Characteristics of the First Domestic Duck-Origin H12N8 Avian Influenza Virus in China
Abstract
1. Introduction
2. Results
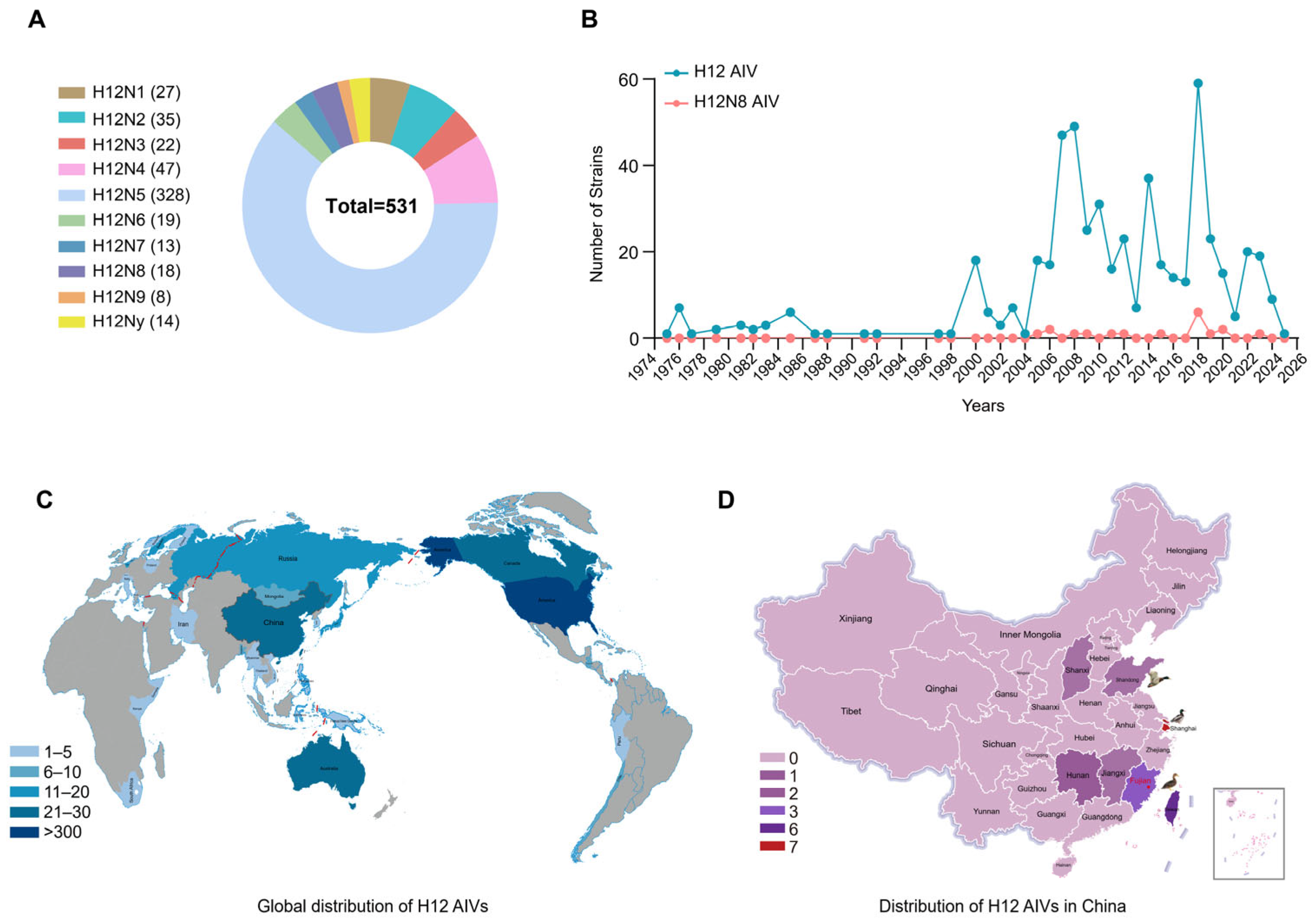
2.1. Global Prevalence of H12 Subtype Viruses
2.2. Isolation and Molecular Characteristics of a Duck-Origin H12N8 Virus
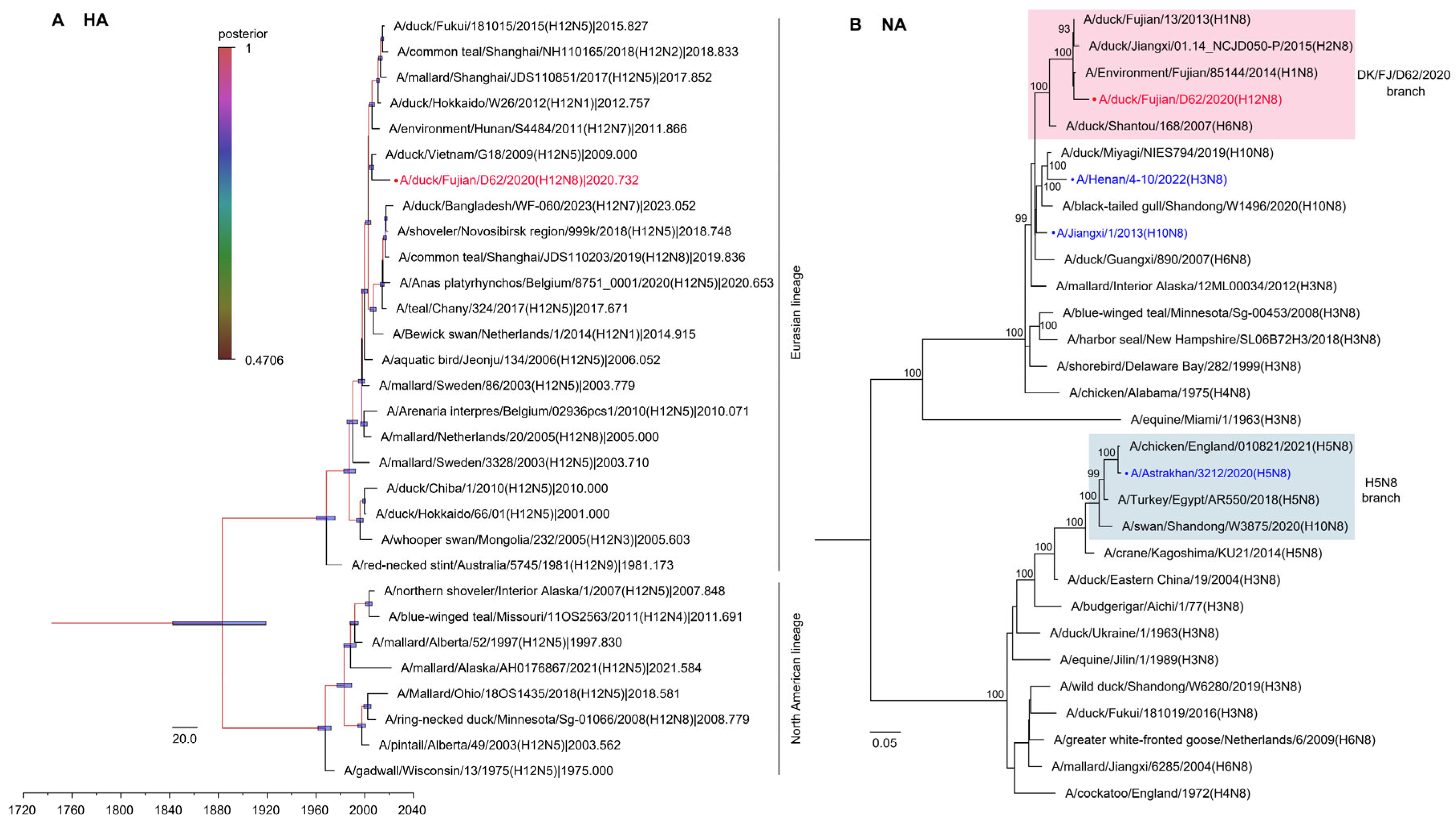
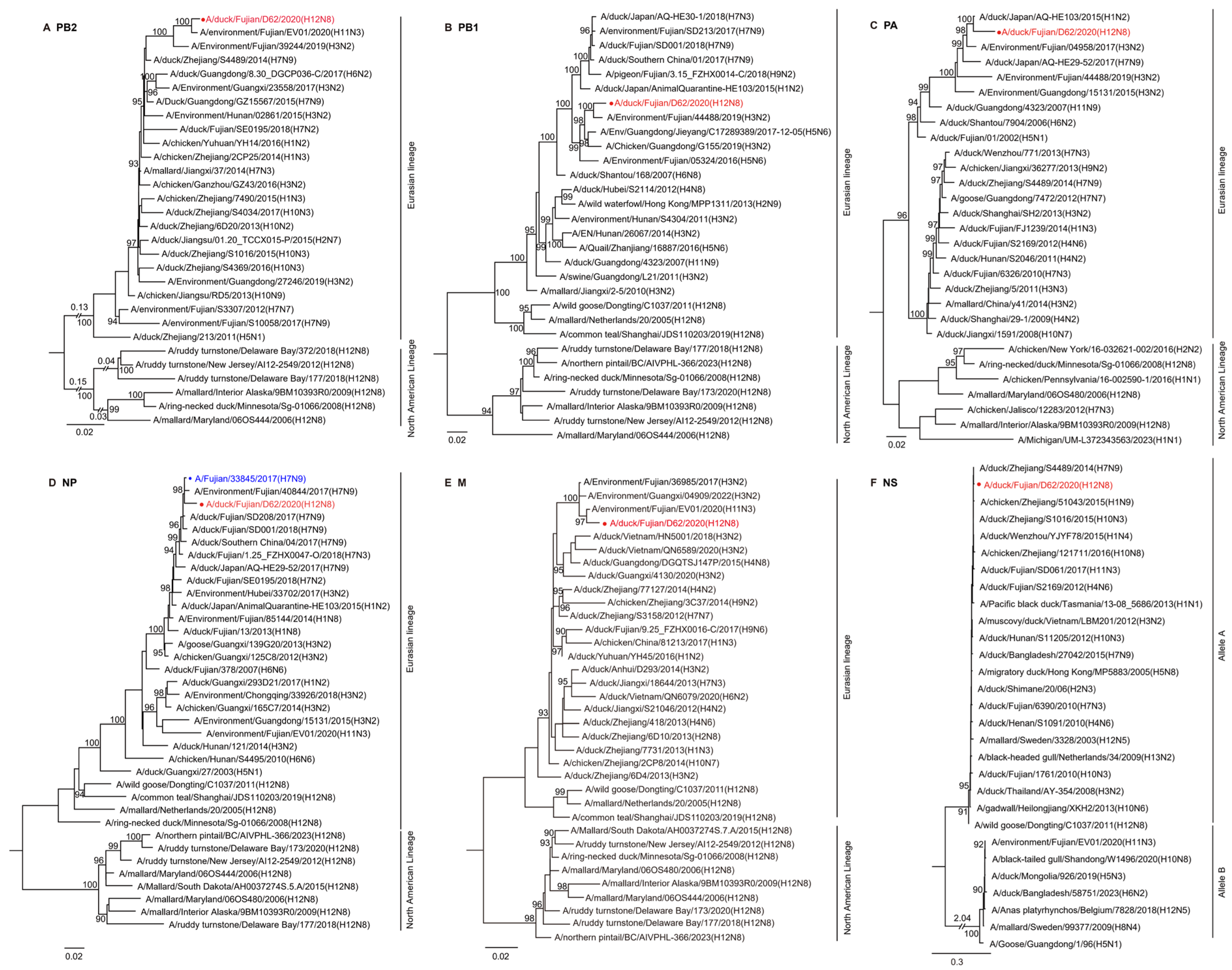
2.3. Phylogenetic Analysis of the H12N8 Virus
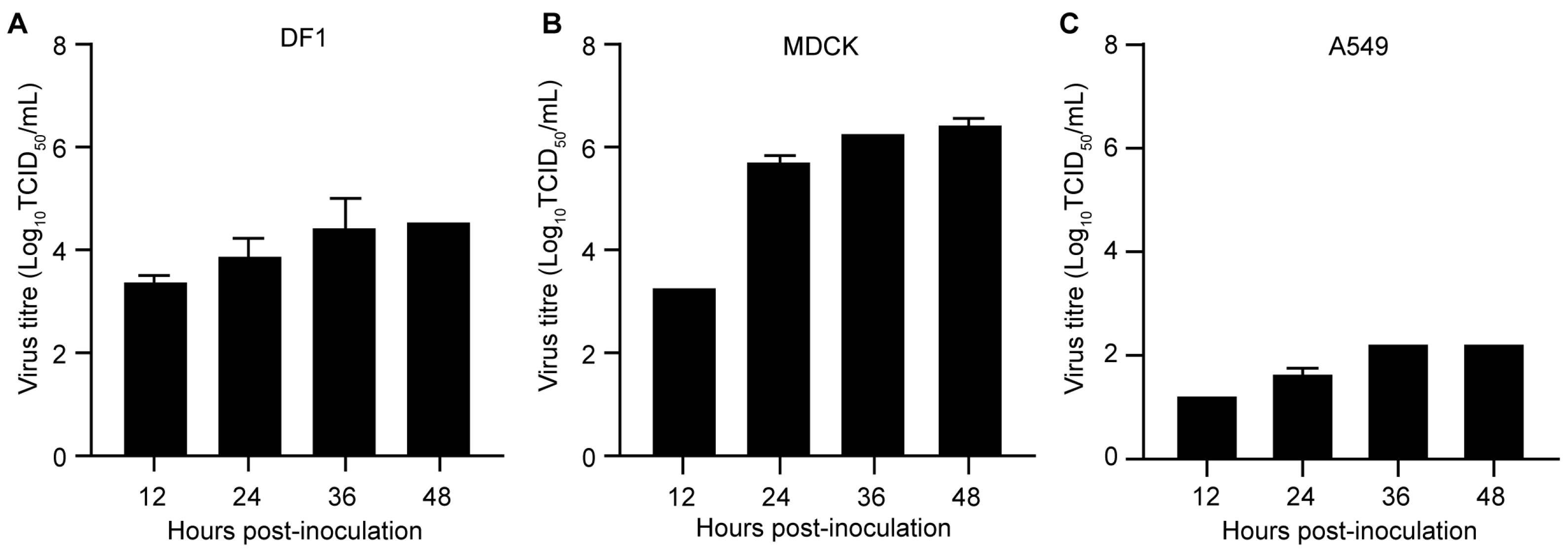
2.4. Replication of the H12N8 Virus in the Mammalian and Avian Cells
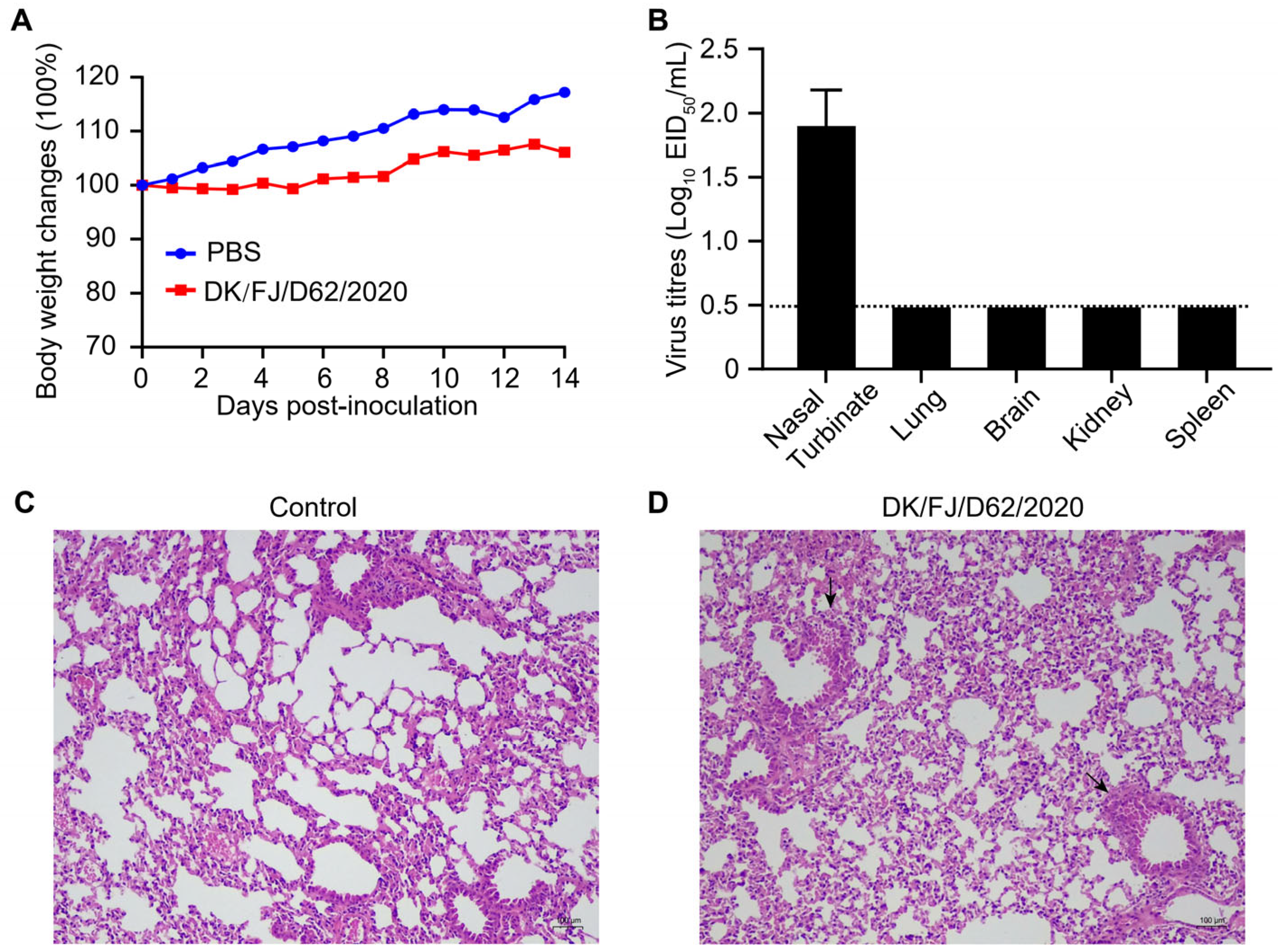
2.5. Replication and Pathogenicity of the H12N8 Virus in Mice
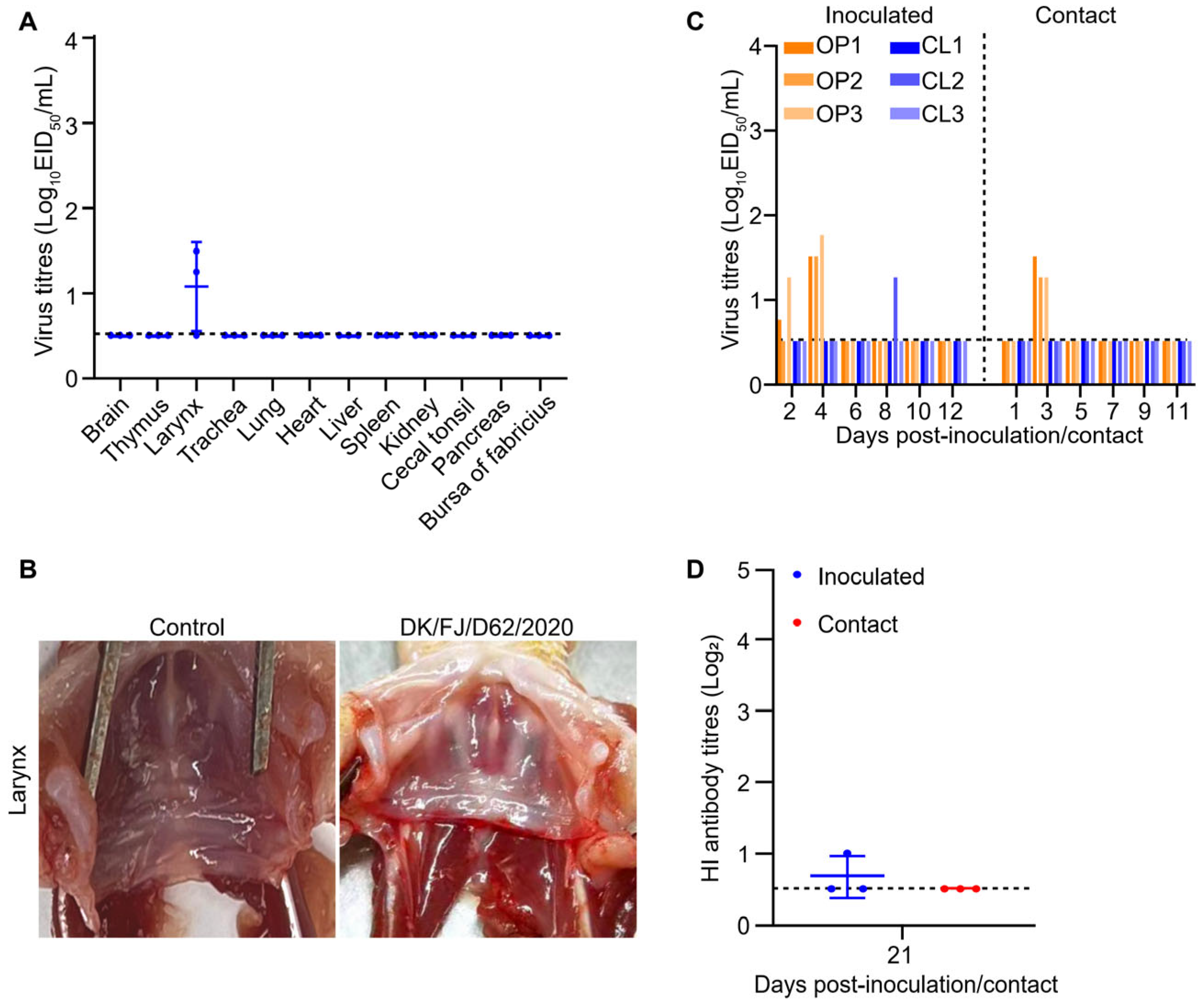
2.6. Replication and Transmission of H12N8 Virus in Ducks
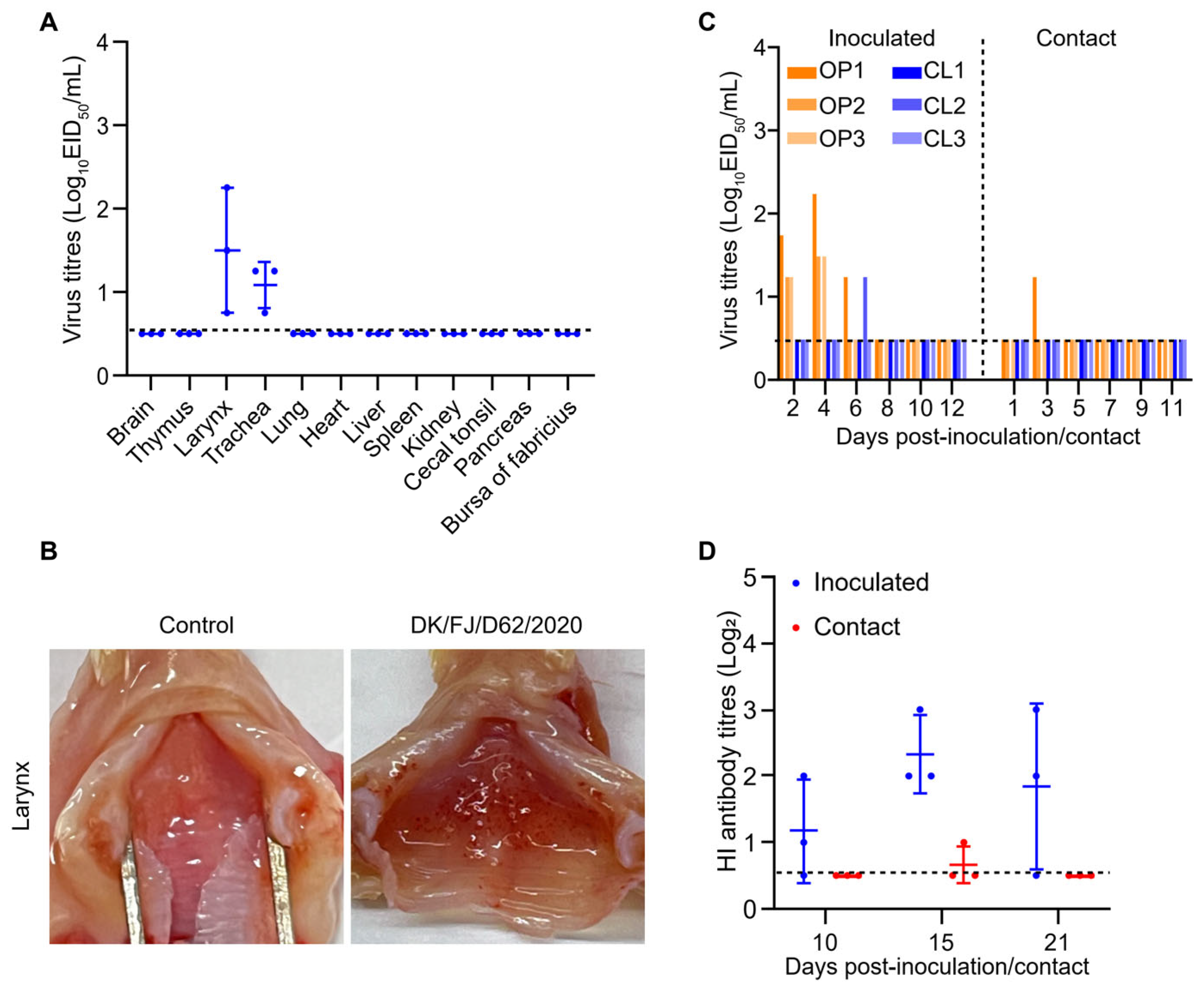
2.7. Replication and Transmission of the H12N8 Virus in Chickens
3. Discussion
4. Materials and Methods
4.1. Ethics Statements and Facility
4.2. Cells, Eggs, and Animals
4.3. Sample Collection and Virus Isolation
4.4. Sequencing and Phylogenetic Analyses of the H12N8 Virus
4.5. Virus Replication Curves in Cells
4.6. Mouse Study
4.7. Duck Study
4.8. Chicken Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tong, S.; Li, Y.; Rivailler, P.; Conrardy, C.; Castillo, D.A.; Chen, L.M.; Recuenco, S.; Ellison, J.A.; Davis, C.T.; York, I.A.; et al. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. USA 2012, 109, 4269–4274. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Zhu, X.; Li, Y.; Shi, M.; Zhang, J.; Bourgeois, M.; Yang, H.; Chen, X.; Recuenco, S.; Gomez, J.; et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013, 9, e1003657. [Google Scholar] [CrossRef] [PubMed]
- Fereidouni, S.; Starick, E.; Karamendin, K.; Genova, C.D.; Scott, S.D.; Khan, Y.; Harder, T.; Kydyrmanov, A. Genetic characterization of a new candidate hemagglutinin subtype of influenza A viruses. Emerg. Microbes Infect. 2023, 12, 2225645. [Google Scholar] [CrossRef] [PubMed]
- Karakus, U.; Mena, I.; Kottur, J.; El Zahed, S.S.; Seoane, R.; Yildiz, S.; Chen, L.; Plancarte, M.; Lindsay, L.; Halpin, R.; et al. H19 influenza A virus exhibits species-specific MHC class II receptor usage. Cell Host Microbe 2024, 32, 1089–1102.e1010. [Google Scholar] [CrossRef]
- Bi, Y.; Chen, Q.; Wang, Q.; Chen, J.; Jin, T.; Wong, G.; Quan, C.; Liu, J.; Wu, J.; Yin, R.; et al. Genesis, Evolution and Prevalence of H5N6 Avian Influenza Viruses in China. Cell Host Microbe 2016, 20, 810–821. [Google Scholar] [CrossRef]
- Cui, P.; Deng, G.; Shi, J.; Kong, H.; Liu, L.; Guan, Y.; Suzuki, Y.; Chen, H. New influenza A(H7N7) viruses detected in live poultry markets in China. Virology 2016, 499, 165–169. [Google Scholar] [CrossRef]
- Cui, P.; Shi, J.; Wang, C.; Zhang, Y.; Xing, X.; Kong, H.; Yan, C.; Zeng, X.; Liu, L.; Tian, G.; et al. Global dissemination of H5N1 influenza viruses bearing the clade 2.3.4.4b HA gene and biologic analysis of the ones detected in China. Emerg. Microbes Infect. 2022, 11, 1693–1704. [Google Scholar] [CrossRef]
- Gu, W.; Shi, J.; Cui, P.; Yan, C.; Zhang, Y.; Wang, C.; Zhang, Y.; Xing, X.; Zeng, X.; Liu, L.; et al. Novel H5N6 reassortants bearing the clade 2.3.4.4b HA gene of H5N8 virus have been detected in poultry and caused multiple human infections in China. Emerg. Microbes Infect. 2022, 11, 1174–1185. [Google Scholar] [CrossRef]
- Lewis, N.S.; Banyard, A.C.; Whittard, E.; Karibayev, T.; Al Kafagi, T.; Chvala, I.; Byrne, A.; Meruyert Akberovna, S.; King, J.; Harder, T.; et al. Emergence and spread of novel H5N8, H5N5 and H5N1 clade 2.3.4.4 highly pathogenic avian influenza in 2020. Emerg. Microbes Infect. 2021, 10, 148–151. [Google Scholar] [CrossRef]
- Shi, J.; Deng, G.; Kong, H.; Gu, C.; Ma, S.; Yin, X.; Zeng, X.; Cui, P.; Chen, Y.; Yang, H.; et al. H7N9 virulent mutants detected in chickens in China pose an increased threat to humans. Cell Res. 2017, 27, 1409–1421. [Google Scholar] [CrossRef]
- Shi, J.; Deng, G.; Ma, S.; Zeng, X.; Yin, X.; Li, M.; Zhang, B.; Cui, P.; Chen, Y.; Yang, H.; et al. Rapid Evolution of H7N9 Highly Pathogenic Viruses that Emerged in China in 2017. Cell Host Microbe 2018, 24, 558–568.e557. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zeng, X.; Cui, P.; Yan, C.; Chen, H. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerg. Microbes Infect. 2023, 12, 2155072. [Google Scholar] [CrossRef] [PubMed]
- Voronina, O.L.; Ryzhova, N.N.; Aksenova, E.I.; Kunda, M.S.; Sharapova, N.E.; Fedyakina, I.T.; Chvala, I.A.; Borisevich, S.V.; Logunov, D.Y.; Gintsburg, A.L. Genetic features of highly pathogenic avian influenza viruses A(H5N8), isolated from the European part of the Russian Federation. Infect. Genet. Evol. 2018, 63, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Du, F.; Xiao, L.; Sun, H.; Lu, L.; Lei, W.; Zheng, J.; Wang, L.; Shu, S.; Li, Y.; et al. Spatiotemporal genotype replacement of H5N8 avian influenza viruses contributed to H5N1 emergence in 2021/2022 panzootic. J. Virol. 2024, 98, e0140123. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, J.; Zeng, X.; Shi, J.; Deng, G.; Zhang, Y.; Wang, Y.; Ma, Q.; Gao, X.; Cui, P.; et al. Novel H7N7 avian influenza viruses detected in migratory wild birds in eastern China between 2018 and 2020. Microbes Infect. 2022, 24, 105013. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, H.; Gao, R.; Zhang, J.; Wang, D.; Xiong, Y.; Fan, G.; Yang, F.; Li, X.; Zhou, J.; et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: A descriptive study. Lancet 2014, 383, 714–721. [Google Scholar] [CrossRef]
- Yang, R.; Sun, H.; Gao, F.; Luo, K.; Huang, Z.; Tong, Q.; Song, H.; Han, Q.; Liu, J.; Lan, Y.; et al. Human infection of avian influenza A H3N8 virus and the viral origins: A descriptive study. Lancet Microbe 2022, 3, e824–e834. [Google Scholar] [CrossRef]
- Shi, J.; Deng, G.; Liu, P.; Zhou, J.; Guan, L.; Li, W.; Li, X.; Guo, J.; Wang, G.; Fan, J.; et al. Isolation and characterization of H7N9 viruses from live poultry markets—Implication of the source of current H7N9 infection in humans. Chin. Sci. Bull. 2013, 58, 1857–1863. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, L.; Luo, Z.; Zou, Y.; Lv, J.; Wang, T. Genomic characteristics and phylogenetic analysis of the first H12N2 influenza A virus identified from wild birds, China. Acta Virol. 2020, 64, 104–110. [Google Scholar] [CrossRef]
- Sharshov, K.; Mine, J.; Sobolev, I.; Kurskaya, O.; Dubovitskiy, N.; Kabilov, M.; Alikina, T.; Nakayama, M.; Tsunekuni, R.; Derko, A.; et al. Characterization and Phylodynamics of Reassortant H12Nx Viruses in Northern Eurasia. Microorganisms 2019, 7, 643. [Google Scholar] [CrossRef]
- Tang, L.; Tang, W.; Ming, L.; Gu, J.; Qian, K.; Li, X.; Wang, T.; He, G. Characterization of Avian Influenza Virus H10-H12 Subtypes Isolated from Wild Birds in Shanghai, China from 2016 to 2019. Viruses 2020, 12, 1085. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Kan, Q.; He, D.; Zhao, Z.; Gong, J.; Jiang, W.; Tang, T.; Li, Y.; Xie, Q.; Li, T.; et al. Phylogeography and Biological Characterizations of H12 Influenza A Viruses. Viruses 2022, 14, 2251. [Google Scholar] [CrossRef] [PubMed]
- Wille, M.; Latorre-Margalef, N.; Tolf, C.; Halpin, R.; Wentworth, D.; Fouchier, R.A.M.; Raghwani, J.; Pybus, O.G.; Olsen, B.; Waldenström, J. Where do all the subtypes go? Temporal dynamics of H8-H12 influenza A viruses in waterfowl. Virus Evol. 2018, 4, vey025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Z.; Yang, H.; Liu, Y.; Li, F.; Wang, L.; Li, X.; Zhu, Y.; Cai, Y.; Bai, Z.; et al. Complete Genome Sequence of an H12N8 Avian Influenza Virus Isolated from Wild Bird Feces in Hunan East Dongting Lake National Nature Reserve. Genome Announc. 2013, 1, e00891-13. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Zhang, H.; Zhao, C.; Zhang, Y.; Shen, J.; Sun, X.; Xu, H.; Xie, Y.; Gao, X.; et al. Prevalence, evolution, replication and transmission of H3N8 avian influenza viruses isolated from migratory birds in eastern China from 2017 to 2021. Emerg. Microbes Infect. 2023, 12, 2184178. [Google Scholar] [CrossRef]
- Lam, T.T.; Wang, J.; Shen, Y.; Zhou, B.; Duan, L.; Cheung, C.L.; Ma, C.; Lycett, S.J.; Leung, C.Y.; Chen, X.; et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature 2013, 502, 241–244. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Li, Z.; Shi, J.; Shinya, K.; Deng, G.; Qi, Q.; Tian, G.; Fan, S.; Zhao, H.; et al. Properties and dissemination of H5N1 viruses isolated during an influenza outbreak in migratory waterfowl in western China. J. Virol. 2006, 80, 5976–5983. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, W.; Yang, L.; Shu, Y. The Epidemiology, Virology, and Pathogenicity of Human Infections with Avian Influenza Viruses. Cold Spring Harb. Perspect. Med. 2021, 11, a038620. [Google Scholar] [CrossRef]
- Sun, H.; Li, H.; Tong, Q.; Han, Q.; Liu, J.; Yu, H.; Song, H.; Qi, J.; Li, J.; Yang, J.; et al. Airborne transmission of human-isolated avian H3N8 influenza virus between ferrets. Cell 2023, 186, 4074–4084.e4011. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, J.; Deng, G.; Guo, J.; Zeng, X.; He, X.; Kong, H.; Gu, C.; Li, X.; Liu, J.; et al. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science 2013, 341, 410–414. [Google Scholar] [CrossRef]
- Li, C.; Chen, H. H7N9 Influenza Virus in China. Cold Spring Harb. Perspect. Med. 2021, 11, a038349. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Deng, G.; Cui, P.; Zeng, X.; Li, B.; Wang, D.; He, X.; Yan, C.; Zhang, Y.; Li, J.; et al. Evolution of H7N9 highly pathogenic avian influenza virus in the context of vaccination. Emerg. Microbes Infect. 2024, 13, 2343912. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Watanabe, T.; Kiso, M.; Nakajima, N.; Yamayoshi, S.; Iwatsuki-Horimoto, K.; Hatta, M.; Yamada, S.; Ito, M.; Sakai-Tagawa, Y.; et al. A Highly Pathogenic Avian H7N9 Influenza Virus Isolated from A Human Is Lethal in Some Ferrets Infected via Respiratory Droplets. Cell Host Microbe 2017, 22, 615–626.e618. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Gu, M.; Liu, D.; Cui, J.; Gao, G.F.; Zhou, J.; Liu, X. Epidemiology, Evolution, and Pathogenesis of H7N9 Influenza Viruses in Five Epidemic Waves since 2013 in China. Trends Microbiol. 2017, 25, 713–728. [Google Scholar] [CrossRef]
- Tian, J.; Bai, X.; Li, M.; Zeng, X.; Xu, J.; Li, P.; Wang, M.; Song, X.; Zhao, Z.; Tian, G.; et al. Highly Pathogenic Avian Influenza Virus (H5N1) Clade 2.3.4.4b Introduced by Wild Birds, China, 2021. Emerg. Infect. Dis. 2023, 29, 1367–1375. [Google Scholar] [CrossRef]
- Burrough, E.R.; Magstadt, D.R.; Petersen, B.; Timmermans, S.J.; Gauger, P.C.; Zhang, J.; Siepker, C.; Mainenti, M.; Li, G.; Thompson, A.C.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024. Emerg. Infect. Dis. 2024, 30, 1335–1343. [Google Scholar] [CrossRef]
- Caserta, L.C.; Frye, E.A.; Butt, S.L.; Laverack, M.; Nooruzzaman, M.; Covaleda, L.M.; Thompson, A.C.; Koscielny, M.P.; Cronk, B.; Johnson, A.; et al. Spillover of highly pathogenic avian influenza H5N1 virus to dairy cattle. Nature 2024, 634, 669–676. [Google Scholar] [CrossRef]
- Guan, L.; Eisfeld, A.J.; Pattinson, D.; Gu, C.; Biswas, A.; Maemura, T.; Trifkovic, S.; Babujee, L.; Presler, R., Jr.; Dahn, R.; et al. Cow’s Milk Containing Avian Influenza A(H5N1) Virus—Heat Inactivation and Infectivity in Mice. N. Engl. J. Med. 2024, 391, 87–90. [Google Scholar] [CrossRef]
- Hu, X.; Saxena, A.; Magstadt, D.R.; Gauger, P.C.; Burrough, E.R.; Zhang, J.; Siepker, C.; Mainenti, M.; Gorden, P.J.; Plummer, P.J.; et al. Genomic characterization of highly pathogenic avian influenza A H5N1 virus newly emerged in dairy cattle. Emerg. Microbes Infect. 2024, 13, 2380421. [Google Scholar] [CrossRef]
- Ly, H. Highly pathogenic avian influenza H5N1 virus infections of dairy cattle and livestock handlers in the United States of America. Virulence 2024, 15, 2343931. [Google Scholar] [CrossRef]
- Nelli, R.K.; Harm, T.A.; Siepker, C.; Groeltz-Thrush, J.M.; Jones, B.; Twu, N.C.; Nenninger, A.S.; Magstadt, D.R.; Burrough, E.R.; Piñeyro, P.E.; et al. Sialic Acid Receptor Specificity in Mammary Gland of Dairy Cattle Infected with Highly Pathogenic Avian Influenza A(H5N1) Virus. Emerg. Infect. Dis. 2024, 30, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Oguzie, J.U.; Marushchak, L.V.; Shittu, I.; Lednicky, J.A.; Miller, A.L.; Hao, H.; Nelson, M.I.; Gray, G.C. Avian Influenza A(H5N1) Virus among Dairy Cattle, Texas, USA. Emerg. Infect. Dis. 2024, 30, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, J.; Guo, J.; Deng, G.; Zhang, Q.; Wang, J.; He, X.; Wang, K.; Chen, J.; Li, Y.; et al. Genetics, receptor binding property, and transmissibility in mammals of naturally isolated H9N2 Avian Influenza viruses. PLoS Pathog. 2014, 10, e1004508. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhang, B.; Shi, J.; Yin, X.; Wang, G.; Cui, P.; Liu, L.; Deng, G.; Jiang, Y.; Li, C.; et al. Amino Acid Mutations A286V and T437M in the Nucleoprotein Attenuate H7N9 Viruses in Mice. J. Virol. 2020, 94, e01530-19. [Google Scholar] [CrossRef]
- Meng, F.; Yang, H.; Qu, Z.; Chen, Y.; Zhang, Y.; Zhang, Y.; Liu, L.; Zeng, X.; Li, C.; Kawaoka, Y.; et al. A Eurasian avian-like H1N1 swine influenza reassortant virus became pathogenic and highly transmissible due to mutations in its PA gene. Proc. Natl. Acad. Sci. USA 2022, 119, e2203919119. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Kong, H.; Jiang, Y.; Gao, Y.; Deng, G.; Shi, J.; Tian, G.; Liu, L.; Liu, J.; et al. H5N1 hybrid viruses bearing 2009/H1N1 virus genes transmit in guinea pigs by respiratory droplet. Science 2013, 340, 1459–1463. [Google Scholar] [CrossRef]
- Thangavel, R.R.; Bouvier, N.M. Animal models for influenza virus pathogenesis, transmission, and immunology. J. Immunol. Methods 2014, 410, 60–79. [Google Scholar] [CrossRef]
- Xing, X.; Shi, J.; Cui, P.; Yan, C.; Zhang, Y.; Zhang, Y.; Wang, C.; Chen, Y.; Zeng, X.; Tian, G.; et al. Evolution and biological characterization of H5N1 influenza viruses bearing the clade 2.3.2.1 hemagglutinin gene. Emerg. Microbes Infect. 2024, 13, 2284294. [Google Scholar] [CrossRef]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Zhao, C.; Gao, X.; Zhang, Y.; Li, J.; Wang, M.; Zhang, H.; Liu, W.; Wang, C.; et al. Molecular characterization, receptor binding property, and replication in chickens and mice of H9N2 avian influenza viruses isolated from chickens, peafowls, and wild birds in eastern China. Emerg. Microbes Infect. 2021, 10, 2098–2112. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
| Protein | Key Amino Acid Mutations | Amino Acid of DK/FJ/D62/2020 |
|---|---|---|
| HA | Q226L | Q |
| G228S | G | |
| NA | R292K | R |
| PB2 | A588V | A |
| G590S | G | |
| Q591K | Q | |
| E627K | E | |
| D701N | D | |
| PB1 | R207K | K |
| I368V | I | |
| H436Y | Y | |
| M677T | T | |
| PA | A515T | T |
| NP | V286A | A |
| N319K | N | |
| M437T | T | |
| M1 | N30D | D |
| T215A | A | |
| M2 | S31N | S |
| NS1 | P42S | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Huang, J.; Zhang, C.; Wang, Y.; Zhang, X.; Liu, S.; Qiang, H.; Wang, H.; Zheng, H.; Zhuang, M.; et al. Characteristics of the First Domestic Duck-Origin H12N8 Avian Influenza Virus in China. Int. J. Mol. Sci. 2025, 26, 2740. https://doi.org/10.3390/ijms26062740
Zhao C, Huang J, Zhang C, Wang Y, Zhang X, Liu S, Qiang H, Wang H, Zheng H, Zhuang M, et al. Characteristics of the First Domestic Duck-Origin H12N8 Avian Influenza Virus in China. International Journal of Molecular Sciences. 2025; 26(6):2740. https://doi.org/10.3390/ijms26062740
Chicago/Turabian StyleZhao, Conghui, Jiacheng Huang, Chunping Zhang, Yang Wang, Xiaoxuan Zhang, Sha Liu, Haoxi Qiang, Huanhuan Wang, Hangyu Zheng, Mingzhi Zhuang, and et al. 2025. "Characteristics of the First Domestic Duck-Origin H12N8 Avian Influenza Virus in China" International Journal of Molecular Sciences 26, no. 6: 2740. https://doi.org/10.3390/ijms26062740
APA StyleZhao, C., Huang, J., Zhang, C., Wang, Y., Zhang, X., Liu, S., Qiang, H., Wang, H., Zheng, H., Zhuang, M., Peng, Y., Chen, F., Zeng, X., Chen, J.-L., & Ma, S. (2025). Characteristics of the First Domestic Duck-Origin H12N8 Avian Influenza Virus in China. International Journal of Molecular Sciences, 26(6), 2740. https://doi.org/10.3390/ijms26062740





