, 88, E68–E75. [Google Scholar]
- Liu, J.; Garcia-Cardena, G.; Sessa, W.C. Palmitoylation of endothelial nitric oxide synthase is necessary for optimal stimulated release of nitric oxide: Implications for caveolae localization. Biochemistry 1996, 35, 13277–13281. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Insull, W., Jr. The pathology of atherosclerosis: Plaque development and plaque responses to medical treatment. Am. J. Med. 2009, 122, S3–S14. [Google Scholar] [CrossRef]
- Agnelli, G.; Belch, J.J.F.; Baumgartner, I.; Giovas, P.; Hoffmann, U. Morbidity and mortality associated with atherosclerotic peripheral artery disease: A systematic review. Atherosclerosis 2020, 293, 94–100. [Google Scholar] [CrossRef]
- Pepin, M.E.; Gupta, R.M. The role of endothelial cells in atherosclerosis: Insights from genetic association studies. Am. J. Pathol. 2024, 194, 499–509. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Le Master, E.; Paul, A.; Lazarko, D.; Aguilar, V.; Ahn, S.J.; Lee, J.C.; Minshall, R.D.; Levitan, I. Caveolin-1 is a primary determinant of endothelial stiffening associated with dyslipidemia, disturbed flow, and ageing. Sci. Rep. 2022, 12, 17822. [Google Scholar] [CrossRef]
- Fernandez-Hernando, C.; Yu, J.; Suarez, Y.; Rahner, C.; Davalos, A.; Lasuncion, M.A.; Sessa, W.C. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. 2009, 10, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Frank, P.G.; Lee, H.; Park, D.S.; Tandon, N.N.; Scherer, P.E.; Lisanti, M.P. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 98–105. [Google Scholar] [CrossRef]
- Fu, Y.; Moore, X.L.; Lee, M.K.; Fernandez-Rojo, M.A.; Parat, M.O.; Parton, R.G.; Meikle, P.J.; Sviridov, D.; Chin-Dusting, J.P. Caveolin-1 plays a critical role in the differentiation of monocytes into macrophages. Arterioscler. Thromb. Vasc. Biol. 2012, 32, e117–e125. [Google Scholar] [CrossRef]
- Tsai, T.H.; Chen, S.F.; Huang, T.Y.; Tzeng, C.F.; Chiang, A.S.; Kou, Y.R.; Lee, T.S.; Shyue, S.K. Impaired cd14 and cd36 expression, bacterial clearance, and toll-like receptor 4-myd88 signaling in caveolin-1-deleted macrophages and mice. Shock 2011, 35, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Kim, H.P.; Nakahira, K.; Ryter, S.W.; Choi, A.M. The heme oxygenase-1/carbon monoxide pathway suppresses tlr4 signaling by regulating the interaction of tlr4 with caveolin-1. J. Immunol. 2009, 182, 3809–3818. [Google Scholar]
- Codrici, E.; Albulescu, L.; Popescu, I.D.; Mihai, S.; Enciu, A.M.; Albulescu, R.; Tanase, C.; Hinescu, M.E. Caveolin-1-knockout mouse as a model of inflammatory diseases. J. Immunol. Res. 2018, 2018, 2498576. [Google Scholar] [CrossRef]
- Puddu, A.; Montecucco, F.; Maggi, D. Caveolin-1 and atherosclerosis: Regulation of ldls fate in endothelial cells. Int. J. Mol. Sci. 2023, 24, 8869. [Google Scholar] [CrossRef] [PubMed]
- Engel, D.; Beckers, L.; Wijnands, E.; Seijkens, T.; Lievens, D.; Drechsler, M.; Gerdes, N.; Soehnlein, O.; Daemen, M.J.; Stan, R.V.; et al. Caveolin-1 deficiency decreases atherosclerosis by hampering leukocyte influx into the arterial wall and generating a regulatory t-cell response. FASEB J. 2011, 25, 3838–3848. [Google Scholar]
- Pisetsky, D.S. Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 2023, 19, 509–524. [Google Scholar] [PubMed]
- Xiang, Y.; Zhang, M.; Jiang, D.; Su, Q.; Shi, J. The role of inflammation in autoimmune disease: A therapeutic target. Front. Immunol. 2023, 14, 1267091. [Google Scholar]
- Di Matteo, A.; Bathon, J.M.; Emery, P. Rheumatoid arthritis. Lancet 2023, 402, 2019–2033. [Google Scholar]
- Tanaka, Y. Recent progress in treatments of rheumatoid arthritis: An overview of developments in biologics and small molecules, and remaining unmet needs. Rheumatology 2021, 60, vi12–vi20. [Google Scholar]
- Ohnuma, K.; Inoue, H.; Uchiyama, M.; Yamochi, T.; Hosono, O.; Dang, N.H.; Morimoto, C. T-cell activation via cd26 and caveolin-1 in rheumatoid synovium. Mod. Rheumatol. 2006, 16, 3–13. [Google Scholar]
- Wei, Y.; Li, Y.; Shu, Y.; Gan, P.R.; Zhu, Y.L.; Xu, J.; Jiang, X.M.; Xia, S.L.; Wang, Y.; Wu, H. The new anti-angiogenesis perspective of rheumatoid arthritis with geniposide: Reducing the extracellular release of hsp70 in huvecs. Int. Immunopharmacol. 2025, 144, 113645. [Google Scholar] [PubMed]
- Kim, T.K.; Na, H.J.; Lee, W.R.; Jeoung, M.H.; Lee, S. Heat shock protein 70-1a is a novel angiogenic regulator. Biochem. Biophys. Res. Commun. 2016, 469, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yu, H.; Lei, Q.; Chen, X.; Tong, Y.; Zhang, Z.; Yang, W.; Guo, Y.; Lin, L. Systemic lupus erythematosus: Pathogenesis and targeted therapy. Mol. Biomed. 2024, 5, 54. [Google Scholar]
- Udhaya Kumar, S.; Thirumal Kumar, D.; Siva, R.; George Priya Doss, C.; Younes, S.; Younes, N.; Sidenna, M.; Zayed, H. Dysregulation of signaling pathways due to differentially expressed genes from the b-cell transcriptomes of systemic lupus erythematosus patients—A bioinformatics approach. Front. Bioeng. Biotechnol. 2020, 8, 276. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.J.; Liu, D.X.; Liu, Z.; Fu, M.; Yang, Q.R.; Sun, H.S. Expression of caveolin family proteins in serum of patients with systemic lupus erythematosus. Lupus 2021, 30, 1819–1828. [Google Scholar]
- Sunderland, D.K.; Sapra, A. Physiology, aqueous humor circulation. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kolb, H. Simple anatomy of the retina. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Jones, B., Nelson, R., Eds.; National Library of Medicine: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Bird, A. How to keep photoreceptors alive. Proc. Natl. Acad. Sci. USA 2007, 104, 2033–2034. [Google Scholar] [CrossRef]
- Vahabikashi, A.; Gelman, A.; Dong, B.; Gong, L.; Cha, E.D.K.; Schimmel, M.; Tamm, E.R.; Perkumas, K.; Stamer, W.D.; Sun, C.; et al. Increased stiffness and flow resistance of the inner wall of schlemm’s canal in glaucomatous human eyes. Proc. Natl. Acad. Sci. USA 2019, 116, 26555–26563. [Google Scholar] [PubMed]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Gu, X.; Reagan, A.M.; McClellan, M.E.; Elliott, M.H. Caveolins and caveolae in ocular physiology and pathophysiology. Prog. Retin. Eye Res. 2017, 56, 84–106. [Google Scholar]
- Enyong, E.N.; Gurley, J.M.; De Ieso, M.L.; Stamer, W.D.; Elliott, M.H. Caveolar and non-caveolar caveolin-1 in ocular homeostasis and disease. Prog. Retin. Eye Res. 2022, 91, 101094. [Google Scholar]
- De Ieso, M.L.; Gurley, J.M.; McClellan, M.E.; Gu, X.; Navarro, I.; Li, G.; Gomez-Caraballo, M.; Enyong, E.; Stamer, W.D.; Elliott, M.H. Physiologic consequences of caveolin-1 ablation in conventional outflow endothelia. Investig. Ophthalmol. Vis. Sci. 2020, 61, 32. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.H.; Ashpole, N.E.; Gu, X.; Herrnberger, L.; McClellan, M.E.; Griffith, G.L.; Reagan, A.M.; Boyce, T.M.; Tanito, M.; Tamm, E.R.; et al. Caveolin-1 modulates intraocular pressure: Implications for caveolae mechanoprotection in glaucoma. Sci. Rep. 2016, 6, 37127. [Google Scholar] [CrossRef]
- Lei, Y.; Song, M.; Wu, J.; Xing, C.; Sun, X. Enos activity in cav1 knockout mouse eyes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2805–2813. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wu, J.; Lei, Y.; Sun, X. Genetic deletion of the nos3 gene in cav1−/− mice restores aqueous humor outflow function. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4976–4987. [Google Scholar] [CrossRef]
- Zhang, L.; Chu, W.; Feng, X.; Li, J.; Ren, Y.; Yang, Y.; Zheng, Z.; Li, H. Caveolin-1 protects retinal ganglion cells in glaucoma by reducing tlr4 and activating the akt/pten signaling pathway. Pathol. Res. Pract. 2024, 262, 155552. [Google Scholar] [CrossRef]
- Shui, Y.B.; Liu, Y.; Huang, A.J.W.; Siegfried, C.J. Sdpr expression in human trabecular meshwork and its potential role in racial disparities of glaucoma. Sci. Rep. 2024, 14, 10258. [Google Scholar] [CrossRef]
- Wang, W.; Lo, A.C.Y. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Kusuhara, S.; Fukushima, Y.; Ogura, S.; Inoue, N.; Uemura, A. Pathophysiology of diabetic retinopathy: The old and the new. Diabetes Metab. J. 2018, 42, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Abel, B.; Willoughby, C.; Jang, S.; Cooper, L.; Xie, L.; Vo-Ransdell, C.; Sowa, G. N-terminal tyrosine phosphorylation of caveolin-2 negates anti-proliferative effect of transforming growth factor beta in endothelial cells. FEBS Lett. 2012, 586, 3317–3323. [Google Scholar] [CrossRef]
- Xu, H.; Qin, B. Increased expression of caveolin-1 in both of the vitreous and the proliferating membranes among the patients with proliferative diabetic retinopathy. Eye 2023, 37, 2152–2153. [Google Scholar] [CrossRef]
- Akram, M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar]
- Hillman, N.; Cox, S.; Noble, A.R.; Gallagher, P.J. Increased numbers of caveolae in retinal endothelium and pericytes in hypertensive diabetic rats. Eye 2001, 15, 319–325. [Google Scholar]
- Sasamoto, Y.; Kiritoshi, S.; Lee, C.A.A.; Fukuda, Y.; Martin, G.; Ksander, B.R.; Frank, M.H.; Frank, N.Y. Caveolin 1 and 2 enhance the proliferative capacity of bcam-positive corneal progenitors. Sci. Rep. 2025, 15, 6672. [Google Scholar]
- Badaut, J.; Blochet, C.; Obenaus, A.; Hirt, L. Physiological and pathological roles of caveolins in the central nervous system. Trends Neurosci. 2024, 47, 651–664. [Google Scholar] [CrossRef]
- Cameron, P.L.; Ruffin, J.W.; Bollag, R.; Rasmussen, H.; Cameron, R.S. Identification of caveolin and caveolin-related proteins in the brain. J. Neurosci. 1997, 17, 9520–9535. [Google Scholar] [CrossRef]
- Boulware, M.I.; Kordasiewicz, H.; Mermelstein, P.G. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J. Neurosci. 2007, 27, 9941–9950. [Google Scholar]
- Zschocke, J.; Manthey, D.; Bayatti, N.; van der Burg, B.; Goodenough, S.; Behl, C. Estrogen receptor alpha-mediated silencing of caveolin gene expression in neuronal cells. J. Biol. Chem. 2002, 277, 38772–38780. [Google Scholar]
- Galbiati, F.; Volonte, D.; Gil, O.; Zanazzi, G.; Salzer, J.L.; Sargiacomo, M.; Scherer, P.E.; Engelman, J.A.; Schlegel, A.; Parenti, M.; et al. Expression of caveolin-1 and -2 in differentiating pc12 cells and dorsal root ganglion neurons: Caveolin-2 is up-regulated in response to cell injury. Proc. Natl. Acad. Sci. USA 1998, 95, 10257–10262. [Google Scholar]
- Ikezu, T.; Ueda, H.; Trapp, B.D.; Nishiyama, K.; Sha, J.F.; Volonte, D.; Galbiati, F.; Byrd, A.L.; Bassell, G.; Serizawa, H.; et al. Affinity-purification and characterization of caveolins from the brain: Differential expression of caveolin-1, -2, and -3 in brain endothelial and astroglial cell types. Brain Res. 1998, 804, 177–192. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Almenar-Queralt, A.; Leem, J.; DerMardirossian, C.; Roth, D.M.; Patel, P.M.; Patel, H.H.; Head, B.P. Caveolin-1 phosphorylation is essential for axonal growth of human neurons derived from ipscs. Front. Cell. Neurosci. 2019, 13, 324. [Google Scholar]
- Trevino, T.N.; Almousawi, A.A.; Robinson, K.F.; Fogel, A.B.; Class, J.; Minshall, R.D.; Tai, L.M.; Richner, J.M.; Lutz, S.E. Caveolin-1 mediates blood-brain barrier permeability, neuroinflammation, and cognitive impairment in SARS-COV-2 infection. J. Neuroimmunol. 2024, 388, 578309. [Google Scholar]
- Gu, Y.; Zheng, G.; Xu, M.; Li, Y.; Chen, X.; Zhu, W.; Tong, Y.; Chung, S.K.; Liu, K.J.; Shen, J. Caveolin-1 regulates nitric oxide-mediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury. J. Neurochem. 2012, 120, 147–156. [Google Scholar]
- Gu, Y.; Dee, C.M.; Shen, J. Interaction of free radicals, matrix metalloproteinases and caveolin-1 impacts blood-brain barrier permeability. Front. Biosci. (Schol. Ed.) 2011, 3, 1216–1231. [Google Scholar]
- Zhao, J.; Huai, J. Role of primary aging hallmarks in alzheimer s disease. Theranostics 2023, 13, 197–230. [Google Scholar]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- Kang, M.J.; Chung, Y.H.; Hwang, C.I.; Murata, M.; Fujimoto, T.; Mook-Jung, I.H.; Cha, C.I.; Park, W.Y. Caveolin-1 upregulation in senescent neurons alters amyloid precursor protein processing. Exp. Mol. Med. 2006, 38, 126–133. [Google Scholar]
- Gaudreault, S.B.; Dea, D.; Poirier, J. Increased caveolin-1 expression in alzheimer’s disease brain. Neurobiol. Aging 2004, 25, 753–759. [Google Scholar]
- Nishiyama, K.; Trapp, B.D.; Ikezu, T.; Ransohoff, R.M.; Tomita, T.; Iwatsubo, T.; Kanazawa, I.; Hsiao, K.K.; Lisanti, M.P.; Okamoto, T. Caveolin-3 upregulation activates beta-secretase-mediated cleavage of the amyloid precursor protein in alzheimer’s disease. J. Neurosci. 1999, 19, 6538–6548. [Google Scholar]
- Islam, M.; Behura, S.K. Role of caveolin-1 in metabolic programming of fetal brain. iScience 2023, 26, 107710. [Google Scholar]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-synuclein in parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Antony, P.M.; Diederich, N.J.; Kruger, R.; Balling, R. The hallmarks of parkinson’s disease. FEBS J. 2013, 280, 5981–5993. [Google Scholar]
- Cha, S.H.; Choi, Y.R.; Heo, C.H.; Kang, S.J.; Joe, E.H.; Jou, I.; Kim, H.M.; Park, S.M. Loss of parkin promotes lipid rafts-dependent endocytosis through accumulating caveolin-1: Implications for parkinson’s disease. Mol. Neurodegener. 2015, 10, 63. [Google Scholar]
- Ha, T.Y.; Choi, Y.R.; Noh, H.R.; Cha, S.H.; Kim, J.B.; Park, S.M. Age-related increase in caveolin-1 expression facilitates cell-to-cell transmission of alpha-synuclein in neurons. Mol. Brain 2021, 14, 122. [Google Scholar]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and other primary brain malignancies in adults: A review. JAMA 2023, 329, 574–587. [Google Scholar]
- van den Bent, M.J.; Geurts, M.; French, P.J.; Smits, M.; Capper, D.; Bromberg, J.E.C.; Chang, S.M. Primary brain tumours in adults. Lancet 2023, 402, 1564–1579. [Google Scholar]
- Martin, W. Evolutionary origins of metabolic compartmentalization in eukaryotes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 847–855. [Google Scholar]
- Moriconi, C.; Civita, P.; Neto, C.; Pilkington, G.J.; Gumbleton, M. Caveolin-1, a key mediator across multiple pathways in glioblastoma and an independent negative biomarker of patient survival. Front. Oncol. 2021, 11, 701933. [Google Scholar]
- Wang, Z.; Chen, G.; Yuan, D.; Wu, P.; Guo, J.; Lu, Y.; Wang, Z. Caveolin-1 promotes glioma proliferation and metastasis by enhancing emt via mediating pai-1 activation and its correlation with immune infiltrates. Heliyon 2024, 10, e24464. [Google Scholar]
- Pu, W.; Nassar, Z.D.; Khabbazi, S.; Xie, N.; McMahon, K.A.; Parton, R.G.; Riggins, G.J.; Harris, J.M.; Parat, M.O. Correlation of the invasive potential of glioblastoma and expression of caveola-forming proteins caveolin-1 and cavin1. J. Neurooncol. 2019, 143, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Pascual, A.; Hale, J.S.; Kordowski, A.; Pugh, J.; Silver, D.J.; Bayik, D.; Roversi, G.; Alban, T.J.; Rao, S.; Chen, R.; et al. Adamdec1 maintains a growth factor signaling loop in cancer stem cells. Cancer Discov. 2019, 9, 1574–1589. [Google Scholar] [CrossRef] [PubMed]
- Hausott, B.; Pircher, L.; Kind, M.; Park, J.W.; Claus, P.; Obexer, P.; Klimaschewski, L. Sprouty2 regulates endocytosis and degradation of fibroblast growth factor receptor 1 in glioblastoma cells. Cells 2024, 13, 1967. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, Y.; Umaru, B.A.; Kanamori, M.; Zama, R.; Shil, S.K.; Miyazaki, H.; Kobayashi, S.; Wannakul, T.; Yang, S.; Tominaga, T.; et al. Nuclear fabp7 regulates cell proliferation of wild-type idh1 glioma through caveolae formation. Mol. Oncol. 2022, 16, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Senetta, R.; Trevisan, E.; Ruda, R.; Maldi, E.; Molinaro, L.; Lefranc, F.; Chiusa, L.; Lanotte, M.; Soffietti, R.; Cassoni, P. Caveolin 1 expression independently predicts shorter survival in oligodendrogliomas. J. Neuropathol. Exp. Neurol. 2009, 68, 425–431. [Google Scholar] [CrossRef]
- Riitano, G.; Manganelli, V.; Capozzi, A.; Mattei, V.; Recalchi, S.; Martellucci, S.; Longo, A.; Misasi, R.; Garofalo, T.; Sorice, M. Lrp6 mediated signal transduction pathway triggered by tissue plasminogen activator acts through lipid rafts in neuroblastoma cells. J. Cell Commun. Signal. 2020, 14, 315–323. [Google Scholar] [CrossRef]

| Lipid Type | Contribution to Lipid Rafts |
|---|---|
| Cholesterol | Maintains fluidity and structural integrity; critical for raft stability. |
| Gangliosides | Facilitate signaling by interacting with proteins and lipids. |
| Glycosphingolipids | Contribute to raft stability and specific protein recruitment. |
| Saturated Phospholipids | Enhance packing density and lipid raft rigidity. |
| Sphingolipids | Provide more ordered packing; interact tightly with cholesterol. |
| Lipid Rafts | Caveolae | References | |
|---|---|---|---|
| Definition | Microdomains of the plasma membrane rich in cholesterol, sphingolipids, and certain proteins. | Flask-shaped invaginations of the plasma membrane enriched in cholesterol, sphingolipids, and caveolins. | [5,85] |
| Shape and Structure | Non-distinct, flat, or slightly curved regions of the membrane. | Flask- or omega-shaped membrane invaginations. | [35,86] |
| Size | 10–200 nm. | Typically, 50–100 nm in diameter. | [87] |
| Protein Markers | Glycosylphosphatidylinositol (GPI)-anchored proteins, flotillins. | Cav-1, -2, -3, and Cavins. | [88,89] |
| Function | Signal transduction, membrane sorting, and lipid/protein trafficking. | Signal transduction, endocytosis, mechanosensing, and cholesterol homeostasis. | [5,90] |
| Dependence on Cholesterol | Cholesterol is essential for maintaining raft integrity and functionality. | Heavily reliant on cholesterol for structural stability and caveolin-membrane interaction. | [91] |
| Biogenesis | Formed dynamically through lipid–lipid and lipid–protein interactions. | Formed through the interaction of caveolins with the membrane lipids and cytoskeletal elements. | [35] |
| Presence in Cells | Ubiquitous in eukaryotic cells. | Found predominantly in adipocytes, endothelial cells, and muscle cells. | [89] |
| Drug/Chemical | Mechanism of Action | Main References |
|---|---|---|
| Brefeldin A | Inhibits Golgi function and vesicular transport, preventing caveolae recycling | [81,92] |
| Cav-based cell permeable peptides (e.g., Cavtratin) | Mimics Cav-1 function, inhibiting excessive signaling | [93,94] |
| Cholesterol oxidase | Oxidizes cholesterol, disrupting lipid raft structure | [95,96] |
| Daidzein | It has been shown to modulate the expression of Cav-1, thereby affecting the biogenesis and function of caveolae | [97,98,99] |
| Dynamin inhibitors (e.g., dynasore) | Block caveolae-mediated endocytosis by inhibiting dynamin | [100,101,102] |
| Filipin | Binds to cholesterol, disrupting lipid raft integrity | [103,104] |
| Lovastatin | Inhibits HMG-CoA reductase, reducing cholesterol synthesis | [105,106] |
| MβCD | Removes cholesterol from lipid rafts, disrupting their structure | [50,107] |
| Nystatin | Binds to cholesterol, disrupting lipid raft structure | [103,108] |
| Metabolic Disorder | Pathophysiology | Link to Cav-1 Dysfunction | References |
|---|---|---|---|
| Cardiovascular Diseases | Dysregulated cholesterol metabolism and endothelial dysfunction. | Cav-1 mutations lead to cholesterol efflux defects and reduced nitric oxide bioavailability, promoting vascular diseases. | [56] |
| Diabetes Mellitus | Impaired glucose metabolism and insulin resistance. | Dysregulation of Cav-1 affects insulin receptor signaling and GLUT4 translocation, exacerbating insulin resistance. | [119,120] |
| Dyslipidemia | Abnormal lipid levels, including elevated LDL and triglycerides. | Altered caveolar lipid homeostasis due to Cav-1 dysregulation affects lipid uptake and efflux in hepatocytes and adipocytes. | [121] |
| Lipodystrophy | Abnormal fat distribution and metabolic derangements. | Loss of caveolae in adipocytes due to Cav-1 mutations cause lipodystrophic phenotypes with insulin resistance. | [122] |
| Non-Alcoholic Fatty Liver Disease (NAFLD) | Hepatic lipid accumulation and steatosis. | Impaired caveolar lipid metabolism influences hepatic lipid storage and triglyceride secretion, driving NAFLD progression. | [123] |
| Obesity | Excess adipose tissue accumulation and systemic inflammation. | Cav-1 dysfunction disrupts adipocyte differentiation and lipid storage, contributing to adipose tissue dysfunction. | [123,124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stea, D.M.; D’Alessio, A. Caveolae: Metabolic Platforms at the Crossroads of Health and Disease. Int. J. Mol. Sci. 2025, 26, 2918. https://doi.org/10.3390/ijms26072918
Stea DM, D’Alessio A. Caveolae: Metabolic Platforms at the Crossroads of Health and Disease. International Journal of Molecular Sciences. 2025; 26(7):2918. https://doi.org/10.3390/ijms26072918
Chicago/Turabian StyleStea, Dante Maria, and Alessio D’Alessio. 2025. "Caveolae: Metabolic Platforms at the Crossroads of Health and Disease" International Journal of Molecular Sciences 26, no. 7: 2918. https://doi.org/10.3390/ijms26072918
APA StyleStea, D. M., & D’Alessio, A. (2025). Caveolae: Metabolic Platforms at the Crossroads of Health and Disease. International Journal of Molecular Sciences, 26(7), 2918. https://doi.org/10.3390/ijms26072918








