Metabolome and Transcriptome Analyses Reveal the Correlation Between Fructan Changes and Phytohormone Regulation During Tuber Sprouting of Helianthus tuberosus L. †
Abstract
:1. Introduction
2. Results
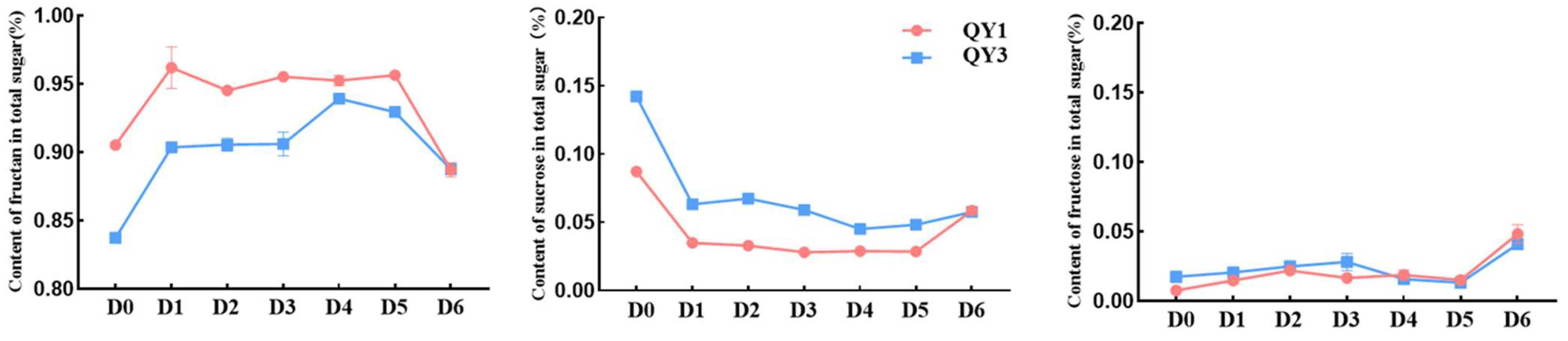
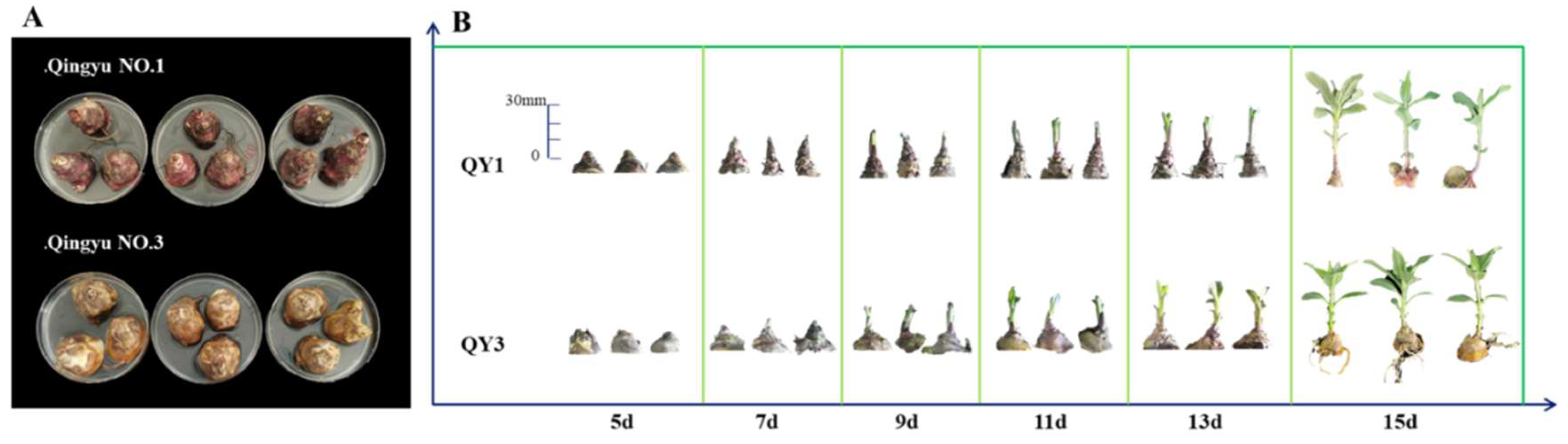
2.1. Dynamics of Carbohydrate Changes During Sprouting
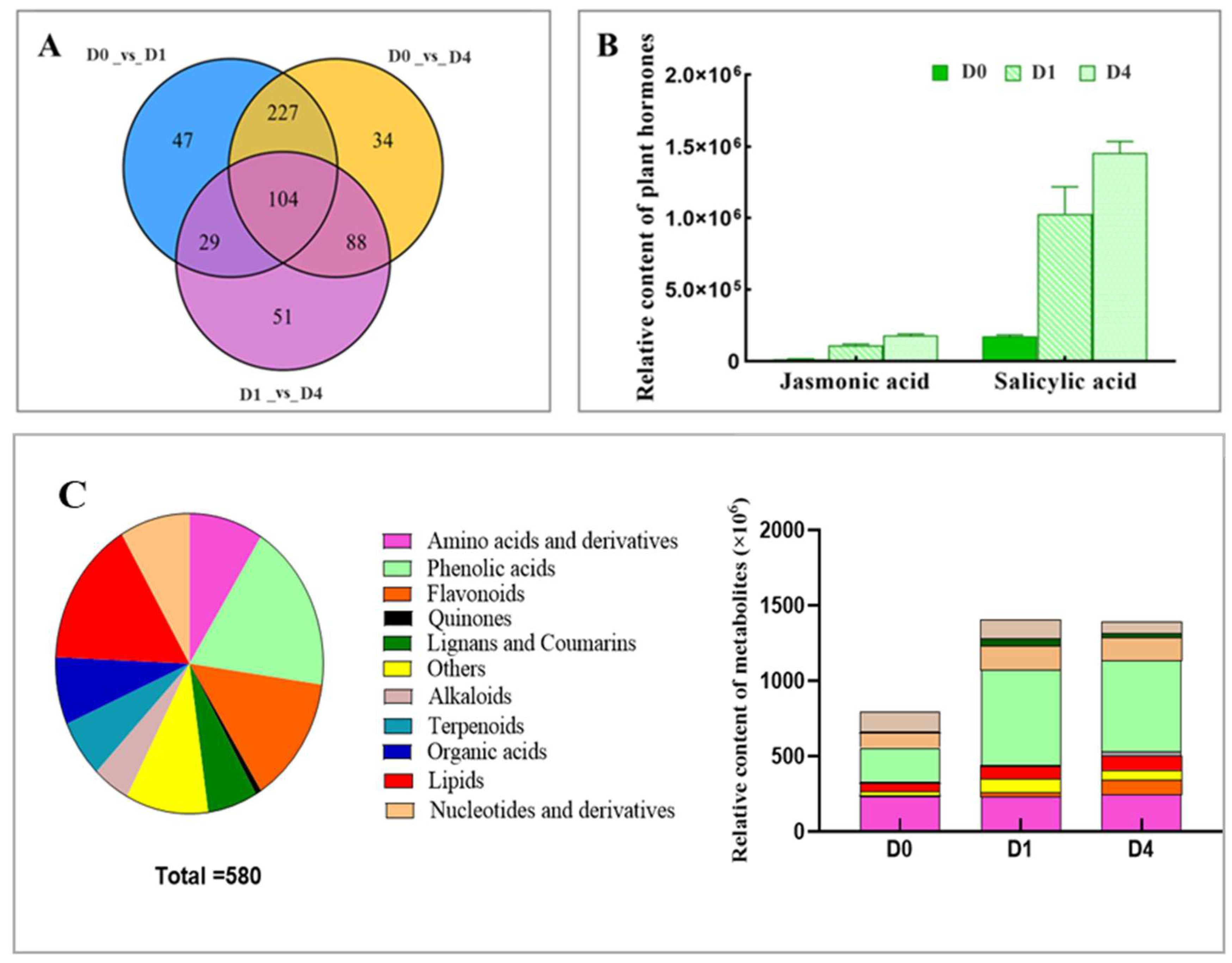
2.2. Analysis of Differential Metabolites
2.3. Analysis of DEGs
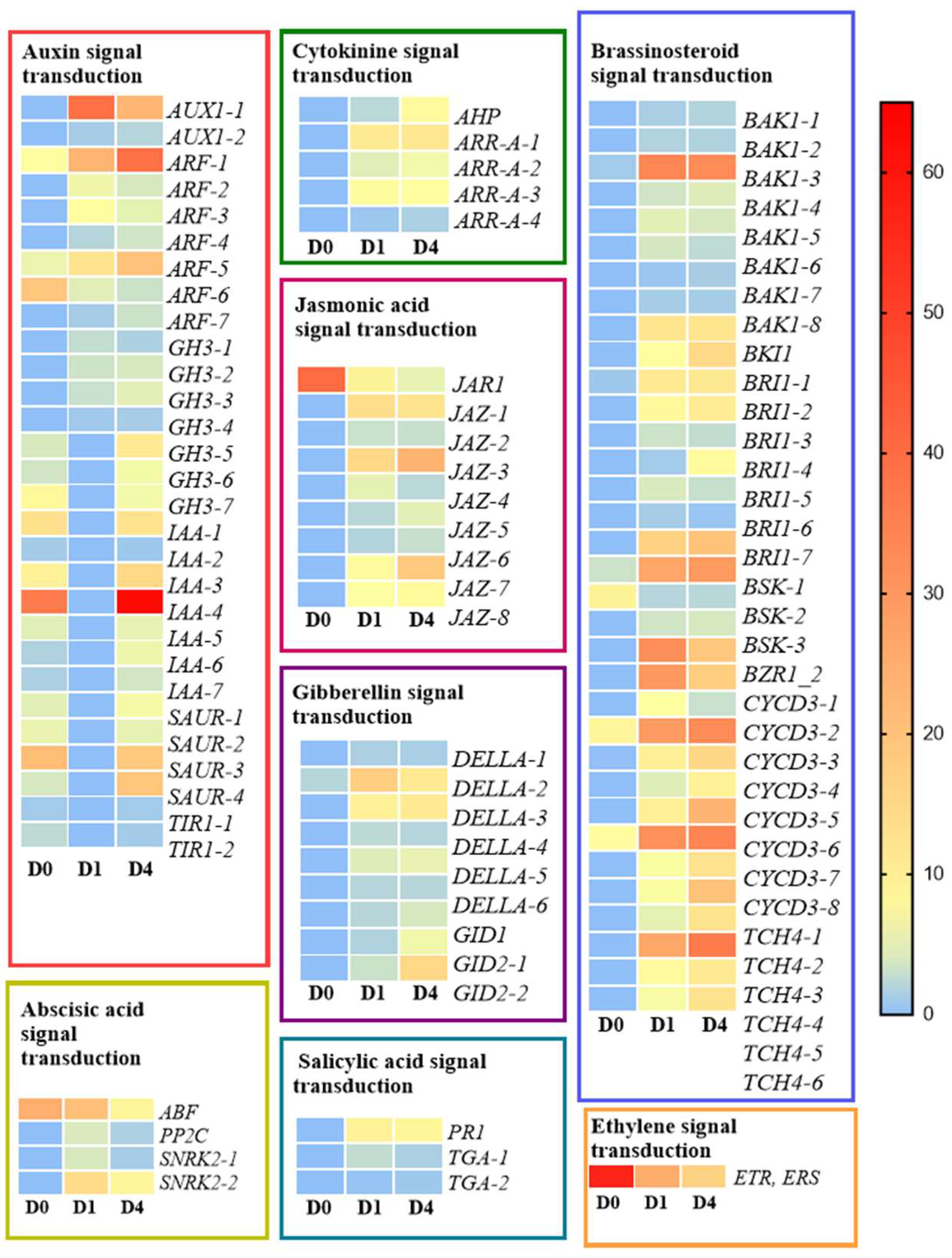
2.4. Combined Analysis of Hormone-Related Differential Metabolites and Differential Genes
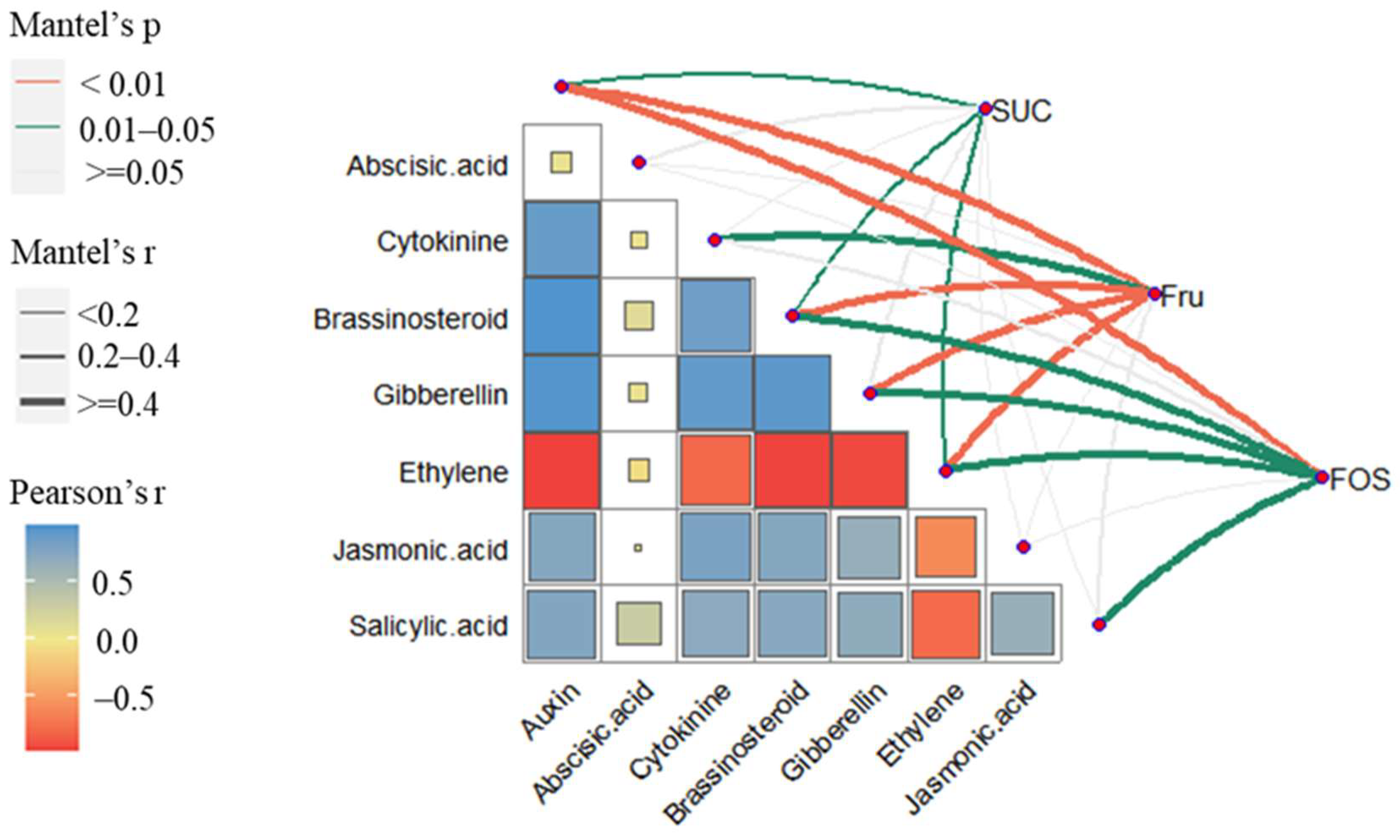
2.5. Correlation Analysis of Carbohydrate Dynamics and Phytohormone-Related DEGs
3. Discussion
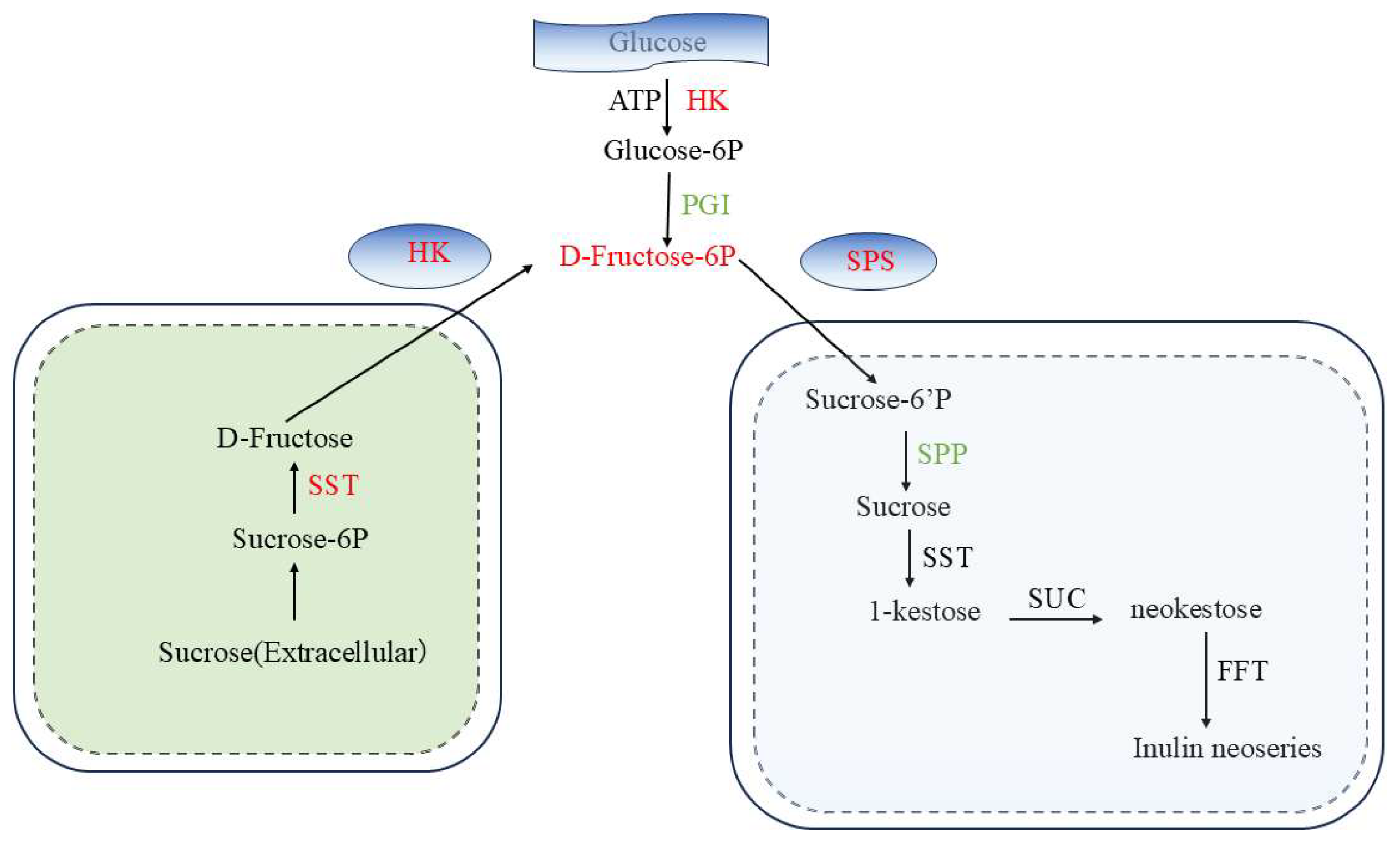
3.1. Dynamics of Fructan Changes
3.2. Phytohormone Regulation Network
3.3. Correlation Analysis of Carbohydrates and Phytohormone-Related DEGs
4. Materials and Methods
4.1. Materials
4.2. Extraction and Determination of Carbohydrates
4.3. Metabolite Analysis Through Ultraperformance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS)
4.4. RNA Extraction and Sequencing
4.5. Transcriptomics and Analysis of Differentially Expressed Genes (DEGs)
4.6. Combined Analysis of Metabolome and Transcriptome of Plant Hormones and Carbohydrates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubel, I.A.; Iraporda, C.; Manrique, G.D.; Genovese, D.B.; Abraham, A.G. Inulin from Jerusalem artichoke (Helianthus tuberosus L.): From its biosynthesis to its application as bioactive ingredient. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100281. [Google Scholar] [CrossRef]
- Radovanovic, A.; Stojceska, V.; Plunkett, A.; Jankovic, S.; Milovanovic, D.; Cupara, S. The use of dry Jerusalem artichoke as a functional nutrient in develo** extruded food with low glycaemic index. Food Chem. 2015, 177, 81–88. [Google Scholar] [CrossRef]
- Li, N.; Wang, C.; Georgiev, M.I.; Bajpai, V.K.; Tundis, R.; Simal-Gandara, J.; Qiao, X. Advances in dietary polysaccharides as anticancer agents: Structure-activity relationship. Trends Food Sci. Technol. 2021, 111, 360–377. [Google Scholar] [CrossRef]
- He, J.; Han, W.; Wang, J.; Qian, Y.; Saito, M.; Bai, W.; Lv, G. Functions of oligosaccharides in improving tomato seeding growth and chilling resistance. J. Plant Growth Regul. 2021, 41, 535–545. [Google Scholar] [CrossRef]
- Bedzo, O.K.; Mandegari, M.; Görgens, J.F. Techno-economic analysis of inulooligosaccharides, protein, and biofuel co-production from Jerusalem artichoke tubers: A biorefinery approach. Biofuels Bioprod. Biorefining 2020, 14, 776–793. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Wang, B. Changes in starch and reducing sugar content of different potato varieties during sprouting. J. Food Sci. Technol. 2014, 51, 1910–1916. [Google Scholar] [CrossRef]
- Sun, X.M.; Wang, L.H.; Li, Y.; Zhao, M.L.; Zhong, Q.W.; Li, L. Study on Fructan Metabolism and Enzyme Activity of Helianthus tuberosus L. Adv. Mater. Res. 2014, 1010, 229–234. [Google Scholar] [CrossRef]
- Márquez-López, R.E.; Loyola-Vargas, V.M.; Santiago-García, P.A. Interaction between fructan metabolism and plant growth regulators. Planta 2022, 255, 49. [Google Scholar] [CrossRef]
- Plummer, J.A.; McPharlin, I.; Kustiati, T. Effects of storage period and gibberellic acid on sprout behaviour and plant growth of potatoes suitable for tropical conditions. In International Symposium on Harnessing the Potential of Horticulture in the Asian-Pacific Region. ISHS Acta Hortic. 2012, 694, 425–429. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Yamamuro, C.; Zhu, J.K.; Yang, Z. Epigenetic modifications and plant hormone action. Mol. Plant 2016, 9, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Men, G.T.; Ma, Y.J.; Lan, K.L.; Yin, C.Y.; Kang, W.Q.; Wang, Z. Changes of endogenous hormones in the tubers of Helianthus tuberosus L during storage. J. North. Agric. 2021, 49, 96–101. [Google Scholar]
- Sorce, C.; Lombardi, L.; Giorgetti, L.; Parisi, B.; Ranalli, P.; Lorenzi, R. Indoleacetic acid concentration and metabolism changes during bud development in tubers of two potato (Solanum tuberosum) cultivars. J. Plant Physiol. 2009, 166, 1023–1033. [Google Scholar] [CrossRef]
- Hartmann, A.; Senning, M.; Hedden, P.; Sonnewald, U.; Sonnewald, S. Reactivation of meristem activity and sprout growth in potato tubers require both cytokinin and gibberellin. Plant Physiol. 2011, 155, 776–796. [Google Scholar] [CrossRef] [PubMed]
- Zubko, E.; Macháčková, I.; Malbeck, J.; Meyer, P. Modification of cytokinin levels in potato via expression of the Petunia hybrida Sho gene. Transgenic Res. 2005, 14, 615–618. [Google Scholar] [CrossRef]
- Cheema, M.U.A.; Rees, D.; Colgan, R.J.; Taylor, M.; Westby, A. The effects of ethylene, 1-MCP and AVG on sprouting in sweetpotato roots. Postharvest Biol. Technol. 2013, 85, 89–93. [Google Scholar] [CrossRef]
- Suttle, J. Dormancy-related changes in cytokinin efficacy and metabolism in potato tubers during postharvest storage. Plant Growth Regul. 2001, 35, 199–206. [Google Scholar] [CrossRef]
- Eriksson, L.; Byrne, T.; Johansson, E.; Trygg, J.; Vikström, C. Multi-and Megavariate Data Analysis Basic Principles and Applications; Umetrics Academy: Umeå, Sweden, 2013; Volume 1. [Google Scholar]
- Chen, Y.; Zhang, R.; Song, Y.; He, J.; Sun, J.; Bai, J.; Abliz, Z. RRLC-MS/MS-based metabonomics combined with in-depth analysis of metabolic correlation network: Finding potential biomarkers for breast cancer. Analyst 2009, 134, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Sherlock, G. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Yamanishi, Y. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2016, 36 (Suppl. S1), D480–D484. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, J.; Ren, W.; Zhou, Z.; Long, X.; Gao, X.; Rengel, Z. Expression of genes related to plant hormone signal transduction in jerusalem artichoke (Helianthus tuberosus L.) seedlings under salt stress. Agronomy 2022, 12, 163. [Google Scholar] [CrossRef]
- Soria, C.B. Main methods of artichoke multiplication and the role of seed reproduction. Acta Hortic. 2020, 1284, 51–56. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Zhou, Z.; Zong, Y.; Wang, L.; Yang, S.; Sun, X. Transcriptome analysis for genes involved in fructan biosynthesis in the Jerusalem artichoke (Helianthus tuberosus L.). Biotechnol. Biotechnol. Equip. 2022, 36, 472–480. [Google Scholar] [CrossRef]
- Jin, Y.; Fei, M.; Rosenquist, S.; Jin, L.; Gohil, S.; Sandström, C.; Sun, C. A dual-promoter gene orchestrates the sucrose-coordinated synthesis of starch and fructan in barley. Mol. Plant 2017, 10, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Wang, J.; Zhou, M.; Ren, X.; Zhan, W.; Sun, Z.; Ende, W.V.D. Characterization of fructan metabolism during Jerusalem artichoke (Helianthus tuberosus L.) germination. Front. Plant Sci. 2018, 9, 1384. [Google Scholar] [CrossRef] [PubMed]
- Sorokan, A.V.; Burkhanova, G.F.; Benkovskaya, G.V.; Maksimov, I.V. Colorado potato beetle microsymbiont Enterobacter BC-8 inhibits defense mechanisms of potato plants using crosstalk between jasmonate-and salicylate-mediated signaling pathways. Arthropod-Plant Interact. 2020, 14, 161–168. [Google Scholar] [CrossRef]
- Ladyzhenskaya, E.P.; Korablyova, N.P. Effect of salicylic acid on the proton translocation activity of plasmalemma of potato tuber cells. Appl. Biochem. Microbiol. 2011, 47, 435–439. [Google Scholar] [CrossRef]
- Li, H.; Cui, D. Physiological functions of jasmonate acid and its signal transduction pathway. Liaoning Agric. Sci. 2010, 6, 32–37. [Google Scholar] [CrossRef]
- Suttle, J.C.; Huckle, L.L.; Lulai, E.C. The effects of dormancy status on the endogenous contents and biological activities of jasmonic acid, n-(jasmonoyl)-isoleucine, and tuberonic acid in potato tubers. Am. J. Potato Res. 2011, 88, 283–293. [Google Scholar] [CrossRef]
- Zhou, Y.; Shou, S.; Jia, C.; Wang, Q. Salicylic acid signal transduction and its role in plant resistance to biological stress. Prog. Nat. Sci. 2007, 17, 305–312. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Lou, C. Jasmonic acid signal transduction and its relationship with abscisic acid signal transduction. Plant Physiol. Commun. 2002, 3, 285–288. [Google Scholar] [CrossRef]
- Song, S.; Liu, J.; Tang, C.; Zhang, W.; Xu, H.; Zhang, Q.; Gao, J. Metabolism and signaling of auxins and their roles in regulating seed dormancy and germination. Chin. Sci. Bull. 2020, 65, 3924–3943. [Google Scholar] [CrossRef]
- Feng, H.Q.; Li, C. Research advances of auxin signal transduction. Biotechnol. Bull. 2018, 34, 24. [Google Scholar] [CrossRef]
- Ma, J.; Xu, T. Non-canonicalauxinsignalingpathwayin plants. Biotechnol. Bull. 2020, 36, 15–22. [Google Scholar] [CrossRef]
- Liu, J. Advances in research on auxin receptors and auxin signaling mechanism. Biotechnol. Bull. 2007, 3, 22–30. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Xu, X.; Yang, K.; Zhao, H.; Li, Y.; Gao, Z. Genome-wide identification and expression analysis of brassinosteroid action-related genes during the shoot growth of moso bamboo. Mol. Biol. Rep. 2019, 46, 1909–1930. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xia, S.; Su, Y.; Wang, H.; Luo, W.; Su, S.; Xiao, L. Brassinolide increases potato root growth in vitro in a dose-dependent way and alleviates salinity stress. BioMed Res. Int. 2016, 8231873. [Google Scholar] [CrossRef]
- Zheng, L.; Ma, J.; Song, C. Genome-wide identification and expression profiling analysis of brassinolide signal transduction genes regulating apple tree architecture. Acta Physiol Plant 2017, 39, 177. [Google Scholar] [CrossRef]
- Tang, W.; Kim, T.W.; Oses-Prieto, J.A.; Sun, Y.; Deng, Z.; Zhu, S.; Wang, Z.Y. BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science 2008, 321, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D. Brassinosteroid signal transduction: From receptor kinase activation to transcriptional networks regulating plant development. Plant Cell 2011, 23, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Bao, F.; Li, J. Promotive effect of brassinosteroids on cell division involves a distinct CycD3-induction pathway in Arabidopsis. Plant J. 2000, 24, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Purugganan, M.M.; Polisensky, D.H.; Antosiewicz, D.M.; Fry, S.C.; Braam, J. Arabidopsis TCH4, regulated by hormones and the environment, encodes a xyloglucan endotransglycosylase. Plant Cell Online 1995, 7, 1555–1567. [Google Scholar] [CrossRef]
- Liu, L.X. Research progress of gibberellin signal transduction pathway. Agric. Eng. Technol. 2021, 41, 66–67. [Google Scholar] [CrossRef]
- Gao, X.H.; Fu, X.D. Research progress for the gibberellin signaling and action on plant growth and development. Biotechnol. Bull. 2018, 34, 1. [Google Scholar] [CrossRef]
- Berry, H.M.; Argueso, C.T. More than growth: Phytohormone-regulated transcription factors controlling plant immunity, plant development and plant architecture. Curr. Opin. Plant Biol. 2022, 70, 102309. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, W.; Geng, W.; Zhao, S.; Pan, Y.; Fan, G.; Liao, K. Transcriptome analysis insight into ethylene metabolism and pectinase activity of apricot (Prunus armeniaca L.) development and ripening. Sci. Rep. 2021, 11, 13569. [Google Scholar] [CrossRef] [PubMed]
- Rehal, P.K.; Tuan, P.A.; Nguyen, T.N.; Cattani, D.J.; Humphreys, D.G.; Ayele, B.T. Genetic variation of seed dormancy in wheat (Triticum aestivum L.) is mediated by transcriptional regulation of abscisic acid metabolism and signaling. Plant Sci. 2022, 324, 111432. [Google Scholar] [CrossRef] [PubMed]
- Huarte, H.R.; Puglia, G.D.; Prjibelski, A.D.; Raccuia, S.A. Seed transcriptome annotation reveals enhanced expression of genes related to ROS homeostasis and ethylene metabolism at alternating temperatures in wild cardoon. Plants 2020, 9, 1225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shui, Z.; Xu, L.; Yang, Y.; Li, Y.; Yuan, X.; Du, J. Gibberellins modulate shade-induced soybean hypocotyl elongation downstream of the mutual promotion of auxin and brassinosteroids. Plant Physiol. Biochem. 2020, 150, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Gao, C.; Zhao, C.; Zhang, L.; Han, M.; An, N.; Ren, X. Effects of brassinosteroid associated with auxin and gibberellin on apple tree growth and gene expression patterns. Hortic. Plant J. 2019, 5, 93–108. [Google Scholar] [CrossRef]
- Kolachevskaya, O.O.; Myakushina, Y.A.; Getman, I.A.; Lomin, S.N.; Deyneko, I.V.; Deigraf, S.V.; Romanov, G.A. Hormonal regulation and crosstalk of auxin/cytokinin signaling pathways in potatoes in vitro and in relation to vegetation or tuberization stages. Int. J. Mol. Sci. 2021, 22, 8207. [Google Scholar] [CrossRef]
- Salopek-Sondi, B.; Šamec, D.; Mihaljević, S.; Smolko, A.; Pavlović, I.; Janković, I.; Ludwig-Müller, J. Influence of stress hormones on the auxin homeostasis in Brassica rapa seedlings. Plant Cell Rep. 2013, 32, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Jin, L.; Jiao, J.; Zhang, X.; Zhang, Y.; Zhao, H.; Liang, M. Expression and purification of plant fructan exohydrolases and their potential applications in fructose production. Int. J. Biol. Macromol. 2018, 108, 9–17. [Google Scholar] [CrossRef]
- Wu, B.; Sun, M.; Zhang, H.; Yang, D.; Lin, C.; Khan, I.; Huang, L. Transcriptome analysis revealed the regulation of gibberellin and the establishment of photosynthetic system promote rapid seed germination and early growth of seedling in pearl millet. Biotechnol. Biofuels 2021, 14, 94. [Google Scholar] [CrossRef]
- Kristo, E.; Foo, A.; Hill, A.R.; Corredig, M. Determination of inulin in milk using high-performance liquid chromatography with evaporative light scattering detection. J. Dairy Sci. 2011, 94, 3316–3321. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Zhou, Z.; Guo, X.; Li, J.; Wang, G.; Sun, X. Metabolome and Transcriptome Analyses Reveal the Correlation Between Fructan Changes and Phytohormone Regulation During Tuber Sprouting of Helianthus tuberosus L. Int. J. Mol. Sci. 2025, 26, 1864. https://doi.org/10.3390/ijms26051864
Wen Y, Zhou Z, Guo X, Li J, Wang G, Sun X. Metabolome and Transcriptome Analyses Reveal the Correlation Between Fructan Changes and Phytohormone Regulation During Tuber Sprouting of Helianthus tuberosus L. International Journal of Molecular Sciences. 2025; 26(5):1864. https://doi.org/10.3390/ijms26051864
Chicago/Turabian StyleWen, Ya, Zhenjie Zhou, Xiaozhu Guo, Juan Li, Gui Wang, and Xuemei Sun. 2025. "Metabolome and Transcriptome Analyses Reveal the Correlation Between Fructan Changes and Phytohormone Regulation During Tuber Sprouting of Helianthus tuberosus L." International Journal of Molecular Sciences 26, no. 5: 1864. https://doi.org/10.3390/ijms26051864
APA StyleWen, Y., Zhou, Z., Guo, X., Li, J., Wang, G., & Sun, X. (2025). Metabolome and Transcriptome Analyses Reveal the Correlation Between Fructan Changes and Phytohormone Regulation During Tuber Sprouting of Helianthus tuberosus L. International Journal of Molecular Sciences, 26(5), 1864. https://doi.org/10.3390/ijms26051864





