Inhibitory Potential of Boscalid and Abamectin Towards Acetylcholinesterase and Butyrylcholinesterase: Computational and In Vitro Studies
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Cholinesterase Inhibition Assay
3.2. Electron–Ion Interaction Potential (EIIP)/Average Quasi-Valence Number (AQVN)
3.3. Unconstrained Conformational Search
3.4. Molecular Docking Studies
3.5. ADMET In Silico Studies
3.6. Metadynamics and Molecular Dynamics Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seufert, V.; Ramankutty, N.; Foley, J. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef] [PubMed]
- European Parliament. Regulation 1107/2009 of the European Parliament and of the Council. 2009. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009R1107&from=EN (accessed on 18 March 2025).
- Perišić, V.; Perišić, V.; Vukajlović, F.; Predojević, D.; Rajičić, V.; Andrić, G.; Kljajić, P. Effects of abamectin on lesser grain borer, Rhyzoperthadominica F. (Coleoptera: Bostrichidae), infestation on some stored grains. Egypt. J. Biol. Pest Control 2020, 30, 116. [Google Scholar] [CrossRef]
- Wolstenholme, A.J.; Rogers, A.T. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology 2005, 131 (Suppl. S1), S85–S95. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.; Abbas, R.Z.; Mehmood, K.; Hussain, R.; Shah, S.; Faheem, M.; Zaheer, T.; Abbas, A.; Morales, B.; Aneva, I.; et al. Assessment of avermectins-induced toxicity in animals. Pharmaceuticals 2022, 15, 332. [Google Scholar] [CrossRef]
- Wen, F.; Zhang, H.; Yu, Z.; Jin, H.; Yang, Q.; Hou, T. Design, synthesis and antifungal/insecticidal evaluation of novel nicotinamide derivatives. Pestic. Biochem. Physiol. 2010, 98, 248–253. [Google Scholar]
- Xiong, L.; Shen, Y.Q.; Jiang, L.N.; Zhu, X.L.; Yang, W.C.; Huang, W.; Yang, G.F. Succinate dehydrogenase: An ideal target for fungicide discovery. In Discovery and Synthesis of Crop Protection Products; Oxford University Press: New York, NY, USA, 2015; pp. 175–194. [Google Scholar]
- Yanicostas, C.; Soussi-Yanicostas, N. SDHI fungicide toxicity and associated adverse outcome pathways: What can zebrafish tell us? Int. J. Mol. Sci. 2021, 22, 12362. [Google Scholar] [CrossRef]
- Song, L.; Zhong, Z.; Han, Y.; Zheng, Q.; Qin, Y.; Wu, Q.; He, X.; Pan, C. Dissipation of sixteen pesticide residues from various applications of commercial formulations on strawberry and their risk assessment under greenhouse conditions. Ecotoxicol. Environ. Saf. 2020, 188, 109842. [Google Scholar]
- Hua, L.T.; Wu, R.L.; Li, C.L.; Wang, C.N.; Li, Y.L.; Xu, F.L. Experimental study on photodegradation and leaching of typical pesticides in greenhouse soil from Shouguang, Shandong Province, East China. Ecol. Process. 2024, 13, 23. [Google Scholar]
- Soreq, H.; Seidman, S. Acetylcholinesterase—New roles for an old actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Dal Ben, D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [PubMed]
- Petrović, S.; Arsić, B.; Zlatanović, I.; Milićević, J.; Glišić, S.; Mitić, M.; Đurović-Pejčev, R.; Stojanović, G. In silico investigation of selected pesticides and their determination in agricultural products using QuEChERS methodology and HPLC-DAD. Int. J. Mol. Sci. 2023, 24, 8003. [Google Scholar] [CrossRef] [PubMed]
- Arsic, B.; Barber, J.; Čikoš, A.; Mladenovic, M.; Stankovic, N.; Novak, P. 16-Membered macrolide antibiotics—A review. Int. J. Antimicrob. Agents 2018, 51, 283–298. [Google Scholar] [PubMed]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision Glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein–ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar]
- Repasky, M.P.; Murphy, R.B.; Banks, J.L.; Greenwood, J.R.; Tubert-Brohman, I.; Bhat, S.; Friesner, R.A. Docking performance of the glide program as evaluated on the Astex and DUD datasets: A complete set of glide SP results and selected results for a new scoring function integrating WaterMap and Glide. J. Comput.-Aided Mol. Des. 2012, 26, 787–799. [Google Scholar]
- Terali, K.; Dalmizrak, O.; Hoti, Q.; Ozer, N. Evaluation of the inhibitory effect of abamectin on mammalian butyrylcholinesterase: Enzyme kinetic and molecular docking studies. J. Environ. Sci. Health B 2018, 53, 713–718. [Google Scholar]
- Wille, T.; Thiermann, H.; Worek, F. Effect of different buffers on kinetic properties of human acetylcholinesterase and the interaction with organophosphates and oximes. Arch. Toxicol. 2011, 85, 193–198. [Google Scholar]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar]
- Liu, K.; Kokubo, H. Exploring the stability of ligand binding modes to proteins by molecular dynamics simulations: A cross-docking study. J. Chem. Inf. Model. 2017, 57, 2514–2522. [Google Scholar]
- Brus, B.; Košak, U.; Turk, S.; Pišlar, A.; Coquelle, N.; Kos, J.; Stojan, J.; Colletier, J.P.; Gobec, S. Discovery, biological evaluation, and crystal structure of a novel nanomolar selective butyrylcholinesterase inhibitor. J. Med. Chem. 2014, 57, 8167–8179. [Google Scholar] [PubMed]
- Vyas, S.; Beck, J.M.; Xia, S.; Zhang, J.; Hadad, C.M. Butyrylcholinesterase and G116H, G116S, G117H, G117N, E197Q and G117H/E197Q mutants: A molecular dynamics study. Chem. Biol. Interact. 2010, 187, 241–245. [Google Scholar]
- Makhaeva, G.F.; Lushchekina, S.V.; Boltneva, N.P.; Sokolov, V.B.; Grigoriev, V.V.; Serebryakova, O.G.; Vikhareva, E.A.; Aksinenko, A.Y.; Barreto, G.E.; Aliev, G.; et al. Conjugates of γ-Carbolines and Phenothiazine as new selective inhibitors of butyrylcholinesterase and blockers of NMDA receptors for Alzheimer Disease. Sci. Rep. 2015, 5, 13164. [Google Scholar]
- Gholami, A.; Minai-Tehrani, D.; Eriksson, L.A. In silico and in vitro studies confirm Ondansetron as a novel acetylcholinesterase and butyrylcholinesterase inhibitor. Sci. Rep. 2023, 13, 643. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar]
- Trobec, T.; Sepčić, K.; Žužek, M.C.; Kladnik, J.; Podjed, N.; Cardoso Páscoa, C.; Turel, I.; Frangež, R. Fine tuning of cholinesterase and glutathione-S-transferase activities by organoruthenium(II) complexes. Biomedicines 2021, 9, 1243. [Google Scholar] [CrossRef]
- Veljkovic, V.; Slavic, I. Simple general-model pseudopotential. Phys. Rev. Lett. 1972, 29, 105–107. [Google Scholar]
- Veljkovic, V.; Veljkovic, N.; Esté, J.A.; Hüther, A.; Dietrich, U. Application of the EIIP/ISM bioinformatics concept in development of new drugs. Curr. Med. Chem. 2007, 14, 441–453. [Google Scholar]
- Arsic, B.; Barber, J.; Cikos, A.; Kadirvel, M.; Kostic, E.; McBain, A.J.; Milicevic, J.; Oates, A.; Regan, A. Computational studies on selected macrolides active against Escherichia coli combined with the NMR study of tylosin A in deuterated chloroform. Molecules 2022, 27, 7280. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar]
- Wichur, T.; Godyń, J.; Góral, I.; Latacz, G.; Bucki, A.; Siwek, A.; Głuch-Lutwin, M.; Mordyl, B.; Śniecikowska, J.; Walczak, M.; et al. Development and crystallography-aided SAR studies of multifunctional BuChE inhibitors and 5-HT6R antagonists with β-amyloid anti-aggregation properties. Eur. J. Med. Chem. 2021, 225, 113792. [Google Scholar]
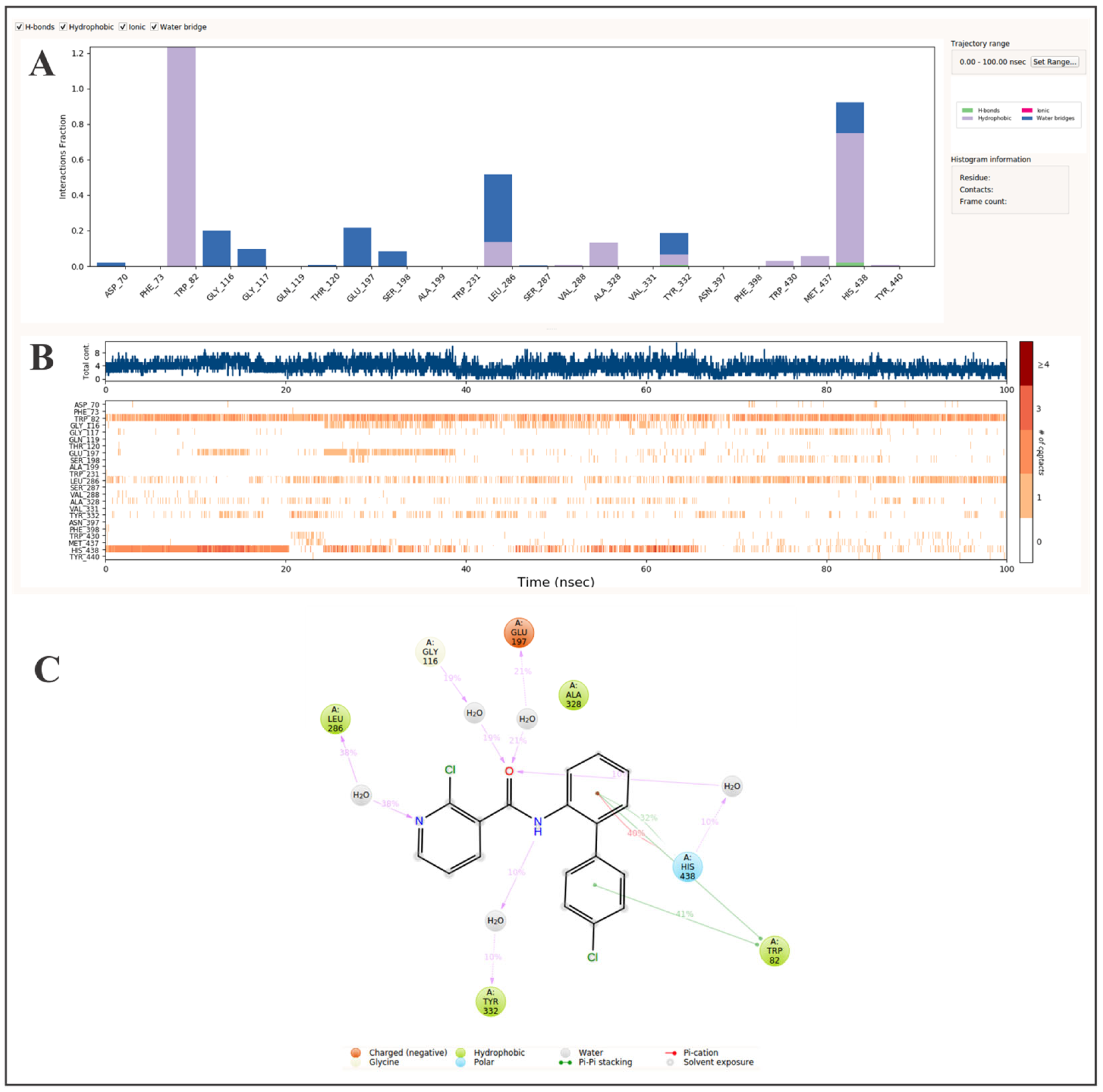

| Compound | Global Minimum (kJ/mol) | Repeats | Glide Score (kJ/mol) | |||
|---|---|---|---|---|---|---|
| hAChE | hBChE | |||||
| Whole Structure | Binding Site | Whole Structure | Binding Site | |||
| Boscalid | 180.8 | 57 | −21.3 | −20.3 | −27.2 | −28.8 |
| Abamectin B1A | 179.0 | 61 | −18.5 | - | - | - |
| Abamectin B1B | 172.8 | 58 | −24.0 | - | - | - |
| Compound | hAChE | hsBChE | hBChE | |||
|---|---|---|---|---|---|---|
| IC50 (µM) | Ki (µM) | IC50 (µM) | Ki (µM) | IC50 (µM) | Ki (µM) | |
| Abamectin (B1A + B1B) | / | n.d. | / | n.d. | / | n.d. |
| Boscalid | / | n.d. | / | n.d. | 308.8 ± 12.6 | n.d. |
| Neostigmine | 4.8 ± 1.4 | n.d. | 62.8 ± 6.3 | n.d. | 173.5 ± 9.2 | n.d. |
| Compound | MW | DM | MV | DHB | AHB | PSA | logP | logS | PCaco | PM | %HOA | VRF | VRT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boscalid | 343.2 | 5.7 | 993.1 | 1 | 3.5 | 42.2 | 4.6 | −5.4 | 3029 | 2 | 100 | 0 | 0 |
| Abamectin B1A | 873.1 | 8.1 | 2515.3 | 2 | 18.5 | 142.4 | 6.3 | −8.3 | 808 | 12 | 77 | 3 | 2 |
| Abamectin B1B | 859.1 | 8.0 | 2486.7 | 2 | 18.6 | 141.2 | 6.1 | −8.0 | 724 | 12 | 75 | 3 | 2 |
| Pesticides | Caco-2 (cm/s) | PPB (%) | CNS | HIA (%) | Metabolic Stability | p-gp Substrate | CYP1A2 Inhibitor | CYP2C9 Inhibitor | CYP2C19 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor | Ames | hERG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boscalid | 86 × 10−6 | 99 | −3.21 | 100 | 0.46 | 0.35 | 0.58 | 0.45 | 0.49 | 0.42 | 0.51 | 0.31 | 0.39 |
| Abamectin B1A | 4 × 10−6 | 97 | −4.89 | 100 | 0.61 | 0.95 | 0.02 | 0.13 | 0.11 | 0.08 | 0.56 | 0.13 | 0.59 |
| Abamectin B1B | 6 × 10−6 | 97 | −4.64 | 100 | 0.60 | 0.94 | 0.02 | 0.13 | 0.11 | 0.08 | 0.55 | 0.13 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arsić, B.; Petrović, S.; Ilić, B.S.; Vrecl, M.; Trobec, T.; Sepčić, K.; Frangež, R.; Glišić, S.M.; Milićević, J.S. Inhibitory Potential of Boscalid and Abamectin Towards Acetylcholinesterase and Butyrylcholinesterase: Computational and In Vitro Studies. Int. J. Mol. Sci. 2025, 26, 2865. https://doi.org/10.3390/ijms26072865
Arsić B, Petrović S, Ilić BS, Vrecl M, Trobec T, Sepčić K, Frangež R, Glišić SM, Milićević JS. Inhibitory Potential of Boscalid and Abamectin Towards Acetylcholinesterase and Butyrylcholinesterase: Computational and In Vitro Studies. International Journal of Molecular Sciences. 2025; 26(7):2865. https://doi.org/10.3390/ijms26072865
Chicago/Turabian StyleArsić, Biljana, Stefan Petrović, Budimir S. Ilić, Milka Vrecl, Tomaž Trobec, Kristina Sepčić, Robert Frangež, Sanja M. Glišić, and Jelena S. Milićević. 2025. "Inhibitory Potential of Boscalid and Abamectin Towards Acetylcholinesterase and Butyrylcholinesterase: Computational and In Vitro Studies" International Journal of Molecular Sciences 26, no. 7: 2865. https://doi.org/10.3390/ijms26072865
APA StyleArsić, B., Petrović, S., Ilić, B. S., Vrecl, M., Trobec, T., Sepčić, K., Frangež, R., Glišić, S. M., & Milićević, J. S. (2025). Inhibitory Potential of Boscalid and Abamectin Towards Acetylcholinesterase and Butyrylcholinesterase: Computational and In Vitro Studies. International Journal of Molecular Sciences, 26(7), 2865. https://doi.org/10.3390/ijms26072865







