High-Throughput Screening of Five Compound Libraries for Anthelmintic Activity and Toxicity Leads to the Discovery of Two Flavonoid Compounds
Abstract
1. Introduction
2. Results
2.1. HTS on C. elegans
2.2. Study on Parasitic Helminths
2.3. Cytotoxicity of the Hits in Liver Spheroids and Intestinal Organoids
2.4. Predictive ADMET
3. Discussion
4. Materials and Methods
4.1. Library and Compounds Management
4.2. High Throughput Screening on C. elegans
4.3. Tests of Susceptibility on Parasitic Helminths
4.4. Spheroids Toxicity Assay
4.5. Mouse Intestinal Organoids’ Tolerance Assay
4.6. In Silico Analysis of the Hits
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChR | Acetylcholine receptor |
| ADMET | Absorption, distribution, metabolism, excretion, toxicity |
| BBB | Blood–brain barrier |
| CC50 | Cytotoxic concentration 50% |
| CYP | Cytochrome P450 |
| DR | Dose response |
| EC50 | Effective dose 50% |
| EHA | Egg-hatching assay |
| EHI | Egg-hatching inhibition |
| FAS | Fatty acid synthetase |
| GI | Gastrointestinal tract |
| GINs | Gastrointestinal nematodes |
| HTS | High-throughput screening |
| LMI | Larval migration inhibition |
| LMIT | Larval migration inhibition test |
| MTD | Maximum tolerated dose |
| SS | Single-slot |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
References
- Morgan, E.R.; Aziz, N.A.A.; Blanchard, A.; Charvet, C.; Claerebout, E.; Geldhof, P.; Greer, A.W.; Hertzberg, H.; Hodgkinson, J.; Höglund, J.; et al. 100 questions in livestock helminthology research. Trends Parasitol. 2019, 35, 52–71. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Vidyashankar, A.N. An inconvenient truth: Global warming and anthelmintic resistance. Vet. Parasitol. 2012, 186, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Vázquez, F.A.; Hosking, B.C. A telephone survey of internal parasite control practices on sheep farms in Spain. Vet. Parasitol. 2013, 192, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Vangheel, M.; Traunspurger, W.; Spann, N. Effects of the antibiotic tetracycline on the reproduction, growth and population growth rate of the nematode Caenorhabditis elegans. Nematology 2014, 16, 19–29. [Google Scholar] [CrossRef]
- Munguía, B.; Saldaña, J.; Nieves, M.; Melian, M.E.; Ferrer, M.; Teixeira, R.; Porcal, W.; Manta, E.; Domínguez, L. Sensitivity of Haemonchus contortus to anthelmintics using different in vitro screening assays: A comparative study. Parasit. Vectors 2022, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Valladares, M.; Robles-Pérez, D.; Martínez-Pérez, J.M.; Cordero-Pérez, C.; Famularo, M.d.R.; Fernández-Pato, N.; González-Lanza, C.; Castañón-Ordóñez, L.; Rojo-Vázquez, F.A. Prevalence of gastrointestinal nematodes and Fasciola hepatica in sheep in the northwest of Spain: Relation to climatic conditions and/or man-made environmental modifications. Parasit. Vectors 2013, 6, 282. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Valladares, M.; Geurden, T.; Bartram, D.J.; Martínez-Pérez, J.M.; Robles-Pérez, D.; Bohórquez, A.; Florez, E.; Meana, A.; Rojo-Vázquez, F.A. Resistance of gastrointestinal nematodes to the most commonly used anthelmintics in sheep, cattle and horses in Spain. Vet. Parasitol. 2015, 211, 228–233. [Google Scholar] [CrossRef]
- Scott, I.; Pomroy, W.E.; Kenyon, P.R.; Smith, G.; Adlington, B.; Moss, A. Lack of efficacy of monepantel against Teladorsagia circumcincta and Trichostrongylus colubriformis. Vet. Parasitol. 2013, 198, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, E.; Gallidis, E.; Ptochos, S. Anthelmintic resistance in sheep in Europe: A selected review. Vet. Parasitol. 2012, 189, 85–88. [Google Scholar] [CrossRef]
- Sepúlveda-Crespo, D.; Reguera, R.M.; Rojo-Vázquez, F.; Balaña-Fouce, R.; Martínez-Valladares, M. Drug discovery technologies: Caenorhabditis elegans as a model for anthelmintic therapeutics. Med. Res. Rev. 2020, 40, 1715–1753. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.R.; Olejnik, N.; Bailey, K.D.; Vaught, C.A.; Sprando, R.L. C. elegans development and activity test detects mammalian developmental neurotoxins. Food Chem. Toxicol. 2018, 121, 583–592. [Google Scholar] [CrossRef]
- Ha, N.M.; Tran, S.H.; Shim, Y.H.; Kang, K. Caenorhabditis elegans as a powerful tool in natural product bioactivity research. Appl. Biol. Chem. 2022, 65, 18. [Google Scholar] [CrossRef]
- O’Reilly, L.P.; Luke, C.J.; Perlmutter, D.H.; Silverman, G.A.; Pak, S.C. C. elegans in high-throughput drug discovery. Adv. Drug Deliv. Rev. 2014, 69–70, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Salinas, G.; Risi, G. Caenorhabditis elegans: Nature and nurture gift to nematode parasitologists. Parasitology 2018, 145, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Risi, G.; Aguilera, E.; Ladós, E.; Suárez, G.; Carrera, I.; Álvarez, G.; Salinas, G. Caenorhabditis elegans infrared-based motility assay identified new hits for nematicide drug development. Vet. Sci. 2019, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Taki, A.C.; Byrne, J.J.; Boag, P.R.; Jabbar, A.; Gasser, R.B. Practical High-Throughput method to screen compounds for anthelmintic activity against Caenorhabditis elegans. Molecules 2021, 26, 4156. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jeon, H. 3D cell cultures toward quantitative high-throughput drug screening. Trends Pharmacol. Sci. 2022, 43, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, V.; Seufferlein, T.; Armacki, M. Intestinal organoids in coculture: Redefining the boundaries of gut mucosa ex vivo modeling. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G693–G704. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, J.; Bertero, A.; Coccini, T.; Baderna, D.; Buzanska, L.; Caloni, F. Organoids are promising tools for species-specific in vitro toxicological studies. J. Appl. Toxicol. 2019, 39, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Wang, J.; Xu, J.; You, F.; Pan, M.; Xu, X.; Weng, J.; Han, X.; Li, S.; Li, Y.; et al. High-throughput three-dimensional spheroid tumor model using a novel stamp-like tool. J. Tissue Eng. 2019, 10, 2041731419889184. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.C.; Hendriks, D.F.G.; Moro, S.M.L.; Ellis, E.; Walsh, J.; Renblom, A.; Fredriksson Puigvert, L.; Dankers, A.C.; Jacobs, F.; Snoeys, J.; et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci. Rep. 2016, 6, 25187. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, W.D.; Panzica-Kelly, J.M.; Chen, S.J.; Strassle, B.; Hasson, C.; Lecureux, L.; Wang, L.; Chen, W.; Sherry, T.; Gan, J.; et al. Development and characterization of rat duodenal organoids for ADME and toxicology applications. Toxicology 2020, 446, 152614. [Google Scholar] [CrossRef]
- Du, Y.; Li, X.; Niu, Q.; Mo, X.; Qui, M.; Ma, T.; Kuo, C.J.; Fu, H. Development of a miniaturized 3D organoid culture platform for ultra-high-throughput screening. J. Mol. Cell Biol. 2020, 12, 630–643. [Google Scholar] [CrossRef]
- Yin, Y.B.; de Jonge, H.R.; Wu, X.; Yin, Y.L. Mini-gut: A promising model for drug development. Drug Discov. Today 2019, 24, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.H.; Behnke, M.S. WRN conditioned media is sufficient for in vitro propagation of intestinal organoids from large farm and small companion animals. Biol. Open 2017, 6, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Blanc, F.; Cherbuy, C.; Egidy, G.; Giuffra, E.; Lacroix-Lamandé, S.; Wiedemann, A. Intestinal organoids in farm animals. Vet. Res. 2021, 52, 33. [Google Scholar] [CrossRef]
- Yang, S.; Hu, H.; Kung, H.; Zou, R.; Dai, Y.; Hu, Y.; Wang, T.; Lv, T.; Yu, J.; Li, F. Organoids: The current status and biomedical applications. MedComm 2023, 4, e274. [Google Scholar] [CrossRef]
- Tenreiro, M.F.; Branco, M.A.; Cotovio, J.P.; Cabral, J.M.S.; Fernandes, T.G.; Diogo, M.M. Advancing organoid design through co-emergence, assembly, and bioengineering. Trends Biotechnol. 2023, 41, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Piplani, M.; Bhagwat, D.P.; Singhvi, G.; Sankaranarayanan, M.; Balana-Fouce, R.; Vats, T.; Chander, S. Plant-based larvicidal agents: An overview from 2000 to 2018. Exp. Parasitol. 2019, 199, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J. Is Caenorhabditis elegans the magic bullet for anthelminthic drug discovery? Trends Parasitol. 2015, 31, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Elfawal, M.A.; Savinov, S.N.; Aroian, R.V. Drug screening for discovery of broad-spectrum agents for soil-transmitted nematodes. Sci. Rep. 2019, 9, 12347. [Google Scholar] [CrossRef] [PubMed]
- Shanley, H.T.; Taki, A.C.; Byrne, J.J.; Nguyen, N.; Wells, T.N.C.; Jabbar, A.; Sleebs, B.E.; Gasser, R.B. A phenotypic screen of the Global Health Priority Box identifies an insecticide with anthelmintic activity. Parasit. Vectors 2024, 17, 131. [Google Scholar] [CrossRef]
- Elfawal, M.A.; Goetz, E.; Kim, Y.; Chen, P.; Savinov, S.N.; Barasa, L.; Thompson, P.R.; Aroian, R.V. High-Throughput Screening of more than 30,000 compounds for anthelmintics against gastrointestinal nematode parasites. ACS Infect. Dis. 2025, 11, 104–120. [Google Scholar] [CrossRef]
- Burns, A.R.; Luciani, G.M.; Musso, G.; Bagg, R.; Yeo, M.; Zhang, Y.; Rajendran, L.; Glavin, J.; Hunter, R.; Redman, E.; et al. Caenorhabditis elegans is a useful model for anthelmintic discovery. Nat. Commun. 2015, 6, 7485. [Google Scholar] [CrossRef] [PubMed]
- Preston, S.; Jiao, Y.; Jabbar, A.; McGee, S.L.; Laleu, B.; Willis, P.; Wells, T.N.C.; Gasser, R.B. Screening of the ‘Pathogen Box’ identifies an approved pesticide with major anthelmintic activity against the Barber’s pole worm. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 329–334. [Google Scholar] [CrossRef]
- Hikiji, W.; Yamaguchi, K.; Saka, K.; Hayashida, M.; Ohno, Y.; Fukunaga, T. Acute fatal poisoning with Tolfenpyrad. J. Forensic Leg. Med. 2013, 20, 962–964. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Hikiji, W.; Takino, M.; Saka, K.; Hayashida, M.; Fukunaga, T.; Ohno, Y. Analysis of tolfenpyrad and its metabolites in plasma in a tolfenpyrad poisoning case. J. Anal. Toxicol. 2012, 36, 529–537. [Google Scholar] [CrossRef][Green Version]
- Preston, S.; Garcia-Bustos, J.; Hall, L.G.; Martin, S.D.; Le, T.G.; Kundu, A.; Ghoshal, A.; Nguyen, N.H.; Jiao, Y.; Ruan, B.; et al. 1-Methyl-1H-pyrazole-5-carboxamide Derivatives Exhibit Unexpected Acute Mammalian Toxicity. J. Med. Chem. 2021, 64, 840–844. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 10 October 2024).
- MedchemExpress.com. Available online: https://www.medchemexpress.com/ (accessed on 10 October 2024).
- DrugBank Online. Available online: https://go.drugbank.com/drugs (accessed on 10 October 2024).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 10 October 2024).
- Turani, O.; Castro, M.J.; Vazzana, J.; Mendioroz, P.; Volpe, M.A.; Gerbino, D.C.; Bouzat, C. Potent Anthelmintic Activity of Chalcones Synthesized by an Effective Green Approach. ChemMedChem 2024, 19, e202400071. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-T.; Fong, T.-H.; Chen, H.-M.; Chang, C.-Y.; Wang, Y.-H.; Chern, C.-Y.; Chen, Y.-H. Toxicity assessments of chalcone and some synthetic chalcone analogues in a zebrafish model. Molecules 2014, 19, 641–650. [Google Scholar] [CrossRef]
- Escala, N.; Valderas-García, E.; Álvarez Bardón, M.; Castilla Gómez de Agüero, V.; López-Pérez, J.L.; Rojo-Vázquez, F.A.; San Feliciano, A.; Martínez-Valladares, M.; Balaña-Fouce, R.; del Olmo, E. Further and new target-based benzimidazole anthelmintics active against Teladorsagia circumcincta. J. Mol. Struct. 2022, 1269, 133735. [Google Scholar] [CrossRef]
- Valderas-García, E.; de la Vega, J.; Álvarez Bardón, M.; Castilla Gómez de Agüero, V.; Escarcena, R.; López-Pérez, J.L.; Rojo-Vázquez, F.A.; San Feliciano, A.; Del Olmo, E.; Balaña-Fouce, R.; et al. Anthelmintic activity of aminoalcohol and diamine derivatives against the gastrointestinal nematode Teladorsagia circumcincta. Vet. Parasitol. 2021, 296, 109496. [Google Scholar] [CrossRef]
- Cambra-Pellejà, M.; Valderas-García, E.; Balaña-Fouce, R.; de la Vega, J.; Del Olmo, E.; Antwi-Ekwuruke, J.; Linnemann, L.; Heepmann, L.; Breloer, M.; Martínez-Valladares, M. Evaluating alternative compounds for strongyloidiasis therapy: Novel insights from larval migration inhibition test. PLoS Negl. Trop. Dis. 2024, 18, e0012532. [Google Scholar] [CrossRef]
- Document #28223, Version 2.0.1, Dec 2016. Stemcell Enteroids Tech Bulletin. Available online: https://cdn.stemcell.com/media/files/techbulletin/TB28223-Intestinal_Epithelial_Organoid_Culture_with_IntestiCult_Organoid_Growth_Medium_(Mouse).pdf?_ga=1.255806536.1701332796.1451577632 (accessed on 6 September 2022).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. admetSAR: A comprehensive source and free tool for assessment of chemical ADMET properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef]
- Iversen, P.W.; Eastwood, B.J.; Sittampalam, G.S.; Cox, K.L. A comparison of assay performance measures in screening assays: Signal window, Z’ factor, and assay variability ratio. J. Biomol. Screen. 2006, 11, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Malo, N.; Hanley, J.A.; Cerquozzi, S.; Pelletier, J.; Nadon, R. Statistical practice in high-throughput screening data analysis. Nat. Biotechnol. 2006, 24, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Wu, Z. Alternative statistical parameter for high-throughput screening assay quality assessment. J. Biomol. Screen. 2007, 12, 229–234. [Google Scholar] [CrossRef]
- Zhang, J.H.; Chung, T.D.Y.; Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of High Throughput Screening assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
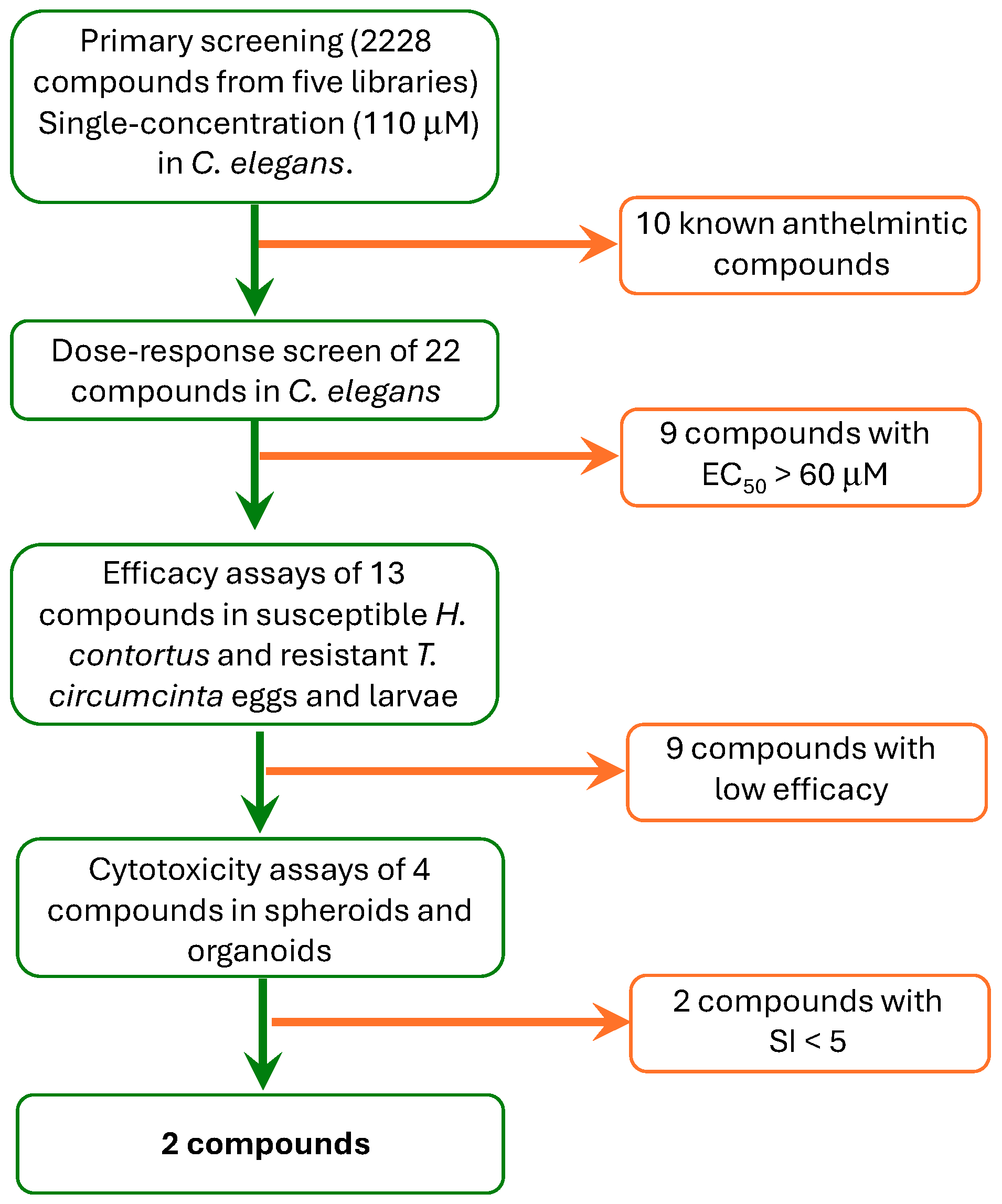

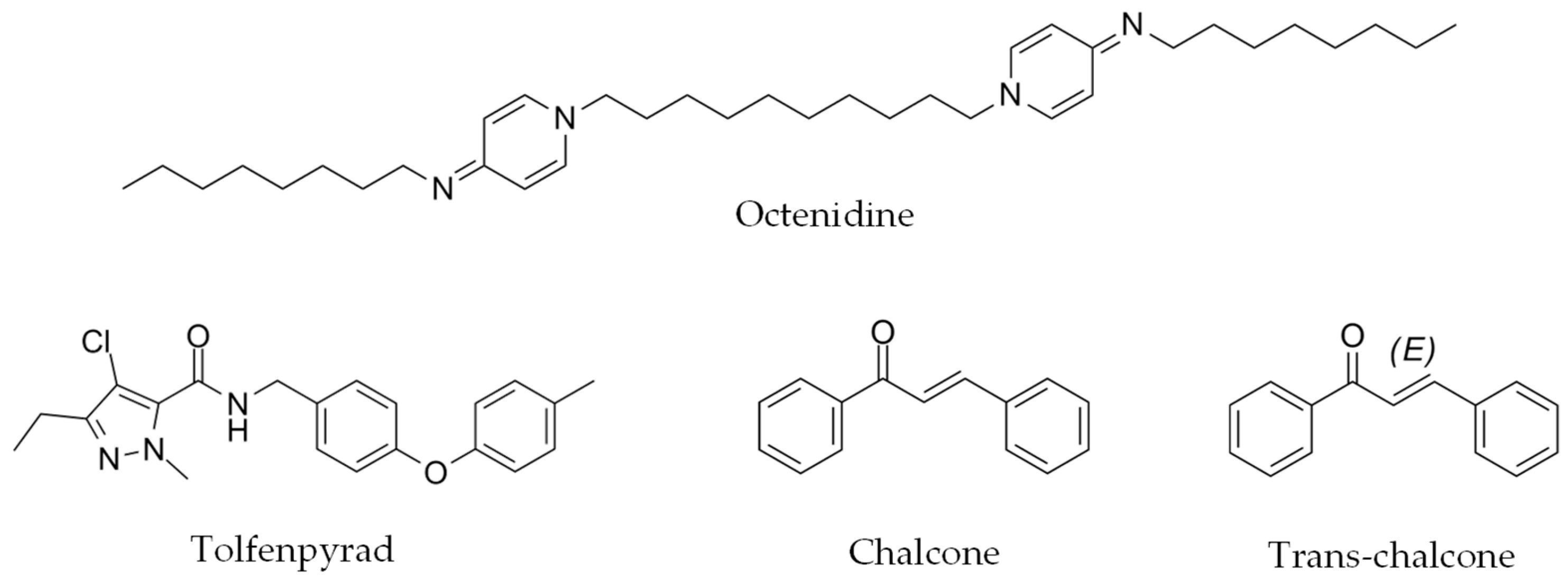
| Compound | Collection | CAS Number | Inhibition (%) 110 μM (0 h) | Inhibition (%) 110 μM (24 h) | EC50 (μM) at 24 h |
|---|---|---|---|---|---|
| Avermectin B1 | Anti-Infection | 71751-41-2 | 81 ± 1 | 42 ± 1 | NT ** |
| Chloroxylenol | 88-04-0 | 100 ± 0 | 100 ± 0 | >60 * | |
| Eprinomectin | 123997-26-2 | 91 ± 3 | 92 ± 3 | NT ** | |
| Ethacridine lactate | 1837-57-6 | 66 ± 26 | 87 ± 6 | 50.1 ± 1.0 | |
| Ethacridine lactate monohydrate | 6402-23-9 | 73 ± 17 | 89 ± 4 | 28.2 ± 1.0 | |
| Fidaxomicin | 873857-62-6 | 82 ± 7 | 89 ± 1 | >60 * | |
| Hygromycin B | 31282-04-9 | 67 ± 28 | 89 ± 5 | >60 * | |
| Ivermectin | 70288-86-7 | 98 ± 1 | 85 ± 14 | NT ** | |
| Levamisole | 14769-73-4 | 87 ± 10 | 61 ± 3 | NT ** | |
| Milbemycin | 51596-10-2 | 100 ± 0 | 86 ± 14 | NT ** | |
| Moxidectin | 113507-06-5 | 99 ± 1 | 85 ± 13 | NT ** | |
| Nitroxoline | 4008-48-4 | 52 ± 32 | 88 ± 2 | >60 * | |
| Octenidine | 70775-75-6 | 84 ± 1 | 96 ± 5 | 6.9 ± 0.5 | |
| Oxyclozanide | 2277-92-1 | 10 ± 9 | 93 ± 5 | NT ** | |
| Pyrantel pamoate | 22204-24-6 | 94 ± 0 | 18 ± 18 | NT ** | |
| Pyrantel tartrate | 33401-94-4 | 71 ± 1 | 26 ± 1 | NT ** | |
| Ribavirin | 36791-04-5 | 91 ± 5 | 76 ± 11 | >60 * | |
| Robenidine | 25875-51-8 | 93 ± 5 | 81 ± 20 | 32.3 ± 2.8 | |
| Salinomycin | 53003-10-4 | 81 ± 9 | 94 ± 1 | 55.3 ± 1.4 | |
| Spiramycin | 8025-81-8 | 43 ± 42 | 81 ± 9 | >60 * | |
| Sulfaquinoxaline | 59-40-5 | 87 ± 1 | 67 ± 6 | >60 * | |
| Tetramisole | 5086-74-8 | 78 ± 2 | 82 ± 3 | NT ** | |
| Tolfenpyrad | 129558-76-5 | 80 ± 2 | 98 ± 2 | 2.4 ± 0.2 | |
| Kuwanon G | Flavonoids | 75629-19-5 | 0 ± 0 | 74 ± 5 | 34.7 ± 1.6 |
| Chalcone | 614-47-1 | 30 ± 30 | 85 ± 15 | 52.4 ± 1.4 | |
| Trans-chalcone | 614-47-1 | 16 ± 17 | 95 ± 5 | 24.9 ± 1.8 | |
| Polygodial | Terpenoid | 6754-20-7 | 0 ± 0 | 93 ± 4 | 33.2 ± 6.4 |
| 3,29-dibenzoyl rarounitriol | 873001-54-8 | 40 ± 22 | 71 ± 19 | 45.4 ± 5.1 | |
| Triptolide | 38748-32-2 | 15 ± 0 | 72 ± 1 | >60 * | |
| Dioscin | Chinese | 19057-60-4 | 12 ± 1 | 79 ± 2 | 37.8 ± 1.3 |
| Propranolol | 318-98-9 | 84 ± 0 | 35 ± 0 | >60 * | |
| Resveratrol | 501-36-0 | 82 ± 8 | 7 ± 7 | 27.7 ± 1.5 |
| Compound | Collection | H. contortus EHI (%) | H. contortus LMIT (%) | T. circumcincta EHI (%) | T. circumcincta LMIT (%) |
|---|---|---|---|---|---|
| Susceptible Strain | Resistant Strain | ||||
| Ethacridine lactate | Anti-Infection | 55.8 ± 5.1 | 4.9 ± 2.2 | 4.0 ± 1.5 | NE * |
| Ethacridine lactate monohydrate | 54.8 ± 11.7 | 0.0 | 0.0 | 0.0 * | |
| Octenidine | 99.5 ± 0.8 | 98.3 ± 0.3 | 99.5 ± 0.9 | 94.8 ± 4.3 | |
| Robenidine | 59.7 ± 0.7 | 3.1 ± 6.9 | 12.5 ± 0.70.0 | NE * | |
| Salinomycin | 2.8 ± 5.1 | 0.0 | NE | NE * | |
| Tolfenpyrad | 96.0 ± 2.9 | 9.0 ± 6.3 | 99.7 ± 0.5 | NE | |
| Kuwanon G | Flavonoids | 9.4 ± 1.8 | 0.0 | NE | NE * |
| Chalcone | 99.4 ± 0.1 | 45.7 ± 4.5 | 100.0 ± 0.0 | 4.7 ± 1.5 | |
| Trans-chalcone | 99.5 ± 0.7 | 20.3 ± 7.5 | 99.9 ± 0.2 | NE | |
| Polygodial | Terpenoid | 0.0 | 0.0 | NE | NE * |
| 3,29-dibenzoyl rarounitriol | 0.0 | 0.0 | 0.0 | 0.0 * | |
| Dioscin | FDA Chinese | NE | 0.0 | NE | 0.0 * |
| Resveratrol | 0.0 | 0.0 | NE | NE * | |
| Thiabendazole | Controls | 100.0 ± 0.0 | - | 100.0 ± 0.0 | - |
| Ivermectine | - | 100.0 ± 0.0 | - | 100.0 ± 0.0 | |
| Ovicidal Effect EC50 (μM) | Citotoxicity CC50 (μM) | Selective Index (SI) CC50/EC50 | ||||||
|---|---|---|---|---|---|---|---|---|
| Compound | H. c | T. c | Sph | Ent | Sph/H. c | Ent/H. c | Sph/T. c | Ent/T. c |
| Octenidine | 2.1 ± 0.1 | 15.1 ± 4.8 | 54.1 ± 2.8 | 14.2 ± 0.4 | 25.8 | 6.8 | 3.6 | 0.9 |
| Tolfenpyrad | 1.5 ± 0.1 | 0.2 ± 0.3 | >50 | <1 | >33.3 | <0.7 | 250 | <5 |
| Chalcone | 3.7 ± 0.1 | 9.8 ± 0.3 | >90 | >50 | >24.3 | >13.5 | >9.2 | >5.1 |
| Trans-chalcone | 3.1 ± 0.2 | 4.9 ± 0.2 | >90 | >50 | >29.0 | >16.1 | >18.4 | >10.2 |
| Thiabendazole | 0.28 ± 0.01 | 1.53 ± 0.06 | - | - | - | - | - | - |
| Compound | MW [g/mol] | Num. Rotable Bonds | Num. H Acceptor | Num. H Donor | Consensus LogP o/w | LogS ESOL * | GI Abs | BBB Pen | CYP Inh |
|---|---|---|---|---|---|---|---|---|---|
| Octenidine | 623.83 ** | 25 | 2 | 0 | 8.12 | −10.20 ** | Low | No | No |
| Tolfenpyrad | 383.87 | 7 | 3 | 1 | 4.23 | −5.27 | High | Yes | Yes |
| Chalcone | 208.26 | 3 | 1 | 0 | 3.29 | −3.43 | High | Yes | Yes |
| Trans-chalcone | 208.26 | 3 | 1 | 0 | 3.29 | −3.43 | High | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galli, G.; Ruiz-Somacarrera, M.; González del Palacio, L.; Melcón-Fernández, E.; González-Pérez, R.; García-Estrada, C.; Martinez-Valladares, M.; Balaña-Fouce, R. High-Throughput Screening of Five Compound Libraries for Anthelmintic Activity and Toxicity Leads to the Discovery of Two Flavonoid Compounds. Int. J. Mol. Sci. 2025, 26, 1595. https://doi.org/10.3390/ijms26041595
Galli G, Ruiz-Somacarrera M, González del Palacio L, Melcón-Fernández E, González-Pérez R, García-Estrada C, Martinez-Valladares M, Balaña-Fouce R. High-Throughput Screening of Five Compound Libraries for Anthelmintic Activity and Toxicity Leads to the Discovery of Two Flavonoid Compounds. International Journal of Molecular Sciences. 2025; 26(4):1595. https://doi.org/10.3390/ijms26041595
Chicago/Turabian StyleGalli, Giulio, Marta Ruiz-Somacarrera, Laura González del Palacio, Estela Melcón-Fernández, Rubén González-Pérez, Carlos García-Estrada, Maria Martinez-Valladares, and Rafael Balaña-Fouce. 2025. "High-Throughput Screening of Five Compound Libraries for Anthelmintic Activity and Toxicity Leads to the Discovery of Two Flavonoid Compounds" International Journal of Molecular Sciences 26, no. 4: 1595. https://doi.org/10.3390/ijms26041595
APA StyleGalli, G., Ruiz-Somacarrera, M., González del Palacio, L., Melcón-Fernández, E., González-Pérez, R., García-Estrada, C., Martinez-Valladares, M., & Balaña-Fouce, R. (2025). High-Throughput Screening of Five Compound Libraries for Anthelmintic Activity and Toxicity Leads to the Discovery of Two Flavonoid Compounds. International Journal of Molecular Sciences, 26(4), 1595. https://doi.org/10.3390/ijms26041595










