Oroxylum indicum (L.) Leaf Extract Attenuates β-Amyloid-Induced Neurotoxicity in SH-SY5Y Cells
Abstract
1. Introduction
2. Results
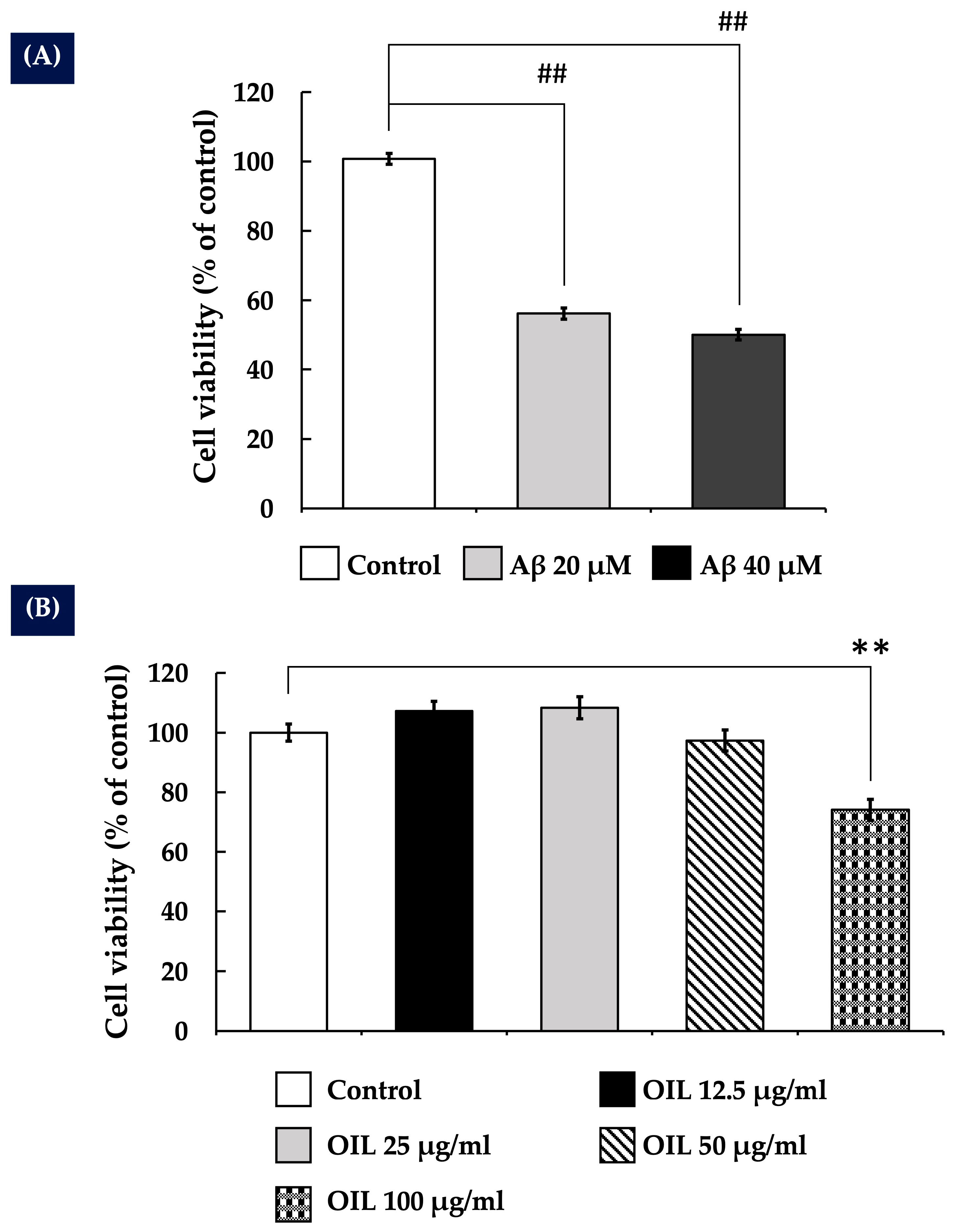
2.1. Impact of Aβ25–35 on the Survival of SH-SY5Y Cells
2.2. Effects of OIL on the Viability of SH-SY5Y Cells
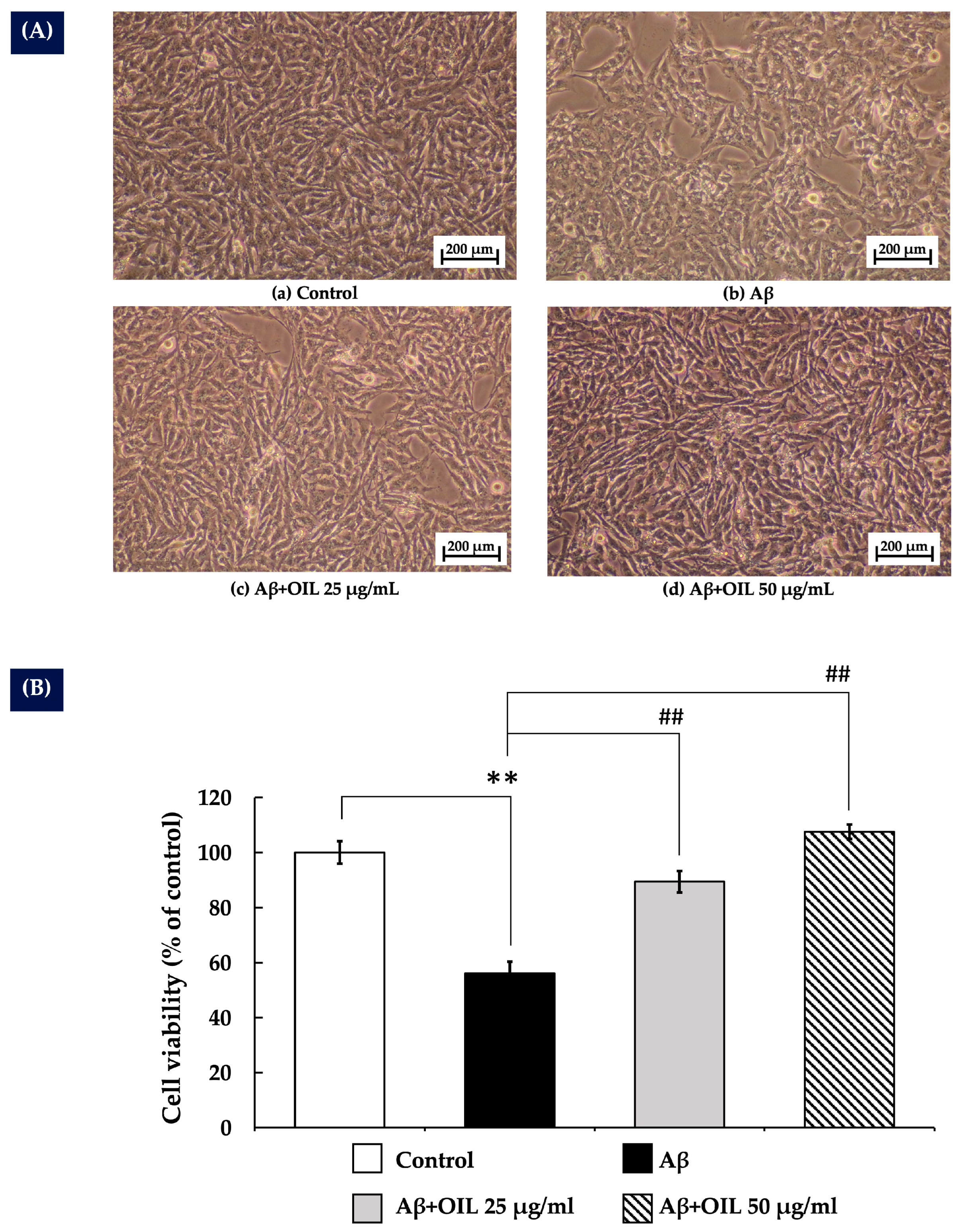
2.3. OIL’s Protective Effect Against Aβ25–35-Induced Cytotoxicity in SH-SY5Y Cells
2.4. OIL Prevents Aβ25−35-Induced ROS Production in SH-SY5Y Cells
2.5. OIL Reduces Caspase-3 Expression Induced by Aβ25–35
2.6. Effects of OIL on Oxidative Stress Status
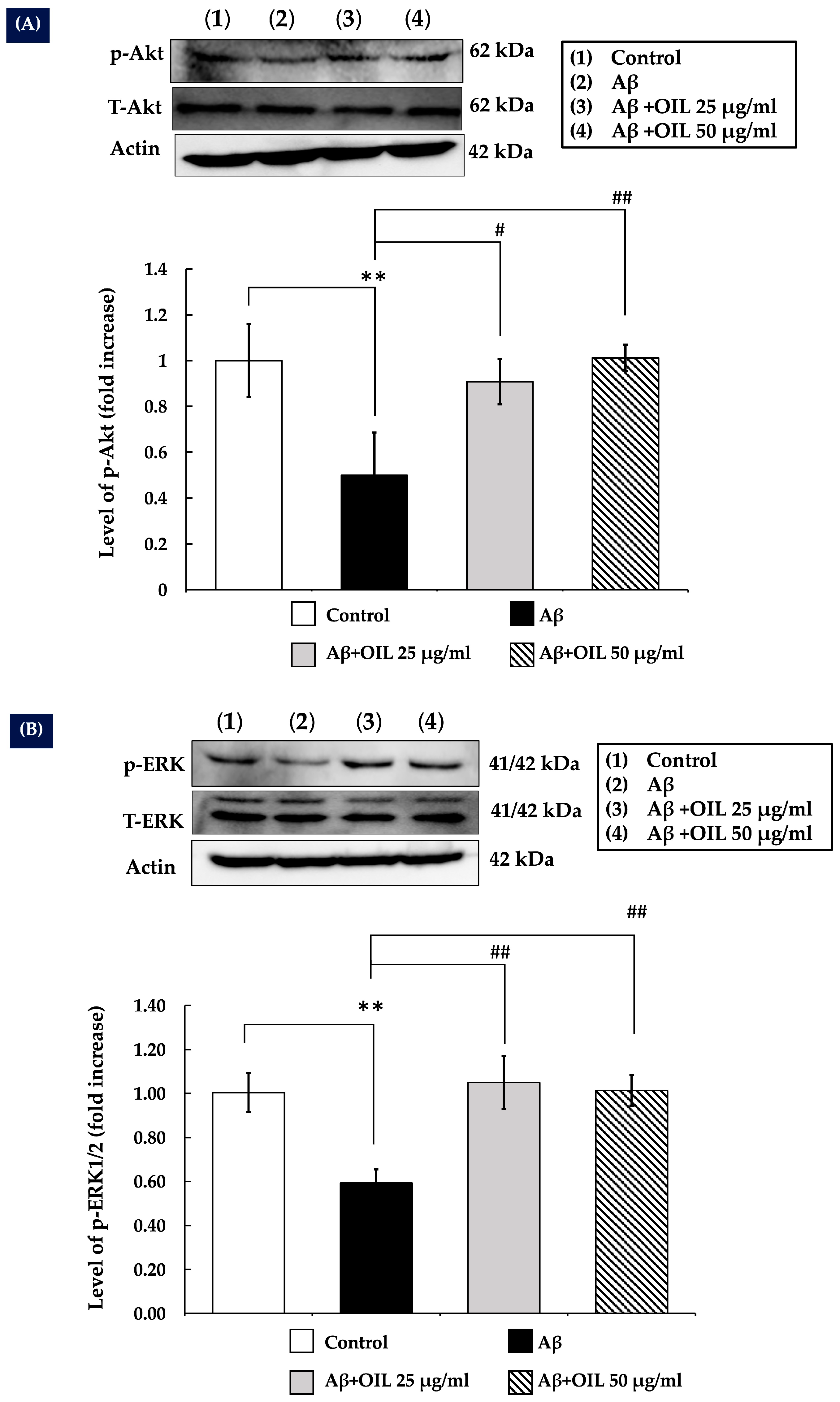
2.7. OIL Increases ERK1/2 and Akt Phosphorylation
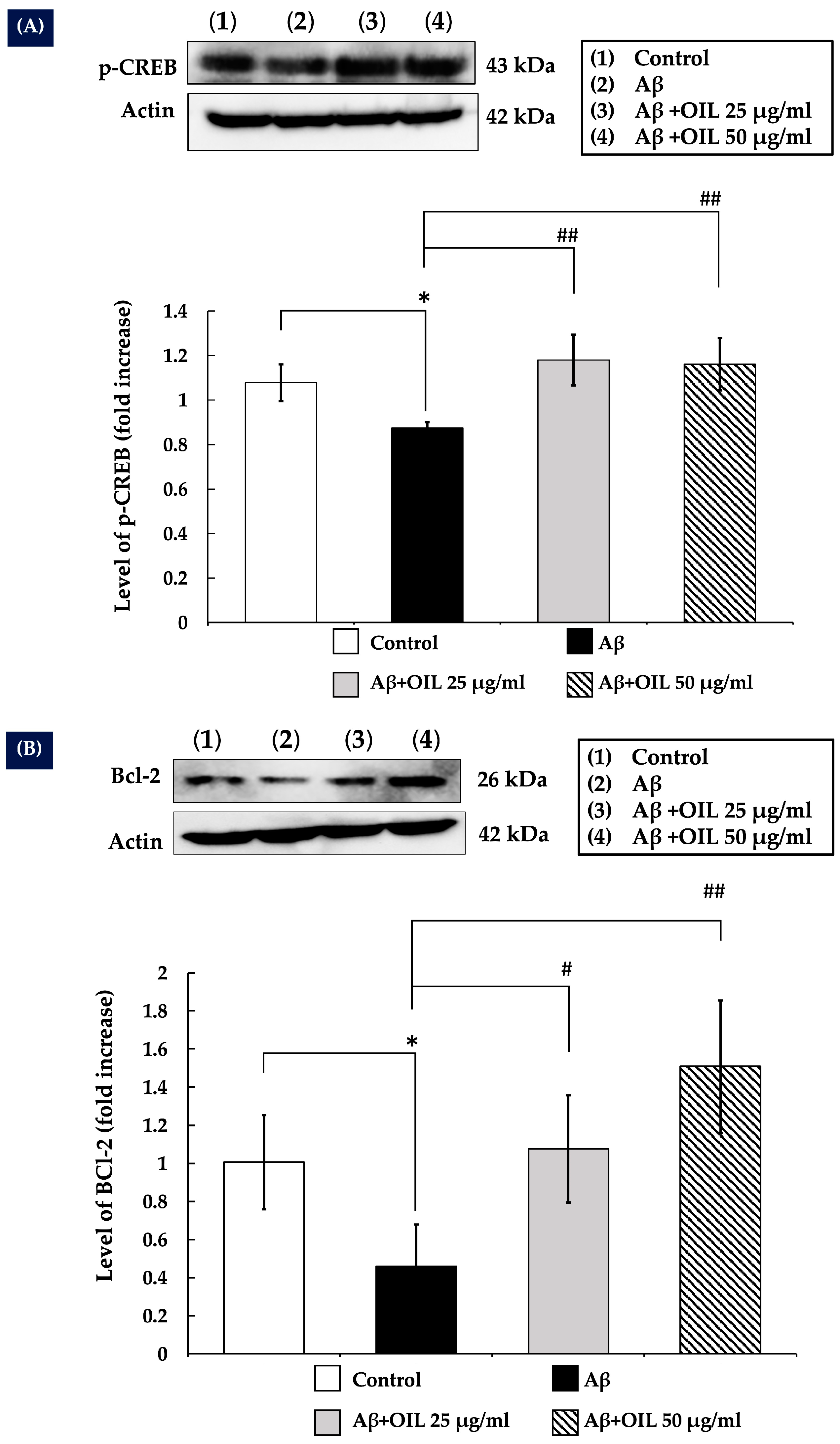
2.8. OIL Enhances CREB Phosphorylation
2.9. OIL Increases Bcl-2 Expression
2.10. Analysis of Flavonoid Content in OIL Extract by HPLC
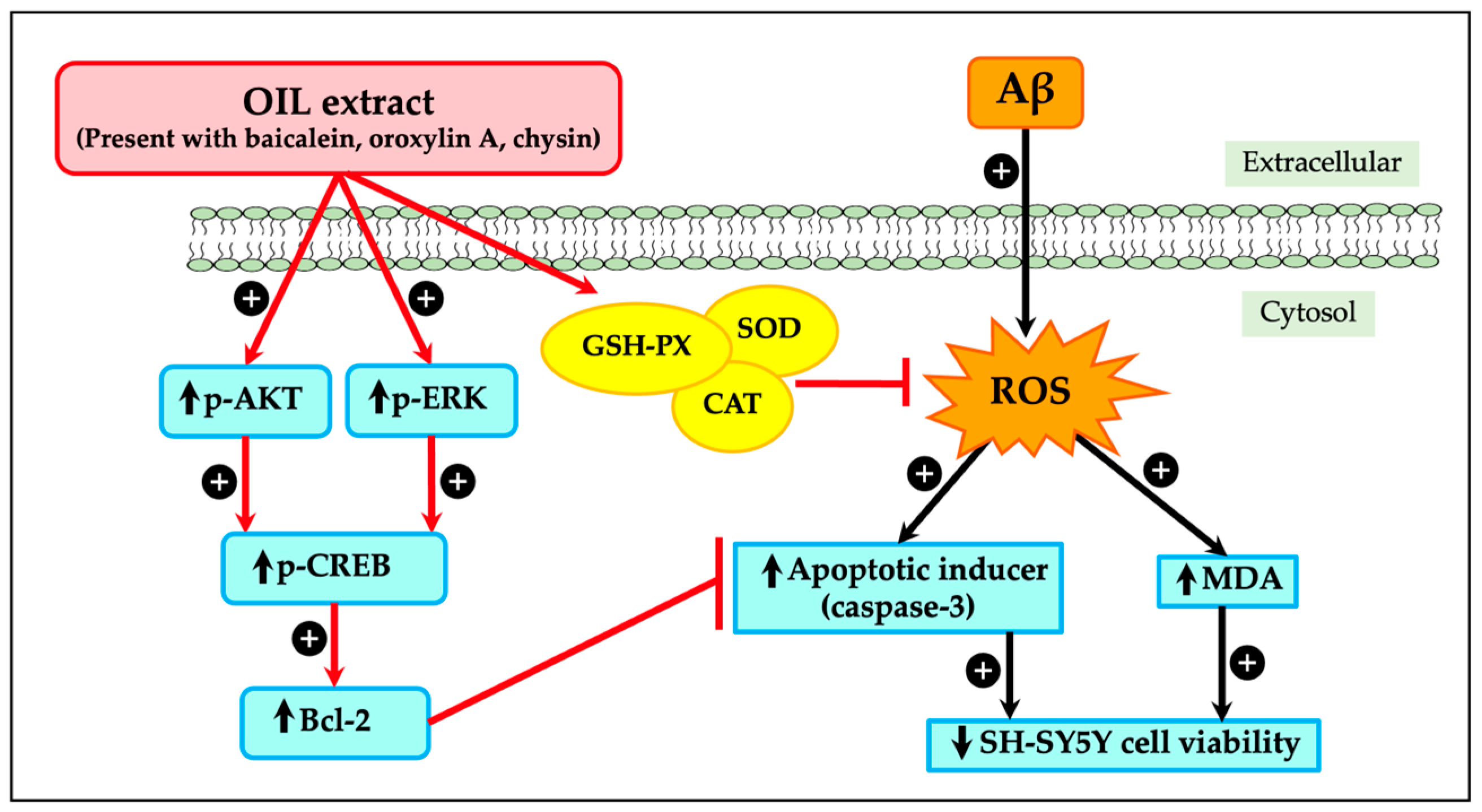
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Antibodies
4.2. Plant Material and Extraction
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Intracellular ROS Assay
4.6. Determination of Oxidative Stress Status
4.7. Western Blotting
4.8. Analysis of Flavonoids Contents in Crude Extracts by HPLC
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yarns, B.C.; Holiday, K.A.; Carlson, D.M.; Cosgrove, C.K.; Melrose, R.J. Pathophysiology of Alzheimer’s Disease. Psychiatr. Clin. North Am. 2022, 45, 663–676. [Google Scholar] [PubMed]
- Alzheimer Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2024, 20, 3708–3821. [Google Scholar]
- Kocahan, S.; Doğan, Z. Mechanisms of Alzheimer’s Disease Pathogenesis and Prevention: The Brain, Neural Pathology, N-Methyl-D-Aspartate Receptors, Tau Protein, and Other Risk Factors. Clin. Psychopharmacol. Neurosci. 2017, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef]
- Azargoonjahromi, A. The duality of amyloid-β: Its role in normal and Alzheimer’s disease states. Mol. Brain 2024, 17, 44. [Google Scholar]
- Zhao, X.; Sun, J.; Xiong, L.; She, L.; Li, L.; Tang, H.; Zeng, Y.; Chen, F.; Han, X.; Ye, S.; et al. β-amyloid binds to microglia Dectin-1 to induce inflammatory response in the pathogenesis of Alzheimer’s disease. Int. J. Biol. Sci. 2023, 19, 3249–3265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Mormino, E.C.; Papp, K.V. Amyloid Accumulation and Cognitive Decline in Clinically Normal Older Individuals: Implications for Aging and Early Alzheimer’s Disease. J. Alzheimers Dis. 2018, 64, S633–S646. [Google Scholar] [CrossRef]
- Butterfield, D.A. Amyloid beta-peptide [1-42]-associated free radical-induced oxidative stress and neurodegeneration in Alzheimer’s disease brain: Mechanisms and consequences. Curr. Med. Chem. 2003, 10, 2651–2659. [Google Scholar] [CrossRef]
- Lawania, R.D.; Mishra, A.; Gupta, R. Oroxylum indicum: A review. Pharm. J. 2010, 2, 304–310. [Google Scholar]
- Dinda, B.; SilSarma, I.; Dinda, M.; Rudrapaul, P. Oroxylum indicum (L.) Kurz, an important Asian traditional medicine: From traditional uses to scientific data for its commercial exploitation. J. Ethnopharmacol. 2015, 161, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Jiwajinda, S.; Santisopasri, V.; Murakami, A.; Kim, O.K.; Kim, H.W.; Ohigashi, H. Suppressive effects of edible Thai plants on superoxide and nitric oxide generation. Asian Pac. J. Cancer Prev. 2002, 3, 215–223. [Google Scholar]
- Mairuae, N.; Cheepsunthorn, P.; Buranrat, B. Antioxidant and anti-inflammatory activities of Oroxylum indicum Kurz (L.) fruit extract in lipopolysaccharide-stimulated BV2 microglial cells. Trop. J. Pharm. Res. 2021, 20, 833–838. [Google Scholar] [CrossRef]
- Mairuae, N.; Cheepsunthorn, P.; Buranrat, B. Oroxylum indicum (L.) Kurz seed extract prevents LPS-mediated BV2 microglial activation through NF-κB nuclear translocation and activation of the Akt/ERK1/2 signaling pathways. Pharmacogn. Mag. 2022, 18, 621–626. [Google Scholar]
- Mairuae, N.; Connor, J.R.; Buranrat, B.; Lee, S.Y. Oroxylum indicum (L.) extract protects human neuroblastoma SH-SY5Y cells against β-amyloid-induced cell injury. Mol. Med. Rep. 2019, 20, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Sreedharan, S.; Pande, A.; Pande, A.; Majeed, M.; Cisneros-Zevallos, L. The neuroprotective effects of Oroxylum indicum extract in SHSY-5Y neuronal cells by upregulating BDNF gene expression under LPS-induced inflammation. Nutrients 2024, 16, 1887. [Google Scholar] [CrossRef]
- Summat, R.; Waiwut, P.; Daodee, S.; Nualkaew, N.; Phemphunananchai, K.; Arsito, P.N.; Chulikhit, Y.; Montakantirat, O.; Khamphukdee, C.; Boonyarat, C. Phytomedicine Potential of Oroxylum indicum Root and Its Constituents: Targeting Alzheimer’s Disease. Plants 2025, 14, 223. [Google Scholar] [CrossRef]
- Wójcik, P.; Jastrzębski, M.K.; Zięba, A.; Matosiuk, D.; Kaczor, A.A. Caspases in Alzheimer’s Disease: Mechanism of Activation, Role, and Potential Treatment. Mol. Neurobiol. 2024, 61, 4834–4853. [Google Scholar] [CrossRef]
- Navarro-Yepes, J.; Zavala-Flores, L.; Anandhan, A.; Wang, F.; Skotak, M.; Chandra, N.; Li, M.; Pappa, A.; Martinez-Fong, D.; Del Razo, L.M.; et al. Antioxidant gene therapy against neuronal cell death. Pharmacol. Ther. 2014, 142, 206–230. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.L. Redox regulation of the intrinsic pathway in neuronal apoptosis. Antioxid. Redox Signal. 2011, 14, 1437–1448. [Google Scholar]
- Hussar, P. Apoptosis Regulators Bcl-2 and Caspase-3. Encyclopedia 2022, 2, 1624–1636. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar]
- Brazil, D.P.; Park, J.; Hemmings, B.A. PKB binding proteins. Getting in on the Akt. Cell 2002, 111, 293–303. [Google Scholar] [PubMed]
- Pugazhenthi, S.; Nesterova, A.; Sable, C.; Heidenreich, K.A.; Boxer, L.M.; Heasley, L.E.; Reusch, J.E. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J. Biol. Chem. 2000, 275, 10761–10766. [Google Scholar]
- Razani, E.; Pourbagheri-Sigaroodi, A.; Safaroghli-Azar, A.; Zoghi, A.; Shanaki-Bavarsad, M.; Bashash, D. The PI3K/Akt signaling axis in Alzheimer’s disease: A valuable target to stimulate or suppress? Cell Stress Chaperones 2021, 26, 871–887. [Google Scholar] [CrossRef]
- Martin-Vega, A.; Cobb, M.H. Navigating the ERK1/2 MAPK Cascade. Biomolecules 2023, 13, 1555. [Google Scholar] [CrossRef]
- Erhardt, P.; Schremser, E.J.; Cooper, G.M. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/ERK pathway. Mol. Cell Biol. 1999, 19, 5308–5315. [Google Scholar]
- Shonai, T.; Adachi, M.; Sakata, K.; Takekawa, M.; Endo, T.; Imai, K.; Hareyama, M. MEK/ERK pathway protects ionizing radiation-induced loss of mitochondrial membrane potential and cell death in lymphocytic leukemia cells. Cell Death Differ. 2002, 9, 963–971. [Google Scholar]
- Chiki, T. Role of cAMP response element binding protein in cardiovascular remodeling: Good, bad, or both? Arterioscler. Thromb. Vasc. Biol. 2006, 26, 449–455. [Google Scholar]
- Fu, X.; Feng, Y.; Shao, B.; Zhang, Y. Activation of the ERK/Creb/Bcl 2 pathway protects periodontal ligament stem cells against hydrogen peroxide induced oxidative stress. Mol. Med. Rep. 2019, 19, 3649–3657. [Google Scholar] [CrossRef]
- Nik Salleh, N.N.H.; Othman, F.A.; Kamarudin, N.A.; Tan, S.C. The Biological Activities and Therapeutic Potentials of Baicalein Extracted from Oroxylum indicum: A Systematic Review. Molecules 2020, 25, 5677. [Google Scholar] [CrossRef]
- Deka, D.C.; Kumar, V.; Prasad, C.; Kumar, K.; Gogoi, B.J.; Singh, L.S.R.B. Oroxylum indicum—A medicinal plant of North East India: An overview of its nutritional, remedial, and prophylactic properties. J. Appl. Pharm Sci. 2013, 3, S104–S112. [Google Scholar]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. Baicalein as a Potent Neuroprotective Agent: A Review. Biomed. Pharmacother. 2017, 95, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; He, G.R.; Sun, L.; Lan, X.; Shi, L.L.; Xuan, Z.H.; Du, G.H. Assessment of the Treatment Effect of Baicalein on a Model of Parkinsonian Tremor and Elucidation of the Mechanism. Life Sci. 2012, 91, 5–13. [Google Scholar] [CrossRef]
- Wei, D.; Tang, J.; Bai, W.; Wang, Y.; Zhang, Z. Ameliorative Effects of Baicalein on an Amyloid-β Induced Alzheimer’s Disease Rat Model: A Proteomics Study. Curr. Alzheimer Res. 2014, 11, 869–881. [Google Scholar] [CrossRef]
- Wong, Y.K.; Chou, M.K.; Shen, Y.C.; Wang, Y.H.; Yen, J.C.; Che, F.; Lin, S.K.; Liao, J.F. Preventive Effect of Baicalein on Methamphetamine-Induced Amnesia in the Passive Avoidance Test in Mice. Pharmacology 2014, 93, 278–285. [Google Scholar] [CrossRef]
- Gao, L.; Li, C.; Yang, R.Y.; Lian, W.W.; Fang, J.S.; Pang, X.C.; Qin, X.M.; Liu, A.L.; Du, G.H. Ameliorative Effects of Baicalein in MPTP-Induced Mouse Model of Parkinson’s Disease: A Microarray Study. Pharmacol. Biochem. Behav. 2015, 33, 155–163. [Google Scholar] [CrossRef]
- Chang, Y.; Lu, C.W.; Lin, T.Y.; Huang, S.K.; Wang, S.J. Baicalein, a Constituent of Scutellaria baicalensis, Reduces Glutamate Release and Protects Neuronal Cells Against Kainic Acid-Induced Excitotoxicity in Rats. Am. J. Chin. Med. 2016, 44, 943–962. [Google Scholar]
- Choi, E.O.; Jeong, J.W.; Park, C.; Hong, S.H.; Kim, G.Y.; Hwang, H.J.; Cho, E.J.; Choi, Y.H. Baicalein Protects C6 Glial Cells Against Hydrogen Peroxide-Induced Oxidative Stress and Apoptosis Through Regulation of the Nrf2 Signaling Pathway. Int. J. Mol. Med. 2016, 37, 798–806. [Google Scholar] [PubMed]
- Hung, K.C.; Huang, H.J.; Wang, Y.T.; Lin, A.M. Baicalein Attenuates α-Synuclein Aggregation, Inflammasome Activation, and Autophagy in the MPP+-Treated Nigrostriatal Dopaminergic System in Vivo. J. Ethnopharmacol. 2016, 194, 522–529. [Google Scholar] [PubMed]
- Liu, C.; Wu, J.; Gu, J.; Xiong, Z.; Wang, F.; Wang, J.; Wang, W.; Chen, J. Baicalein Improves Cognitive Deficits Induced by Chronic Cerebral Hypoperfusion in Rats. Pharmacol. Biochem. Behav. 2007, 86, 423–430. [Google Scholar] [PubMed]
- Li, Y.; Zhao, J.; Hölscher, C. Therapeutic Potential of Baicalein in Alzheimer’s Disease and Parkinson’s Disease. CNS Drugs. 2017, 31, 639–652. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeon, S.J.; Son, K.H.; Jung, J.W.; Lee, S.; Yoon, B.H.; Choi, J.W.; Cheong, J.H.; Ko, K.H.; Ryu, J.H. Effect of the Flavonoid, Oroxylin A, on Transient Cerebral Hypoperfusion-Induced Memory Impairment in Mice. Pharmacol. Biochem. Behav. 2006, 85, 658–668. [Google Scholar]
- Mishra, A.; Mishra, P.S.; Bandopadhyay, R.; Khurana, N.; Angelopoulou, E.; Paudel, Y.N.; Piperi, C. Neuroprotective Potential of Chrysin: Mechanistic Insights and Therapeutic Potential for Neurological Disorders. Molecules 2021, 26, 6456. [Google Scholar] [CrossRef]


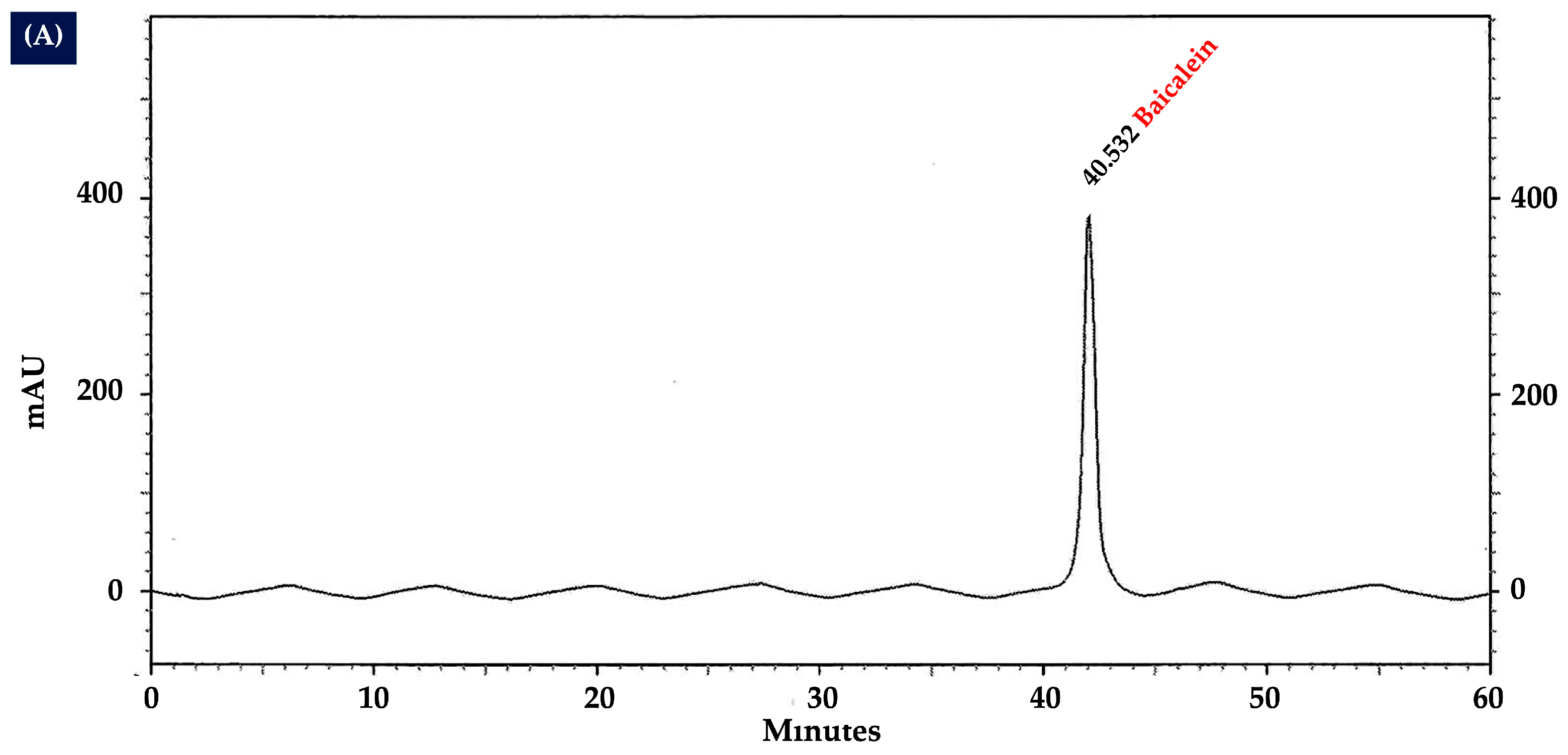
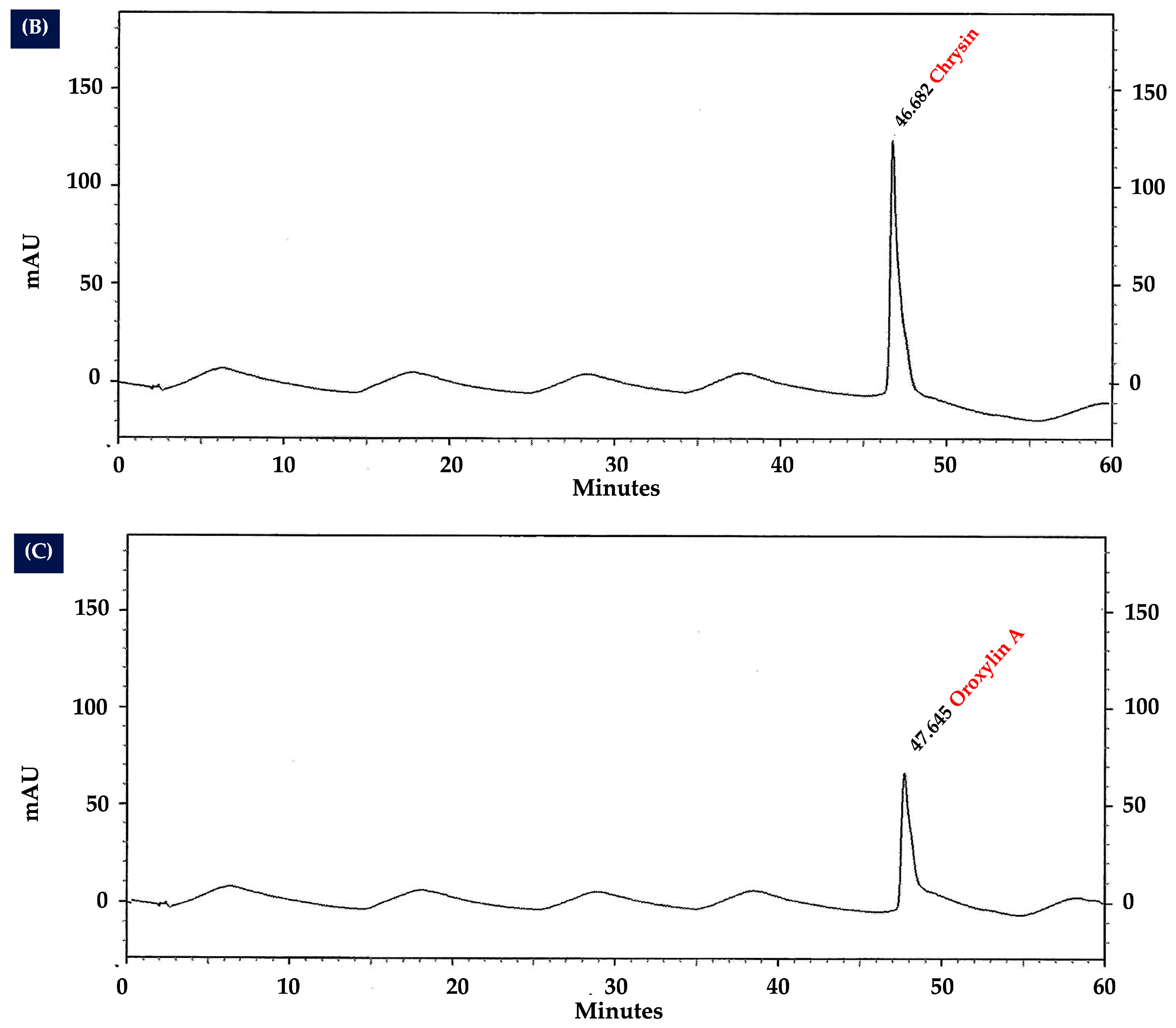
| Treatment Groups | MDA (nmol/mg Protein) | CAT Activity (U/mg Protein) | SOD Activity (% Inhibition/ mg Protein) | GSH-Px Activity (u/mg Protein) |
|---|---|---|---|---|
| Control | 0.569 | 26.6655 | 44.670 | 0.3449 |
| Aβ | 0.929 a | 30.0036 | 37.640 a | 0.2103 a |
| Aβ + OIL 25 m/mL | 0.882 | 37.7973 * | 44.289 | 0.4716 * |
| Aβ + OIL 50 m/mL | 0.547 * | 60.4452 ** | 48.139 * | 0.6103 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palachai, N.; Buranrat, B.; Noisa, P.; Mairuae, N. Oroxylum indicum (L.) Leaf Extract Attenuates β-Amyloid-Induced Neurotoxicity in SH-SY5Y Cells. Int. J. Mol. Sci. 2025, 26, 2917. https://doi.org/10.3390/ijms26072917
Palachai N, Buranrat B, Noisa P, Mairuae N. Oroxylum indicum (L.) Leaf Extract Attenuates β-Amyloid-Induced Neurotoxicity in SH-SY5Y Cells. International Journal of Molecular Sciences. 2025; 26(7):2917. https://doi.org/10.3390/ijms26072917
Chicago/Turabian StylePalachai, Nut, Benjaporn Buranrat, Parinya Noisa, and Nootchanat Mairuae. 2025. "Oroxylum indicum (L.) Leaf Extract Attenuates β-Amyloid-Induced Neurotoxicity in SH-SY5Y Cells" International Journal of Molecular Sciences 26, no. 7: 2917. https://doi.org/10.3390/ijms26072917
APA StylePalachai, N., Buranrat, B., Noisa, P., & Mairuae, N. (2025). Oroxylum indicum (L.) Leaf Extract Attenuates β-Amyloid-Induced Neurotoxicity in SH-SY5Y Cells. International Journal of Molecular Sciences, 26(7), 2917. https://doi.org/10.3390/ijms26072917






