Evaluating Drought Tolerance in Codonopsis pilosula Seedlings: Combining Growth, Physiology, Yield, and Tolerance Indices
Abstract
1. Introduction
2. Results
2.1. Variations in Stress-Resistant Physiological Indicators of Different C. pilosula Cultivar Seedlings
2.2. Yield and Its Attributes of C. pilosula Cultivar Seedlings
2.3. Correlation Analysis of C. pilosula Cultivar Seedlings
2.4. Drought Tolerance Indices and Comprehensive Factor Analysis
3. Discussion
3.1. Drought Stress Affects the Growth Rate of C. pilosula Seedlings
3.2. Drought Stress Affects Physiological Changes in C. pilosula Seedlings
3.3. Drought Stress Affects Yield and Quality in C. pilosula Seedlings
3.4. Comprehensive Evaluation of Different Cultivars of C. pilosula Seedlings Under Drought Stress
4. Materials and Methods
4.1. The Experimental Area
4.2. Experimental Design and Treatments
4.3. The Studied Traits
4.4. Drought Tolerance Indices
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Principal Components | Eigenvalue | Contribution Rate (%) | Cumulative Contribution Rate (%) |
|---|---|---|---|
| 1 | 8.529 | 38.768 | 38.768 |
| 2 | 5.175 | 23.523 | 62.290 |
| 3 | 2.579 | 11.722 | 74.013 |
| 4 | 2.285 | 10.385 | 84.398 |
| 5 | 1.642 | 7.464 | 91.862 |
| Drought Indices | Load | Weight Value | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| ESI | −0.547 | −0.009 | −0.168 | −0.588 | −0.026 | 0.037 |
| RSI-Plant height | −0.334 | 0.707 | −0.426 | 0.072 | 0.413 | 0.057 |
| RSI-Number of blades | 0.225 | 0.543 | −0.743 | 0.100 | −0.164 | 0.052 |
| RSI-Root length | 0.647 | 0.359 | −0.108 | 0.084 | 0.526 | 0.048 |
| RSI-Single fresh weight | −0.643 | 0.466 | 0.242 | −0.370 | 0.229 | 0.054 |
| RWCSI | 0.000 | −0.245 | 0.444 | 0.773 | 0.211 | 0.057 |
| CSI | −0.039 | −0.365 | −0.520 | 0.443 | 0.317 | 0.056 |
| EASI-CAT | 0.242 | −0.099 | −0.673 | 0.138 | −0.374 | 0.048 |
| EASI-POD | −0.209 | 0.225 | −0.008 | −0.665 | 0.669 | 0.061 |
| EASI-APX | 0.445 | −0.096 | 0.781 | −0.191 | −0.230 | 0.051 |
| EASI-SOD | −0.406 | −0.649 | 0.244 | 0.317 | 0.445 | 0.061 |
| PSI | 0.563 | 0.638 | 0.145 | −0.292 | −0.258 | 0.052 |
| STI | −0.699 | 0.658 | 0.140 | 0.228 | −0.075 | 0.045 |
| MPI | −0.744 | 0.615 | 0.119 | 0.213 | −0.088 | 0.044 |
| TOL | 0.991 | 0.017 | 0.037 | −0.025 | 0.093 | 0.023 |
| YI | −0.196 | 0.908 | 0.18 | 0.307 | −0.022 | 0.046 |
| GMP | −0.684 | 0.671 | 0.139 | 0.230 | −0.089 | 0.046 |
| YSI | 0.934 | 0.306 | 0.066 | 0.034 | 0.092 | 0.031 |
| SSI | 0.941 | 0.295 | 0.051 | 0.038 | 0.087 | 0.030 |
| % reduction | 0.940 | 0.294 | 0.050 | 0.044 | 0.083 | 0.030 |
| YSSI | −0.984 | 0.077 | 0.018 | 0.061 | −0.103 | 0.025 |
| YPSI | 0.558 | 0.764 | 0.196 | 0.219 | 0.030 | 0.045 |
References
- Ahmed, K.; Shabbir, G.; Ahmb, M.; Shah, K.N. Phenotyping for drought resistance in bread wheat using physiological and biochemical traits. Sci. Total Environ. 2020, 729, 139082. [Google Scholar] [CrossRef] [PubMed]
- Mean, F.H.; Yusop, Z.; Yusof, F. Drought analysis and water resource availability using standardised precipitation evapotranspiration index. Atmos. Res. 2018, 201, 102–115. [Google Scholar] [CrossRef]
- Min, H.; Chen, C.; Wei, S.; Shang, X.; Sun, M.; Xia, R.; Liu, X.; Hao, D.; Chen, H.; Xie, Q. Identification of drought tolerant mechanisms in maize seedlings based on transcriptome analysis of recombination inbred lines. Front. Plant Sci. 2016, 26, 1080. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Committee. Edition of Chinese Pharmacopoeia; Chemical Industry Press: Beijing, China, 2020; Volume 1, pp. 293–294. [Google Scholar]
- Liang, Z.S. The development of the homology of medicine and food industry of exciting fire. Econ. Trade 2024, 7, 19–20. [Google Scholar]
- He, J.Y.; Zhu, S.; Komatsu, K.; Goda, Y.; Cai, S.Q. Genetic polymorphism of medicinally-used Codonopsis species in an internal transcribed spacer sequence of nuclear ribosomal DNA and its application to authenticate Codonopsis radix. J. Nat. Med. 2014, 68, 112–124. [Google Scholar] [CrossRef]
- Liang, Y.C.; Wei, G.F.; Ning, K.; Li, M.Z.; Zhang, G.Z.; Luo, L.; Zhao, G.H.; Wei, J.H.; Liu, Y.P.; Dong, L.L.; et al. Increase in carbohydrate content and variation in microbiome are related to the drought tolerance of Codonopsis pilosula. Plant Physiol. Biochem. 2021, 165, 19–35. [Google Scholar] [CrossRef]
- Shin, J.; Kathuria, A.; Lee, Y.S. Effect of hydrophilic and hydrophobic cyclodextrins on the release of encapsulated allyl isothiocyanate (AITC) and their potential application for plastic film extrusion. Appl. Polym. 2019, 136, 48137. [Google Scholar] [CrossRef]
- Xiao, S.X.; Lei, Z.H.; Song, C.X.; Wang, X.F.; Wang, Y.L.; Zhao, Y.; Feng, Z.; Meng, R.L. Effects of different seedling grades on yield and commercial quality of Codonopsis pilosula. Agric. Biotech. 2020, 9, 18–21. [Google Scholar]
- Ahmed, M.; Aslam, M.; Hassan, E.; Hayat, R.; Ahmed, S. Biochemical, physiological and agronomic response of wheat to changing climate of rainfed Pakistan. Pak. J. Bot. 2018, 51, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.G.M.; Sajjad, M.; Li, M.J.; Azmat, M.A.; Rizwan, M.; Maqsood, R.H.; Khan, S.H. Selection criteria for drought-tolerant bread wheat genotypes at seedling stage. Sustainability 2019, 11, 2584. [Google Scholar] [CrossRef]
- Ahmed, K.; Shahid, S.; Chung, E.; Wang, X.; Harun, S. Climate change uncertainties in seasonal drought severity-area-frequency curves: Case of arid region of Pakistan. J. Hydrol. 2019, 570, 473–485. [Google Scholar] [CrossRef]
- Wang, X.; Mao, Z.; Zhang, J.; Hemat, M.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Osmolyte accumulation plays important roles in the drought priming induced tolerance to post-anthesis drought stress in winter wheat (Triticum aestivum L.). Environ. Exp. Bot. 2019, 166, 103804. [Google Scholar] [CrossRef]
- Liao, Q.; Gu, S.; Kang, S.; Du, T.; Tong, L.; Wood, J.D.; Ding, R. Mild water and salt stress improve water use efficiency by decreasing stomatal conductance via osmotic adjustment in field maize. Sci. Total Environ. 2022, 805, 150364. [Google Scholar] [CrossRef]
- Shi, H.R.; Wang, B.; Yang, P.J.; Li, Y.B.; Miao, F. Differences in sugar accumulation and mobilization between sequential and non-sequential senescence wheat cultivars under natural and drought conditions. PLoS ONE 2016, 11, e0166155. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Zhao, X.Q.; Zhai, L.Y.; Shao, K.T.; Jiang, K.W.; Shen, C.C.; Chen, K.; Wang, S.; Wang, Y.; Xu, J.L. Genetic bases of the stomata-related traits revealed by a genome-wide association analysis in rice (Oryza sativa L.). Front. Genet. 2020, 11, 00611. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, A.; Ali, S.; Javed, M.A.; Iqbal, R.; Khan, M.N.; Çiğ, F.; Sabagh, A.E.; Abujamel, T.; Harakeh, S.; Ercisli, S.; et al. Breeding for water-use efficiency in wheat: Progress, challenges and prospects. Mol. Biol. Rep. 2024, 51, 429. [Google Scholar] [CrossRef] [PubMed]
- Toulotte, J.M.; Pantazopoulou, C.K.; Sanclemente, M.A.; Voesenek, L.A.C.J.; Sasidharan, R. Water stress resilient cereal crops: Lessons from wild relatives. J. Integr. Plant Biol. 2022, 64, 412–430. [Google Scholar] [CrossRef]
- Verma, H.; Sarma, R.N. Identification of markers for root traits related to drought tolerance using traditional rice germplasm. Mol. Biotechnol. 2021, 63, 1280–1292. [Google Scholar] [CrossRef] [PubMed]
- Pandey, B.B.; Ratnakumar, P.; Kiran, B.U.; Dudhe, M.Y.; Lakshmi, G.S.; Ramesh, K.; Guhey, A. Identifying traits associated with terminal drought tolerance in sesame (Sesamum indicum L.) genotypes. Front. Plant Sci. [CrossRef]
- Yemata, G.; Bekele, T. Evaluation of sesame (Sesamum indicum L.) varieties for drought tolerance using agromorphological traits and drought tolerance indices. PeerJ 2024, 12, e16840. [Google Scholar] [CrossRef]
- Anwaar, H.A.; Perveen, R.; Mansha, M.Z.; Abid, M.; Sarwar, Z.M.; Aatif, H.M.; Umar, U.u.d.; Sajid, M.; Usman Aslam, H.M.; Alam, M.M.; et al. Assessment of grain yield indices in response to drought stress in wheat (Triticum aestivum L.). Saudi J. Biol. Sci. 2020, 27, 1818–1823. [Google Scholar] [CrossRef]
- Meng, T.T.; Zhao, T.T.; Leng, F.F.; Chen, J.X.; Wang, Y.G. Changes of key soil factors, biochemistry and bacterial species composition during seasons in the rhizosphere and roots of Codonopsis pilosula (tangshen). Agronomy 2023, 13, 1545. [Google Scholar] [CrossRef]
- Sperdouli, I. Heavy Metal Toxicity Effects on Plants. Toxics 2022, 10, 715. [Google Scholar] [CrossRef]
- Orimoloye, I.R.; Belle, J.A.; Orimoloye, Y.M.; Olusola, A.O.; Ololade, O.O. Drought: A Common Environmental Disaster. Atmosphere 2022, 13, 111. [Google Scholar] [CrossRef]
- He, Z.H.; Zhang, P.; Jia, H.T.; Zhang, S.L.; Nishawy, E.; Sun, X.P.; Dai, M.Q. Regulatory mechanisms and breeding strategies for crop drought resistance. New Crops 2024, 1, 100029. [Google Scholar] [CrossRef]
- Li, J.H.; Huang, Y.; Yu, X.S.; Wu, Q.Q.; Man, X.X.; Diao, Z.H.; You, H.; Shen, J.B.; Cai, Y. Identification and Application of CLE Peptides for Drought Resistance in Solanaceae Crops. J. Agric. Food Chem. 2024, 72, 13869–13884. [Google Scholar] [CrossRef]
- Deng, Y.R.; Yang, J.W.; Wei, J.J.; Liu, S.Y.; Yang, L.; Wang, X.F.; Zhang, N.; Si, H.J. stu-miR159a negatively regulates anthocyanin-specific MYB transcription factor to mediate drought stress tolerance in potato. Hortic. Plant J. 2024. [Google Scholar] [CrossRef]
- Toker, C.; Canci, H.; Yildirim, T. Evaluation of perennial wild Cicer species for drought resistance. Genet. Resour. Crop. Evol. 2007, 54, 1781–1786. [Google Scholar] [CrossRef]
- Mwadzingeni, L.; Shimelis, H.; Dube, E.; Liang, M.D.; Tsilo, T.J. Breeding wheat for drought tolerance: Progress and technologies. J. Integr. Agric. 2016, 15, 935–943. [Google Scholar] [CrossRef]
- Khan, R.; Ma, X.H.; Shah, S.; Wu, X.Y.; Shaheen, A.; Xiao, L.X.; Wu, Y.H.; Wang, S.S. Drought-hardening improves drought tolerance in Nicotiana tabacum at physiological, biochemical, and molecular levels. BMC Plant Biol. 2020, 20, 486. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Hou, E.; Ma, C.; Li, G.; Lin, R.; Zhao, Y.; Wang, X. Comparative transcriptome analysis of the peanut semi-dwarf mutant 1 reveals regulatory mechanism involved in plant height. Gene 2021, 791, 145722. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, X.; Salvat, F.; Wang, Y.; Yan, X.; Zhou, Z.; Lin, J. Salt-adaptive strategies in oil seed crop Ricinus communis early seedlings (cotyledon vs. true leaf) revealed from proteomics analysis. Ecotoxicol. Environ. Saf. 2019, 171, 12–25. [Google Scholar] [CrossRef]
- Zhi, Y.; Cai, X.; Wang, J.; Jia, M.; Wang, Z. Photosynthesis promotion mechanisms of artificial humic acid depend on plant types: A hydroponic study on C3 and C4 plants. Sci. Total Environ. 2024, 917, 170404. [Google Scholar] [CrossRef]
- Henry, R.J.; Furtado, A.; Rangan, P. Pathways of photosynthesis in non-leaf tissues. Biology 2020, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, G.; Xi, B.; Gan, J.; Wen, D.; Cao, F.; Suo, F.; Li, J.; Ma, B.; Guo, B. Response mechanism of growth and gypenosides content for Gynostemma longipes cultivated at two altitude habitats to fine root morphological characteristics. Front. Plant Sci. 2023, 14, 1143745. [Google Scholar] [CrossRef]
- Tripathi, S.; Tiwari, K.; Mahra, S.; Victoria, J.; Rana, S.; Tripathi, D.K.; Sharma, S. Nanoparticles and root traits: Mineral nutrition, stress tolerance and interaction with rhizosphere microbiota. Planta 2024, 260, 34. [Google Scholar] [CrossRef]
- Makonya, G.M.; Ogola, J.B.O.; Muasya, A.M.; Crespo, O.; Maseko, S.; Valentine, A.J.; Ottosen, C.O.; Rosenqvist, E.; Chimphango, S.B.M. Intermittent moisture supply induces drought priming responses in some heat-tolerant chickpea genotypes. Crop Sci. 2020, 60, 2527–2542. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, H.; Ai, X.; Dong, Q.; Shi, X.; Zhao, X.; Zhong, C.; Yu, H. Improving chilling tolerance of peanut seedlings by enhancing antioxidant-modulated ROS scavenging ability, alleviating photosynthetic inhibition, and mobilizing nutrient absorption. Plant Biol. 2024, 26, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, D.; Shahid Iqbal, M.; Li, H.; Faisal Nazir, M.; Khalid, S.; Sarraz, Z.; Hu, D.; Baojun, C.; Geng, X.; Tajo, S.; et al. Differential seedling growth and tolerance indices reflect drought tolerance in cotton. BMC Plant Biol. 2022, 22, 331. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, Y.; Wang, X.; Wang, B.; Xiao, F.; He, K. Physiological response and drought resistance evaluation of Gleditsia sinensis seedlings under drought-rehydration state. Sci. Rep. 2023, 13, 19963. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Han, Z.; Feng, H.; Wang, Y.; Kang, J.; Han, X.; Wang, L.; Wang, C.; Li, H.; et al. Analysis of physiological indicators associated with drought tolerance in wheat under drought and re-watering conditions. Antioxidants 2022, 11, 2266. [Google Scholar] [CrossRef] [PubMed]
- Altinkut, A.; Kazan, K.; Ipekci, Z.; Gozukirmizi, N. Tolerance to paraquat is correlated with the traits associated with water stress tolerance in segregating F2 populations of barley and wheat. Euphytica 2001, 121, 81–86. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.Q.; Song, L.L.; Jacobs, D.F.; Mei, L.; Liu, P.; Jin, S.H.; Wu, J.S. Physiological response to drought stress in Camptotheca acuminata seedlings from two provenances. Front. Plant Sci. 2015, 6, 361. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, D.; Xiang, F.; Zhang, Z. Differentially expressed miRNAs potentially involved in the regulation of defense mechanism to drought stress in maize seedlings. Int. J. Plant Sci. 2009, 170, 605122. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Febrianti Salsinha, Y.C.; Rini, D.S.; Indradewa, D.; Rachmawati, D.; Alam, T.; Purwestri, Y.A. Exogenously applied Casuarina equisetifolia leaf extracts act as an osmoprotectant on proline accumulation under drought stress in local rice from Indonesia. Front. Plant Sci. 2023, 14, 1210241. [Google Scholar] [CrossRef]
- Efeoglu, B.; Ekmekci, Y.; Cicek, N. Physiological responses of three maize cultivars to drought stress and recovery. S. Afr. J. Bot. 2009, 75, 34–42. [Google Scholar] [CrossRef]
- Bano, Q.; Ilyas, N.; Bano, A.; Zafar, N.; Akram, A.; Hassan, F. Effect of Azospirillum inoculation on maize (Zea mays L.) under drought stress. Pak. J. Bot. 2013, 45, 13–20. [Google Scholar]
- Aranjuelo, I.; Molero, G.; Erice, G.; Avice, J.C.; Nogués, S. Plant physiology and proteomics reveals the leaf response to drought in alfalfa (Medicago sativa L.). J. Exp Bot. 2011, 62, 111–123. [Google Scholar] [CrossRef]
- Kissen, R.; Øverby, A.; Winge, P.; Bones, A.M. Allyl-isothiocyanate treatment induces a complex transcriptional reprogramming including heat stress, oxidative stress and plant defence responses in Arabidopsis thaliana. BMC Genom. 2016, 17, 740. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Qin, K.; Song, X.; Zhang, Q.; Zhou, Y.; Xia, X.; Yu, J. BZR1 transcription factor regulates heat stress tolerance through FERONIA receptor-like kinase mediated reactive oxygen species signaling in tomato. Plant Cell Physiol. 2018, 59, 2239–2254. [Google Scholar] [CrossRef]
- Prerostova, S.; Dobrev, P.; Kramna, B.; Gaudinova, A.; Knirsch, V.; Spichal, L.; Zatloukal, M.; Vankova, R. Heat acclimation and inhibition of cytokinin degradation positively affect heat stress tolerance of Arabidopsis. Front. Plant Sci. 2020, 11, 87. [Google Scholar] [CrossRef]
- Medina, E.; Kim, S.H.; Yun, M.; Choi, W.G. Recapitulation of the function and role of ROS generated in response to heat stress in plants. Plants 2021, 10, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.S.; Samsampour, D.; Ebrahimi, M.; Abadía, J.; Khanahmadi, M. Effect of drought stress on growth parameters, osmolyte contents, antioxidant enzymes and glycyrrhizin synthesis in licorice (Glycyrrhiza glabra L.) grown in the field. Phytochemisty 2018, 156, 124–134. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Blum, A. Crop responses to drought and the interpretation of adaptation. Plant Growth Regul. 1996, 20, 135–148. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Passioura, J. The drought environment: Physical, biological and agricultural perspectives. J. Exp Bot. 2007, 58, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Rao, I.M.; Beebe, S.E.; Polania, J.; Grajales, M.; Cajiao, C.; Ricaurte, J.; García, R.; Rivera, M. Evidence for genotypic differences among elite lines of common bean in the ability to remobilize photosynthate to increase yield under drought. J. Agric. Sci. 2017, 155, 857–875. [Google Scholar] [CrossRef]
- Dinneny, J.R. Developmental responses to water and salinity in root systems. Annu. Rev. Cell Dev. Biol. 2019, 35, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Polania, J.; Poschenrieder, C.; Rao, I.; Beebe, S. Root traits and their potential links to plant ideotypes to improve drought resistance in common bean. Theor. Exp. Plant Physiol. 2017, 29, 143–154. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for adaptation to drought. Front. Physiol. 2013, 4, 35. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.J.; Guo, F.X.; Chen, Y.; Liu, L.L.; Cheng, Y.Z.; Jiao, X.S.; Zhang, B.Q.; Bai, G.; Jin, J.Q. Effect of organic fertilizer application on the medicinal character, yield and disease resistance of Angelica sinensis. Acta Pratacult. Sin. 2021, 30, 189–199. [Google Scholar]
- Cui, L.G.; Guo, F.X.; Chen, Y.; Xu, B.; Li, R.X. Effects of cultivation methods on the propagating characteristics of Angelica sinensis SP2 induced by space flight. J. Nucl. Agric. Sci. 2024, 38, 813–821. [Google Scholar]
- Zhou, B.P.; Peng, Y.W.; Zhao, C.L.; Ding, C.W.; Miao, G.X.; Liu, X.J.; Wang, S. Analysis on climate change characteristics of Longxi in the upstream of Wei River in recent 61 years. J. Anhui Agric. Sci. 2018, 46, 125–128. [Google Scholar] [CrossRef]
- Asmare, B.; Mekuriaw, Y. Assessment of livestock production system and feed balance in watersheds of North Achefer district, Ethiopia. J. Agric. Environ. Int. Dev. 2017, 11, 574. [Google Scholar] [CrossRef]
- González, L.; González-Vilar, M. Determination of relative water content. In Handbook of Plant Ecophysiology Techniques; Springer: Dordrecht, The Netherlands, 2001; pp. 207–212. ISBN 978-0-7923-7053-6. [Google Scholar]
- Guo, F.X.; Zhang, M.X.; Chen, Y.; Zhang, W.H.; Xu, S.J.; Wang, J.H.; An, L.Z. Relation of several antioxidant enzymes to rapid freezing resistance in suspension cultured cells from alpine Chorispora bungeana. Cryobiology 2006, 52, 241–250. [Google Scholar] [CrossRef]
- Guo, F.X.; Xiao, W.J.; Chen, Y.; Zhang, Y.J.; Chen, Y.Z.; Liu, L.L.; Gao, X. Initiation of early bolting by pre-enhancing anthocyanin and catalase activity in Angelica sinensis tender leaf during medicine formation cultivation year. Russ. J. Plant Physiol. 2021, 68, 763–773. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, S.P.; Liu, S.D.; Ma, H.J.; Chen, J.; Shen, Q.; Ge, C.W.; Zhang, X.M.; Pang, C.Y.; Zhao, X.H. The compensation effects of physiology and yield in cotton after drought stress. J. Plant Physiol. 2018, 224–225, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Chen, Q.; Chen, Q.; Jiang, M.; Qu, Y. Yield-based drought tolerance index evaluates the drought tolerance of cotton germplasm lines in the interaction of genotype-by-environment. PeerJ 2023, 11, e14367. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhi, M.; Khorasani, S.K.; Ebrahimi, A. Evaluation of drought tolerance indices for screening some of super sweet maize (Zea mays L. var. saccharata) inbred lines. Agrivita J. Agric. Sci. 2020, 42, 435–448. [Google Scholar] [CrossRef]
- Adhikari, N.R.; Sharma, S.; Gairhe, J.; Bhandari, R.M.; Poudel, S. Evaluation of drought tolerant rice cultivars using drought tolerant indices under water stress and irrigated condition. Am. J. Clim. Chang. 2019, 8, 228–236. [Google Scholar] [CrossRef]
- Kouighat, M.; Nabloussi, A.; Kettani, R.; Fakhour, S.; El Fechtali, M.; Hamdani, A. Drought-tolerant sesame mutant lines assessed by physiological traits and stress indices under water deficit conditions. J. Agric. Food Res. 2023, 14, 100842. [Google Scholar] [CrossRef]
- Zare, M. Evaluation of drought tolerance indices for the selection of Iranian barley (Hordeum vulgare) cultivars. Afr. J. Biotechnol. 2012, 11, 15975–15981. [Google Scholar] [CrossRef]
- Choukan, R.; Taherkhani, I.; Ghanadha, M.; Khodarahmi, M. Evaluation of drought tolerance in grain maize hybrids using drought tolerance indices. Seed Plant Improvment J. 2008, 24, 543–562. Available online: https://api.semanticscholar.org/CorpusID:111322998 (accessed on 8 September 2024).
- Malinowska, M.; Donnison, I.; Robson, P. Morphological and physiological traits that explain yield response to drought stress in Miscanthus. Agronomy 2020, 10, 1194. [Google Scholar] [CrossRef]
- Wang, H.Y.; Chen, Y.; Guo, F.X.; Dong, P.B.; Liang, W.; Cheng, J.L. Improvement in the quality and productivity of Codonopsis pilosula seedlings by dazomet soil fumigation. Sci. Rep. 2024, 14, 5407–5420. [Google Scholar] [CrossRef]
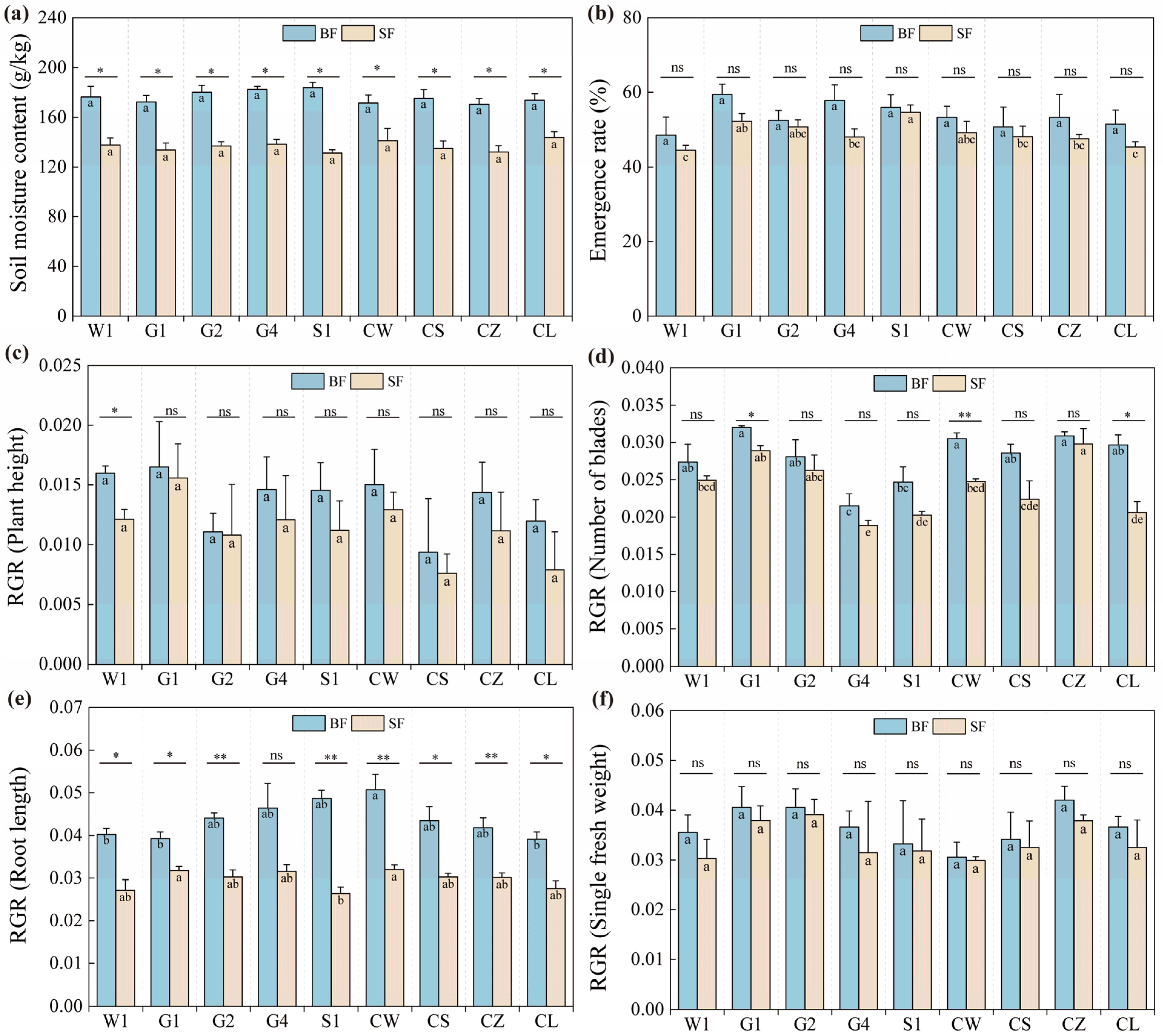
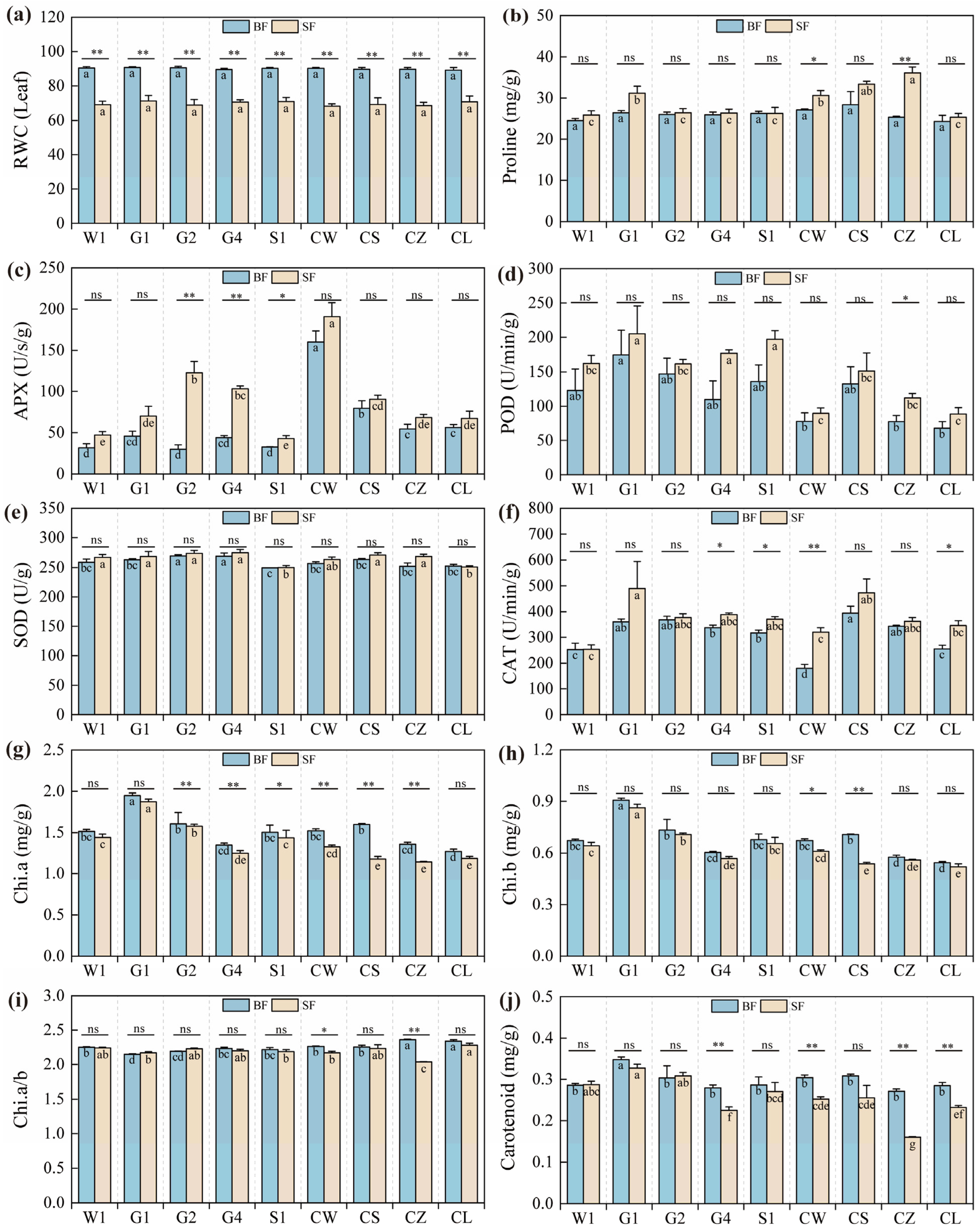
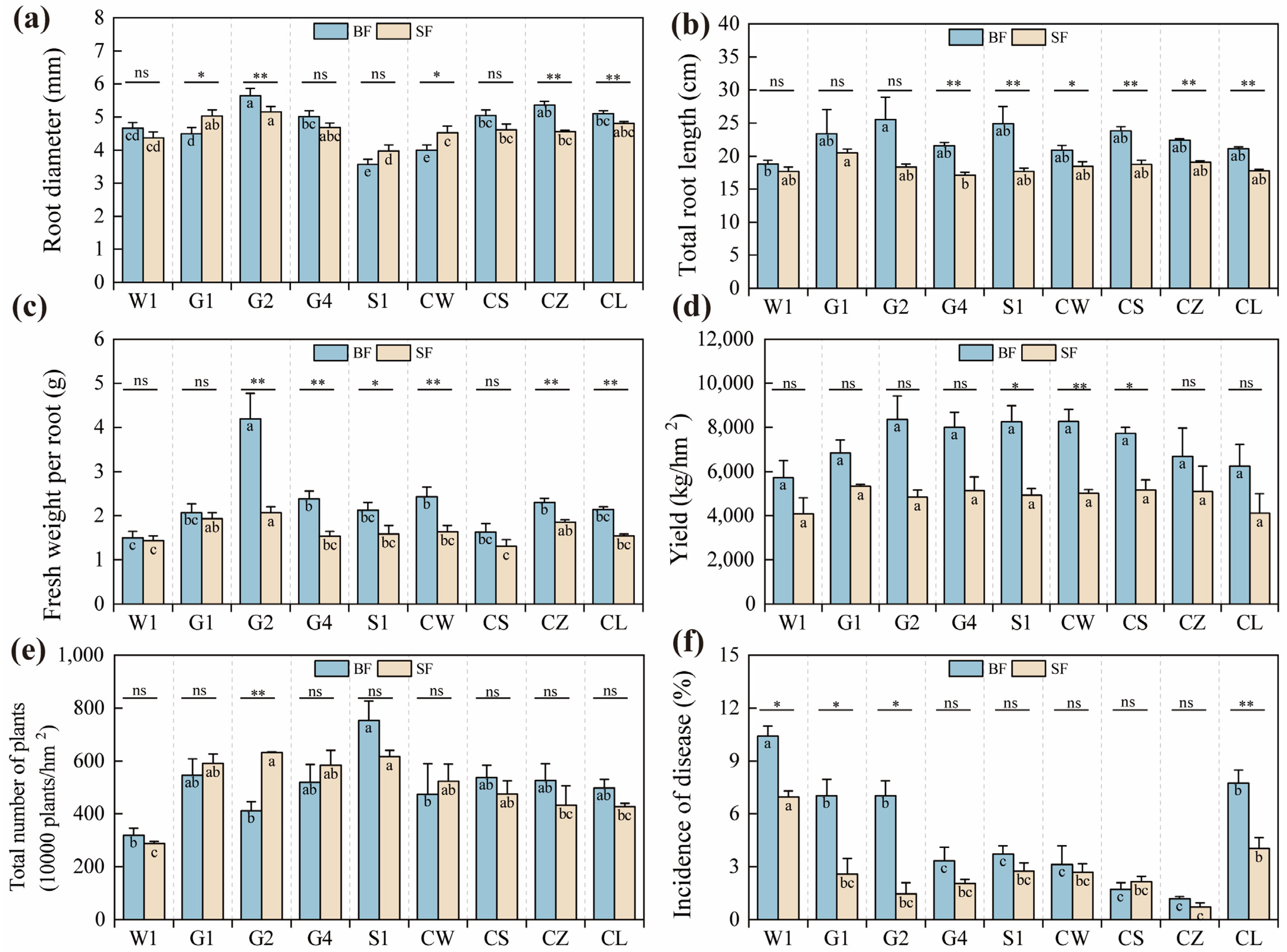


| Source | df | MS/Sig. | ||||
|---|---|---|---|---|---|---|
| Emergence Rate | RGR (Plant Height) | RGR (Number of Blades) | RGR (Root Length) | RGR (Single Fresh Weight) | ||
| W | 1 | 305.422 ** | 8.138 × 10−5 * | 0.000 ** | 0.002 ** | 0.000 ns |
| C | 8 | 56.263 ns | 3.240 × 10−5 ns | 6.704 × 10−5 ** | 2.811 × 10−5 ns | 7.693 × 10−5 ns |
| Block | 2 | 19.454 ns | 0.000 ** | 1.121 × 10−5 ns | 0.000 ** | 0.000 ns |
| W × C | 8 | 11.319 ns | 2.572 × 10−6 ns | 9.737 × 10−6 ns | 6.083 × 10−5 ns | 4.734 × 10−6 ** |
| Error | 34 | 36.978 | 1.647 × 10−5 | 6.393 × 10−6 | 2.754 × 10−5 | 6.584 × 10−5 |
| Source | df | MS/Sig. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RWC (Leaf) | Proline | APX | POD | SOD | CAT | Chi.a | Chi.b | Chi.a/b | Carotenoid | ||
| W | 1 | 5564.354 ** | 122.949 ** | 12,072.268 ** | 14,939.910 ** | 491.478 ** | 54,924.322 ** | 0.266 ** | 0.031 ** | 0.043 * | 0.021 ** |
| C | 8 | 2.285 ns | 31.844 ** | 10,082.467 ** | 9004.174 ** | 380.612 ** | 26,088.905 ** | 0.262 ** | 0.065 ** | 0.011 ** | 0.007 ** |
| Block | 2 | 0.635 ns | 1.929 ns | 3.508 ns | 5157.167 * | 68.423 ns | 663.737 ns | 0.000 ns | 0.000 ns | 0.001 ns | 1.993 × 10−5 ns |
| W × C | 8 | 2.577 ns | 18.031 ** | 1199.301 ** | 600.412 ns | 38.736 ns | 3868.050 ns | 0.022 * | 0.004 * | 0.017 ** | 0.002 ** |
| Error | 34 | 12.356 | 4.566 | 208.483 | 1105.257 | 52.773 | 3076.853 | 0.008 | 0.002 | 0.001 | 0.001 |
| Source | df | MS/Sig. | |||||
|---|---|---|---|---|---|---|---|
| Root Diameter | Total Root Length | Fresh Weight per Root | Total Number of Plants | Yield | Incidence of Disease | ||
| W | 1 | 0.215 ns | 224.714 ** | 5.799 ** | 44.262 ns | 83,999,917.780 ** | 66.168 ** |
| C | 8 | 1.320 ** | 10.012 ns | 1.423 ** | 61,324.448 ** | 2,585,914.143 ns | 32.410 ** |
| Block | 2 | 0.532 * | 3.350 ns | 0.263 ns | 3484.338 ns | 2,171,003.736 ns | 3.077 ns |
| W × C | 8 | 0.362 * | 6.618 ns | 0.570 * | 17,607.675 ns | 962,622.148 ns | 6.691 ** |
| Error | 34 | 0.141 | 6.811 | 0.194 | 13,991.329 | 2,377,142.101 | 1.640 |
| Drought Indices | C. pilosula Cultivars | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| W1 | G1 | G2 | G4 | S1 | CW | CS | CZ | CL | |
| ESI | 0.92 | 0.88 | 0.97 | 0.83 | 0.98 | 0.92 | 0.95 | 0.89 | 0.88 |
| RSI—Plant height | 0.73 | 0.94 | 0.98 | 0.81 | 0.74 | 0.85 | 0.79 | 0.75 | 0.59 |
| RSI—Number of blades | 0.91 | 0.90 | 0.93 | 0.87 | 0.80 | 0.79 | 0.76 | 0.96 | 0.64 |
| RSI—Root length | 0.61 | 0.79 | 0.63 | 0.62 | 0.41 | 0.55 | 0.64 | 0.67 | 0.65 |
| RSI—Single fresh weight | 0.84 | 0.93 | 0.96 | 0.85 | 0.96 | 0.98 | 0.95 | 0.90 | 0.88 |
| RWCSI | 0.76 | 0.79 | 0.76 | 0.79 | 0.79 | 0.76 | 0.77 | 0.76 | 0.79 |
| CSI | 0.95 | 0.96 | 0.98 | 0.93 | 0.96 | 0.88 | 0.74 | 0.88 | 0.94 |
| EASI-CAT | 1.00 | 0.64 | 0.97 | 0.85 | 0.83 | 0.21 | 0.80 | 0.95 | 0.64 |
| EASI-POD | 0.68 | 0.82 | 0.90 | 0.39 | 0.55 | 0.86 | 0.86 | 0.55 | 0.69 |
| EASI-APX | 0.50 | 0.47 | −2.09 | −0.35 | 0.69 | 0.81 | 0.86 | 0.75 | 0.81 |
| EASI-SOD | 0.97 | 0.98 | 0.98 | 0.98 | 1.00 | 0.97 | 0.97 | 0.93 | 1.01 |
| PSI | 1.05 | 1.18 | 1.02 | 1.02 | 1.00 | 1.13 | 1.18 | 1.42 | 1.04 |
| STI | 0.43 | 0.68 | 0.75 | 0.76 | 0.75 | 0.77 | 0.74 | 0.63 | 0.48 |
| MPI | 4904.36 | 6089.67 | 6600.33 | 6568.97 | 6590.75 | 6648.67 | 6451.06 | 5895.11 | 5182.95 |
| TOL | 1645.61 | 1508.67 | 3531.33 | 2871.72 | 3324.17 | 3262.67 | 2579.22 | 1597.11 | 2129.43 |
| YI | 0.56 | 0.73 | 0.66 | 0.70 | 0.67 | 0.68 | 0.70 | 0.69 | 0.56 |
| GMP | 48,34.85 | 6042.77 | 6359.78 | 6410.12 | 6377.73 | 6445.43 | 6320.84 | 5840.77 | 5072.41 |
| YSI | 0.71 | 0.78 | 0.58 | 0.64 | 0.60 | 0.61 | 0.67 | 0.76 | 0.66 |
| SSI | 0.85 | 0.65 | 1.24 | 1.06 | 1.19 | 1.16 | 0.98 | 0.70 | 1.00 |
| % reduction | 28.73 | 22.04 | 42.21 | 35.87 | 40.28 | 39.40 | 33.32 | 23.86 | 34.08 |
| YSSI | 0.64 | 0.66 | 1.00 | 0.91 | 0.97 | 0.97 | 0.86 | 0.67 | 0.74 |
| YPSI | 1629.17 | 2290.51 | 1534.25 | 1848.48 | 1633.07 | 1692.80 | 1935.80 | 2148.96 | 1526.49 |
| Drought Indices | C. pilosula Cultivars | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| W1 | G1 | G2 | G4 | S1 | CW | CS | CZ | CL | |
| ESI | 0.600 | 0.333 | 0.933 | 0.000 | 1.000 | 0.600 | 0.800 | 0.400 | 0.333 |
| RSI—Plant height | 0.359 | 0.897 | 1.000 | 0.564 | 0.385 | 0.667 | 0.513 | 0.410 | 0.000 |
| RSI—Number of blades | 0.844 | 0.813 | 0.906 | 0.719 | 0.500 | 0.469 | 0.375 | 1.000 | 0.000 |
| RSI—Root length | 0.526 | 1.000 | 0.579 | 0.553 | 0.000 | 0.368 | 0.605 | 0.684 | 0.632 |
| RSI—Single fresh weight | 0.000 | 0.643 | 0.857 | 0.071 | 0.857 | 1.000 | 0.786 | 0.429 | 0.286 |
| RWCSI | 0.000 | 1.000 | 0.000 | 1.000 | 1.000 | 0.000 | 0.333 | 0.000 | 1.000 |
| CSI | 0.875 | 0.917 | 1.000 | 0.792 | 0.917 | 0.583 | 0.000 | 0.583 | 0.833 |
| EASI-CAT | 1.000 | 0.544 | 0.962 | 0.810 | 0.785 | 0.000 | 0.747 | 0.937 | 0.544 |
| EASI-POD | 0.569 | 0.843 | 1.000 | 0.000 | 0.314 | 0.922 | 0.922 | 0.314 | 0.588 |
| EASI-APX | 0.878 | 0.868 | 0.000 | 0.590 | 0.942 | 0.983 | 1.000 | 0.963 | 0.983 |
| EASI-SOD | 0.500 | 0.625 | 0.625 | 0.625 | 0.875 | 0.500 | 0.500 | 0.000 | 1.000 |
| PSI | 0.119 | 0.429 | 0.048 | 0.048 | 0.000 | 0.310 | 0.429 | 1.000 | 0.095 |
| STI | 0.000 | 0.735 | 0.941 | 0.971 | 0.941 | 1.000 | 0.912 | 0.588 | 0.147 |
| MPI | 0.000 | 0.680 | 0.972 | 0.954 | 0.967 | 1.000 | 0.887 | 0.568 | 0.160 |
| TOL | 0.932 | 1.000 | 0.000 | 0.326 | 0.102 | 0.133 | 0.471 | 0.956 | 0.693 |
| YI | 0.000 | 1.000 | 0.588 | 0.824 | 0.647 | 0.706 | 0.824 | 0.765 | 0.000 |
| GMP | 0.000 | 0.750 | 0.947 | 0.978 | 0.958 | 1.000 | 0.923 | 0.625 | 0.147 |
| YSI | 0.650 | 1.000 | 0.000 | 0.300 | 0.100 | 0.150 | 0.450 | 0.900 | 0.400 |
| SSI | 0.661 | 1.000 | 0.000 | 0.305 | 0.085 | 0.136 | 0.441 | 0.915 | 0.407 |
| % reduction | 0.668 | 1.000 | 0.000 | 0.314 | 0.096 | 0.139 | 0.441 | 0.910 | 0.403 |
| YSSI | 0.000 | 0.056 | 1.000 | 0.750 | 0.917 | 0.917 | 0.611 | 0.083 | 0.278 |
| YPSI | 0.134 | 1.000 | 0.010 | 0.421 | 0.139 | 0.218 | 0.536 | 0.815 | 0.000 |
| Comprehensive evaluation index | 0.413 | 0.783 | 0.598 | 0.553 | 0.597 | 0.558 | 0.616 | 0.603 | 0.420 |
| Comprehensive sorting | 9 | 1 | 4 | 7 | 5 | 6 | 3 | 2 | 8 |
| Cultivar Number | Cultivar Name | Breeding Methods | Abbreviation |
|---|---|---|---|
| 2006-92-02 | Weidang No. 1 | Pedigree selection | W1 |
| 2011-D07-3 | Gandang No. 1 | Mass selection | G1 |
| 2011-D07-1 | Gandang No. 2 | Mass selection | G2 |
| 2011-D07-7 | Gandang No. 4 | Mass selection | G4 |
| 2011-13-3 | Space-bred No. 1 | Mutation breeding | S1 |
| 2011-2-1 | Wild patterned Codonopsis | Domestication breeding | CW |
| 2011-2-2 | Succulent patterned Codonopsis | Domestication breeding | CS |
| 2011-1-1 | CL | Pedigree Selection | CZ |
| 2011-1-2 | CZ | Pedigree Selection | CL |
| Drought Tolerance Indices | Formula | Reference | |
|---|---|---|---|
| 1 | Stress tolerance index, STI | [1] | |
| 2 | Mean productivity index, MPI | [75] | |
| 3 | Stress tolerance, TOL | [23] | |
| 4 | Yield index, YI | [76] | |
| 5 | Geometric mean productivity, GMP | [77] | |
| 6 | Yield stability index, YSI | [78] | |
| 7 | Stress intensity, SI | [79] | |
| 8 | Stress susceptibility index, SSI | [23] | |
| 9 | % reduction | [80] | |
| 10 | Yield potential score index, YPSI | [81] | |
| 11 | Yield stress score index, YSSI | [81] | |
| 12 | Emergence rate stability index, ESI | ||
| 13 | RWC stability index, RWCSI | ||
| 14 | Proline content stability index, PSI | ||
| 15 | Chlorophyll stability index, CSI | ||
| 16 | RGR stability index, RSI-X | ||
| 17 | Enzyme activity stability index, EASI-X | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Chen, Y.; Guo, F.; Wu, D.; Liang, W.; Dong, P.; Cheng, J. Evaluating Drought Tolerance in Codonopsis pilosula Seedlings: Combining Growth, Physiology, Yield, and Tolerance Indices. Int. J. Mol. Sci. 2025, 26, 1600. https://doi.org/10.3390/ijms26041600
Wang H, Chen Y, Guo F, Wu D, Liang W, Dong P, Cheng J. Evaluating Drought Tolerance in Codonopsis pilosula Seedlings: Combining Growth, Physiology, Yield, and Tolerance Indices. International Journal of Molecular Sciences. 2025; 26(4):1600. https://doi.org/10.3390/ijms26041600
Chicago/Turabian StyleWang, Hongyan, Yuan Chen, Fengxia Guo, Di Wu, Wei Liang, Pengbin Dong, and Jiali Cheng. 2025. "Evaluating Drought Tolerance in Codonopsis pilosula Seedlings: Combining Growth, Physiology, Yield, and Tolerance Indices" International Journal of Molecular Sciences 26, no. 4: 1600. https://doi.org/10.3390/ijms26041600
APA StyleWang, H., Chen, Y., Guo, F., Wu, D., Liang, W., Dong, P., & Cheng, J. (2025). Evaluating Drought Tolerance in Codonopsis pilosula Seedlings: Combining Growth, Physiology, Yield, and Tolerance Indices. International Journal of Molecular Sciences, 26(4), 1600. https://doi.org/10.3390/ijms26041600






