Biotransformation of Thiochroman Derivatives Using Marine-Derived Fungi: Isolation, Characterization, and Antimicrobial Activity
Abstract
1. Introduction
2. Results and Discussion
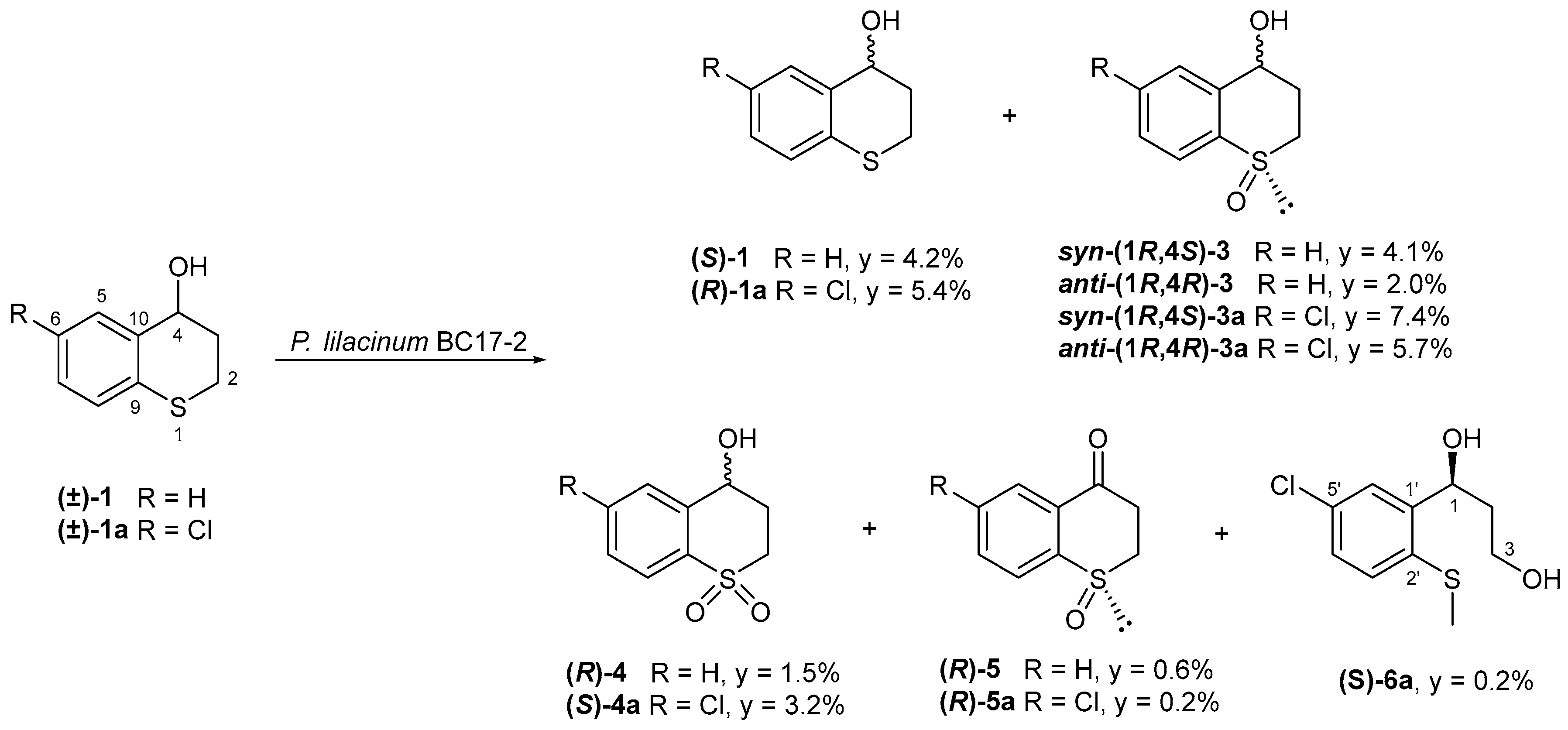
2.1. Biotransformation of (±)-Thiochroman-4-Ol (1) and (±)-6-Chlorothiochroman-4-ol (1a) by P. lilacinum BC17-2
2.2. Biotransformation of (±)-Thiochroman-4-Ol (1) and (±)-6-Chlorothiochroman-4-ol (1a) by E. maritima BC17
2.3. Antimicrobial Assays
3. Materials and Methods
3.1. General Experimental Methods
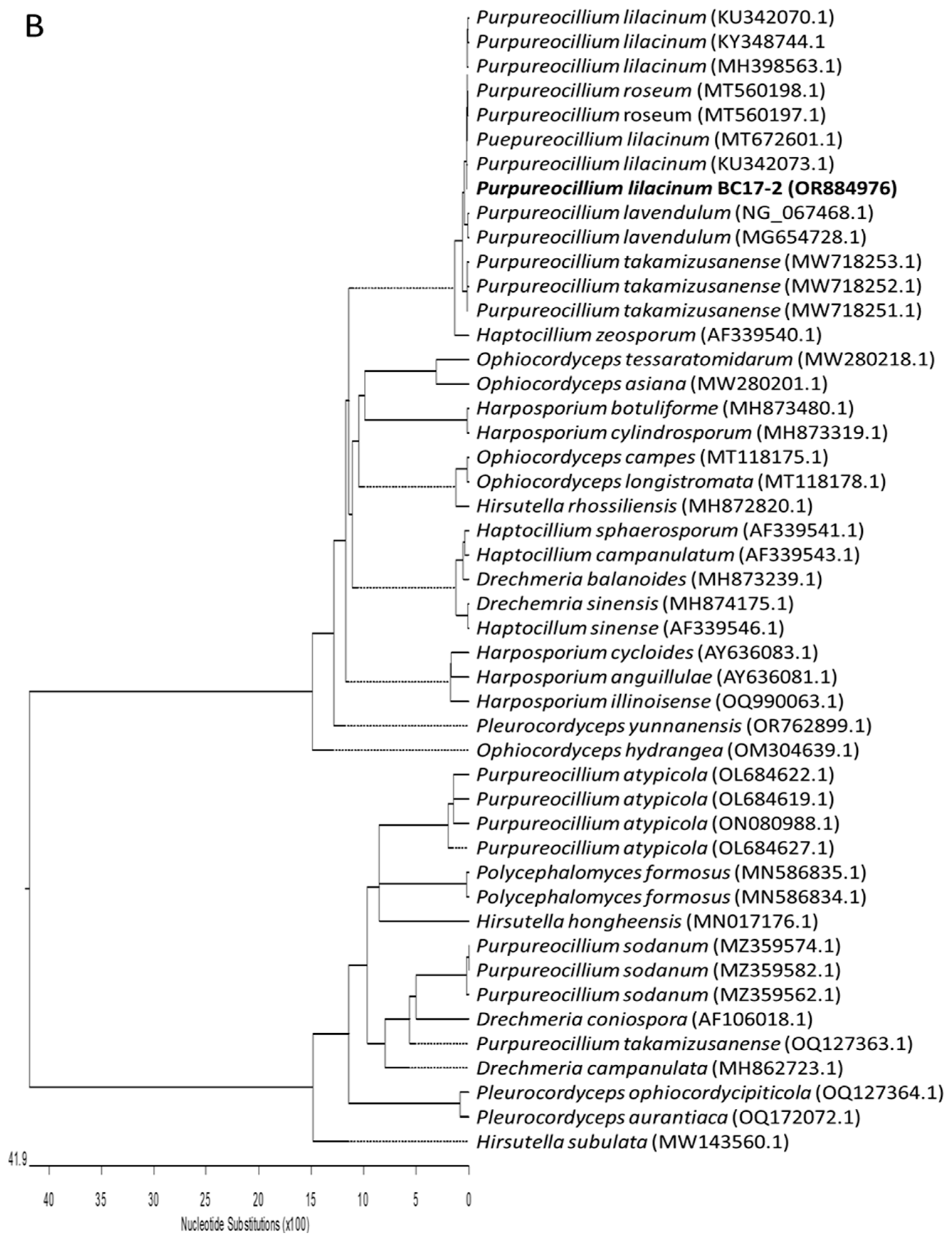
3.2. Fungal Material and Identification
3.3. Synthesis of Racemic Substrates (1, 1a)
3.4. Biotransformation Experiments
3.4.1. General Method
3.4.2. Biotransformation of (±)-Thiochroman-4-Ol (1) by P. lilacinum BC17-2
3.4.3. Biotransformation of (±)-6-Chlorothiochroman-4-Ol (1a) by P. lilacinum BC17-2
3.4.4. Biotransformation of (±)-Thiochroman-4-Ol (1) by E. maritima BC17
3.4.5. Biotransformation of (±)-6-Chlorothiochroman-4-Ol (1a) by E. maritima BC17
3.5. General Procedure for the Preparation of Mosher’s Esters
3.6. Computational Details of ECD Calculations
3.7. In Vitro Antimicrobial Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muñoz Solano, D.; Hoyos, P.; Hernáiz, M.J.; Alcántara, A.R.; Sánchez-Montero, J.M. Industrial biotransformations in the synthesis of building blocks leading to enantiopure drugs. Bioresour. Technol. 2012, 115, 196–207. [Google Scholar] [CrossRef]
- Virués-Segovia, J.R.; Muñoz-Mira, S.; Durán-Patrón, R.; Aleu, J. Marine-derived fungi as biocatalysts. Front. Microbiol. 2023, 14, 1125639. [Google Scholar] [CrossRef]
- Ahumada-Rudolph, R.; Novoa, V.; Sáez, K.; Martínez, M.; Rudolph, A.; Torres-Diaz, C.; Becerra, J. Marine fungi isolated from Chilean fjord sediments can degrade oxytetracycline. Environ. Monit. Assess. 2016, 188, 468. [Google Scholar] [CrossRef]
- Batista-García, R.A.; Sutton, T.; Jackson, S.A.; Tovar-Herrera, O.E.; Balcázar-López, E.; Sánchez-Carbente, M.D.R.; Sánchez-Reyes, A.; Dobson, A.D.W.; Folch-Mallol, J.L. Characterization of lignocellulolytic activities from fungi isolated from the deep-sea sponge Stelletta normani. PLoS ONE 2017, 12, e0173750. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.; Qiu, H.; Li, S.; Zhang, T. Biological degradation of Aflatoxin B1 by Emericellopsis sp. 1912 and Sarocladium sp. 10A. Lect. Notes Electr. Eng. 2018, 444, 525–532. [Google Scholar] [CrossRef]
- BenIsrael, M.; Habtewold, J.Z.; Khosla, K.; Wanner, P.; Aravena, R.; Parker, B.L.; Haack, E.A.; Tsao, D.T.; Dunfield, K.E. Identification of degrader bacteria and fungi enriched in rhizosphere soil from a toluene phytoremediation site using DNA stable isotope probing. Int. J. Phytoremediat. 2021, 23, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Cavello, I.A. Study of the production of alkaline keratinases in submerged cultures as an alternative for solid waste treatment generated in leather technology. J. Microbiol. Biotechnol. 2013, 23, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, S.; Faseela, P.; Josh, M.K.S.; Balachandran, S.; Devi, R.S.; Benjamin, S. Fungal biodegradation of phthalate plasticizer in situ. Biodegradation 2013, 24, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Bondock, S.; Metwally, M.A. Thiochroman-4-ones: Synthesis and reactions. J. Sulfur. Chem. 2008, 29, 623–653. [Google Scholar] [CrossRef]
- Chouchène, N.; Toumi, A.; Boudriga, S.; Edziri, H.; Sobeh, M.; Abdelfattah, M.A.O.; Askri, M.; Knorr, M.; Strohmann, C.; Brieger, L.; et al. Antimicrobial activity and DFT studies of a novel set of spiropyrrolidines tethered with thiochroman-4-one/chroman-4-one scaffolds. Molecules 2022, 27, 582. [Google Scholar] [CrossRef]
- Zhong, Y.; Han, X.; Li, S.; Qi, H.; Song, Y.; Qiao, X. Design, synthesis, antifungal activity and molecular docking of thiochroman-4-one derivatives. Chem. Pharm. Bull. 2017, 65, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xiao, L.; Chi, J.; Tang, Z.; Li, J.; Tan, S.; Li, P. Design, synthesis, and bioactivity evaluation of novel thiochromanone derivatives containing an oxime or oxime ether moiety. J. Heterocycl. Chem. 2021, 58, 2124–2131. [Google Scholar] [CrossRef]
- Xiao, L.; Yu, L.; Li, P.; Chi, J.; Tang, Z.; Li, J.; Tan, S.; Wang, X. Design, synthesis, and bioactivity evaluation of new thiochromanone derivatives containing a carboxamide moiety. Molecules 2021, 26, 4391. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xiao, L.; Li, P.; Chi, J.; Li, J.; Tan, S. Synthesis and bioactivity evaluation of novel thiochroman-4-one derivatives incorporating carboxamide and 1, 3, 4-thiadiazole thioether moieties. J. Chem. 2022, 2022, 5354088. [Google Scholar] [CrossRef]
- Pinedo-Rivilla, C.; Collado, I.G.; Aleu, J. Metabolism of antifungal thiochroman-4-ones by Trichoderma viride and Botrytis cinerea. J. Nat. Prod. 2018, 81, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Demirayak, S.; Yurttas, L.; Gundogdu-Karaburun, N.; Karaburun, A.C.; Kayagil, I. New chroman-4-one/thiochroman-4-one derivatives as potential anticancer agents. Saudi Pharm. J. 2017, 25, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Le, T.C.; Berlin, K.D.; Benson, S.D.; Eastman, M.A.; Bell-Eunice, G.; Nelson, A.C.; Benbrook, D.M. Heteroarotinoids with anti-cancer activity against ovarian cancer cells. Open Med. Chem. J. 2007, 1, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Hammam, A.E.-F.G.; Fahmy, A.F.M.; Amr, A.-G.E.; Mohamed, A.M. Synthesis of novel tricyclic heterocyclic compounds as potential anticancer agents using chromanone and thiochromanone as synthons. ChemInform 2003, 34, 1985–1993. [Google Scholar] [CrossRef]
- Song, J.; Pan, R.; Li, G.; Su, W.; Song, X.; Li, J.; Liu, S. Synthesis and anticancer activities of thiosemicarbazones derivatives of thiochromanones and related scaffolds. Med. Chem. Res. 2020, 29, 630–642. [Google Scholar] [CrossRef]
- Zhang, D.; Ji, X.; Gao, R.; Wang, H.; Meng, S.; Zhong, Z.; Li, Y.; Jiang, J.; Li, Z. Synthesis and antiviral activities of a novel class of thioflavone and flavonoid analogues. Acta Pharm. Sin. B 2012, 2, 575–580. [Google Scholar] [CrossRef]
- Ortiz, C.; Echeverri, F.; Robledo, S.; Lanari, D.; Curini, M.; Quiñones, W.; Vargas, E. Synthesis and evaluation of antileishmanial and cytotoxic activity of benzothiopyrane derivatives. Molecules 2020, 25, 800. [Google Scholar] [CrossRef]
- Vargas, E.; Echeverri, F.; Upegui, Y.A.; Robledo, S.M.; Quiñones, W. Hydrazone derivatives enhance antileishmanial activity of thiochroman-4-ones. Molecules 2018, 23, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.; Echeverri, F.; Vélez, I.D.; Robledo, S.M.; Quiñones, W. Synthesis and evaluation of thiochroman-4-one derivatives as potential leishmanicidal agents. Molecules 2017, 22, 2041. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Kumar, R.; Sehrawat, R. Synthesis and antibacterial activity of some new 2,3-dimethoxy-3-hydroxy-2-(1-phenyl-3-aryl-4-pyrazolyl)chromanones. Eur. J. Med. Chem. 2009, 44, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Holland, H.L.; Manoharan, T.S.; Schweizer, F. Preparation of homochiral chroman-4-ols and thiochroman-4-ols by microbial biotransformation. Tetrahedron Asymmetry 1991, 2, 335–338. [Google Scholar] [CrossRef]
- Virués-Segovia, J.R.; Millán, C.; Pinedo, C.; González-Rodríguez, V.E.; Papaspyrou, S.; Zorrilla, D.; Mackenzie, T.A.; Ramos, M.C.; de la Cruz, M.; Aleu, J.; et al. New eremophilane-type sesquiterpenes from the marine sediment-derived fungus Emericellopsis maritima BC17 and their cytotoxic and antimicrobial activities. Mar. Drugs 2023, 21, 634. [Google Scholar] [CrossRef] [PubMed]
- Luangsa-ard, J.; Houbraken, J.; van Doorn, T.; Hong, S.-B.; Borman, A.M.; Hywel-Jones, N.L.; Samson, R.A. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol. Lett. 2011, 321, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Ursini, C.V.; Dias, G.H.M.; Rodrigues, J.A.R. Ruthenium-catalyzed reduction of racemic tricarbonyl(H6-aryl ketone)chromium complexes using transfer hydrogenation: A simple alternative to the resolution of planar chiral organometallics. J. Organomet. Chem. 2005, 690, 3176–3186. [Google Scholar] [CrossRef]
- Stepanenko, V.; De Jesús, M.; Correa, W.; Bermúdez, L.; Vázquez, C.; Guzmán, I.; Ortiz-Marciales, M. Chiral spiroaminoborate ester as a highly enantioselective and efficient catalyst for the borane reduction of furyl, thiophene, chroman, and thiochroman-containing ketones. Tetrahedron Asymmetry 2009, 20, 2659–2665. [Google Scholar] [CrossRef] [PubMed]
- Seco, J.M.; Quiñoá, E.; Riguera, R. A practical guide for the assignment of the absolute configuration of alcohols, amines and carboxylic acids by NMR. Tetrahedron Asymmetry 2001, 12, 2915–2925. [Google Scholar] [CrossRef]
- Kersey, I.D.; Fishwick, C.W.G.; Findlay, J.B.C.; Ward, P. Neighbouring group effects in a pummerer-type rearrangement: A facile entry into 3,1-benzoxathiins. Tetrahedron 1995, 51, 6819–6834. [Google Scholar] [CrossRef]
- Ohkuma, T.; Utsumi, N.; Tsutsumi, K.; Watanabe, M.; Murata, K.; Arai, N.; Kurono, N. Asymmetric hydrogenation of aromatic heterocyclic ketones catalyzed by the MsDPEN–Cp* Ir (III) complex. Heterocycles 2010, 80, 141. [Google Scholar] [CrossRef]
- Gan, S.; Yin, J.; Yao, Y.; Liu, Y.; Chang, D.; Zhu, D.; Shi, L. Metal- and additive-free oxygen-atom transfer reaction: An efficient and chemoselective oxidation of sulfides to sulfoxides with cyclic diacyl peroxides. Org. Biomol. Chem. 2017, 15, 2647–2654. [Google Scholar] [CrossRef] [PubMed]
- Kišić, A.; Stephan, M.; Mohar, B. ansa-Ruthenium(II) complexes of R2NSO2DPEN-(CH2)n(η6-aryl) conjugate ligands for asymmetric transfer hydrogenation of aryl ketones. Adv. Synth. Catal. 2015, 357, 2540–2546. [Google Scholar] [CrossRef]
- Katsuki, T.; Matsumoto, K.; Yamaguchi, T. Asymmetric oxidation of cyclic sulfides catalyzed by an aluminum(salalen) complex as the catalyst. Heterocycles 2008, 76, 191. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density—Functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Casida, M.E.; Jamorski, C.; Casida, K.C.; Salahub, D.R. Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: Characterization and correction of the time-dependent local density approximation ionization threshold. J. Chem. Phys. 1998, 108, 4439–4449. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Mennucci, B.; Cancès, E.; Tomasi, J. Evaluation of solvent effects in isotropic and anisotropic dielectrics and in ionic solutions with a unified integral equation method: Theoretical bases, computational implementation, and numerical applications. J. Phys. Chem. B 1997, 101, 10506–10517. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100: Wayne, PA, USA, 2020. [Google Scholar]
- EUropean Committee on Antimicrobial Susceptibility Testing (EUCAST). Methods for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts. Version 7.4. Available online: https://www.eucast.org/astoffungi/methodsinantifungalsusceptibilitytesting/susceptibility_testing_of_yeasts (accessed on 15 February 2024).
- ISO 20776-1; International Organization for Standardization (ISO). Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Broth Micro-Dilution Reference Method for Testing the In Vitro Activity of Antimicrobial Agents Against Rapidly Growing Aerobic Bacteria, 2nd ed. ISO: Geneva, Switzerland, 2019.



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virués-Segovia, J.R.; Pinedo-Rivilla, C.; Muñoz-Mira, S.; Ansino, M.; González-Rodríguez, V.E.; Ezzanad, A.; Galán-Sánchez, F.; Durán-Patrón, R.; Aleu, J. Biotransformation of Thiochroman Derivatives Using Marine-Derived Fungi: Isolation, Characterization, and Antimicrobial Activity. Int. J. Mol. Sci. 2025, 26, 908. https://doi.org/10.3390/ijms26030908
Virués-Segovia JR, Pinedo-Rivilla C, Muñoz-Mira S, Ansino M, González-Rodríguez VE, Ezzanad A, Galán-Sánchez F, Durán-Patrón R, Aleu J. Biotransformation of Thiochroman Derivatives Using Marine-Derived Fungi: Isolation, Characterization, and Antimicrobial Activity. International Journal of Molecular Sciences. 2025; 26(3):908. https://doi.org/10.3390/ijms26030908
Chicago/Turabian StyleVirués-Segovia, Jorge R., Cristina Pinedo-Rivilla, Salvador Muñoz-Mira, Matilde Ansino, Victoria E. González-Rodríguez, Abdellah Ezzanad, Fátima Galán-Sánchez, Rosa Durán-Patrón, and Josefina Aleu. 2025. "Biotransformation of Thiochroman Derivatives Using Marine-Derived Fungi: Isolation, Characterization, and Antimicrobial Activity" International Journal of Molecular Sciences 26, no. 3: 908. https://doi.org/10.3390/ijms26030908
APA StyleVirués-Segovia, J. R., Pinedo-Rivilla, C., Muñoz-Mira, S., Ansino, M., González-Rodríguez, V. E., Ezzanad, A., Galán-Sánchez, F., Durán-Patrón, R., & Aleu, J. (2025). Biotransformation of Thiochroman Derivatives Using Marine-Derived Fungi: Isolation, Characterization, and Antimicrobial Activity. International Journal of Molecular Sciences, 26(3), 908. https://doi.org/10.3390/ijms26030908









