Early Intervention in Herpes Simplex Virus-1 Replication in Vitro with Allenic Macrolide Archangiumide
Abstract
1. Introduction
2. Results
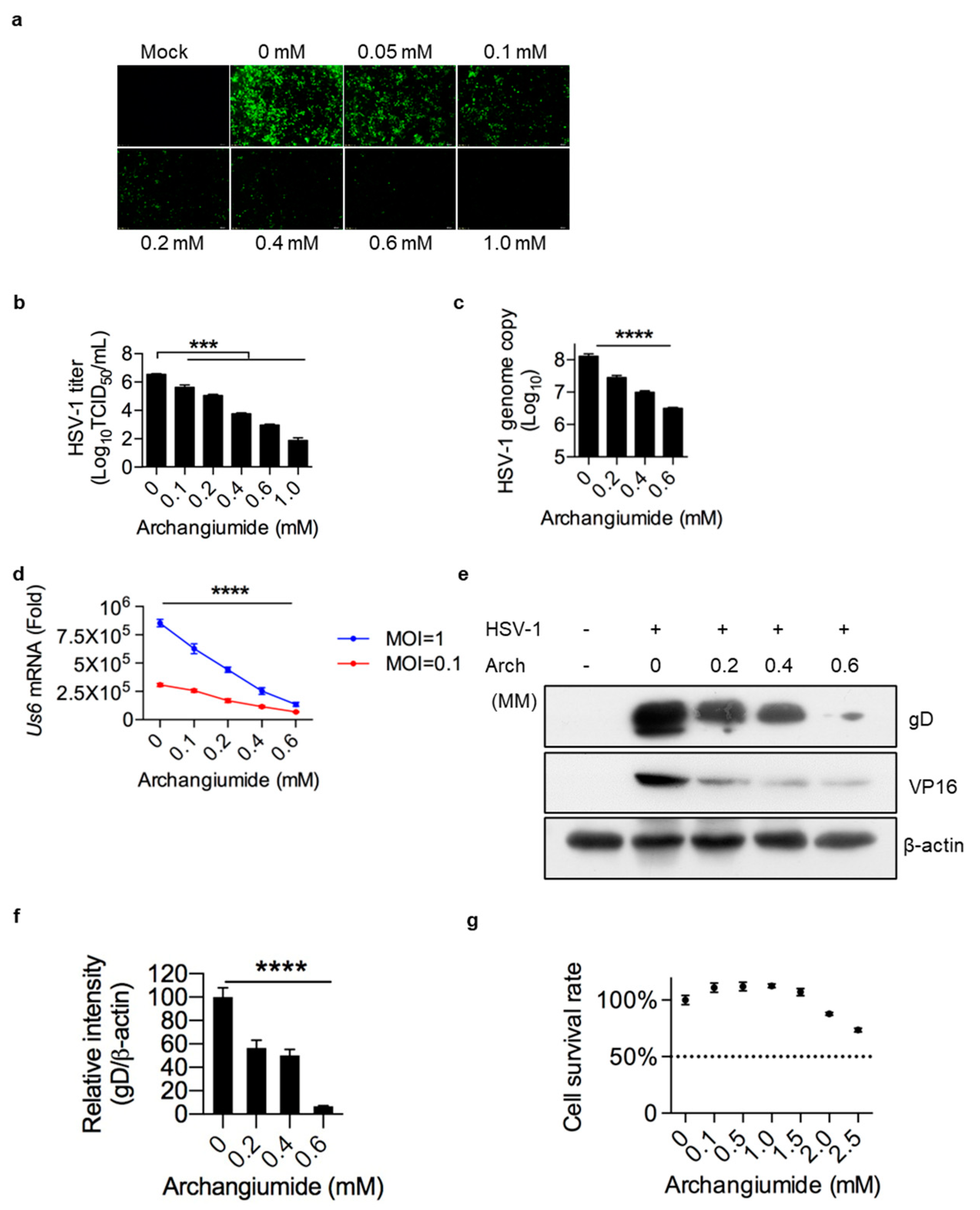
2.1. Archangiumide Significantly Suppressed HSV-1 Replication
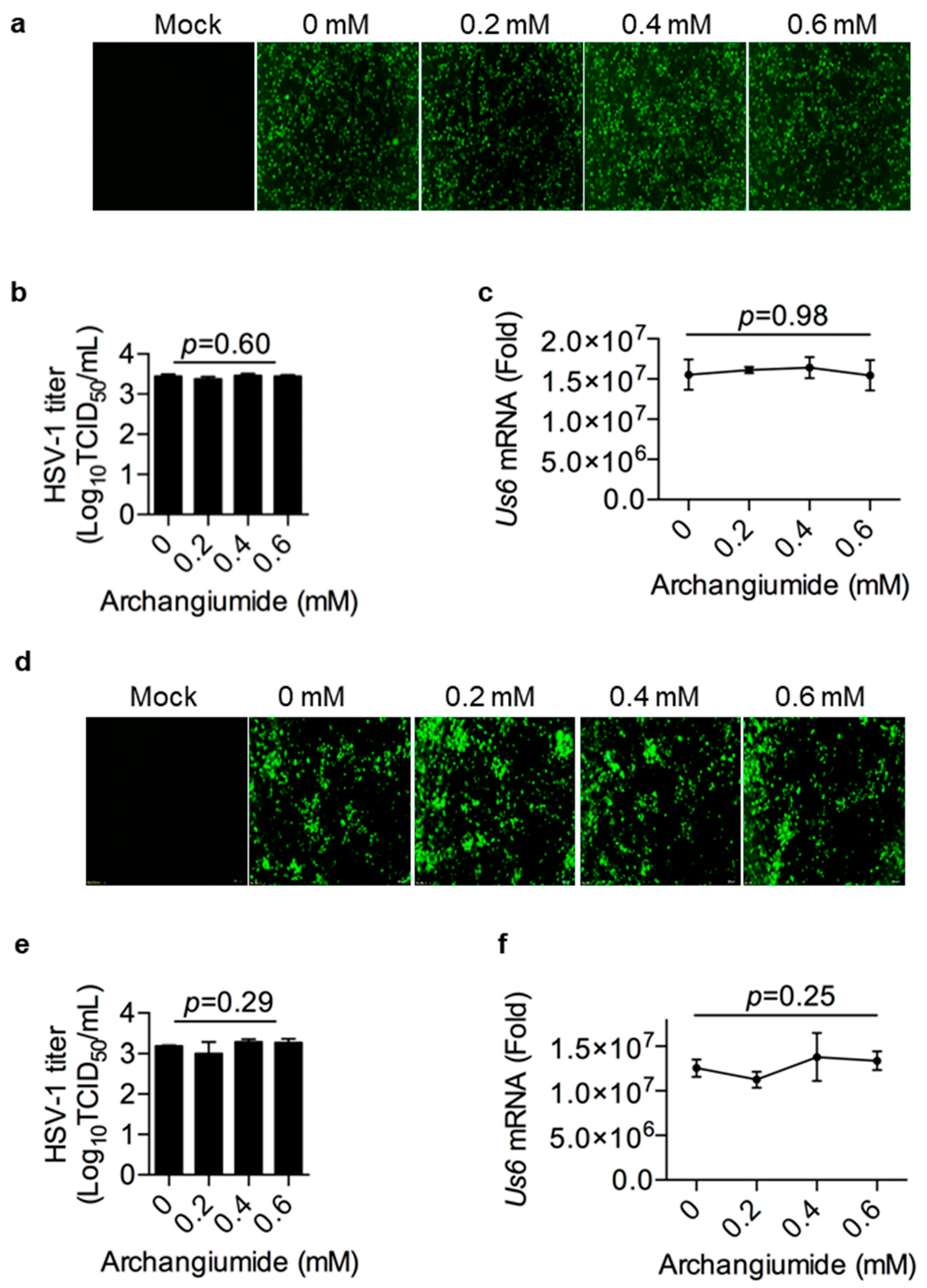
2.2. Archangiumide Was Not Able to Inactivate HSV-1 Directly
2.3. Archangiumide Did Not Block HSV-1’s Attachment to the Cell, nor Penetration
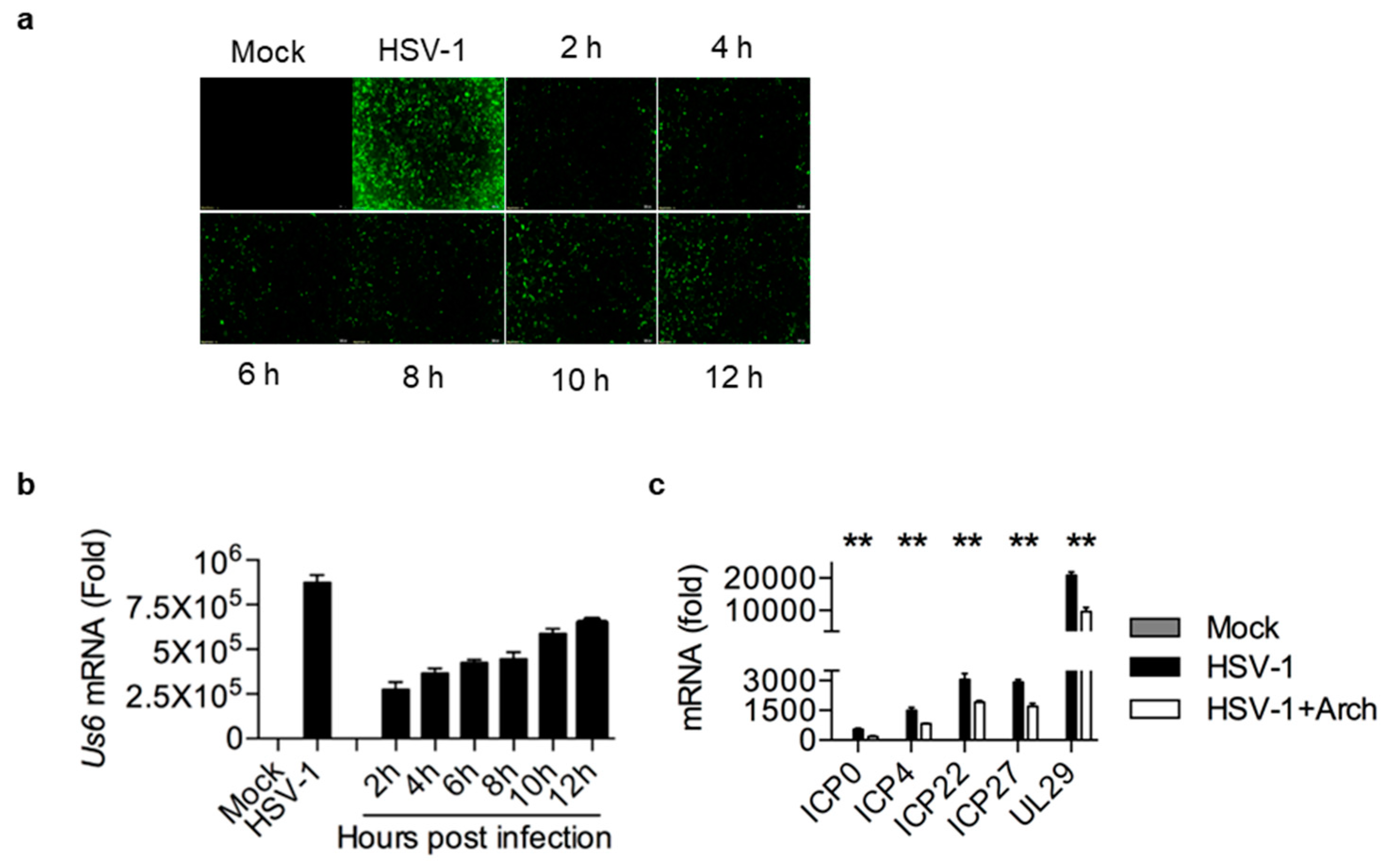
2.4. Archangiumide Suppressed Early Replication of Intracellular HSV-1
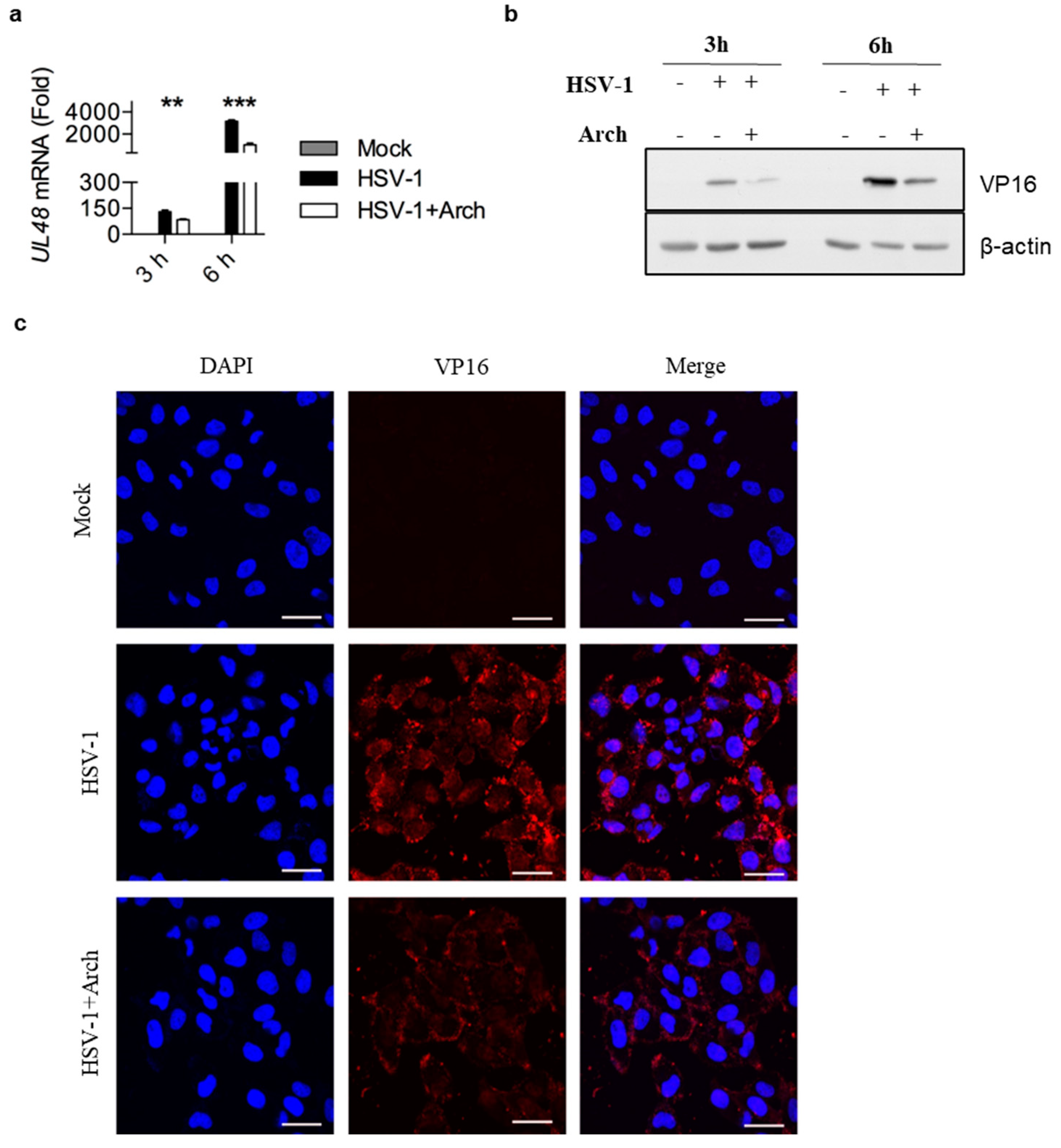
2.5. Archangiumide Blocked VP16 Nuclear Translocation to Suppress Early Replication
3. Discussion
4. Materials and Methods
4.1. Isolation of Archangiumide
4.2. Cells and Virus
4.3. TCID50 and Genome Copy
4.4. Cell Survival Rate
4.5. Real-Time qPCR
4.6. Western Blot
4.7. Immunofluorescence
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dinos, G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.; Martens, E.; Pereira, D. Nature nurtures the design of new semi-synthetic macrolide antibiotics. J. Antibiot. 2017, 70, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.G.; Clark, R.B. Discovery of Macrolide Antibiotics Effective against Multi-Drug Resistant Gram-Negative Pathogens. Acc. Chem. Res. 2021, 54, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jung, S.; Kim, M.; Park, S.; Yang, H.J.; Lee, E. Global Trends in the Proportion of Macrolide-Resistant Mycoplasma pneumoniae Infections: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2220949. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-Q.; Wang, J.-J.; Li, Y.-L.; Zhuo, L.; Zhang, A.; Sui, H.-Y.; Li, X.-J.; Shen, T.; Yin, Y.; Wu, Z.-H.; et al. Combining NMR-Based Metabolic Profiling and Genome Mining for the Accelerated Discovery of Archangiumide, an Allenic Macrolide from the Myxobacterium Archangium violaceum SDU8. Org. Lett. 2021, 23, 2114–2119. [Google Scholar] [CrossRef]
- Sutro, J.L.; Furstner, A. Total Synthesis of the Allenic Macrolide (+)-Archangiumide. J. Am. Chem. Soc. 2024, 146, 2345–2350. [Google Scholar] [CrossRef] [PubMed]
- Batiha GE, S.; Zayed, M.A.; Awad, A.A.; Shaheen, H.M.; Mustapha, S.; Herrera-Calderon, O.; Pagnossa, J.P.; Algammal, A.M.; Zahoor, M.; Adhikari, A.; et al. Management of SARS-CoV-2 Infection: Key Focus in Macrolides Efficacy for COVID-19. Front. Med. 2021, 8, 642313. [Google Scholar] [CrossRef] [PubMed]
- Pani, A.; Lauriola, M.; Romandini, A.; Scaglione, F. Macrolides and viral infections: Focus on azithromycin in COVID-19 pathology. Int. J. Antimicrob. Agents 2020, 56, 106053. [Google Scholar] [CrossRef]
- Khoshnood, S.; Shirani, M.; Dalir, A.; Moradi, M.; Haddadi, M.H.; Sadeghifard, N.; Birjandi, F.S.; Yashmi, I.; Heidary, M. Antiviral effects of azithromycin: A narrative review. Biomed. Pharmacother. 2022, 147, 112682. [Google Scholar] [CrossRef] [PubMed]
- Shushni, M.A.M.; Singh, R.; Mentel, R.; Lindequist, U. Balticolid: A new 12-membered macrolide with antiviral activity from an ascomycetous fungus of marine origin. Mar. Drugs 2011, 9, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Amin, I.; Vajeeha, A.; Younas, S.; Afzal, S.; Shahid, M.; Nawaz, R.; Khan, M.U.; Idrees, M. HSV-1 Infection: Role of Viral Proteins and Cellular Receptors. Crit. Rev. Eukaryot. Gene Expr. 2019, 29, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and virulence of herpes simplex virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Wilson, D.W. HSV-1 Cytoplasmic Envelopment and Egress. Int. J. Mol. Sci. 2020, 21, 5969. [Google Scholar] [CrossRef] [PubMed]
- Treml, J.; Gazdova, M.; Smejkal, K.; Sudomova, M.; Kubatka, P.; Hassan, S.T.S. Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development. Viruses 2020, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Geng, S.; Suo, Y.; Wei, X.; Cai, Q.; Wu, B.; Zhou, X.; Shi, Y.; Wang, B. Critical Role of Regulatory T Cells in the Latency and Stress-Induced Reactivation of HSV-1. Cell Rep. 2018, 25, 2379–2389.e2373. [Google Scholar] [CrossRef] [PubMed]
- Pietila, M.K.; Bachmann, J.J.; Ravantti, J.; Pelkmans, L.; Fraefel, C. Cellular state landscape and herpes simplex virus type 1 infection progression are connected. Nat. Commun. 2023, 14, 4515. [Google Scholar] [CrossRef]
- Reske, A.; Pollara, G.; Krummenacher, C.; Chain, B.M.; Katz, D.R. Understanding HSV-1 entry glycoproteins. Rev. Med. Virol. 2007, 17, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Wysocka, J.; Herr, W. The herpes simplex virus VP16-induced complex: The makings of a regulatory switch. Trends Biochem. Sci. 2003, 28, 294–304. [Google Scholar] [CrossRef]
- Herrera, F.J.; Triezenberg, S.J. VP16-dependent association of chromatin-modifying coactivators and underrepresentation of histones at immediate-early gene promoters during herpes simplex virus infection. J. Virol. 2004, 78, 9689–9696. [Google Scholar] [CrossRef] [PubMed]
- Triezenberg, S.J.; Kingsbury, R.C.; McKnight, S.L. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 1988, 2, 718–729. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.; Qi, X.; Zhao, K.; Pang, X.; Lin, X.; Liao, S.; Yang, B.; Zhou, X.; Liu, S.; et al. p-Terphenyls as Anti-HSV-1/2 Agents from a Deep-Sea-Derived Penicillium sp. J. Nat. Prod. 2021, 84, 2822–2831. [Google Scholar] [CrossRef] [PubMed]
- Benassi-Zanqueta, E.; Marques, C.F.; Valone, L.M.; Pellegrini, B.L.; Bauermeister, A.; Ferreira, I.C.P.; Lopes, N.P.; Nakamura, C.V.; Filho, B.P.D.; Natali, M.R.M.; et al. Evaluation of anti-HSV-1 activity and toxicity of hydroethanolic extract of Tanacetum parthenium (L.) Sch.Bip. (Asteraceae). Phytomedicine 2019, 55, 249–254. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hu, J.-Q.; Feng, W.-H.; Wu, C.; Gao, L. Early Intervention in Herpes Simplex Virus-1 Replication in Vitro with Allenic Macrolide Archangiumide. Int. J. Mol. Sci. 2025, 26, 1537. https://doi.org/10.3390/ijms26041537
Li Y, Hu J-Q, Feng W-H, Wu C, Gao L. Early Intervention in Herpes Simplex Virus-1 Replication in Vitro with Allenic Macrolide Archangiumide. International Journal of Molecular Sciences. 2025; 26(4):1537. https://doi.org/10.3390/ijms26041537
Chicago/Turabian StyleLi, You, Jia-Qi Hu, Wen-Hai Feng, Changsheng Wu, and Li Gao. 2025. "Early Intervention in Herpes Simplex Virus-1 Replication in Vitro with Allenic Macrolide Archangiumide" International Journal of Molecular Sciences 26, no. 4: 1537. https://doi.org/10.3390/ijms26041537
APA StyleLi, Y., Hu, J.-Q., Feng, W.-H., Wu, C., & Gao, L. (2025). Early Intervention in Herpes Simplex Virus-1 Replication in Vitro with Allenic Macrolide Archangiumide. International Journal of Molecular Sciences, 26(4), 1537. https://doi.org/10.3390/ijms26041537





