The Transcription Factor CcMYB330 Regulates Capsaicinoid Biosynthesis in Pepper Fruits
Abstract
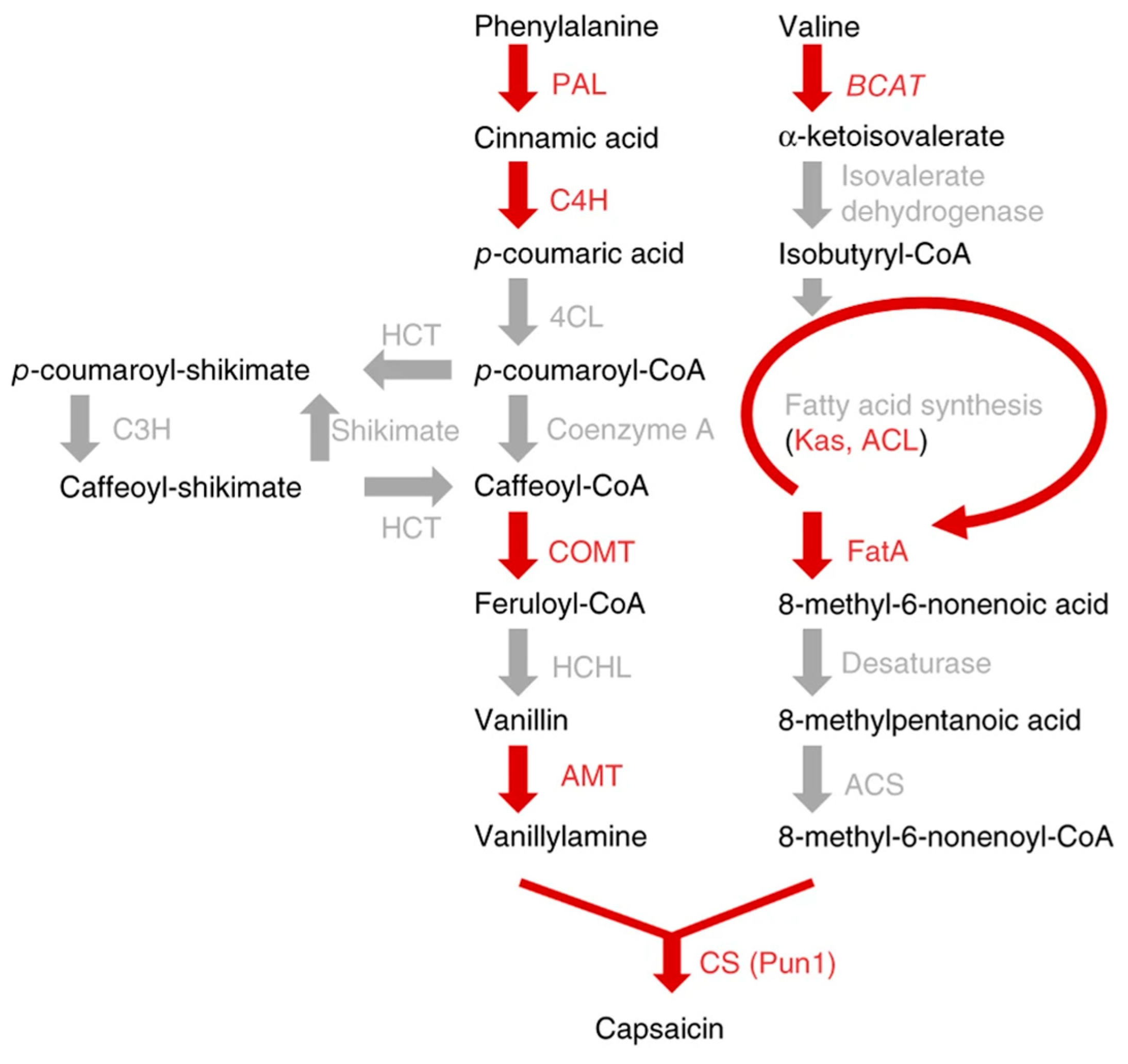
1. Introduction
2. Results
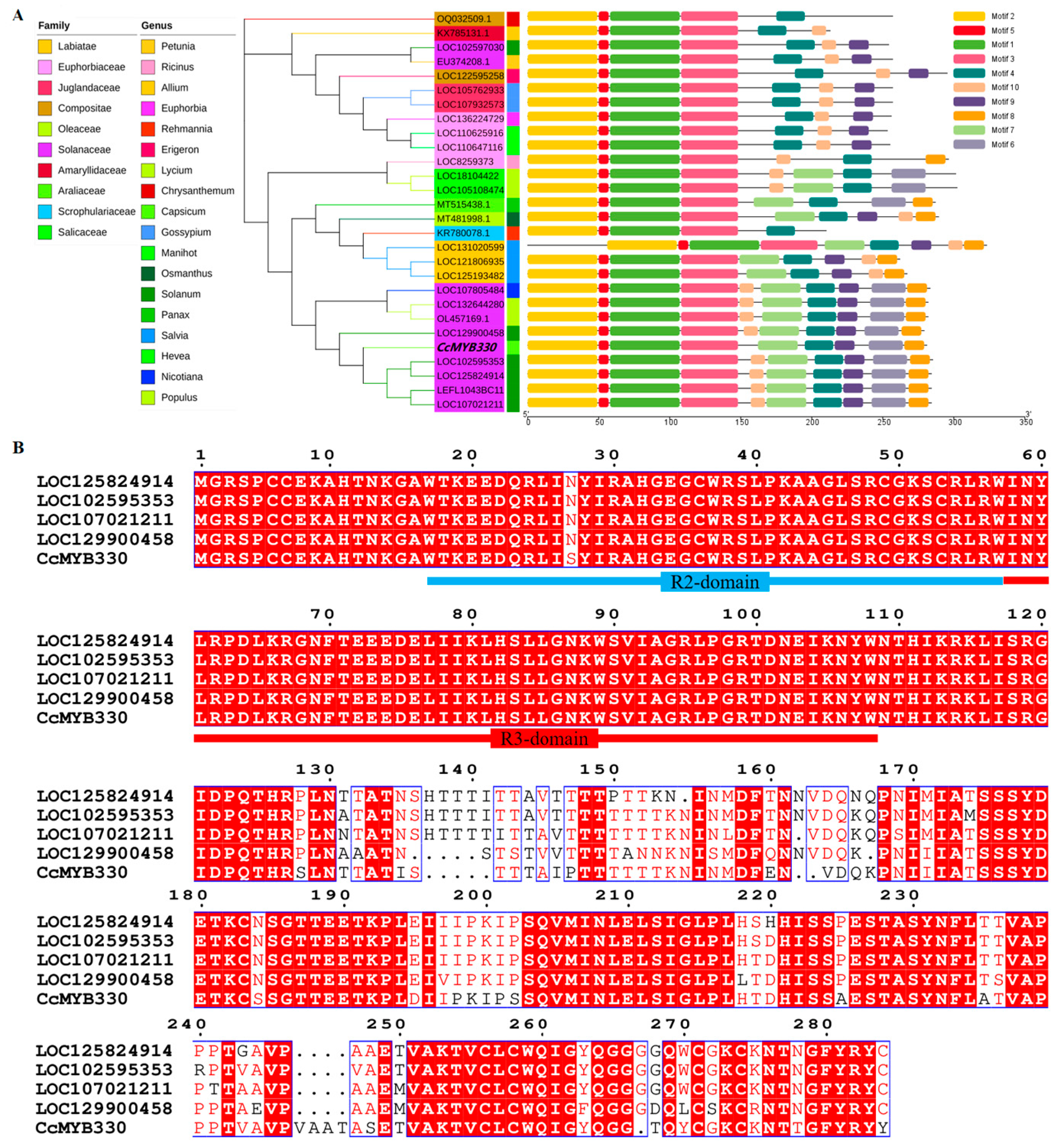
2.1. Cloning and Sequence Analysis of the CcMYB330
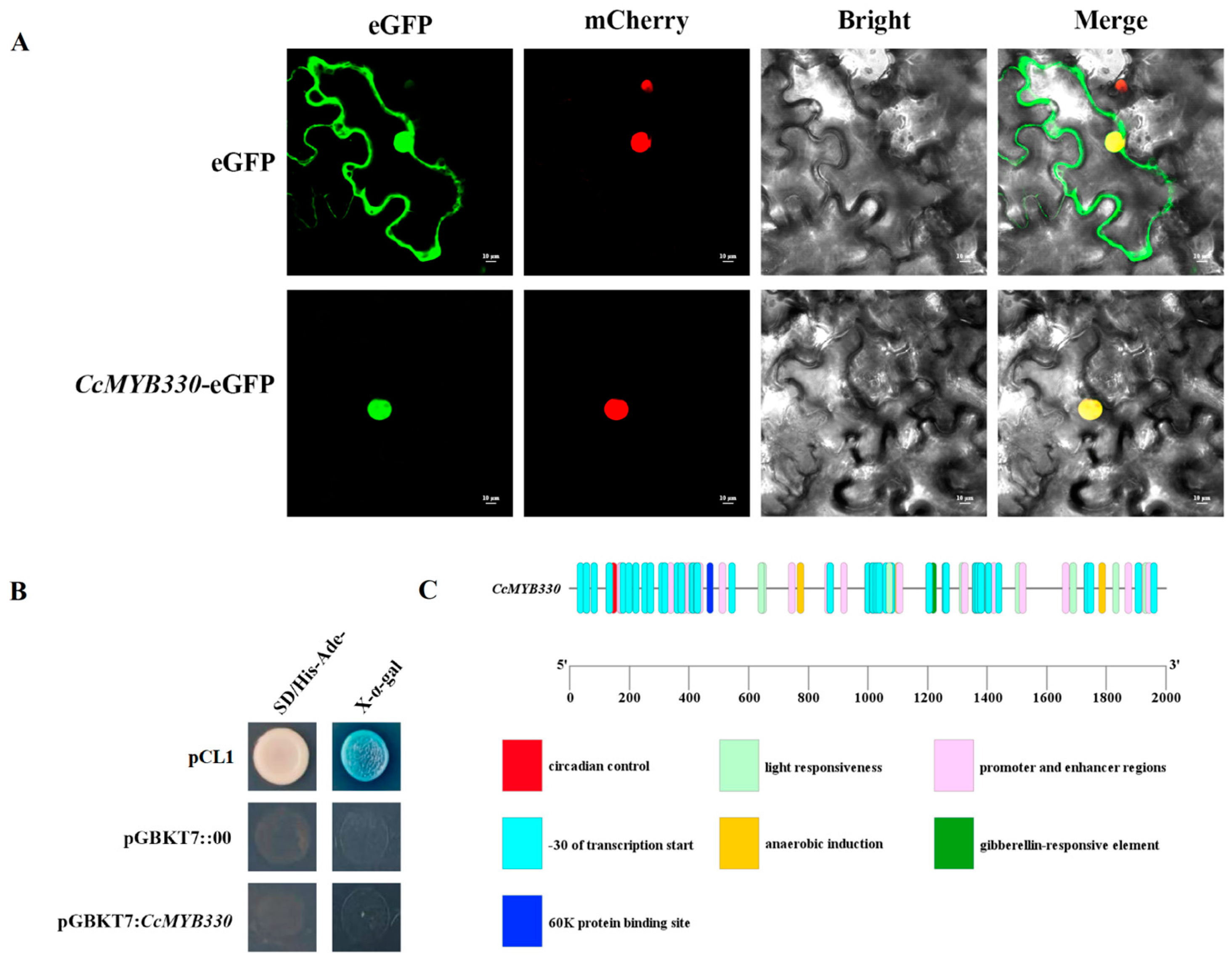
2.2. Characterization of CcMYB330
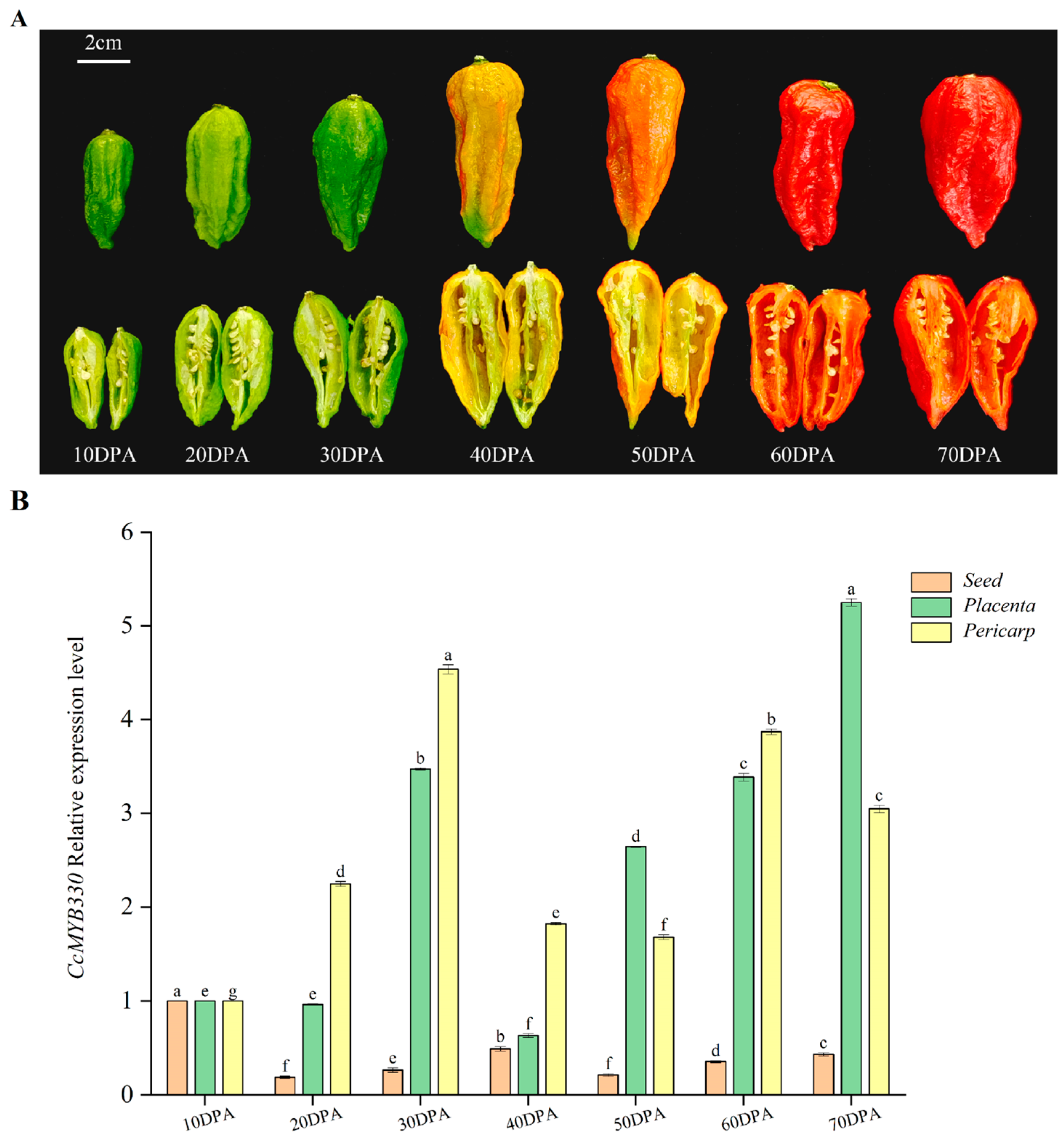
2.3. Expression Analysis of CcMYB330
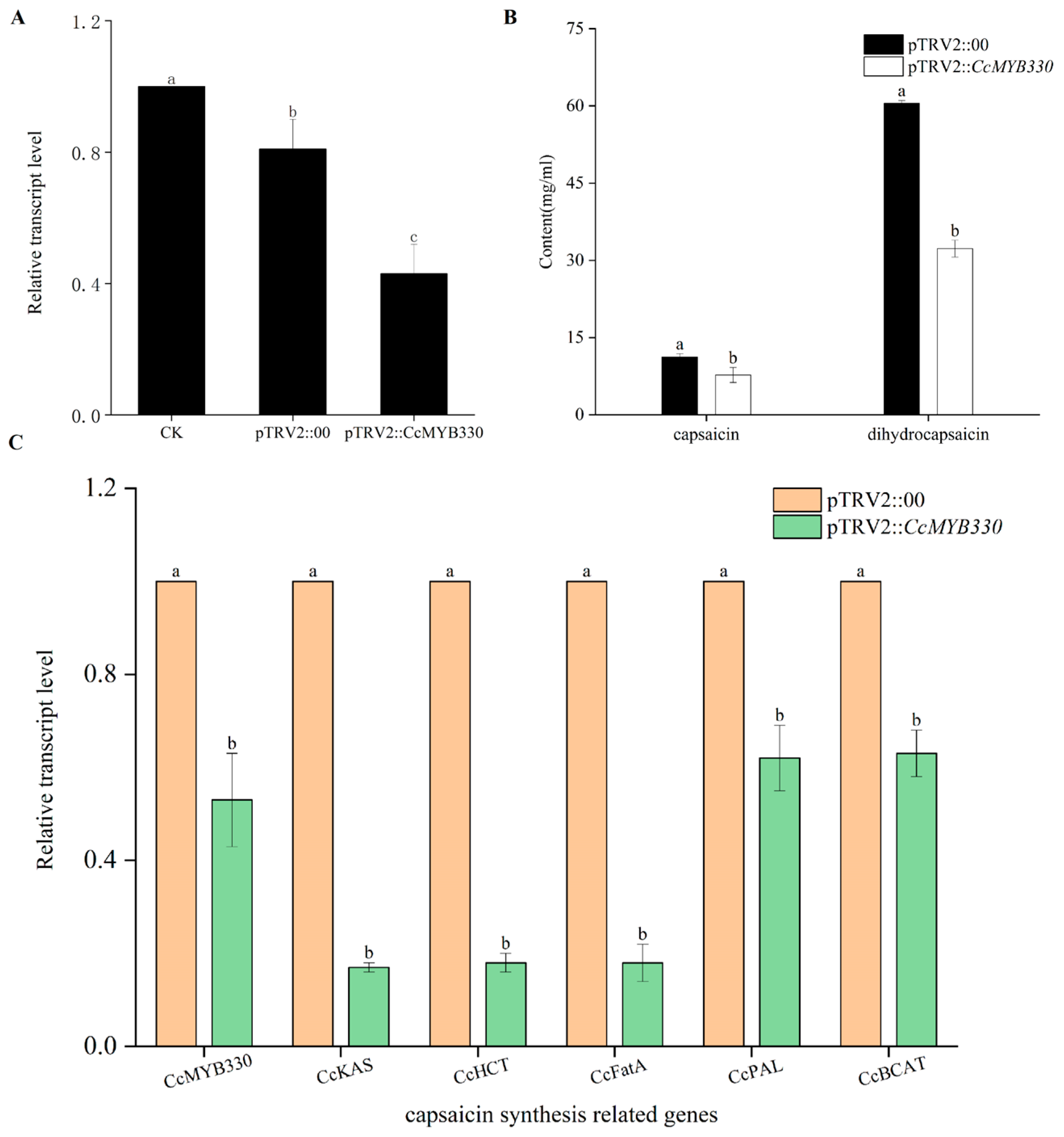
2.4. CcMYB330 Silencing Reduces Capsaicin Accumulation in Pepper Fruits
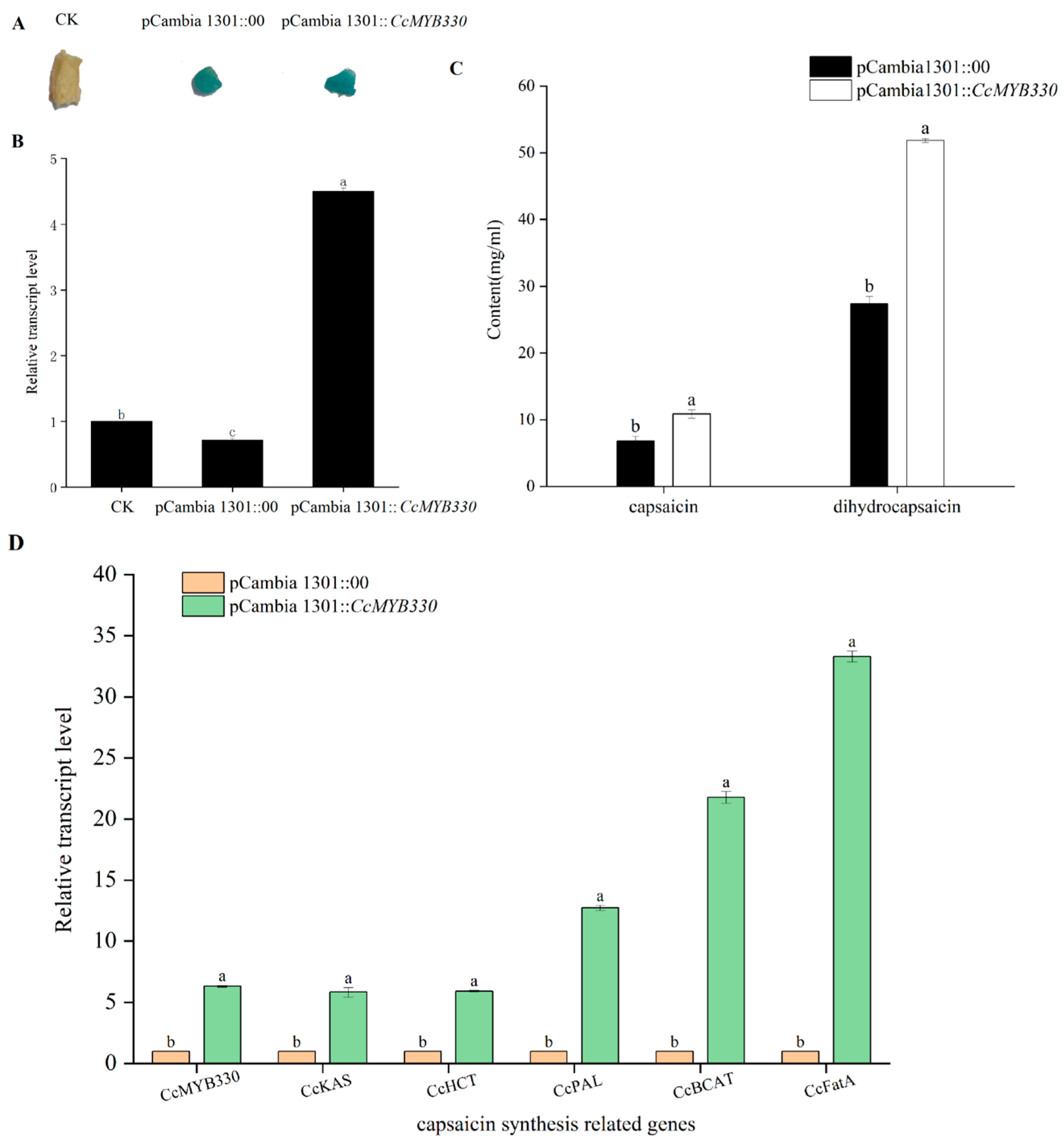
2.5. Transient Overexpression of CcMYB330 Increase Capsaicin Accumulation in Pepper Fruits
2.6. CcMYB330 Binding to the CcPAL Promoter
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Cloning and Sequence Analysis of CcMYB330
4.3. Subcellular Localization
4.4. Transcriptional Activity Analysis of CcMYB330
4.5. Gene Expression Analysis of CcMYB330
4.6. VIGS Identification of CcMYB330 in Pepper
4.7. Transient Overexpression Identification of CcMYB330 in Pepper
4.8. Extraction and Determination of Capsaicinoids
4.9. Yeast One-Hybrid Experiment
4.10. Electrophoretic Mobility Shift Assay (EMSA)
4.11. Dual Luciferase Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, W.; Zhang, L.; Yang, Y.; Xiang, H.; Yang, P. Plant secondary metabolites-mediated plant defense against bacteria and fungi pathogens. Plant Physiol. Biochem. PPB 2024, 217, 109224. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Zhou, C.; Li, X.; Zou, X.; Ou, L.; Tao, Y. The changes of rhizosphere microbial communities in pepper varieties with different capsaicinoids. Front. Microbiol. 2024, 15, 1430682. [Google Scholar] [CrossRef]
- Orzuna-Orzuna, J.F.; Godina-Rodríguez, J.E.; Garay-Martínez, J.R.; Lara-Bueno, A. Capsaicin as a Dietary Additive for Dairy Cows: A Meta-Analysis on Performance, Milk Composition, Digestibility, Rumen Fermentation, and Serum Metabolites. Animals 2024, 14, 1075. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, B.; Mitchell, J.; Major, E.; Podwojniak, A.; Brancaccio, H.; Rusinak, K.; King, M.; Tahir, H. The efficacy of topical 8% capsaicin patches for the treatment of postsurgical neuropathic pain: A systematic review. Pain Manag. 2024, 14, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Andretta, E.; Costa, A.; Ventura, E.; Quintiliani, M.; Damiano, S.; Giordano, A.; Morrione, A.; Ciarcia, R. Capsaicin Exerts Antitumor Activity in Mesothelioma Cells. Nutrients 2024, 16, 3758. [Google Scholar] [CrossRef]
- Wang, J.; Shi, T.; Yang, X.; Han, W.; Zhou, Y. Environmental risk assessment on capsaicin used as active substance for antifouling system on ships. Chemosphere 2014, 104, 85–90. [Google Scholar] [CrossRef]
- Milde, R.; Schnabel, A.; Ditfe, T.; Hoehenwarter, W.; Proksch, C.; Westermann, B.; Vogt, T. Chemical Synthesis of Trans 8-Methyl-6-Nonenoyl-CoA and Functional Expression Unravel Capsaicin Synthase Activity Encoded by the Pun1 Locus. Molecules 2022, 27, 6878. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, M.; Yeom, S.-I.; Kim, Y.-M.; Lee, J.M.; Lee, H.-A.; Seo, E.; Choi, J.; Cheong, K.; Kim, K.-T.; et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat. Genet. 2014, 46, 270–278. [Google Scholar] [CrossRef]
- Han, K.; Jang, S.; Lee, J.H.; Lee, D.G.; Kwon, J.K.; Kang, B.C. A MYB transcription factor is a candidate to control pungency in Capsicum annuum. TAG Theor. Appl. Genet. 2019, 132, 1235–1246. [Google Scholar] [CrossRef]
- Arce-Rodríguez, M.L.; Ochoa-Alejo, N. An R2R3-MYB Transcription Factor Regulates Capsaicinoid Biosynthesis. Plant Physiol. 2017, 174, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhou, X.; Chen, C.; Chen, C.; Chen, K.; Chen, M.; Liu, S.; Chen, G.; Cao, B.; Cao, F.; et al. Coexpression network analysis reveals an MYB transcriptional activator involved in capsaicinoid biosynthesis in hot peppers. Hortic. Res. 2020, 7, 162. [Google Scholar] [CrossRef]
- Wen, J.; Lv, J.; Zhao, K.; Zhang, X.; Li, Z.; Zhang, H.; Huo, J.; Wan, H.; Wang, Z.; Zhu, H.; et al. Ethylene-Inducible AP2/ERF Transcription Factor Involved in the Capsaicinoid Biosynthesis in Capsicum. Front. Plant Sci. 2022, 13, 832669. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Fang, K.; Shan, Q.; He, L.; Dai, X.; Zou, X.; Liu, F. Genome-Wide Analysis of the MYB-Related Transcription Factor Family in Pepper and Functional Studies of CaMYB37 Involvement in Capsaicin Biosynthesis. Int. J. Mol. Sci. 2022, 23, 11667. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, W.; Zhang, L.; Wu, D.; Fu, G.; Yang, M.; Wu, K.; Wu, Z.; Deng, Q.; Zhu, J.; et al. Negative regulation of CcPAL2 gene expression by the repressor transcription factor CcMYB4-12 modulates lignin and capsaicin biosynthesis in Capsicum chinense fruits. Int. J. Biol. Macromol. 2024, 280, 135592. [Google Scholar] [CrossRef]
- Geng, P.; Zhang, S.; Liu, J.; Zhao, C.; Wu, J.; Cao, Y.; Fu, C.; Han, X.; He, H.; Zhao, Q. MYB20, MYB42, MYB43, and MYB85 Regulate Phenylalanine and Lignin Biosynthesis during Secondary Cell Wall Formation. Plant Physiol. 2019, 182, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Duan, A.Q.; Deng, Y.J.; Tan, S.S.; Xu, Z.S.; Xiong, A.S. A MYB activator, DcMYB11c, regulates carrot anthocyanins accumulation in petiole but not taproot. Plant Cell Environ. 2023, 46, 2794–2809. [Google Scholar] [CrossRef]
- Han, Y.; Yang, R.; Zhang, X.; Wang, Q.; Wang, Y.; Li, Y.; Prusky, D.; Bi, Y. MYB24, MYB144, and MYB168 positively regulate suberin biosynthesis at potato tuber wounds during healing. Plant J. 2024, 119, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Wen, J.; Zhu, H.; Zou, X. The hottest pepper variety in China. Genet. Resour. Crop Evol. 2009, 56, 605–608. [Google Scholar] [CrossRef]
- Das, S.; Priyadarshani, N.; Basak, P.; Maitra, P.; Bhattacharya, S.; Bhattacharya, S.S. Capsaicin derived from endemic chili landraces combats Shigella pathogen: Insights on intracellular inhibition mechanism. Microb. Pathogenesis. 2023, 181, 106210. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Mei, T.S.; Yee, W.X.; Hilles, A.R.; Alelwani, W.; Bannunah, A.M. Synthesis of Capsaicin Loaded Silver Nanoparticles Using Green Approach and Its Anti-Bacterial Activity Against Human Pathogens. J. Biomed. Nanotechnol. 2021, 17, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Chabaane, Y.; Arce, C.M.; Glauser, G.; Benrey, B. Altered capsaicin levels in domesticated chili pepper varieties affect the interaction between a generalist herbivore and its ectoparasitoid. J. Pest Sci. 2021, 95, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niu, Y.; Zheng, Y. Multiple Functions of MYB Transcription Factors in Abiotic Stress Responses. Int. J. Mol. Sci. 2021, 22, 6125. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Z.; Khan, S.; Qayyum, A.; Rehman, A.; Du, X. Unveiling the power of MYB transcription factors: Master regulators of multi-stress responses and development in cotton. Int. J. Biol. Macromol. 2024, 276, 133885. [Google Scholar] [CrossRef]
- An, C.; Sheng, L.; Du, X.; Wang, Y.; Zhang, Y.; Song, A.; Jiang, J.; Guan, Z.; Fang, W.; Chen, F.; et al. Overexpression of CmMYB15 provides chrysanthemum resistance to aphids by regulating the biosynthesis of lignin. Hortic. Res. 2019, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Niyitanga, S.; He, Q.; Li, H.; Wei, H.; Ishimwe, C.; Qi, J.; Fang, P.; Xu, J.; Tao, A.; Zhang, L. Genome-wide identification and expression analysis of MYB transcription factors involved in fiber formation in white jute (Corchorus capsularis). Ind. Crops Prod. 2023, 197, 116539. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, X.; Yang, L.; Zhu, X.; Du, Y.; Fang, Z. A R2R3-MYB transcription factor, FeR2R3-MYB, positively regulates anthocyanin biosynthesis and drought tolerance in common buckwheat (Fagopyrum esculentum). Plant Physiol. Biochem. 2024, 217, 109254. [Google Scholar] [CrossRef] [PubMed]
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. MYB transcription factor genes as regulators for plant responses: An overview. Physiol. Mol. Biol. Plants 2013, 19, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhu, Z.; Chen, C.; Chen, G.; Cao, B.; Chen, C.; Lei, J. Jasmonate-Inducible R2R3-MYB Transcription Factor Regulates Capsaicinoid Biosynthesis and Stamen Development in Capsicum. J. Agric. Food Chem. 2019, 67, 4444–4455. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, W.; Zhang, L.; Wu, D.; Sun, P.; Huang, C.; Fu, G.; Deng, Q.; Wang, Z.; Cheng, S. MYB24 Negatively Regulates the Biosynthesis of Lignin and Capsaicin by Affecting the Expression of Key Genes in the Phenylpropanoid Metabolism Pathway in Capsicum chinense. Molecules 2023, 28, 2644. [Google Scholar] [CrossRef]
- Sun, B.; Chen, C.; Song, J.; Zheng, P.; Wang, J.; Wei, J.; Cai, W.; Chen, S.; Cai, Y.; Yuan, Y.; et al. The Capsicum MYB31 regulates capsaicinoid biosynthesis in the pepper pericarp. Plant Physiol. Biochem. PPB 2022, 176, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Jeeatid, N.; Suriharn, B.; Bosland, P.W.; Techawongstien, S. Light intensity affects capsaicinoid accumulation in hot pepper (Capsicum chinense Jacq.) cultivars. Hortic. Environ. Biotechnol. 2017, 58, 103–110. [Google Scholar] [CrossRef]
- Ancona-Escalante, W.d.R.; Baas-Espinola, F.M.; Castro-Concha, L.A.; Vázquez-Flota, F.A.; Zamudio-Maya, M.; de Miranda-Ham, M.L. Induction of capsaicinoid accumulation in placental tissues of Capsicum chinense Jacq. requires primary ammonia assimilation. Plant Cell Tissue Organ Cult. (PCTOC) 2013, 113, 565–570. [Google Scholar] [CrossRef]
- He, G.; Zhang, R.; Jiang, S.; Wang, H.; Ming, F. The MYB transcription factor RcMYB1 plays a central role in rose anthocyanin biosynthesis. Hortic. Res. 2023, 10, uhad080. [Google Scholar] [CrossRef]
- Xing, M.; Xin, P.; Wang, Y.; Han, C.; Lei, C.; Huang, W.; Zhang, Y.; Zhang, X.; Cheng, K.; Zhang, X. A negative feedback regulatory module comprising R3-MYB repressor MYBL2 and R2R3-MYB activator PAP1 fine-tunes high light-induced anthocyanin biosynthesis in Arabidopsis. J. Exp. Bot. 2024, 75, 7381–7400. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, J.; Zeng, T.; Xu, Z.; Luo, J.; Zheng, R.; Wang, Y.; Wang, C. TcMYB8, a R3-MYB Transcription Factor, Positively Regulates Pyrethrin Biosynthesis in Tanacetum cinerariifolium. Int. J. Mol. Sci. 2022, 23, 12186. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Z.; Yang, Y.; Zhou, X.; Lei, D.; He, R.; Zhang, Y.; Zhang, J.; Lin, Y.; Wang, Y.; et al. R2R3-MYB transcription factor PbMYB5-like positively regulates the biosynthesis of phenylalanine-related metabolites in pear (Pyrus bretschneideri). J. Agric. Food Res. 2024, 18, 101328. [Google Scholar] [CrossRef]
- Wang, W.; Hu, S.; Yang, J.; Zhang, C.; Zhang, T.; Wang, D.; Cao, X.; Wang, Z. A Novel R2R3-MYB Transcription Factor SbMYB12 Positively Regulates Baicalin Biosynthesis in Scutellaria baicalensis Georgi. Int. J. Mol. Sci. 2022, 23, 15452. [Google Scholar] [CrossRef]
- Liu, W.; Mu, H.; Yuan, L.; Li, Y.; Li, Y.; Li, S.; Ren, C.; Duan, W.; Fan, P.; Dai, Z.; et al. VvBBX44 and VvMYBA1 form a regulatory feedback loop to balance anthocyanin biosynthesis in grape. Hortic. Res. 2023, 10, uhad176. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Q.; Yan, C.; Sun, Q.; Wang, J.; Li, C.; Yuan, C.; Mou, Y.; Shan, S. The bHLH transcription factor AhbHLH121 improves salt tolerance in peanut. Int. J. Bio. Macromol. 2023, 256, 128492. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Deng, B.; Yuan, H.; Zhang, B.; Liu, J.; Meng, J.; Chang, M. Transcription factor FfMYB15 regulates the expression of cellulase gene FfCEL6B during mycelial growth of Flammulina filiformis. Microb. Cell Factories 2022, 21, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, X.; Yu, X.; Wang, F.; Long, J.; Shen, W.; Jiang, D.; Zhao, X. The MYB transcription factor CiMYB42 regulates limonoids biosynthesis in citrus. BMC Plant Biol. 2020, 20, 316. [Google Scholar] [CrossRef]
- GB/T21266-2007; Determination of Total Capsaicinoid Content and Representation of Pungency Degree in Capsicum and Its Products. Standardization Administration of China: Beijing, China, 2007.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Zhang, M.; Fang, G.; Li, M.; Zhang, R.; Xie, Q.; Han, S.; Lv, J.; Deng, M. The Transcription Factor CcMYB330 Regulates Capsaicinoid Biosynthesis in Pepper Fruits. Int. J. Mol. Sci. 2025, 26, 1438. https://doi.org/10.3390/ijms26041438
Cheng H, Zhang M, Fang G, Li M, Zhang R, Xie Q, Han S, Lv J, Deng M. The Transcription Factor CcMYB330 Regulates Capsaicinoid Biosynthesis in Pepper Fruits. International Journal of Molecular Sciences. 2025; 26(4):1438. https://doi.org/10.3390/ijms26041438
Chicago/Turabian StyleCheng, Hong, Mingxian Zhang, Guining Fang, Mengjuan Li, Ruihao Zhang, Qiaoli Xie, Shu Han, Junheng Lv, and Minghua Deng. 2025. "The Transcription Factor CcMYB330 Regulates Capsaicinoid Biosynthesis in Pepper Fruits" International Journal of Molecular Sciences 26, no. 4: 1438. https://doi.org/10.3390/ijms26041438
APA StyleCheng, H., Zhang, M., Fang, G., Li, M., Zhang, R., Xie, Q., Han, S., Lv, J., & Deng, M. (2025). The Transcription Factor CcMYB330 Regulates Capsaicinoid Biosynthesis in Pepper Fruits. International Journal of Molecular Sciences, 26(4), 1438. https://doi.org/10.3390/ijms26041438





