The Impact of Tetracycline on the Soil Microbiome and the Rhizosphere of Lettuce (Lactuca sativa L.)
Abstract
1. Introduction
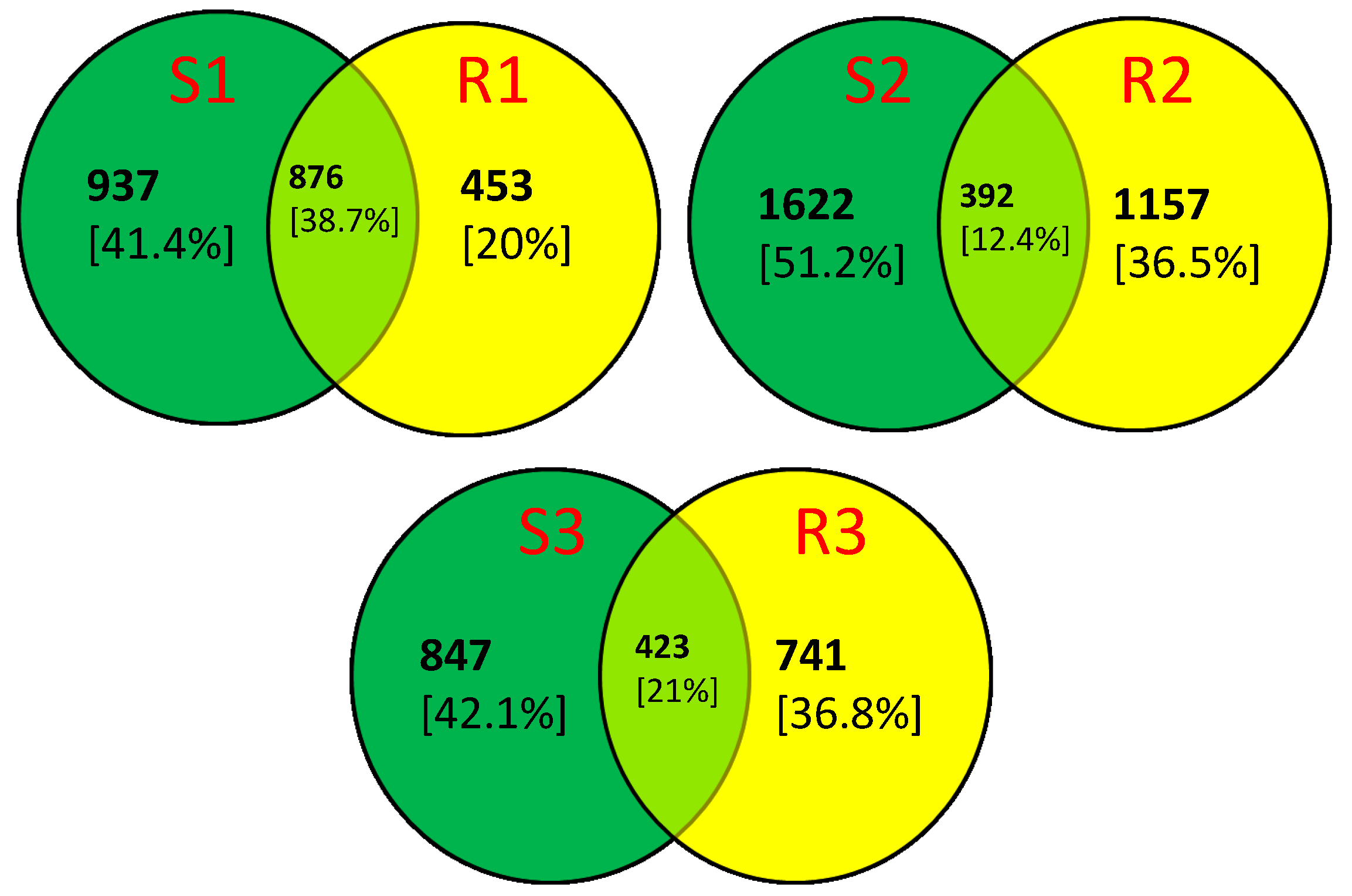
2. Results and Discussion
3. Materials and Methods
3.1. Lettuce Growing Conditions
3.2. DNA Isolation from Soil and Rhizosphere
3.3. Metagenomic Analysis of Soil and Rhizosphere Samples
4. Conclusions
- Tetracycline at concentrations of 5 and 25 mg/kg significantly alters the soil microbiome at various taxonomic levels, and this effect is noticeable even after applying the lowest concentration of the antibiotic.
- The presence of M. tuberculosis in soil treated with 5 mg/kg tetracycline may suggest that tetracycline affects the microbiome in a way that favors the survival of this pathogen in the environment. This finding requires further investigation to understand the mechanisms allowing M. tuberculosis to persist in soil environments, the potential transfer to plant tissues, and public health risks.
- The addition of tetracycline did not induce significant changes in the taxonomic profile of the rhizosphere microorganisms. This suggests that plants may influence the stabilization of the rhizosphere microbiome.
- The increased abundance of Firmicutes bacteria after tetracycline treatment in soil and rhizosphere suggests that this taxon should be particularly monitored in plants growing in antibiotic-contaminated environments.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berg, G.; Rybakova, D.; Grube, M.; Köberl, M. The plant microbiome explored: Implications for experimental botany. J. Exp. Bot. 2016, 67, 995–1002. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Dastogeer, K.M.; Tumpa, F.H.; Sultana, A.; Akter, M.A.; Chakraborty, A. Plant microbiome–an account of the factors that shape community composition and diversity. Curr. Plant Biol. 2020, 23, 100161. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Cernava, T.; Wassermann, B.; Abdelfattah, A.; Soto-Giron, M.J.; Toledo, G.V.; Virtanen, S.M.; Knip, M.; Hyöty, H.; Berg, G. The edible plant microbiome: Evidence for the occurrence of fruit and vegetable bacteria in the human gut. Gut Microbes 2023, 15, 2258565. [Google Scholar] [CrossRef]
- Khaneghah Mousavi, A.; Abhari, K.; Eş, I.; Soares, M.B.; Oliveira, R.B.; Hosseini, H.; Rezaei, M.; Balthazar, C.F.; Silva, R.; Cruz, A.G.; et al. Interactions between probiotics and pathogenic microorganisms in hosts and foods: A review. Trends Food Sci. Technol. 2020, 95, 205–218. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Li, Z.; Guo, S.; Li, K.; Xu, P.; Ok, Y.S.; Jones, D.L.; Zou, J. Antibiotics and antibiotic resistance genes in agricultural soils: A systematic analysis. Crit. Rev. Environ. Sci. Technol. 2023, 53, 847–864. [Google Scholar] [CrossRef]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef]
- Ahlawat, S.; Asha; Sharma, K.K. Gut-organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Banerjee, S.; van der Heijden, M.G.A. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef]
- CDDEP. The State of the World’s Antibiotics 2021—A Global Analysis of Antimicrobial Aesistance and Its Drivers; Center for Disease Dynamics, Economics & Policy: Washington, DC, USA, 2021. [Google Scholar]
- Tian, M.; He, X.; Feng, Y.; Wang, W.; Chen, H.; Gong, M.; Liu, D.; Clarke, J.L.; van Eerde, A. Pollution by antibiotics and antimicrobial resistance in livestock and poultry manure in China, and countermeasures. Antibiotics 2021, 10, 539. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment-degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Marutescu, L.G.; Jaga, M.; Postolache, C.; Barbuceanu, F.; Milita, N.M.; Romascu, L.M.; Schmitt, H.; de Roda Husman, A.M.; Sefeedpari, P.; Glaeser, S.; et al. Insights into the impact of manure on the environmental antibiotic residues and resistance pool. Front. Microbiol. 2022, 13, 965132. [Google Scholar] [CrossRef]
- Xu, L.; Wang, W.; Xu, W. Effects of tetracycline antibiotics in chicken manure on soil microbes and antibiotic resistance genes (ARGs). Environ. Geochem. Health 2022, 44, 273–284. [Google Scholar] [CrossRef]
- Heuer, H.; Schmitt, H.; Smalla, K. Antibiotic resistance gene spread due to manure application on agricultural fields. Curr. Opin. Microbiol. 2011, 14, 236–243. [Google Scholar] [CrossRef]
- De La Torre, A.; Iglesias, I.; Carballo, M.; Ramírez, P.; Muñoz, M.J. An approach for mapping the vulnerability of European Union soils to antibiotic contamination. Sci. Total Environ. 2012, 414, 672–679. [Google Scholar] [CrossRef]
- Fang, L.; Chen, C.; Li, S.; Ye, P.; Shi, Y.; Sharma, G.; Sarkar, B.; Shaheen, S.M.; Lee, S.S.; Xiao, R.; et al. A comprehensive and global evaluation of residual antibiotics in agricultural soils: Accumulation, potential ecological risks, and attenuation strategies. Ecotoxicol. Environ. Saf. 2023, 262, 115175. [Google Scholar] [CrossRef]
- Zalewska, M.; Błażejewska, A.; Czapko, A.; Popowska, M. Antibiotics and antibiotic resistance genes in animal manure—Consequences of its application in agriculture. Front. Microbiol. 2021, 12, 610656. [Google Scholar] [CrossRef]
- Cedeño-Muñoz, J.S.; Aransiola, S.A.; Reddy, K.V.; Ranjit, P.; Victor-Ekwebelem, M.O.; Oyedele, O.J.; Pérez-Almeida, I.B.; Maddela, N.R.; Rodríguez-Díaz, J.M. Antibiotic resistant bacteria and antibiotic resistance genes as contaminants of emerging concern: Occurrences, impacts, mitigations and future guidelines. Sci. Total Environ. 2024, 952, 175906. [Google Scholar] [CrossRef]
- Rasheela, A.R.P.; Khalid, M.F.; Abumaali, D.A.; Alatalo, J.M.; Ahmed, T. Impact of abiotic stressors on soil microbial communities: A focus on antibiotics and their interactions with emerging pollutants. Soil Syst. 2025, 9, 2. [Google Scholar] [CrossRef]
- Xie, W.-Y.; Shen, Q.; Zhao, F.J. Antibiotics and antibiotic resistance from animal manures to soil: A review. Eur. J. Soil Sci. 2018, 69, 181–195. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A Review. Michrochem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Han, B.; Ma, L.; Yu, Q.; Yang, J.; Su, W.; Hilal, M.G.; Li, X.; Zhang, S.; Li, H. The source, fate and prospect of antibiotic resistance genes in soil: A review. Front. Microbiol. 2022, 13, 976657. [Google Scholar] [CrossRef]
- Shen, C.; He, M.; Zhang, J.; Liu, J.; Su, J.; Dai, J. Effects of the coexistence of antibiotics and heavy metals on the fate of antibiotic resistance genes in chicken manure and surrounding soil. Ecotoxicol. Environ. Saf. 2023, 263, 115367. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Wu, Q.; Zhang, J.; Zhang, F.; Yang, X.; Wu, H.; Zeng, H.; Chen, M.; Ding, Y. Staphylococcus aureus isolated from retail meat and meat products in China: Incidence, antibiotic resistance and genetic diversity. Front. Microbiol. 2018, 9, 2767. [Google Scholar] [CrossRef]
- Sobiczewski, P. Bakterie w środowisku roślin-wrogowie i sprzymierzeńcy. Kosmos 2022, 70, 685–696. (In Polish) [Google Scholar] [CrossRef]
- Compant, S.; Cambon, M.C.; Vacher, C.; Mitter, B.; Samad, A.; Sessitsch, A. The plant endosphere world-bacterial life within plants. Environ. Microbiol. 2021, 23, 1812–1829. [Google Scholar] [CrossRef]
- Bahmani, K.; Shahbazi, Y.; Nikousefat, Z. Monitoring and risk assessment of tetracycline residues in foods of animal origin. Food Sci. Biotechnol. 2020, 29, 441–448. [Google Scholar] [CrossRef]
- Zhou, X.; Qiao, M.; Su, J.Q.; Wang, Y.; Cao, Z.H.; Cheng, W.D.; Zhu, Y.G. Turning pig manure into biochar can effectively mitigate antibiotic resistance genes as organic fertilizer. Sci. Total. Environ. 2019, 649, 902–908. [Google Scholar] [CrossRef]
- Krupka, M.; Olkowska, E.; Klimkowicz-Pawlas, A.; Łęczyński, L.; Tankiewicz, M.; Michalczyk, D.J.; Wolska, L.; Piotrowicz-Cieślak, A.I. The impact of soil and water pollutants released from poultry farming on the growth and development of two plant species. Agriculture 2024, 14, 87. [Google Scholar] [CrossRef]
- Talwar, C.; Nagar, S.; Kumar, R.; Scaria, J.; Lal, R.; Negi, R.K. Defining the environmental adaptations of genus Devosia: Insights into its expansive short peptide transport system and positively selected senes. Sci. Rep. 2020, 10, 1151. [Google Scholar] [CrossRef]
- Bayer-Santos, E.; Ceseti, L.d.M.; Farah, C.S.; Alvarez-Martinez, C.E. Distribution, function and regulation of type 6 secretion systems of Xanthomonadales. Front. Microbiol. 2019, 10, 1635. [Google Scholar] [CrossRef]
- Crossman, L.C.; Gould, V.C.; Dow, J.M.; Vernikos, G.S.; Okazaki, A.; Sebaihia, M.; Saunders, D.; Arrowsmith, C.; Carver, T.; Peters, N.; et al. The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol. 2008, 9, R74. [Google Scholar] [CrossRef]
- Looney, W.J.; Narita, M.; Mühlemann, K. Stenotrophomonas maltophilia: An emerging opportunist human pathogen. Lancet Infect. Dis. 2009, 9, 312–323. [Google Scholar] [CrossRef]
- Kruczyńska, A.; Kuźniar, A.; Podlewski, J.; Słomczewski, A.; Grządziel, J.; Marzec-Grządziel, A.; Gałązka, A.; Wolińska, A. Bacteroidota structure in the face of varying agricultural practices as an important indicator of soil quality—A culture independent approach. Agri. Ecosyst. Environ. 2023, 342, 108252. [Google Scholar] [CrossRef]
- Wolińska, A.; Kuźniar, A.; Zielenkiewicz, U.; Izak, D.; Szafranek-Nakonieczna, A.; Banach, A.; Błaszczyk, M. Bacteroidetes as a sensitive biological indicator of agricultural soil usage revealed by a culture-independent approach. Appl. Soil Ecol. 2017, 119, 128–137. [Google Scholar] [CrossRef]
- Kolton, M.; Erlacher, A.; Berg, G.; Cytryn, E. The Flavobacterium Genus in the Plant Holobiont: Ecological, Physiological, and Applicative Insights. In Microbial Models: From Environmental to Industrial Sustainability; Castro-Sowinski, S., Ed.; Springer: Singapore, 2016; pp. 189–207. ISBN 978-981-10-2554-9. [Google Scholar]
- Huang, J.; Mi, J.; Yan, Q.; Wen, X.; Zhou, S.; Wang, Y.; Ma, B.; Zou, Y.; Liao, X.; Wu, Y. Animal manures application increases the abundances of antibiotic resistance genes in soil-lettuce system associated with shared bacterial distributions. Sci. Total Environ. 2021, 787, 147667. [Google Scholar] [CrossRef]
- Sung, J.Y.; Kim, T.S.; Shin, S.; Roh, E.Y.; Yoon, J.H.; Kim, E.C. Flavobacterium ceti from blood samples of a Korean patient with alcoholic liver cirrhosis. Ann. Lab. Med. 2015, 35, 384–386. [Google Scholar] [CrossRef]
- Tian, G.Z.; Piao, D.R.; Zhao, H.Y.; Jiang, H.; Cui, B.Y.; Li, J.Y. A Flavobacterium lindanitolerans strain isolated from the ascites sample of a Chinese patient with EV71 virus infection. Biomed. Environ. Sci. 2011, 24, 694–696. [Google Scholar] [CrossRef]
- Park, S.K.; Ryoo, N. A case of Flavobacterium ceti meningitis. Ann. Lab. Med. 2016, 36, 614–616. [Google Scholar] [CrossRef]
- Mosayebi, Z.; Movahedian, A.H.; Soori, T. Flavobacterium sepsis outbreak due to contaminated distilled water in a neonatal intensive care unit. J. Hosp. Infect. 2011, 78, 214–215. [Google Scholar] [CrossRef]
- Minkina, T.; Sushkova, S.; Delegan, Y.; Bren, A.; Mazanko, M.; Kocharovskaya, Y.; Filonov, A.; Rajput, V.D.; Mandzhieva, S.; Rudoy, D.; et al. Effect of chicken manure on soil microbial community diversity in poultry keeping areas. Environ. Geochem. Health 2023, 45, 9303–9319. [Google Scholar] [CrossRef]
- Parente, C.E.T.; Brito, E.M.S.; Caretta, C.A.; Cervantes-Rodríguez, E.A.; Fábila-Canto, A.P.; Vollú, R.E.; Seldin, L.; Malm, O. Bacterial diversity changes in agricultural soils influenced by poultry litter fertilization. Braz. J. Microbiol. 2021, 52, 675–686. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Mao, W.; Wang, C.; Yin, S. Functional potential differences between Firmicutes and Proteobacteria in response to manure amendment in a reclaimed soil. Can. J. Microbiol. 2020, 66, 689–697. [Google Scholar] [CrossRef]
- Laconi, A.; Mughini-Gras, L.; Tolosi, R.; Grilli, G.; Trocino, A.; Carraro, L.; Di Cesare, F.; Cagnardi, P.; Piccirillo, A. Microbial community composition and antimicrobial resistance in agricultural soils fertilized with livestock manure from conventional farming in Northern Italy. Sci. Total Environ. 2021, 760, 143404. [Google Scholar] [CrossRef]
- Wessén, E.; Hallin, S.; Philippot, L. Differential responses of bacterial and archaeal groups at high taxonomical ranks to soil management. Soil Biol. Biochem. 2010, 42, 1759–1765. [Google Scholar] [CrossRef]
- Wolińska, A.; Górniak, D.; Zielenkiewicz, U.; Kuźniar, A.; Izak, D.; Banach, A.; Błaszczyk, M. Actinobacteria structure in autogenic, hydrogenic and lithogenic cultivated and non-cultivated soils: A culture-independent approach. Agronomy 2019, 9, 598. [Google Scholar] [CrossRef]
- Seshadri, R.; Roux, S.; Huber, K.J.; Wu, D.; Yu, S.; Udwary, D.; Call, L.; Nayfach, S.; Hahnke, R.L.; Pukall, R.; et al. Expanding the genomic encyclopedia of Actinobacteria with 824 isolate reference genomes. Cell Genom. 2022, 2, 100213. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, X.; Guo, D.; Zhao, J.; Yan, L.; Feng, G.; Gao, Q.; Yu, H.; Zhao, L. Soil pH is the primary factor driving the distribution and function of microorganisms in farmland soils in northeastern China. Ann. Microbiol. 2019, 69, 1461–1473. [Google Scholar] [CrossRef]
- Fan, H.; Wu, S.; Dong, W.; Li, X.; Dong, Y.; Wang, S.; Zhu, Y.-G.; Zhuang, X. Characterization of tetracycline-resistant microbiome in soil-plant systems by combination of H218O-based DNA-Stable isotope probing and metagenomics. J. Hazard. Mater. 2021, 420, 126440. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, J.; Liu, Y.; Wang, X.; Zhang, B.; Zhang, W.; Chen, T.; Liu, G.; Xue, L.; Cui, X. Nocardioides: “Specialists” for hard-to-degrade pollutants in the environment. Molecules 2023, 28, 7433. [Google Scholar] [CrossRef]
- Benedek, T.; Pápai, M.; Gharieb, K.; Bedics, A.; Táncsics, A.; Tóth, E.; Daood, H.; Maróti, G.; Wirth, R.; Menashe, O.; et al. Nocardioides carbamazepini sp. nov., an ibuprofen degrader isolated from a biofilm bacterial community enriched on carbamazepine. Syst. Appl. Microbiol. 2022, 45, 126339. [Google Scholar] [CrossRef]
- Ouyang, W.-Y.; Su, J.-Q.; Richnow, H.H.; Adrian, L. Identification of dominant sulfamethoxazole-degraders in pig farm-impacted soil by DNA and protein stable isotope probing. Environ. Int. 2019, 126, 118–126. [Google Scholar] [CrossRef]
- Pápai, M.; Benedek, T.; Táncsics, A.; Bornemann, T.L.V.; Plewka, J.; Probst, A.J.; Hussein, D.; Maróti, G.; Menashe, O.; Kriszt, B. Selective enrichment, identification, and isolation of diclofenac, ibuprofen, and carbamazepine degrading bacteria from a groundwater biofilm. Environ. Sci. Pollut. Res. Int. 2023, 30, 44518–44535. [Google Scholar] [CrossRef] [PubMed]
- Crofts, T.S.; Wang, B.; Spivak, A.; Gianoulis, T.A.; Forsberg, K.J.; Gibson, M.K.; Johnsky, L.A.; Broomall, S.M.; Rosenzweig, C.N.; Skowronski, E.W. Shared strategies for β-lactam catabolism in the soil microbiome. Nat. Chem. Biol. 2018, 14, 556–564. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, X.; Du, P.; Li, G.; Li, L.; Li, Z. Susceptibility profiles of Nocardia spp. to antimicrobial and antituberculotic agents detected by a microplate Alamar Blue assay. Sci. Rep. 2017, 7, 43660. [Google Scholar] [CrossRef]
- WHO. Fact Sheets: Tuberculosis 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 1 June 2024).
- Velayati, A.A.; Farnia, P.; Mozafari, M.; Malekshahian, D.; Farahbod, A.M.; Seif, S.; Rahideh, S.; Mirsaeidi, M. Identification and genotyping of Mycobacterium tuberculosis isolated from water and soil samples of a metropolitan city. Chest 2015, 147, 1094–1102. [Google Scholar] [CrossRef]
- Fine, A.E.; Bolin, C.A.; Gardiner, J.C.; Kaneene, J.B. A study of the persistence of Mycobacterium bovis in the environment under natural weather conditions in Michigan, USA. Vet. Med. Int. 2011, 2011, 765430. [Google Scholar] [CrossRef]
- Mtetwa, H.N.; Amoah, I.D.; Kumari, S.; Bux, F.; Reddy, P. The source and fate of Mycobacterium tuberculosis complex in wastewater and possible routes of transmission. BMC Public Health 2022, 22, 145. [Google Scholar] [CrossRef]
- Singh, R.; Dwivedi, S.P.; Gaharwar, U.S.; Meena, R.; Rajamani, P.; Prasad, T. Recent updates on drug resistance in Mycobacterium tuberculosis. J. Appl. Microbiol. 2020, 128, 1547–1567. [Google Scholar] [CrossRef]
- Rosenblueth, M.; Martinez-Romero, J.C.; Reyes-Prieto, M.; Rogel, M.A.; Martinez-Romero, E. Environmental mycobacteria: A threat to human health? DNA Cell Biol. 2011, 30, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Richter, E. Mycobacterium holsaticum sp. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Varghese, B.; Enani, M.; Shoukri, M.; AlThawadi, S.; AlJohani, S.; Al-Hajoj, S. Emergence of rare species of Nontuberculous mycobacteria as potential pathogens in Saudi Arabian clinical setting. PLoS Negl. Trop. Dis. 2017, 11, e0005288. [Google Scholar] [CrossRef]
- de Lima, C.A.M.; Gomes, H.M.; Oelemann, M.A.C.; Ramos, J.P.; Caldas, P.C.; Campos, C.E.D.; Da Pereira, M.A.S.; Montes, F.F.O.; de Oliveira, M.d.S.C.; Suffys, P.N. Nontuberculous mycobacteria in respiratory samples from patients with pulmonary tuberculosis in the state of Rondônia, Brazil. Mem. Inst. Oswaldo Cruz 2013, 108, 457–462. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Fan, W.; Sun, S.; Fan, X. The impact of Mycobacterium tuberculosis complex in the environment on one health approach. Front. Public Health 2022, 10, 994745. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, G.; Wang, Y.; Jin, F.; Wang, H.; Yu, Z.; Li, L.; Li, X.; Gao, J.; Xu, W. A rare strain Actinomadura geliboluensis was first isolated from the bronchoalveolar lavage fluid of a patient with pneumonia. Infect. Drug Resist. 2023, 16, 3101–3108. [Google Scholar] [CrossRef]
- Blaser, M.J. Antibiotic use and its consequences for the normal microbiome. Science 2016, 352, 544–545. [Google Scholar] [CrossRef]
- Geng, J.; Ni, Q.; Sun, W.; Li, L.; Feng, X. The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother. 2022, 147, 112678. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, Q.; Luo, Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ. Pollut. 2010, 158, 2992–2998. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Kolpin, D.W.; Halling-Sørensen, B.; Tolls, J. Are veterinary medicines causing environmental risks? Environ. Sci. Technol. 2003, 37, 286A–294A. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef]
- Hughes, D.; Andersson, D.I. Selection of resistance at lethal and non-lethal antibiotic concentrations. Curr. Opin. Microbiol. 2012, 15, 555–560. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pomati, F.; Orlandi, C.; Clerici, M.; Luciani, F.; Zuccato, E. Effects and interactions in an environmentally relevant mixture of pharmaceuticals. Toxicol. Sci. 2008, 102, 129–137. [Google Scholar] [CrossRef]
- Subirats, J.; Domingues, A.; Topp, E. Does dietary consumption of antibiotics by humans promote antibiotic resistance in the gut microbiome? J. Food Prot. 2019, 82, 1636–1642. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, Z.; Tan, L.; Wang, X.; Xue, Y.; Wang, S.; Wang, Q.; Das, R.; Lin, H.; Hou, J.; et al. Gut resistomes, microbiota and antibiotic residues in Chinese patients undergoing antibiotic administration and healthy individuals. Sci. Total Environ. 2020, 705, 135674. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, M.; Dai, J.; Sun, Y.; Zeng, Z. Application of manure containing tetracyclines slowed down the dissipation of tet resistance genes and caused changes in the composition of soil bacteria. Ecotoxicol. Environ. Saf. 2018, 147, 455–460. [Google Scholar] [CrossRef]
- Blau, K.; Jacquiod, S.; Sørensen, S.J.; Su, J.Q.; Zhu, Y.G.; Smalla, K.; Jechalke, S. Manure and doxycycline affect the bacterial community and its resistome in lettuce rhizosphere and bulk soil. Front. Microbiol. 2019, 10, 725. [Google Scholar] [CrossRef]
- Harirchi, S.; Sar, T.; Ramezani, M.; Aliyu, H.; Etemadifar, Z.; Nojoumi, S.A.; Yazdian, F.; Awasthi, M.K.; Taherzadeh, M.J. Bacillales: From Taxonomy to Biotechnological and Industrial Perspectives. Microorganisms 2022, 10, 2355. [Google Scholar] [CrossRef]
- Peng, M.; Tabashsum, Z.; Millner, P.; Parveen, S.; Biswas, D. Influence of manure application on the soil bacterial microbiome in integrated crop-livestock farms in Maryland. Microorganisms 2021, 9, 2586. [Google Scholar] [CrossRef]
- Mujakić, I.; Piwosz, K.; Koblížek, M. Phylum Gemmatimonadota and its role in the environment. Microorganisms 2022, 10, 151. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, J.; Gao, L.; Kong, F.; Shen, G.; Wang, R.; Gao, J.; Zhang, J. The effects of tetracycline residues on the microbial community structure of tobacco soil in pot experiment. Sci. Rep. 2020, 10, 8804. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Zhang, B.; Li, B.; Du, H.; Wu, Z.; Rashid, A.; Mensah, C.O.; Lei, M. Transmission mechanisms of antibiotic resistance genes in arsenic-contaminated soil under sulfamethoxazole stress. Environ. Pollut. 2023, 326, 121488. [Google Scholar] [CrossRef]
- Chepsergon, J.; Moleleki, L.N. Rhizosphere bacterial interactions and impact on plant health. Curr. Opin. Microbiol. 2023, 73, 102297. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O. Rhizosphere microbiome modulators: Contributions of nitrogen fixing bacteria towards sustainable agriculture. Int. J. Environ. Res. Public Health 2018, 15, 574. [Google Scholar] [CrossRef]
- Ali, M.A.; Naveed, M.; Mustafa, A.; Abbas, A. The good, the bad, and the ugly of rhizosphere microbiome. In Probiotics and Plant Health; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 253–290. ISBN 978-981-10-3472-5. [Google Scholar]
- Soto-Giron, M.J.; Kim, J.-N.; Schott, E.; Tahmin, C.; Ishoey, T.; Mincer, T.J.; DeWalt, J.; Toledo, G. The edible plant microbiome represents a diverse genetic reservoir with functional potential in the human host. Sci. Rep. 2021, 11, 24017. [Google Scholar] [CrossRef]
- Rastogi, G.; Sbodio, A.; Tech, J.J.; Suslow, T.V.; Coaker, G.L.; Leveau, J.H.J. Leaf microbiota in an agroecosystem: Spatiotemporal variation in bacterial community composition on field-grown lettuce. ISME J. 2012, 6, 1812–1822. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database. Available online: https://www.fao.org/faostat/en/#home (accessed on 25 May 2024).
- Zhang, M.; Cai, Z.; Zhang, G.; Zhang, D.; Pan, X. Abiotic mechanism changing tetracycline resistance in root mucus layer of floating plant: The role of antibiotic-exudate complexation. J. Hazard. Mater. 2021, 416, 125728. [Google Scholar] [CrossRef]
- Babalola, O.O.; Emmanuel, O.C.; Adeleke, B.S.; Odelade, K.A.; Nwachukwu, B.C.; Ayiti, O.E.; Adegboyega, T.T.; Igiehon, N.O. Rhizosphere microbiome cooperations: Strategies for sustainable crop production. Curr. Microbiol. 2021, 78, 1069–1085. [Google Scholar] [CrossRef] [PubMed]
- Schreiter, S.; Ding, G.-C.; Heuer, H.; Neumann, G.; Sandmann, M.; Grosch, R.; Kropf, S.; Smalla, K. Effect of the soil type on the microbiome in the rhizosphere of field-grown lettuce. Front. Microbiol. 2014, 5, 144. [Google Scholar] [CrossRef]
- Žiarovská, J.; Urbanová, L.; Moravčíková, D.; Artimová, R.; Omelka, R.; Medo, J. Varieties of lettuce forming distinct microbial communities inhabiting roots and rhizospheres with various responses to osmotic stress. Horticulturae 2022, 8, 1174. [Google Scholar] [CrossRef]
- Wolfgang, A.; Zachow, C.; Müller, H.; Grand, A.; Temme, N.; Tilcher, R.; Berg, G. Understanding the impact of cultivar, seed origin, and substrate on bacterial diversity of the sugar beet rhizosphere and suppression of soil-borne pathogens. Front. Plant Sci. 2020, 11, 560869. [Google Scholar] [CrossRef]
- Rolfe, S.A.; Griffiths, J.; Ton, J. Crying out for help with root exudates: Adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr. Opin. Microbiol. 2019, 49, 73–82. [Google Scholar] [CrossRef]
- Huang, F.; Lei, M.; Li, W. The rhizosphere and root selections intensify fungi-bacteria interaction in abiotic stress-resistant plants. PeerJ 2024, 12, e17225. [Google Scholar] [CrossRef]
- Pandiyan, K.; Kushwaha, P.; Kashyap, P.L.; Bagul, S.Y.; Karthikeyan, N.; Saxena, A.K. Phyllosphere microbiome: Modern prospectus and application. In Microbiomes and Plant Health; Elsevier: Amsterdam, The Netherlands, 2021; pp. 345–366. ISBN 9780128197158. [Google Scholar]
- Li, X.; Rui, J.; Xiong, J.; Li, J.; He, Z.; Zhou, J.; Yannarell, A.C.; Mackie, R.I. Functional potential of soil microbial communities in the maize rhizosphere. PLoS ONE 2014, 9, e112609. [Google Scholar] [CrossRef]
- Mahdi, I.; Fahsi, N.; Hijri, M.; Sobeh, M. Antibiotic resistance in plant growth promoting bacteria: A comprehensive review and future perspectives to mitigate potential gene invasion risks. Front. Microbiol. 2022, 13, 999988. [Google Scholar] [CrossRef]
- Jones, A.M.; Dodd, M.E.; Webb, A.K. Burkholderia cepacia: Current clinical issues, environmental controversies and ethical dilemmas. Eur. Respir. J. 2001, 17, 295–301. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, S.K. Changes in antibiotic-resistance genes induced by the grazing effect in three Cladoceran species. Microorganisms 2021, 9, 1959. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, M.-H.; Park, J.; Kim, Y.J.; Kim, E.; Heo, E.J.; Kim, S.H.; Kim, G.; Shin, H.; Kim, S.H.; et al. Seasonal changes in the microbial communities on lettuce (Lactuca sativa L.) in Chungcheong-do, South Korea. J. Microbiol. Biotechnol. 2023, 33, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Wu, J.; Hua, Q.; Bao, C. Distribution and transfer of antibiotic resistance genes in different soil-plant systems. Environ. Sci. Pollut. Res. Int. 2022, 29, 59159–59172. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Peng, K.; Jiang, L.; Zhang, D.; Song, D.; Chen, G.; Xu, H.; Li, Y.; Luo, C. Alleviated antibiotic-resistant genes in the rhizosphere of agricultural soils with low antibiotic concentration. J. Agric. Food Chem. 2020, 68, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.C.; Yum, S.J.; Jeon, D.Y.; Jeong, H.G. Analysis of the microbiota on lettuce (Lactuca sativa L.) cultivated in South Korea to identify foodborne pathogens. J. Microbiol. Biotechnol. 2018, 28, 1318–1331. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-A.; Lee, D.H.; Heu, S. The interaction of human enteric pathogens with plants. Plant Pathol. J. 2014, 30, 109–116. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Q.; Zhang, Z.; Zhou, S.; Jin, M.; Zhu, D.; Yang, X.; Qian, H.; Lu, T. Plants select antibiotic resistome in rhizosphere in early stage. Sci. Total Environ. 2023, 858, 159847. [Google Scholar] [CrossRef]
- Ji, X.; Shen, Q.; Liu, F.; Ma, J.; Xu, G.; Wang, Y.; Wu, M. Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai; China. J. Hazard. Mater. 2012, 235–236, 178–185. [Google Scholar] [CrossRef]
- Preacher, K.J. Calculation for the Chi-Square Test: An Interactive Calculation Tool for Chi-Square Tests of Goodness of Fit and Independence [Computer Software]. Available online: http://quantpsy.org (accessed on 3 March 2025).


| Sample | Kingdom | Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|---|---|
| S1 | 100 | 99.99 | 99.96 | 99.47 | 96.15 | 76.45 | 9.607 |
| S2 | 100 | 99.93 | 99.86 | 98.78 | 94.24 | 80.43 | 6.89 |
| S3 | 100 | 99.98 | 99.86 | 98.88 | 95.08 | 82.86 | 6.78 |
| Order | Family | Genus | Number of Reads |
|---|---|---|---|
| S1 | |||
| Bacteroidota | Flavobacterium | Flavobacterium pectinovorum | 4716 |
| Proteobacteria | Bradyrhizobium | Bradyrhizobium japonicum | 324 |
| Actinobacteriota | Arthrobacter | Arthrobacter crystallopoietes | 193 |
| Proteobacteria | Rhodanobacter | Rhodanobacter spathiphylli | 165 |
| Firmicutes | Sporosarcina | Sporosarcina ureae | 144 |
| Cyanobacteria | Chloroplast | Lactuca sativa | 121 |
| Proteobacteria | Luteimonas | Lysobacter pocheonensis | 113 |
| Bacteroidota | Flavobacterium | Flavobacterium arsenitoxidans | 108 |
| S2 | |||
| Firmicutes | Sporosarcina | Sporosarcina ureae | 1011 |
| Actinobacteriota | Arthrobacter | Arthrobacter crystallopoietes | 381 |
| Actinobacteriota | Actinomadura | Mycobacterium tuberculosis | 222 |
| Proteobacteria | Bradyrhizobium | Bradyrhizobium japonicum | 203 |
| Firmicutes | Sporosarcina | Sporosarcina psychrophila | 147 |
| Firmicutes | Oceanobacillus | Oceanobacillus indicireducens | 137 |
| Myxococcota | Polyangiaceae | Sorangium cellulosum | 129 |
| Actinobacteriota | Actinomadura | Actinomadura geliboluensis | 118 |
| S3 | |||
| Firmicutes | Sporosarcina | Sporosarcina ureae | 581 |
| Actinobacteriota | Arthrobacter | Arthrobacter crystallopoietes | 216 |
| Firmicutes | Sporosarcina | Sporosarcina psychrophila | 139 |
| Firmicutes | Oceanobacillus | Oceanobacillus indicireducens | 104 |
| Proteobacteria | Bradyrhizobium | Bradyrhizobium japonicum | 90 |
| Actinobacteriota | Agromyces | Agromyces neolithicus | 80 |
| Proteobacteria | Stenotrophomonas | Stenotrophomonas maltophilia | 73 |
| Actinobacteriota | Mycobacterium | Mycobacterium holsaticum | 68 |
| Order | S1 | S2 | S3 | SG1 | GG2 | CG3 |
| Rhizobiales | 13.16 ± 0.97 | 7.51 ± 0.57 | 6.70 ± 0.40 | A | A | B |
| Micropepsales | 7.46 ± 0.56 | <1 ± 0.09 | 6.54 ± 0.39 | A | B | A |
| Burkholderiales | 7.29 ± 0.89 | 3.68 ± 0.41 | 3.94 ± 0.54 | A | A | A |
| Flavobacteriales | 6.8 ± 0.85 | <1 ± 0.08 | <1 ± 0.08 | A | B | B |
| Chitinophagales | 5.34 ± 0.4 | <1 ± 0.09 | <1 ± 0.10 | A | B | B |
| Opitutales | 3.87 ± 0.37 | <1 ± 0.09 | <1 ± 0.08 | A | B | B |
| Sphingobacteriales | 3.42 ± 1.18 | <1 ± 0.03 | <1 ± 0.06 | A | B | B |
| Xanthomodales | 3.39 ± 0.78 | <1 ± 0.03 | <1 ± 0.04 | A | B | B |
| Bacillales | <1 ± 0.06 | 10.25 ± 0.33 | 12.58 ± 0.37 | B | A | A |
| Micrococcales | <1 ± 0.06 | 6.66 ± 0.43 | 6.54 ± 0.36 | B | A | A |
| Thermomicrobiales | <1 ± 0.11 | 5.06 ± 0.99 | 5.36 ± 0.37 | B | A | A |
| Gemmatimonadales | <1 ± 0.07 | 3.78 ± 0.19 | 3.5 ± 0.06 | B | A | A |
| Gitt-GS | <1 ± 0.05 | 2.87 ± 1.1 | <1 ± 0.06 | B | B | A |
| Propionibacteriales | <1 ± 0.05 | <1 ± 0.44 | 3.92 ± 0.25 | B | A | B |
| Family | S1 | S2 | S3 | SG1 | GG2 | CG3 |
| Rhizobiaceae | 4.56 ± 0.48 | 2.79 ± 0.39 | <1 ± 0.10 | A | B | C |
| Micropepsaceae | 7.46 ± 0.56 | <1 ± 0.10 | <1 ± 0.05 | A | B | B |
| Flavobacteriaceae | 6.68 ± 1.59 | <1 ± 0.04 | <1 ± 0.04 | A | B | B |
| Chitiniphagaceae | 4.59 ± 0.09 | <1 ± 0.07 | <1 ± 0.08 | A | B | B |
| Devosiaceae | 3.97 ± 0.82 | <1 ± 0.06 | <1 ± 0,04 | A | B | B |
| Comamonadaceae | 3.84 ± 0.31 | <1 ± 0.06 | <1 ± 0.04 | A | B | B |
| Opitutaceae | 3.81 ± 0.18 | <1 ± 0.06 | <1 ± 0.05 | A | B | B |
| Sphinhobacteriaceae | 3.13 ± 0.12 | <1 ± 0.05 | <1 ± 0.03 | A | B | B |
| Planococcaceae | <1 ± 0.1 | 5.93 ± 0.89 | 7.31 ± 0.88 | B | A | A |
| Bacillaceae | <1 ± 0.07 | 4.3 ± 0.26 | 5.26 ± 0.27 | B | A | A |
| Nocardioidaceae | <1 ± 0.1 | 3.86 ± 0.48 | 3.91 ± 0.06 | B | A | A |
| Gemmatimonadaceae | <1 ± 0.06 | 3.77 ± 0.71 | 3.51 ± 0.2 | B | A | A |
| Gitt-GS | <1 ± 0.06 | 2.87 ± 0.60 | 2.99 ± 0.35 | B | A | A |
| Micrococcaceae | <1 ± 0.07 | 2.57 ± 0.06 | 2.58 ± 0.24 | B | A | A |
| Sample | Kingdom | Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|---|---|
| R1 | 100 | 100 | 99.97 | 99.34 | 95.87 | 76.67 | 13.6 |
| R2 | 100 | 99.97 | 99.94 | 99.26 | 95.00 | 76.72 | 5.35 |
| R3 | 100 | 99.89 | 99.84 | 99.13 | 95.96 | 79.05 | 13.43 |
| Order | R1 | R2 | R3 | G1 | G2 | G3 |
| Rhizobiales | 14.94 ± 0.65 | 13.43 ± 0.74 | 11.69 ± 0.44 | A | AB | B |
| Micropepsales | 4.14 ± 0.35 | 4.63 ± 1.09 | <1 ± 0.09 | A | A | B |
| Burkholderiales | 4.94 ± 0.81 | 5.29 ± 0.13 | 6.52 ± 0.62 | A | AB | B |
| Flavobacteriales | 10.4 ± 0.48 | 13.24 ± 0.45 | 10.46 ± 1.72 | A | B | A |
| Chitinophagales | 4.45 ± 1.00 | 4.95 ± 1.15 | 4.35 ± 0.85 | A | A | A |
| Opitutales | <1 ± 0.07 | <1 ± 0.09 | <1 ± 0.10 | A | B | A |
| Sphingobacteriales | <1 ± 0.07 | 4.38 ± 0.17 | <1 ± 0.06 | A | B | A |
| Xanthomodales | <1 ± 0.06 | <1 ± 0.07 | <1 ± 0.12 | A | A | A |
| Bacillales | <1 ± 0.06 | <1 ± 0.08 | 5.05 ± 0.39 | A | A | B |
| Micrococcales | 3.79 ± 0.24 | <1 ± 0.08 | 3.37 ± 0.75 | A | B | A |
| Gemmatimonadales | <1 ± 0.08 | <1 ± 0.07 | <1 ± 0.10 | A | A | A |
| Candidatus | <1 ± 0.06 | 3.72 ± 1.1 | <1 ± 0.08 | A | B | A |
| Propionibacteriales | <1 ± 0.10 | <1 ± 0.2 | 3.02 ± 0.41 | A | A | B |
| Caulobacterales | 3.45 ± 0.63 | 4.96 ± 1.97 | 3.09 ± 0.56 | A | B | A |
| Family | R1 | R2 | R3 | G1 | GG2 | GG3 |
| Rhizobiaceae | 5.13 ± 0.34 | 3.31 ± 0.67 | 3.79 ± 0.37 | A | AB | B |
| Micropepsaceae | 4.14 ± 0.27 | 4.63 ± 0.96 | <1 ± 0.14 | A | A | B |
| Flavobacteriaceae | 10.16 ± 0.94 | 10.13 ± 0.13 | 10.18 ± 1.96 | A | A | A |
| Chitiniphagaceae | 4.12 ± 1.03 | 3.79 ± 0.21 | 3.71 ± 0.39 | A | A | A |
| Devosiaceae | 4.52 ± 0.71 | 4.55 ± 0.75 | 3.36 ± 0.43 | A | A | A |
| Comamonadaceae | 2.97 ± 0.12 | <1 ± 0.13 | 3.47 ± 0.92 | A | B | A |
| Sphinhobacteriaceae | <1 ± 0.05 | 4.07 ± 0.52 | <1 ± 0.05 | B | A | B |
| Planococcaceae | <1 ± 0.17 | <1 ± 0.19 | 2.78 ± 0.36 | B | B | A |
| Nocardioidaceae | 3.00 ± 0.12 | 3.01 ± 0.05 | 3.18 ± 0.66 | A | B | A |
| Gemmatimonadaceae | <1 ± 0.12 | 3.77 ± 1.21 | 3.51 ± 0.5 | B | A | A |
| Xanthobacteraceae | 3.25 ± 0.39 | <1 ± 0.14 | 2.97 ± 0.25 | A | B | A |
| Comamonadaceae | 2.97 ± 0.37 | <1 ± 0.10 | <1 ± 0.08 | A | B | B |
| Caulobacteriaceae | <1 ± 0.23 | 4.73 ± 1.95 | 2.73 ± 0.64 | B | A | A |
| Order | Family | Genus | Number of Reads |
|---|---|---|---|
| R1 | |||
| Bacteroidota | Flavobacterium | Flavobacterium pectinovorum | 4662 |
| Actinobacteriota | Arthrobacter | Arthrobacter crystallopoietes | 265 |
| Proteobacteria | Bradyrhizobium | Bradyrhizobium japonicum | 217 |
| Bacteroidia | Flavobacterium | Flavobacterium akiainvivens | 152 |
| Proteobacteria | Rhodanobacter | Rhodanobacter spathiphylli | 139 |
| Actinobacteriota | Flexivirga | Flexivirga alba | 114 |
| Proteobacteria | Rhodanobacter | Rhodanobacter fulvus | 109 |
| Proteobacteria | Luteimonas | Lysobacter pocheonensis | 97 |
| R2 | |||
| Bacteroidota | Flavobacterium | Flavobacterium pectinovorum | 3625 |
| Firmicutes | Sporosarcina | Sporosarcina ureae | 293 |
| Proteobacteria | Bradyrhizobium | Bradyrhizobium japonicum | 139 |
| Actinobacteriota | Arthrobacter | Arthrobacter crystallopoietes | 128 |
| Proteobacteria | Rhodanobacter | Rhodanobacter spathiphylli | 122 |
| Actinobacteriota | Agromyces | Agromyces neolithicus | 88 |
| Cyanobacteria | Chloroplast | Lactuca sativa | 84 |
| Proteobacteria | Methylophilus | Methylomonas clara | 68 |
| R3 | |||
| Bacteroidota | Flavobacterium | Flavobacterium pectinovorum | 1360 |
| Firmicutes | Sporosarcina | Sporosarcina ureae | 168 |
| Proteobacteria | Bradyrhizobium | Bradyrhizobium japonicum | 158 |
| Cyanobacteria | Chloroplast | Lactuca sativa | 145 |
| Actinobacteriota | Flexivirga | Flexivirga alba | 118 |
| Proteobacteria | Beijerinckiaceae | Chelatococcus asaccharovorans | 99 |
| Planctomycetota | Paludisphaer | Paludisphaera borealis | 86 |
| Actinobacteriota | Nocardioides | Nocardioides tritolerans | 81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupka, M.; Wolska, L.; Piechowicz, L.; Głowacka, K.; Piotrowicz-Cieślak, A.I. The Impact of Tetracycline on the Soil Microbiome and the Rhizosphere of Lettuce (Lactuca sativa L.). Int. J. Mol. Sci. 2025, 26, 2854. https://doi.org/10.3390/ijms26072854
Krupka M, Wolska L, Piechowicz L, Głowacka K, Piotrowicz-Cieślak AI. The Impact of Tetracycline on the Soil Microbiome and the Rhizosphere of Lettuce (Lactuca sativa L.). International Journal of Molecular Sciences. 2025; 26(7):2854. https://doi.org/10.3390/ijms26072854
Chicago/Turabian StyleKrupka, Magdalena, Lidia Wolska, Lidia Piechowicz, Katarzyna Głowacka, and Agnieszka I. Piotrowicz-Cieślak. 2025. "The Impact of Tetracycline on the Soil Microbiome and the Rhizosphere of Lettuce (Lactuca sativa L.)" International Journal of Molecular Sciences 26, no. 7: 2854. https://doi.org/10.3390/ijms26072854
APA StyleKrupka, M., Wolska, L., Piechowicz, L., Głowacka, K., & Piotrowicz-Cieślak, A. I. (2025). The Impact of Tetracycline on the Soil Microbiome and the Rhizosphere of Lettuce (Lactuca sativa L.). International Journal of Molecular Sciences, 26(7), 2854. https://doi.org/10.3390/ijms26072854






