Anti-Steatotic Effect of Opuntia stricta var. dillenii Prickly Pear Extracts on Murine and Human Hepatocytes
Abstract
1. Introduction
2. Results
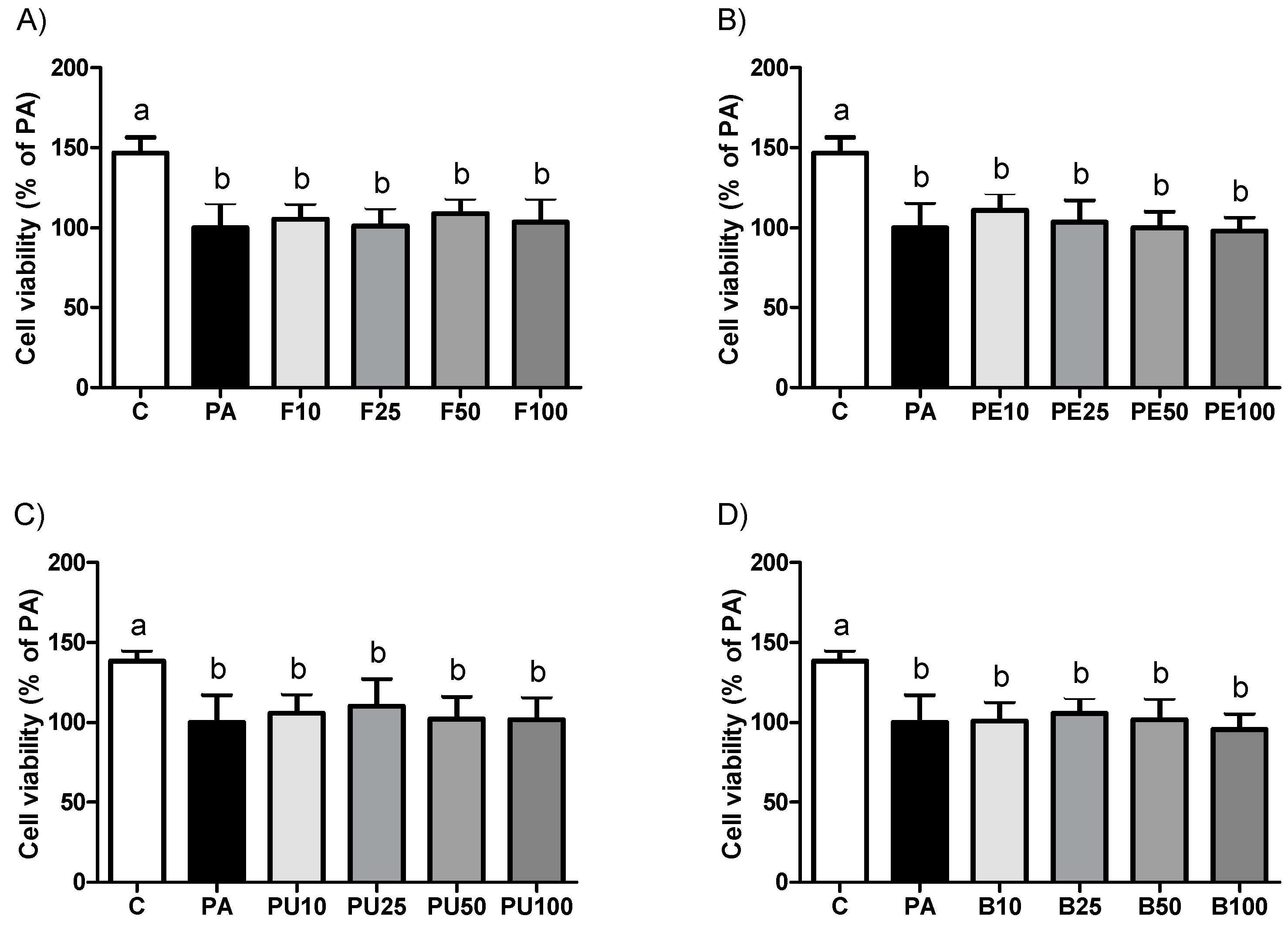
2.1. Cell Viability in AML12 Hepatocytes Treated with Opuntia stricta var. dillenii Extracts
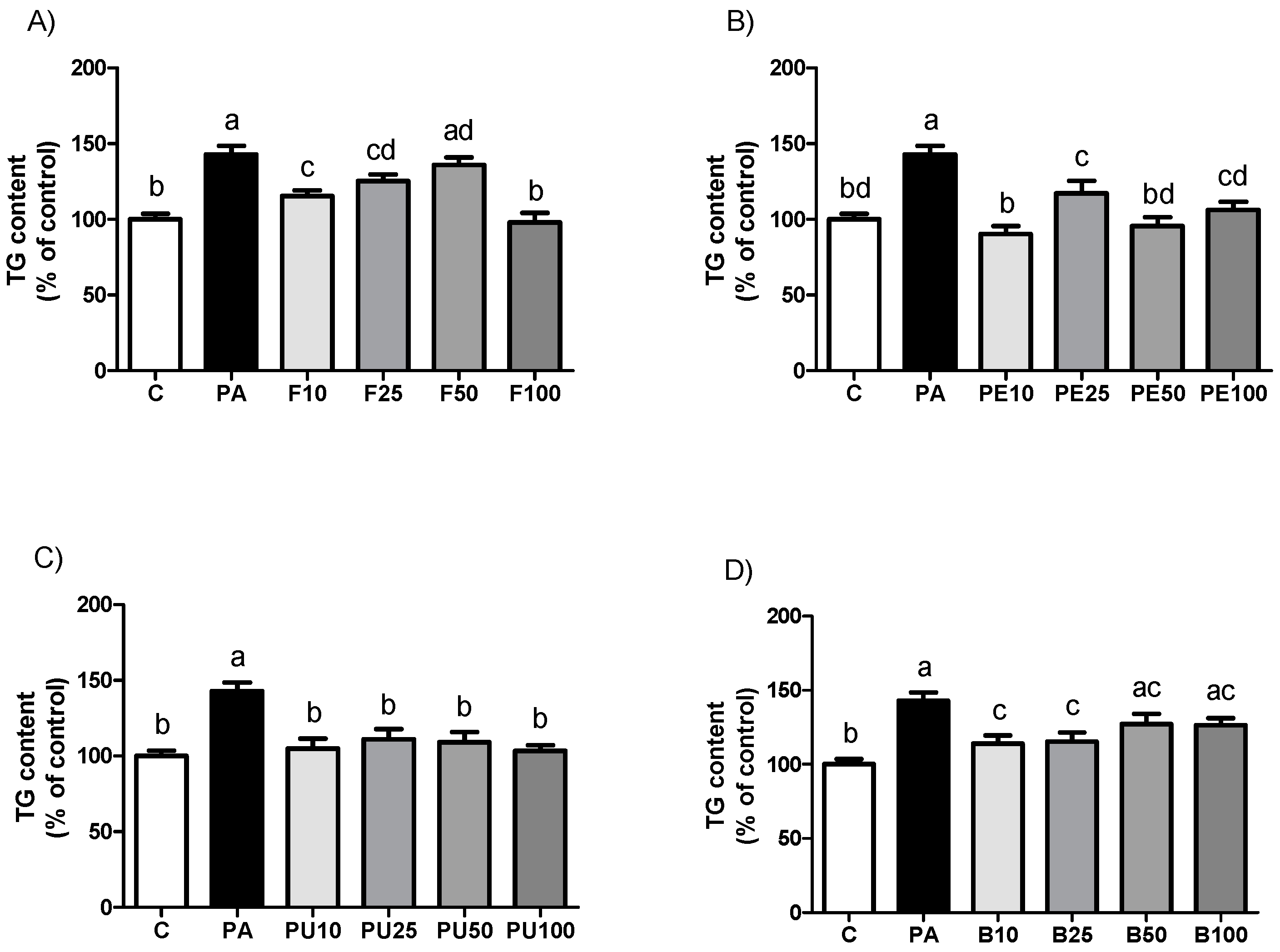
2.2. Triglyceride Levels in AML12 Hepatocytes Treated with Opuntia stricta var. dillenii Extracts
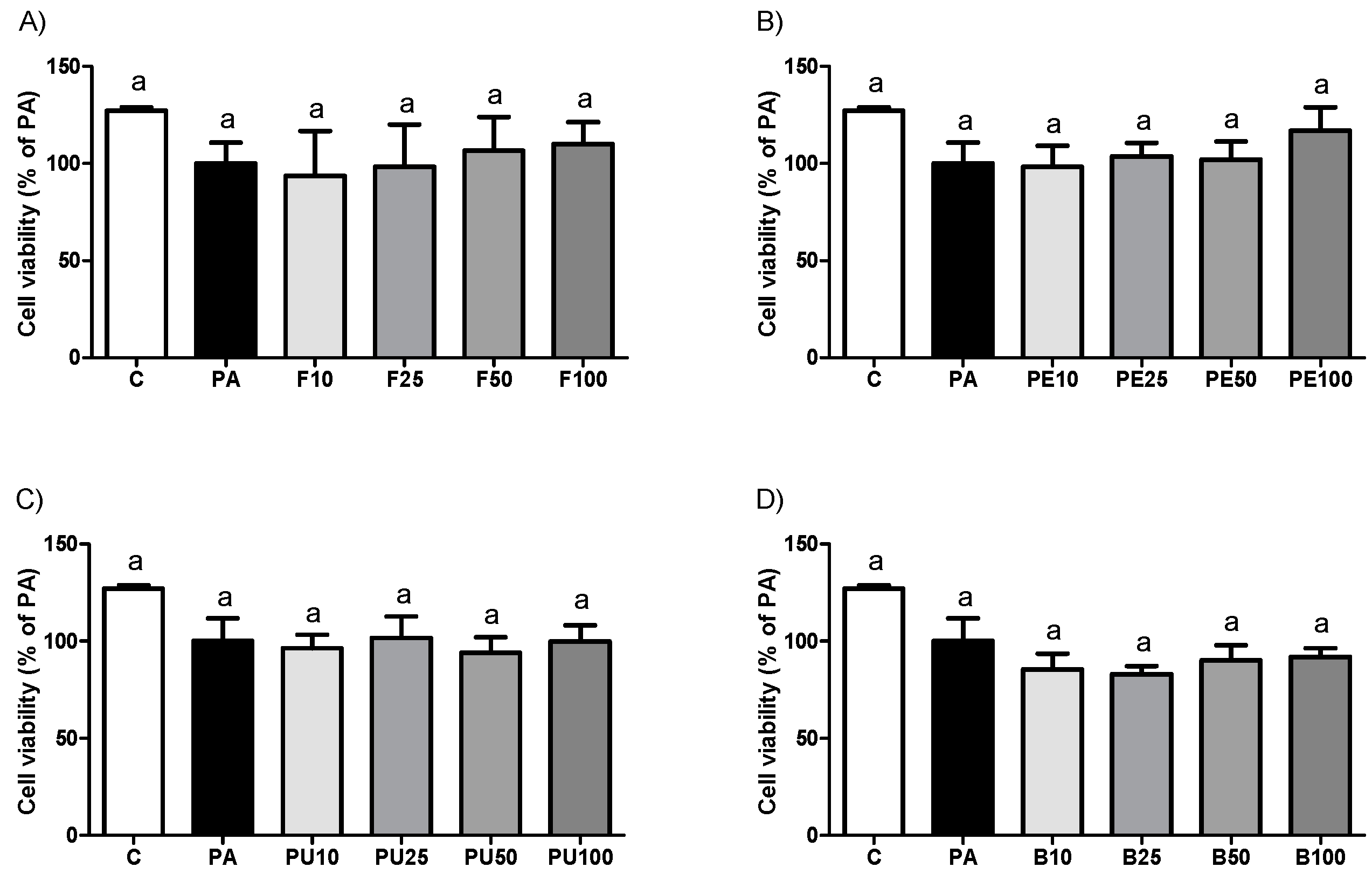
2.3. Cell Viability in HepG2 Hepatocytes Treated with Opuntia stricta var. dillenii Extracts
2.4. Triglyceride Levels in HepG2 Hepatocytes Treated with Opuntia stricta var. dillenii Extracts
2.5. Effects of Opuntia stricta var. dillenii Extracts on Proteins Involved in Triglyceride Metabolism
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Opuntia stricta var. dillenii Extracts
4.3. Bioactive Compound Quantification by HPLC
4.4. Cell Culture Maintenance
4.5. Experimental Design
4.6. Cell Viability Assay
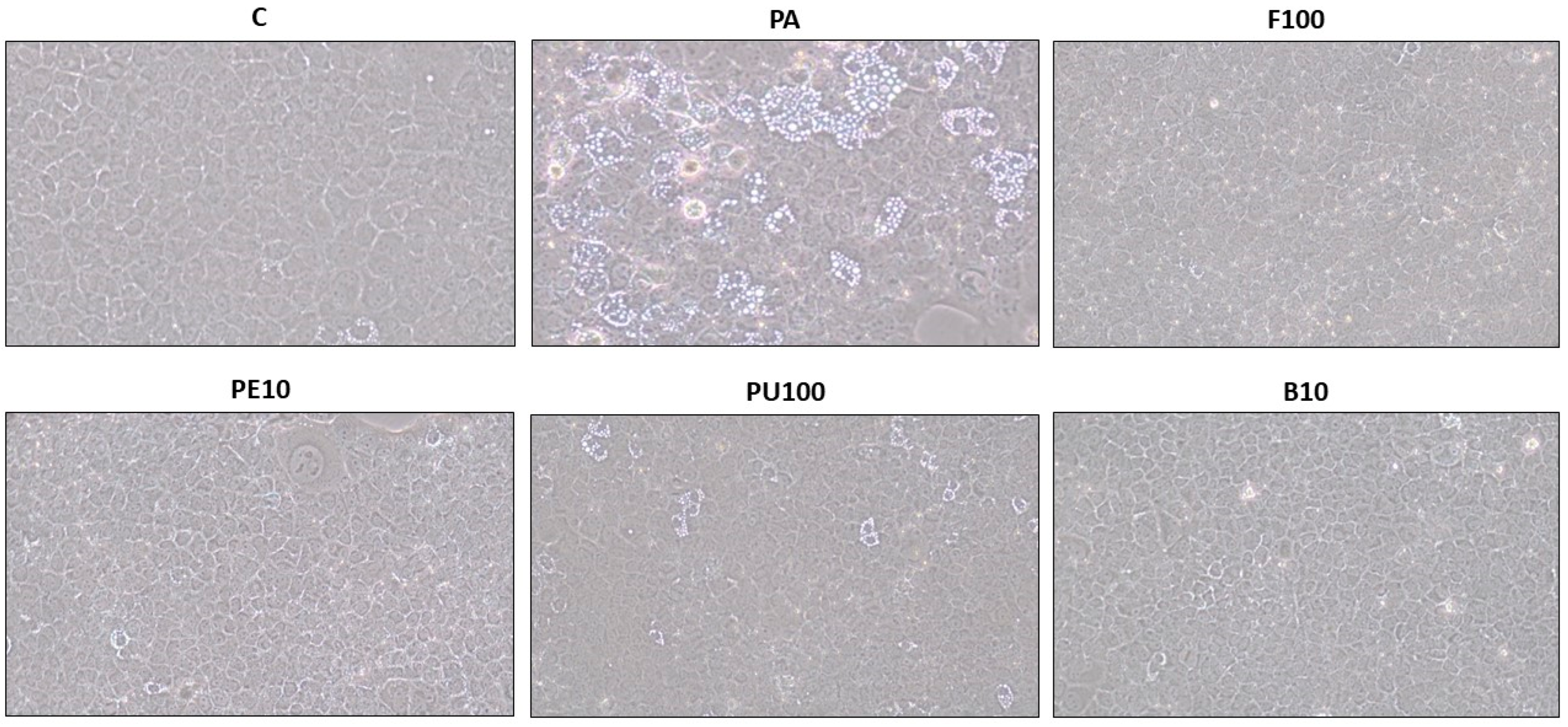
4.7. Optical Microscopy Analysis of Steatotic AML12 and HepG2 Hepatocytes
4.8. Determination of Triglyceride Levels
4.9. Protein Immunodetection
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.; Zheng, M. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [PubMed]
- Guo, X.; Yin, X.; Liu, Z.; Wang, J. Non-Alcoholic Fatty Liver Disease (NAFLD) Pathogenesis and Natural Products for Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 15489. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.D.W.; George, J.; Qiao, L. From MAFLD to hepatocellular carcinoma and everything in between. Chin. Med. J. 2022, 135, 547–556. [Google Scholar] [PubMed]
- Mantovani, A.; Dalbeni, A. Treatments for NAFLD: State of Art. Int. J. Mol. Sci. 2021, 22, 2350. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Barros, L.; Ferreira, I.C.; Rodriguez-Lopez, A.D.; Garcia-Castello, E.M. Ultrasound and Microwave Assisted Extraction of Opuntia Fruit Peels Biocompounds: Optimization and Comparison Using RSM-CCD. Molecules 2019, 24, 3618. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Opuntia (Cactaceae) plant compounds, biological activities and prospects—A comprehensive review. Food Res. Int. 2018, 112, 328–344. [Google Scholar]
- de Araujo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Underutilized plants of the Cactaceae family: Nutritional aspects and technological applications. Food Chem. 2021, 362, 130196. [Google Scholar]
- Aispuro-Hernandez, E.; Vergara-Jimenez, M.J.; Cardenas-Torres, F.I.; Martinez-Tellez, M.A.; Ontiveros, N. Cactaceae plants as sources of active bioavailable phytochemicals. Food Funct. 2022, 13, 9720–9733. [Google Scholar]
- Besne-Eseverri, I.; Trepiana, J.; Gomez-Zorita, S.; Antunes-Ricardo, M.; Cano, M.P.; Portillo, M.P. Beneficial Effects of Opuntia spp. on Liver Health. Antioxidants 2023, 12, 1174. [Google Scholar] [CrossRef]
- Díaz, M.D.S.S.; de La Rosa, A.P.B.; Héliès-Toussaint, C.; Guéraud, F.; Nègre-Salvayre, A. Opuntia spp.: Characterization and Benefits in Chronic Diseases. Oxid Med. Cell. Longev 2017, 2017, 8634249. [Google Scholar]
- Madrigal-Santillan, E.; Portillo-Reyes, J.; Madrigal-Bujaidar, E.; Sanchez-Gutierrez, M.; Izquierdo-Vega, J.A.; Izquierdo-Vega, J.; Delgado-Olivares, L.; Vargas-Mendoza, N.; Alvarez-Gonzalez, I.; Morales-Gonzalez, A.; et al. Opuntia spp. in Human Health: A Comprehensive Summary on Its Pharmacological, Therapeutic and Preventive Properties. Part 2. Plants 2022, 11, 2333. [Google Scholar] [CrossRef] [PubMed]
- Loukili, E.H.; Merzouki, M.; Taibi, M.; Elbouzidi, A.; Hammouti, B.; Kumar Yadav, K.; Khalid, M.; Addi, M.; Ramdani, M.; Kumar, P.; et al. Phytochemical, biological, and nutritional properties of the prickly pear, Opuntia dillenii: A review. Saudi Pharm. J. 2024, 32, 102167. [Google Scholar] [PubMed]
- Lu, W.; Chiu, C.; Chan, Y.; Mulio, A.T.; Li, P. Recent Research on Different Parts and Extracts of Opuntia dillenii and Its Bioactive Components, Functional Properties, and Applications. Nutrients 2023, 15, 2962. [Google Scholar] [CrossRef] [PubMed]
- Bouhrim, M.; Ouassou, H.; Choukri, M.; Mekhfi, H.; Ziyyat, A.; Legssyer, A.; Aziz, M.; Bnouham, M. Hepatoprotective effect of Opuntia dillenii seed oil on CCl4 induced acute liver damage in rat. Asian Pac. J. Trop. Biomed. 2018, 8, 254–260. [Google Scholar]
- Bouhrim, M.; Bencheikh, N.; Imtara, H.; Daoudi, N.E.; Mechchate, H.; Ouassou, H.; Kharchoufa, L.; Elachouri, M.; Mekhfi, H.; Ziyyat, A.; et al. Protective Effect of Opuntia dillenii (Ker Gawl.) Haw. Seed Oil on Gentamicin-Induced Nephrotoxicity: A Biochemical and Histological Analysis. Sci. World 2021, 2021, 2173012. [Google Scholar]
- Shirazinia, R.; Golabchifar, A.A.; Rahimi, V.B.; Jamshidian, A.; Samzadeh-Kermani, A.; Hasanein, P.; Hajinezhad, M.; Askari, V.R. Protective Effect of Opuntia dillenii Haw Fruit against Lead Acetate-Induced Hepatotoxicity: In Vitro and In Vivo Studies. Evid.-Based Complement. Altern. Med. 2021, 2021, 6698345. [Google Scholar]
- Besne-Eseverri, I.; Martin, M.A.; Lobo, G.; Cano, M.P.; Portillo, M.P.; Trepiana, J. Antioxidant and Anti-Inflammatory Effects of Opuntia Extracts on a Model of Diet-Induced Steatosis. Antioxidants 2024, 13, 1416. [Google Scholar] [CrossRef]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef]
- Gómez-López, I.; Lobo-Rodrigo, G.; Portillo, M.P.; Cano, M.P. Characterization, Stability, and Bioaccessibility of Betalain and Phenolic Compounds from Opuntia stricta var. dillenii Fruits and Products of Their Industrialization. Foods 2021, 10, 1593. [Google Scholar]
- Bouazza, A.; Bitam, A.; Amiali, M.; Bounihi, A.; Yargui, L.; Koceir, E.A. Effect of fruit vinegars on liver damage and oxidative stress in high-fat-fed rats. Pharm. Biol. 2016, 54, 260–265. [Google Scholar]
- Navarro, G.; Gomez-Autet, M.; Morales, P.; Rebassa, J.B.; Llinas Del Torrent, C.; Jagerovic, N.; Pardo, L.; Franco, R. Homodimerization of CB(2) cannabinoid receptor triggered by a bivalent ligand enhances cellular signaling. Pharmacol. Res. 2024, 208, 107363. [Google Scholar] [PubMed]
- Gonzalez-Arceo, M.; Trepiana, J.; Aguirre, L.; Ibarruri, J.; Martinez-Sanz, M.; Cebrian, M.; Recio, I.; Portillo, M.P.; Gomez-Zorita, S. Anti-Steatotic Effects of Chlorella vulgaris, Nannochloropsis gaditana and Gracilaria vermiculophylla Algae Extracts in AML-12 Hepatocytes. Nutrients 2023, 15, 1960. [Google Scholar] [CrossRef] [PubMed]
- Trepiana, J.; Krisa, S.; Renouf, E.; Portillo, M.P. Resveratrol Metabolites Are Able to Reduce Steatosis in Cultured Hepatocytes. Pharmaceuticals 2020, 13, 285. [Google Scholar] [CrossRef]
- Ren, Y.; Shi, X.; Mu, J.; Liu, S.; Qian, X.; Pei, W.; Ni, S.; Zhang, Z.; Li, L.; Zhang, Z. Chronic exposure to parabens promotes non-alcoholic fatty liver disease in association with the changes of the gut microbiota and lipid metabolism. Food Funct. 2024, 15, 1562–1574. [Google Scholar]
- Bai, Y.; Zhang, T.; Hu, Z.; Zhang, Y.; Wang, D.; Zhou, M.; Zhang, Y.; Zhang, F.; Kong, X. Sesamin ameliorates nonalcoholic hepatic steatosis by inhibiting CD36-mediated hepatocyte lipid accumulation in vitro and in vivo. Biochem. Pharmacol. 2024, 224, 116240. [Google Scholar]
- Nagarajan, S.R.; Paul-Heng, M.; Krycer, J.R.; Fazakerley, D.J.; Sharland, A.F.; Hoy, A.J. Lipid and glucose metabolism in hepatocyte cell lines and primary mouse hepatocytes: A comprehensive resource for in vitro studies of hepatic metabolism. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E578–E589. [Google Scholar]
- Zhu, X.; Bian, H.; Wang, L.; Sun, X.; Xu, X.; Yan, H.; Xia, M.; Chang, X.; Lu, Y.; Li, Y.; et al. Berberine attenuates nonalcoholic hepatic steatosis through the AMPK-SREBP-1c-SCD1 pathway. Free Radic. Biol. Med. 2019, 141, 192–204. [Google Scholar]
- Wang, J.; Zhang, F.; Yang, W.; Gao, D.; Yang, L.; Yu, C.; Chen, C.; Li, X.; Zhang, J. FGF1 ameliorates obesity-associated hepatic steatosis by reversing IGFBP2 hypermethylation. FASEB J. 2023, 37, e22881. [Google Scholar]
- Chen, L.; Duan, Y.; Wei, H.; Ning, H.; Bi, C.; Zhao, Y.; Qin, Y.; Li, Y. Acetyl-CoA carboxylase (ACC) as a therapeutic target for metabolic syndrome and recent developments in ACC1/2 inhibitors. Expert Opin. Investig. Drugs 2019, 28, 917–930. [Google Scholar]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar]
- Gomez-Maqueo, A.; Antunes-Ricardo, M.; Welti-Chanes, J.; Cano, M.P. Digestive Stability and Bioaccessibility of Antioxidants in Prickly Pear Fruits from the Canary Islands: Healthy Foods and Ingredients. Antioxidants 2020, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, I.; Eseberri, I.; Cano, M.P.; Portillo, M.P. Anti-Obesity Effect of Different Opuntia stricta var. dillenii’s Prickly Pear Tissues and Industrial By-Product Extracts in 3T3-L1 Mature Adipocytes. Nutrients 2024, 16, 499. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Didier, N.; Denton, M. Determination of cell number in monolayer cultures. Anal. Biochem. 1986, 159, 109–113. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar]
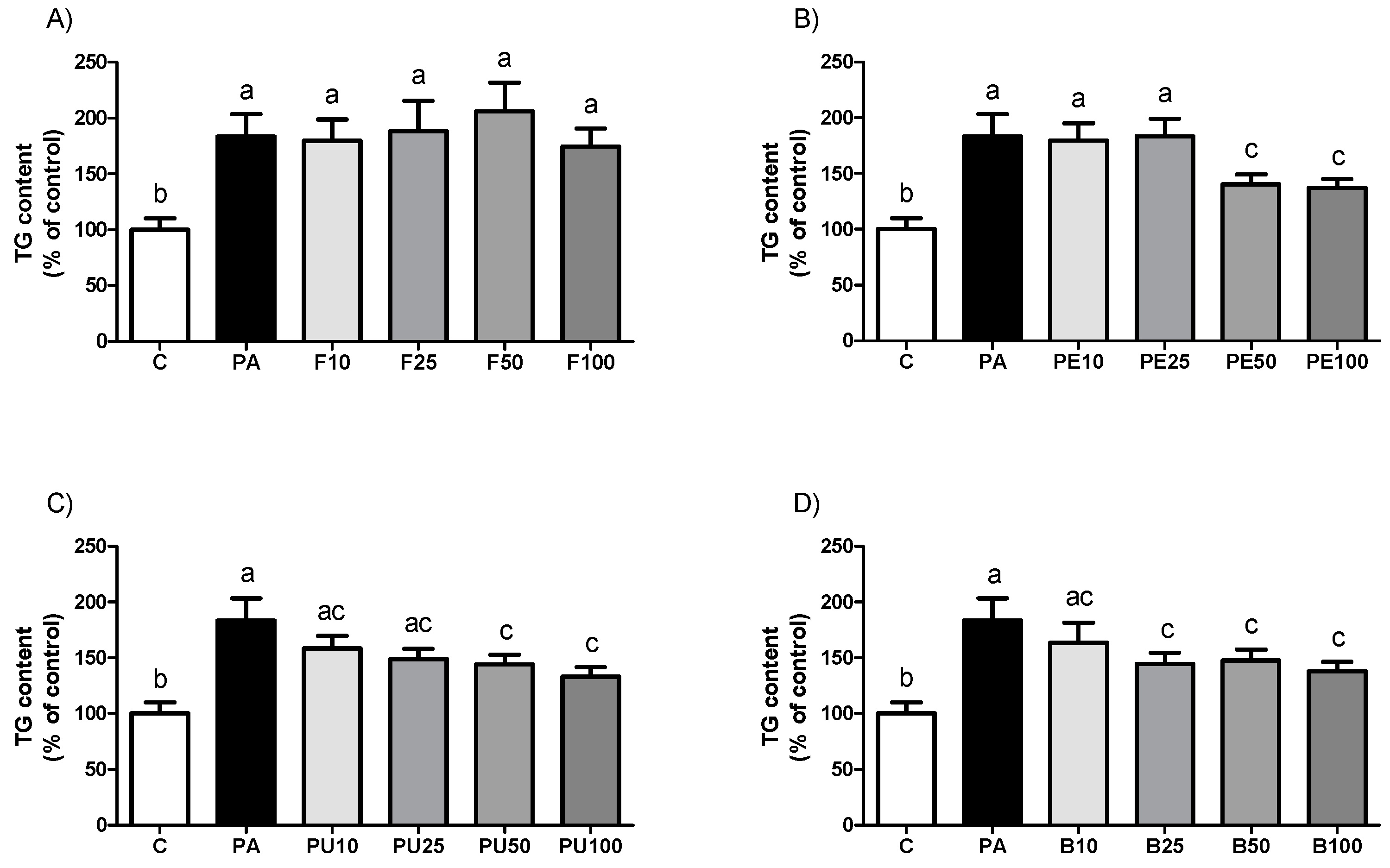
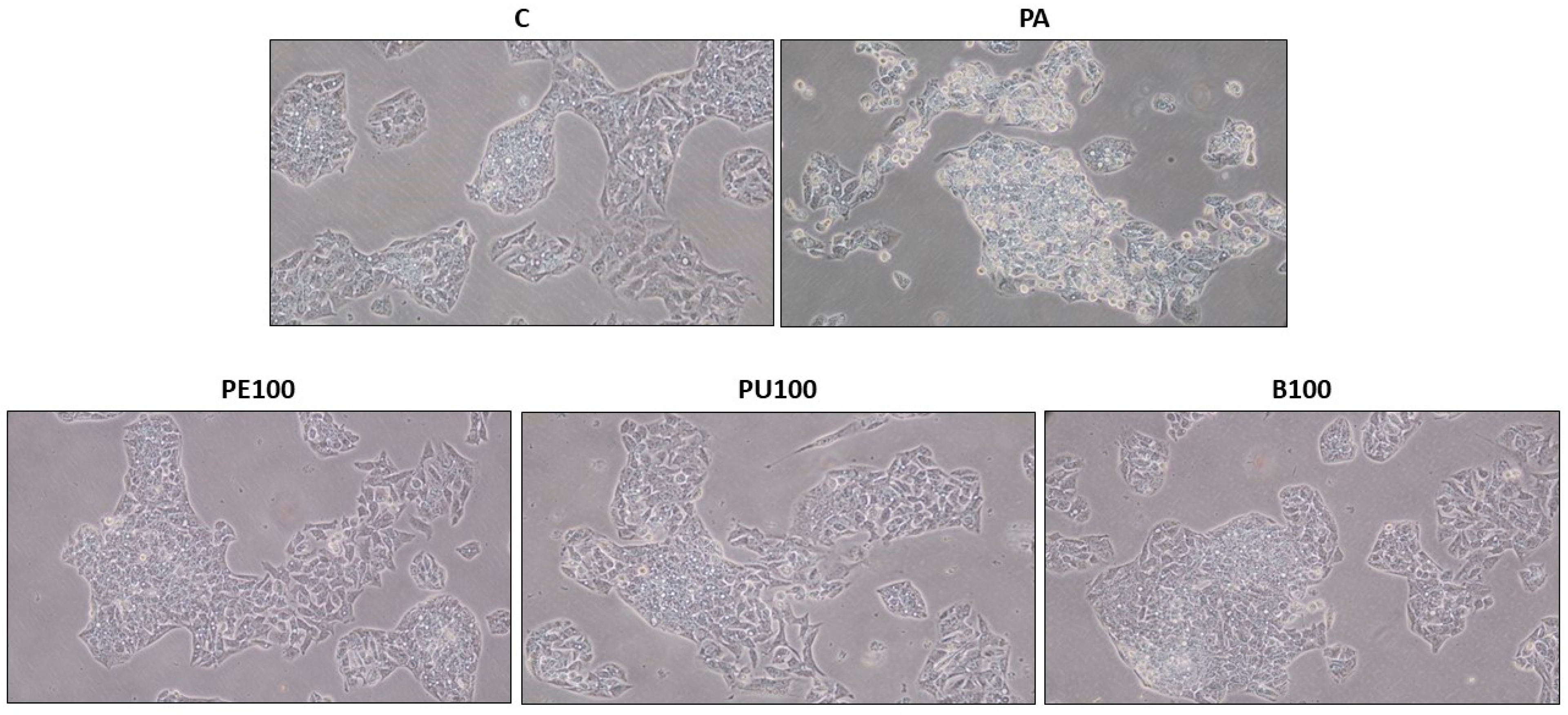
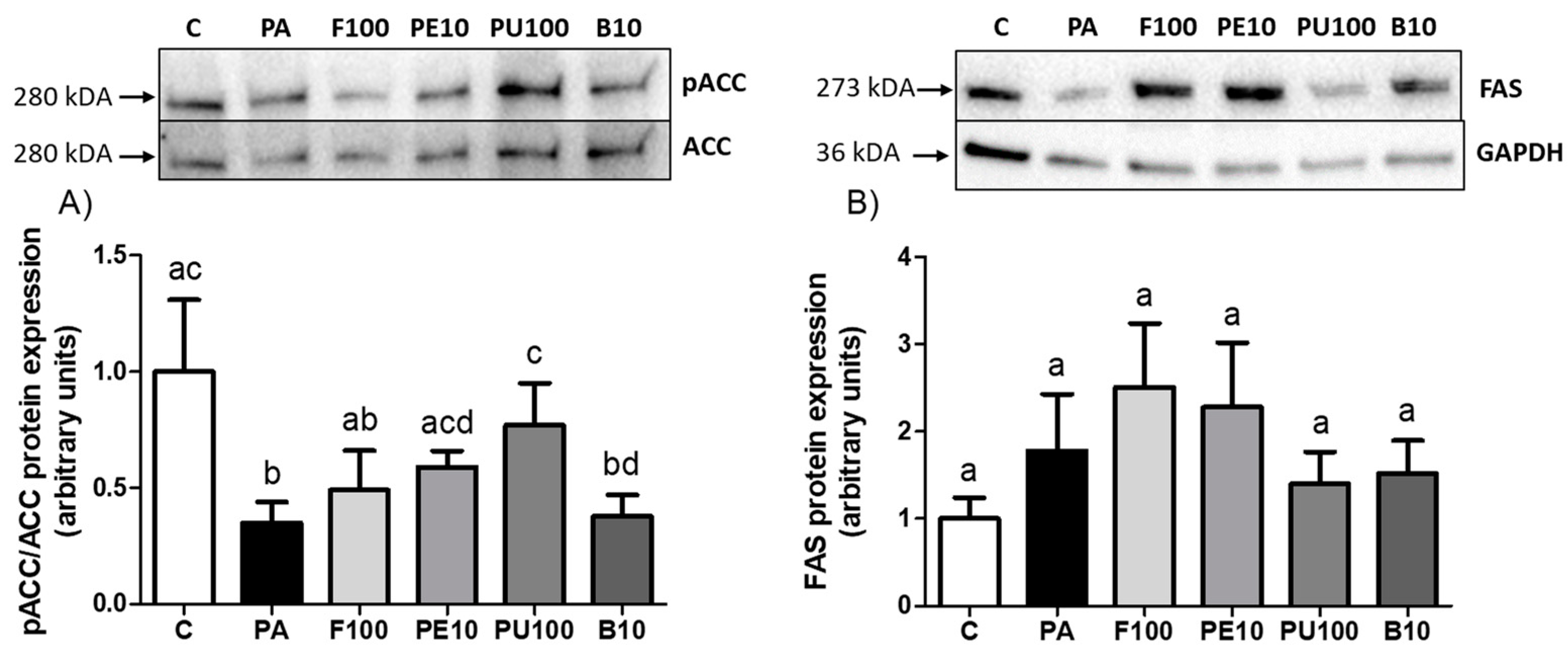
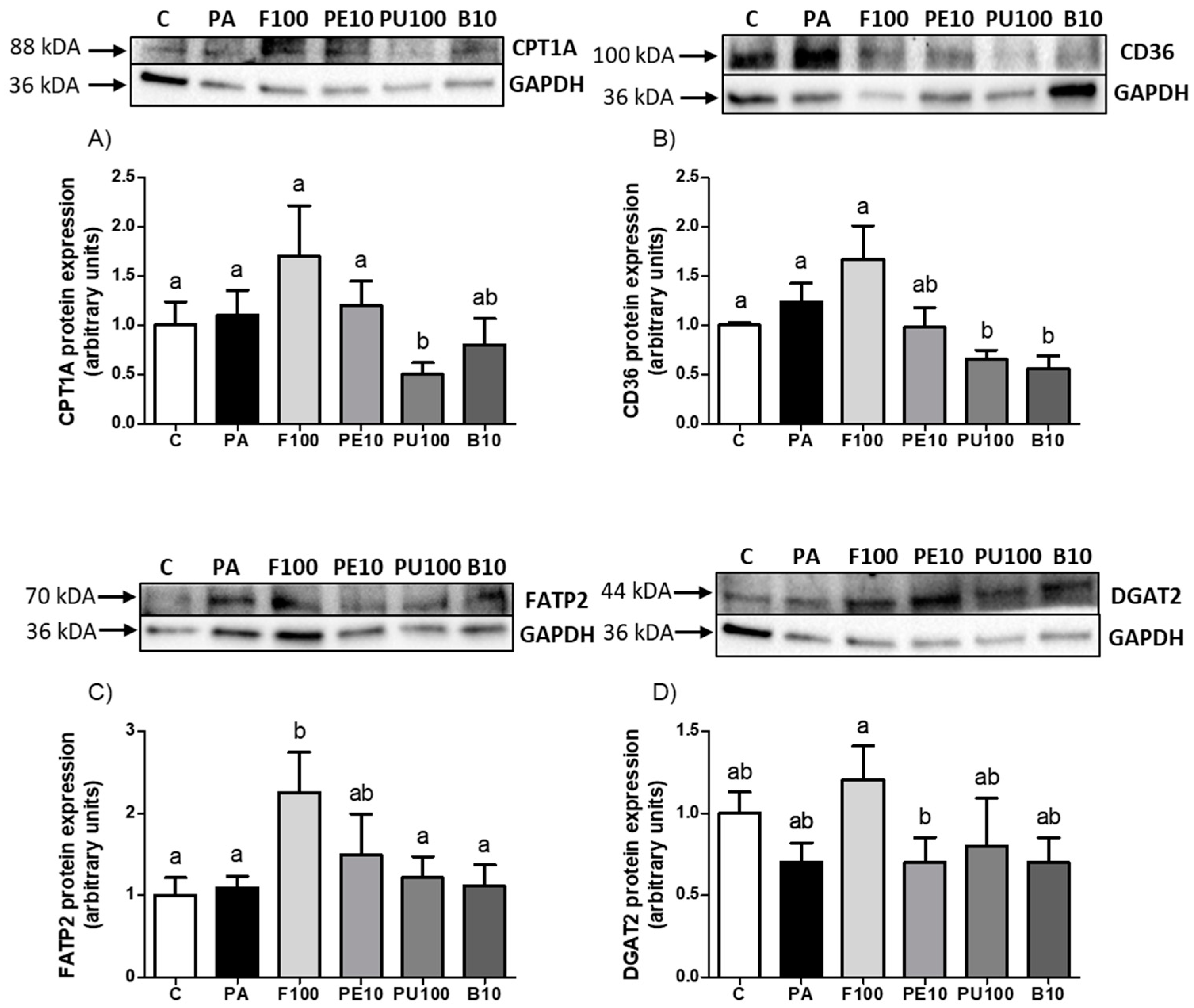
| Extracts | Triglyceride Content Reduction (%) |
|---|---|
| Whole fruit (10 µg/mL) | 16.9 ± 10.1 |
| Whole fruit (25 µg/mL) | 9.4 ± 11.9 |
| Whole fruit (100 µg/mL) | 29.5 ± 12.6 |
| Peel (10 µg/mL) | 34.7 ± 10.0 |
| Peel (25 µg/mL) | 17.8 ± 6.1 |
| Peel (50 µg/mL) | 34.3 ± 1.2 |
| Peel (100 µg/mL) | 25.4 ± 5.0 |
| Pulp (10 µg/mL) | 24.6 ± 11.5 |
| Pulp (25 µg/mL) | 21.5 ± 2.4 |
| Pulp (50 µg/mL) | 21.9 ± 9.9 |
| Pulp (100 µg/mL) | 26.3 ± 5.2 |
| Bagasse (10 µg/mL) | 19.8 ± 7.5 |
| Bagasse (25 µg/mL) | 18.6 ± 7.4 |
| Extracts | Triglyceride Content Reduction (%) |
|---|---|
| Peel (50 µg/mL) | 14.5 ± 14.4 |
| Peel (100 µg/mL) | 16.3 ± 14.2 |
| Pulp (50 µg/mL) | 13.9 ± 13.5 |
| Pulp (100 µg/mL) | 21.0 ± 11.6 |
| Bagasse (25 µg/mL) | 15.8 ± 9.0 |
| Bagasse (50 µg/mL) | 12.8 ± 11.2 |
| Bagasse (100 µg/mL) | 17.1 ± 14.0 |
| Compound | Family | Opuntia stricta var. dillenii | |||
|---|---|---|---|---|---|
| Whole Fruit | Peel | Pulp | Bagasse | ||
| Piscidic acid | Phenolic acid | 1.64 ± 0.09 | 2.33 ± 0.33 | 0.62 ± 0.05 | 1.54 ± 0.05 |
| Betanin | Betalain | 2.97 ± 0.01 | 2.99 ± 0.05 | 2.91 ± 0.23 | 0.84 ± 0.02 |
| Isobetanin | Betalain | 1.85 ± 0.00 | 1.65 ± 0.04 | 2.28 ± 0.19 | 0.77 ± 0.02 |
| Betanidin | Betalain | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.01 | 0.02 ± 0.00 |
| 6′-O-sinapoyl-O-gompherin | Betalain | 0.13 ± 0.00 | 0.14 ± 0.00 | 0.08 ± 0.00 | 0.01 ± 0.00 |
| 2′-O-apiosyl-4-O-phyllocactin | Betalain | 1.29 ± 0.02 | 1.22 ± 0.02 | 1.61 ± 0.18 | 0.58 ± 0.16 |
| 5″-O-E-sinapoyl-2′-apyosil-phyllocactin | Betalain | 3.14 ± 0.00 | 3.23 ± 0.13 | 2.6 ± 0.07 | n.d. |
| Neobetanin | Betalain | 1.95 ± 0.02 | 0.82 ± 0.00 | 3.26 ± 0.05 | 1.03 ± 0.07 |
| Quercetin-3-O-rhamnosyl-rutinoside (QG3) | Flavonoid | 0.04 ± 0.00 | 0.07 ± 0.00 | n.d. | 0.02 ± 0.00 |
| Quercetin glycoside (QG1)-Quercetin hexosyl pentosyl rhamnoside | Flavonoid | 0.04 ± 0.00 | 0.08 ± 0.00 | n.d. | 0.02 ± 0.00 |
| Quercetin glycoside (QG2)-Quercetin hexose pentoside | Flavonoid | 0.02 ± 0.00 | 0.02 ± 0.00 | n.d. | n.d. |
| Isorhamnetin glucosyl-rhamnosyl-rhamnoside (IG1) | Flavonoid | 0.02 ± 0.00 | 0.03 ± 0.00 | n.d. | 0.01 ± 0.00 |
| Isorhamnetin glucosyl-rhamnosyl-pentoside (IG2) | Flavonoid | 0.29 ± 0.00 | 0.52 ± 0.02 | 0.05 ± 0.00 | 0.18 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besné-Eseverri, I.; Trepiana, J.; Boutaleb, L.; Martín, M.Á.; Krisa, S.; Lobo, M.G.; Cano, M.P.; Portillo, M.P. Anti-Steatotic Effect of Opuntia stricta var. dillenii Prickly Pear Extracts on Murine and Human Hepatocytes. Int. J. Mol. Sci. 2025, 26, 2864. https://doi.org/10.3390/ijms26072864
Besné-Eseverri I, Trepiana J, Boutaleb L, Martín MÁ, Krisa S, Lobo MG, Cano MP, Portillo MP. Anti-Steatotic Effect of Opuntia stricta var. dillenii Prickly Pear Extracts on Murine and Human Hepatocytes. International Journal of Molecular Sciences. 2025; 26(7):2864. https://doi.org/10.3390/ijms26072864
Chicago/Turabian StyleBesné-Eseverri, Irene, Jenifer Trepiana, Lina Boutaleb, María Ángeles Martín, Stéphanie Krisa, María Gloria Lobo, M. Pilar Cano, and María P. Portillo. 2025. "Anti-Steatotic Effect of Opuntia stricta var. dillenii Prickly Pear Extracts on Murine and Human Hepatocytes" International Journal of Molecular Sciences 26, no. 7: 2864. https://doi.org/10.3390/ijms26072864
APA StyleBesné-Eseverri, I., Trepiana, J., Boutaleb, L., Martín, M. Á., Krisa, S., Lobo, M. G., Cano, M. P., & Portillo, M. P. (2025). Anti-Steatotic Effect of Opuntia stricta var. dillenii Prickly Pear Extracts on Murine and Human Hepatocytes. International Journal of Molecular Sciences, 26(7), 2864. https://doi.org/10.3390/ijms26072864









