Abstract
In the present study, we investigated the association of genetic predisposition with specific dimensions of dementia pathophysiology for global and domain-specific cognitive decline in older adults. The sample was drawn from the Hellenic Longitudinal Investigation of Aging and Diet (HELIAD) study, comprising 512 cognitively normal individuals over 64 years of age, with a mean follow-up of 2.9 years. Cognitive function was evaluated through a neuropsychological test battery, while genetic predisposition was assessed based on two distinct Polygenic Risk Scores (PRS) for amyloid-beta 42 (Aβ42) and white matter hyperintensities (WMH). The association of each PRS with the cognitive decline rate was examined using generalized estimating equation models. In the whole sample, higher PRSs Aβ42 (β = −0.042) and WMH (β =−0.029) were associated with a higher rate of global cognitive decline per year, an association which remained significant in age, sex, and education subgroups. Moreover, higher PRSs Aβ42 and WMH were related to significant memory decline only in females, older, and highly educated participants. Thus, while the association of both PRSs with global cognitive decline over time was independent of age, sex, or education, the relationship of the specific PRSs with the memory decline rate appeared to vary depending on these factors.
1. Introduction
Existing data suggest that approximately 75% of new dementia cases are related to Alzheimer’s disease (AD), which is the most common neurodegenerative disease affecting one-third of individuals over 85 years old [1]. The accumulation of β-amyloid (Aβ) plaques and neurofibrillary tangles has been shown to contribute significantly to the pathophysiology of AD [2], being the most well-known underlying mechanism of cognitive decline. Apart from amyloidosis, white matter hyperintensities (WMH) have been associated with cognitive decline [3] and dementia [4,5]. Recently, the previous hypothesis that WMH were solely related to the vascular aspect of dementia [6] has been considered insufficient to explain the intricate relationship between WMH and dementia [7], as non-vascular mechanisms might be involved as well.
The consensus today is that the development of dementia is determined by both environmental and genetic factors. The most common type of dementia, AD, is considered a polygenic disease [8], as the presence of multiple polymorphisms in specific proteins, such as the apolipoprotein E (ApoE) [9], might increase the likelihood of developing the disease and, thus, dementia. In fact, the heritability of AD is high ranging, from 60% to 80% [10]. Relevant genome-wide association studies (GWAS) have revealed common single nucleotide polymorphisms (SNPs) associated with an increased risk of developing AD-type dementia [11] and have been used to calculate Polygenic Risk Scores (PRSs). Therefore, PRSs have been used as predictors of the risk for disease [12].
Apart from predictors of disease risk, relevant PRSs have also been investigated as possible indicators of cognitive decline [13]. An AD PRS might even exhibit a stronger relationship with cognitive decline compared to a PRS specific to cognitive function [14]. Existing studies have demonstrated distinct differences across specific cognitive domains. For instance, Gustavson et al. [15] and Darst et al. [16] have shown that the association of AD-PRSs and higher rates of cognitive decline (in memory and executive function) are driven by the effect of APOE. Xu et al. [17] have concluded that the predictive capacity of an AD-PRS in cognitive decline is independent of age and APOE only in the domain of executive function. In contrast, other studies [18,19] have highlighted that AD-PRSs are not able to predict cognitive decline over time in an aging population, asserting that these are solely associated with baseline cognitive ability. Thus, the existing literature presents conflicting data concerning the association of PRS related to dementia and longitudinal cognitive decline.
At the same time, data regarding the possible relationship between genetic propensity for specific pathways related to dementia pathophysiology and global or domain-specific cognitive decline are quite limited. From the aforementioned studies, only two [16,17] have explored PRSs related to specific pathways in the context of Aβ metabolism, endocytosis, Tau pathology, and immune responses without promising results. In fact, a PRS for Aβ metabolism was associated with a decline solely in the delayed recall score after excluding APOE [16], while all relationships in the other study were driven by the effect of APOE [17].
Therefore, we aimed to fill that literature gap by investigating the possible correlation of two PRSs, which are specific to distinct aspects of dementia pathophysiology (PRS Aβ42 and PRS WMH) and the rate of cognitive decline in cognitively normal (CN) older adults, as well as whether such an association might be influenced by age, sex, and cognitive reserve (CR), as proxied by the number of years of formal education. Our hypothesis was that increased risk for amyloidosis and WMH might be related to global and domain-specific cognitive decline in a population-based sample of older adults.
2. Results
2.1. Baseline Clinical and Socio-Demographic Characteristics
In total, 512 participants in the Hellenic Longitudinal Investigation of Aging and Diet (HELIAD) study, along with the available genomic data, were included in our analyses. The participants were followed longitudinally over time, with a mean follow-up of 2.9 years.
The baseline clinical and socio-demographic characteristics of all participants divided into classes of low/high PRS Aβ42 and PRS WMH can be found in Table 1. Those who had a higher PRS Aβ42 were older (p = 0.03) and had a greater global cognition (GC) score (p = 0.042) compared to those in the low PRS Aβ42 group. Clinical and demographic characteristics at baseline did not differ between PRS WMH groups.

Table 1.
Participants’ baseline characteristics by groups of polygenic risk scores.
The comparison of the clinical and socio-demographic characteristics when classifying participants according to global cognitive (GC) performance can be found in Table 2. Individuals with higher GC scores were younger and had more education years (p < 0.001) in comparison to participants belonging to the low GC group.

Table 2.
Participants’ baseline characteristics by global cognition at baseline.
2.2. PRSs and Cognitive Decline
Compared to the low PRS groups, the high PRS Aβ42 group was associated with a 4.2% higher standard deviation (SD) decline per year in the global composite score (Table 3), while the high PRS WMH group was related to a 2.9% higher SD decline per year, as presented in Figure 1. The results regarding individual cognitive domains were not significant. Analyses were adjusted for age, sex, CR, and APOE genotype, as well as the first two principal components (PC1, PC2) of genetic ancestry.

Table 3.
Results from independent adjusted GEE models concerning the association between baseline PRSs and differential rates of change of cognitive composite scores in the whole sample.
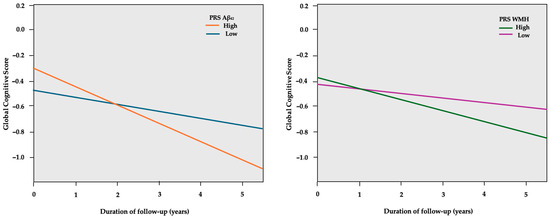
Figure 1.
GEE predicted global cognitive scores (y-axis) over the course of follow-up in years (x-axis), separately for the low and high PRS Aβ42 groups. The model is adjusted for age, sex, education years, PC1, PC2, and APOE genotype.
In sex-stratified GEE models, higher PRS groups in both males and females were associated with a decline in global cognition (Table 4). However, higher PRSs were related to significant memory decline only in females. A higher PRS Aβ42 was related to a 3.8% higher SD decline per year, as shown in Figure 2, while higher PRS WMH was related to a 3.0% higher SD decline in memory per year.

Table 4.
Results from sex-stratified independent adjusted GEE models concerning the association between baseline PRSs and differential rates of change of cognitive composite scores.
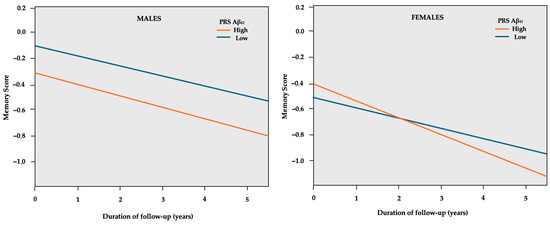
Figure 2.
GEE predicted memory z-scores (y-axis) over the course of follow-up in years (x-axis) separately for the low and high PRS Aβ42 in sex subgroups. The model is adjusted for age, education years, PC1, PC2, and APOE genotype.
After age stratification, higher PRS groups in both younger and older participants were associated with a decline in global cognition (Table 5). PRSs were related to significant memory decline only in the older age group (higher PRS Aβ42 was related to a 3.9% higher SD decline per year and higher PRS WMH to 2.7%, respectively).

Table 5.
Results from age-stratified independent adjusted GEE models concerning the association between baseline PRSs and differential rates of change of cognitive composite scores.
In CR-stratified models, a decline in global cognition was associated with higher PRS groups regardless of CR status (Table 6). Concerning the specific cognitive domains, PRSs were related to significant memory decline only in the high CR group (consisting of individuals with over 6 years of formal education). Specifically, a higher PRS Aβ42 was related to a 4.6% higher SD memory decline, while higher PRS WMH led to a 3.2% higher SD memory decline, as shown in Figure 3.

Table 6.
Results from independent CR-stratified adjusted GEE models concerning the association between baseline PRSs and differential rates of change of cognitive composite scores.
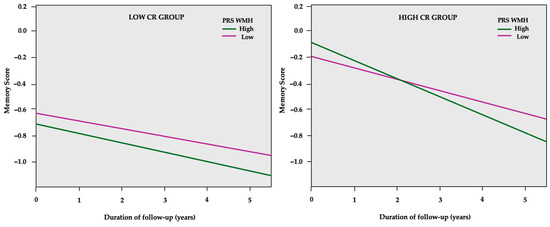
Figure 3.
GEE predicted memory z-scores (y-axis) over the course of follow-up in years (x-axis) separately for the low and high PRS WMH in cognitive reserve (CR) subgroups. The model is adjusted for age, sex, PC1, PC2, and APOE genotype.
All the aforementioned p-values were corrected using the Benjamini–Hochberg procedure for multiple testing correction (as described in Section 4.5).
3. Discussion
In this population-based study, including individuals over 64 years of age, we investigated the predictive capacity of PRS Aβ42 and PRS WMH on the rate of cognitive decline over a 2.9-year (on average) follow-up period. We observed a relationship between higher genetic predisposition (as indicated by PRSs) for Aβ accumulation and WMH burden and an increased rate of global cognitive decline over time among individuals who were cognitively normal at baseline. Notably, in the whole study sample, we did not detect a statistically significant association with any of the cognitive subdomains assessed.
In stratified analyses, high PRS Aβ42 and high PRS WMH remained significantly associated with faster global cognitive decline independently of sex, age, or CR. Furthermore, we found an association between high PRSs and faster memory decline, which was detectable only among women, older individuals, and high-CR participants. Thus, stratification analysis has provided age, sex, and CR-dependent differences in memory function.
To date, and to our knowledge, no previous study has investigated the possible association of a PRS, which is specific for Aβ accumulation or WMH burden and longitudinal cognitive change, either in terms of global cognition or specific cognitive domains. The majority of studies have only used PRSs specific for AD, which were constructed using SNPs associated with late-onset AD derived from relevant GWAS. An AD PRS has been found to be related to longitudinal cognitive decline (global or domain-specific), independently of the APOE genotype, in six studies [16,20,21,22,23,24]. The associations observed concern mainly the subdomains of memory and executive function. In contrast, other studies have not demonstrated any significant association between a PRS specific for AD and cognitive decline [18,19,25], or the observed association was driven by the influence of the APOE genotype [14,15,26]. Ritchie et al. [18] further elaborated that AD PRS was associated only with the baseline cognitive status but not the rate of cognitive decline.
Only two of the aforementioned studies have used PRSs specific for pathways related to dementia, such as PRSs for Aβ clearance and metabolism, which are conceptually closer to our study. In particular, Xu et al. [16] have shown that a PRS related to Aβ metabolism was associated with steeper memory (delayed recall) decline, an association which was controlled for APOE. In contrast, Darst et al. [15] concluded that the association between a pathway-specific PRS for Aβ clearance (consisting of 21 relevant SNPs apart from APOE) and cognitive decline was driven by the inclusion of APOE in the specific PRS. Furthermore, genetic predisposition to a higher WMH burden expressed using PRSs has been related to AD incidence in a limited number of studies [23,27], with no study investigating a possible relationship with the cognitive decline rate. The observed inconsistencies may stem from variations in sample characteristics among different studies as well as the inclusion of different SNPs in relevant GWASs in order to estimate genetic predisposition and calculate relevant PRSs for dementia. In fact, our population is South European, while most studies have included central and north European populations (in the UK and Sweden) with probable differences in genetic architecture.
The memory subdomain has been consistently associated with aging [28], while sex differences in memory performance have not been thoroughly investigated. In our study, elevated PRSs were significantly linked to memory decline only in females. Existing evidence suggests that women exhibit stronger memory skills compared to men, a trend that appears to remain consistent throughout the lifespan [29,30,31]. Some studies have shown a more pronounced memory decline in women during their eighth decade [32,33,34], which could be a possible explanation for our findings, as in our study sample, the mean age was 73.4 years. Moreover, women with an existing cognitive impairment have been shown to progress more quickly to MCI and dementia than men [35,36]. Thus, our findings are in accordance with the above assumption, as higher genetic predisposition for both Aβ42 and WMH are risk factors for dementia development. Sex-based variations in genetic risk between males and females have also been observed [37], as, for instance, women carrying two APOE ɛ4 alleles have been shown to demonstrate poorer memory performance between the ages of 65 and 69 compared to their male counterparts with the same genotype [38,39]. However, these findings have not yet been extended in the context of polygenic risk. In any case, opposing studies propose that males and females experience similar rates of cognitive decline [40]. Thus, further investigation is required to clarify the decline of sex differences in memory within the context of dementia.
Interestingly, as far as CR is concerned, we observed that individuals possessing a higher CR exhibited a more pronounced memory decline. In general, CR, as depicted by education years, is considered a protective factor for dementia development, as the vast majority of existing literature acknowledges its protective effect, highlighting a slower rate of memory decline in persons with high CR [41,42]. However, several studies are in accordance with our findings, showing a more rapid memory decline in patients with higher educational attainment [43,44,45], while a few studies have reported no difference in the rate of cognitive decline [46,47]. Our findings may indicate that the mechanism by which higher CR affects cognitive function in the dementia continuum is by delaying the onset of symptoms rather than reducing the rate of cognitive decline.
Our study presents some limitations. As real-time measurements of Aβ42 from CSF or PET scans were not available, as well as MRI scans, we were not able to assess the actual predictive capacity of PRSs for amyloid accumulation and the presence of WMHs. Moreover, the assessment of genetic predisposition through PRSs was based solely on common variants identified in GWASs, thus overlooking other biological factors known to influence or predict Aβ42 and WMH burden (rare variants, haplotypes, and epigenetic elements). Additionally, the duration of follow-up for this study was relatively short (2.9 years) in relation to the evolution of dementia, which occurs over a longer timeframe. Last but not least, the average educational level of our cohort was 7.1 years, which may restrict the generalizability of our results.
The present study also has several strengths. Firstly, we are unaware of another study investigating the effects of high genetic propensity for Aβ42 and WMH on the cognitive decline rate longitudinally. Moreover, the PRS approach offers several advantages, as it reduces potential errors associated with different methods of measuring CSF Aβ42 across different centers, as well as the concerns related to PET and MRI scans in terms of procedure and cost (e.g., selection of the Aβ42-PET positive threshold, especially in cases of low amyloid accumulation). Furthermore, the neuropsychological testing included thorough assessments for specific subdomains of cognition conducted by experts through detailed interviews.
4. Materials and Methods
4.1. Participants and Procedures
The study participants were derived from the HELIAD study, which is an epidemiological study of aging in the elderly Greek population focusing on dementia and other neuropsychiatric disorders. The recruitment process involved the random selection of individuals over 64 years old from an Athens suburb (Marousi) and the city of Larissa, as well as its rural surroundings, using local municipality registries.
Overall, 1986 individuals completed the baseline evaluation from 2011 to 2015, while 1226 participants completed the follow-up evaluation from 2013 to 2019. For our analyses, we only included HELIAD participants who (i) had available cognitive follow-up data, (ii) were not genetically related, (iii) did not have a baseline diagnosis of dementia or amnestic mild cognitive impairment (aMCI), and (iv) had available genotypic data (concerning the APOE genotype as well as PRS Aβ42 and PRS WMH). The final analytic sample consisted of 512 individuals.
All participants had provided informed consent before taking part in the study. All study procedures were approved by the Institutional Ethics Review Boards of the University of Thessaly and the National and Kapodistrian University of Athens. Information concerning medical and family history, lifestyle, and demographics (including age, sex, and education years) were collected during face-to-face interviews from the participants or their caregivers (first-degree relatives or spouses) when needed. Details about the design and key features of the HELIAD study and data collection procedures have been previously provided [48,49].
4.2. Neuropsychological Assessment
In both HELIAD visits (baseline and first follow-up) a thorough and detailed neuropsychological assessment was performed by trained neuropsychologists. A neuropsychological test battery, which is described in detail in the Supplementary Materials (Section S1.1), was used to evaluate global cognition and specific cognitive domains including memory, executive function, visuospatial ability, language, and attention/processing speed.
Each cognitive test score was converted to a z-score, using the mean and standard deviation values from the subset of participants without dementia or mild cognitive impairment (MCI) diagnosis. The z-scores of all tests within a specific domain were first averaged and then normalized again by their mean and standard deviation (SD) to create a domain-specific score. Finally, a z-score for global cognition was obtained by normalization of the averaged domain z-scores, with higher scores indicating better cognitive performance.
4.3. Genotyping and Imputation
Genome-wide genotyping was conducted using the Illumina Infinium Global Screening Array at the Life & Brain facilities in Bonn, Germany, the ‘Centre National de Recherche en Génétique Humaine’ (CNRGH) in Evry, France) and the Erasmus Medical Center in Rotterdam, Netherlands [12] as part of the European Alzheimer & Dementia Biobank (EADB) project. Calling was generated by the CNRGH in Evry, France, using the data generated by all centers involved in genotyping. Detailed information regarding the genotyping and imputation in the HELIAD study have been provided in a previously published work [50,51] and can be found in the Supplementary Materials (Section S1.2).
4.4. Polygenic Risk Score Estimation
Genetic predisposition for amyloid accumulation was modeled through a PRS, constructed by aggregating the effects of common genetic variants associated with cerebrospinal fluid (CSF) levels of Aβ42 [52]. In particular, the PRS Aβ42 was developed based on the summary statistics of a GWAS for CSF Aβ42 [24], in which each single nucleotide polymorphism (SNP) was associated with CSF Aβ42 levels at a certain p-value threshold. For each participant, we computed different PRSs for CSF Aβ42 based on a prior set of 10 p-value GWAS thresholds (PT) (i.e., 5 × 10−5, 0.0001, 0.001, 0.05, 0.01, 0.1, 0.2, 0.3, 0.4, 0.5). Given that Aβ42 levels in CSF are inversely related to the accumulation of Aβ42 in the brain, PRS values were multiplied by –1 to align a higher score with a higher genetic predisposition for increased Aβ42 levels in the brain.
Similarly, genetic propensity for a higher WMH burden was assessed through a PRS calculated using data from a meta-analysis of GWASs related to WMH volume, which was conducted by the CHARGE consortium [53]. The meta-analysis incorporated summary statistics from 23 population-based studies (n = 24,182), encompassing a total of 21,666 European individuals. Individuals with a history of stroke, brain tumors, or head trauma, as well as brain infarctions impacting the gray matter identified through MRI, were excluded. The methodology we followed for the calculation of the PRSs can be found in the Supplementary Materials (Section S1.3).
4.5. Statistical Analysis
The statistical analyses were performed using SPSS 28.0. Participant characteristics were expressed as mean values ± SD for continuous variables or as percentages for categorical variables. To compare the baseline socio-demographic characteristics of participants, we ranked the baseline PRSs and global cognitive scores into two equal groups (low-high) based on the median values for each variable. These groups were compared using Pearson’s chi-squared test for categorical variables, while analysis of variance (ANOVA) was performed for the comparison of continuous variables. The level of significance was set at p < 0.05.
PRSs were treated as dichotomous variables, with the median used as a cut-off (0: low PRS and 1: high PRS). The values of the medians were 0.028 for PRS Aβ42 and −0.025 for PRS WMH, respectively. Regarding stratified analyses, we performed sex (males vs. females), age (younger vs. older group using the median of 72.68 years as cut-off), and CR stratification (using 6 education years as cut-off, which corresponds to the cut-off for primary/elementary education in Greece).
For our investigation, we specifically chose the PTs that exhibited the highest accuracy in classifying AD/aMCI cases versus non-AD/aMCI cases in our cohort, as done in previous studies [50,51,54]. Therefore, we chose the pT < 0.1 for PRS Aβ42 and pT < 0.3 for PRS WMH (as shown in Section S1.4, Tables S1 and S2 of the Supplementary Materials).
We used generalized estimating equations (GEE) models to examine whether PRSs might be associated with differential rates of cognitive change over time. The reason why we chose the specific models is the fact that GEE models extend the generalized linear model, allowing for analysis of repeated measurements (thus, the relationship between the predictor and the outcome remains linear). In particular, GEE models take into consideration the multiple visits per individual, as well as the fact that the characteristics of the same individual subjected to repeated measurements over time might be correlated [55]. We treated each participant’s baseline and follow-up evaluations as a cluster.
We constructed six consecutive GEE models for each PRS. GEE analyses featured the main effects of each PRS (as a dichotomous variable) and time from baseline, as well as PRS by time interaction terms. Specifically, PRS Aβ42/PRS WMH, time (follow-up duration in years from baseline assessment), as well as PRS x follow-up duration interaction were the main predictors in independent models. The global and domain-specific cognitive z-scores (memory, executive function, visuospatial ability, language, and attention) were used as the dependent scale variables. A significant interaction term would indicate differential rates of cognitive change as a function of baseline PRS.
Therefore, the value of ‘β’, which is the regression coefficient of GEE models, corresponds to the difference in the cognitive change rate between the high PRS and the low PRS group (as PRSs were treated as dichotomous variables, using the low PRS group as reference) per year of follow-up. The difference is expressed as a percentage of one unit of SD of the relevant cognitive score. All analyses were adjusted for age, sex, CR, and APOE genotype, as well as the first two principal components (PC1, PC2) of genetic ancestry.
To explore potential disparities regarding the impact of PRS on different sexes, ages, and CRs, we performed subgroup analyses using the same approach described above. Given that we examined multiple PRSs and multiple cognitive domains, we performed multiple testing corrections. We have corrected p-values using the Benjamini–Hochberg procedure [56]. The false discovery rate (FDR) was controlled at <5%.
5. Conclusions
In conclusion, in our study, two PRSs related to different aspects of dementia pathophysiology (i.e., amyloid deposition and WMHs) independently predicted a more rapid rate of global cognitive decline in a sample of adults older than 64 years old. Therefore, the specific genetic predictors might be used to recognize individuals with an increased genetic risk of cognitive decline before the onset of clinical symptoms, who may be promising candidates for clinical trials concerning new dementia treatments. Moreover, the two PRSs were also associated with memory decline; nevertheless, the specific relationships were sex-, age-, and CR-dependent. Thus, these factors should be taken into account when investigating genetic predictors for dementia. In any case, further longitudinal studies are needed to validate the aforementioned genetic predictors, elucidate the impact of genetic predisposition on pathways related to dementia pathophysiology and cognitive decline, and clarify specific sex and CR differences.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26030910/s1, S1.1: Neuropsychological Evaluation [57,58,59,60,61,62,63,64,65]; S1.2: Genotype Imputation in HELIAD [66,67,68,69,70,71]; S1.3: Polygenic Risk Score Calculation; S1.4: Polygenic Risk Score Thresholds [72,73]; Table S1: Number of SNPs included at each PRS Aβ42 calculated at different GWAS p-value thresholds. AUC area together with p value of each PRS derived from a logistic regression with outcome aMCI/AD status, adjusted for APOE e4 genotype, PC1 and PC2. Table S2: Number of SNPs included at each PRS WMH calculated at different GWAS p-value thresholds. AUC area together with p value of each PRS derived from a logistic regression with outcome aMCI/AD status, adjusted for APOE e4 genotype, PC1 and PC2.
Author Contributions
Conceptualization, S.N.S., A.H., G.K. and N.S.; Μethodology, S.N.S., N.M., A.H. and N.S.; Formal analysis, S.N.S. and N.M.; Investigation, S.N.S.; Writing—original draft preparation, S.N.S. and I.G.; Writing—review and editing, N.M., S.C., E.M., E.N., A.H., A.R., M.Y., M.H.K., E.D., G.H., P.S. and N.S.; Supervision, N.S.; Project administration, N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The HELIAD study conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Boards of the National and Kapodistrian University of Athens (Approval Code: 256) and the University of Thessaly (Approval Code: 138).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the study’s principal investigator, N.S., upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tahami Monfared, A.A.; Byrnes, M.J.; White, L.A.; Zhang, Q. Alzheimer’s Disease: Epidemiology and Clinical Progression. Neurol. Ther. 2022, 11, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991, 1, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Shi, J. White matter hyperintensities volume and cognition: A meta-analysis. Front. Aging Neurosci. 2022, 14, 949763. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Y.; Ou, Y.N.; Shen, X.N.; Qu, Y.; Ma, Y.H.; Wang, Z.T.; Dong, Q.; Tan, L.; Yu, J.T. White matter hyperintensities and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 36 prospective studies. Neurosci. Biobehav. Rev. 2021, 120, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Prins, N.D.; Scheltens, P. White matter hyperintensities, cognitive impairment and dementia: An update. Nat. Rev. Neurol. 2015, 11, 157–165. [Google Scholar] [CrossRef]
- Marnane, M.; Al-Jawadi, O.O.; Mortazavi, S.; Pogorzelec, K.J.; Wang, B.W.; Feldman, H.H.; Hsiung, G.Y.; Alzheimer’s Disease Neuroimaging Initiative. Periventricular hyperintensities are associated with elevated cerebral amyloid. Neurology 2016, 86, 535–543. [Google Scholar] [CrossRef]
- Marden, J.R.; Mayeda, E.R.; Walter, S.; Vivot, A.; Tchetgen Tchetgen, E.J.; Kawachi, I.; Glymour, M.M. Using an Alzheimer Disease Polygenic Risk Score to Predict Memory Decline in Black and White Americans Over 14 Years of Follow-up. Alzheimer Dis. Assoc. Disord. 2016, 30, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Wingo, T.S.; Lah, J.J.; Levey, A.I.; Cutler, D.J. Autosomal recessive causes likely in early-onset Alzheimer disease. Arch. Neurol. 2012, 69, 59–64. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Gatz, M.; Reynolds, C.A.; Fratiglioni, L.; Johansson, B.; Mortimer, J.A.; Berg, S.; Fiske, A.; Pedersen, N.L. Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry 2006, 63, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Sims, R.; Hill, M.; Williams, J. The multiplex model of the genetics of Alzheimer’s disease. Nat. Neurosci. 2020, 23, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.R.; Foley, S.F.; Baker, E.; Bracher-Smith, M.; Holmans, P.; Stergiakouli, E.; Linden, D.E.J.; Caseras, X.; Jones, D.K.; Escott-Price, V. Pathway-specific polygenic scores for Alzheimer’s disease are associated with changes in brain structure in younger and older adults. Brain Commun. 2023, 5, fcad229. [Google Scholar] [CrossRef]
- Zhao, W.; Smith, J.A.; Wang, Y.Z.; Chintalapati, M.; Ammous, F.; Yu, M.; Moorjani, P.; Ganna, A.; Gross, A.; Dey, S.; et al. Polygenic Risk Scores for Alzheimer’s Disease and General Cognitive Function Are Associated with Measures of Cognition in Older South Asians. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Gustavson, D.E.; Reynolds, C.A.; Hohman, T.J.; Jefferson, A.L.; Elman, J.A.; Panizzon, M.S.; Neale, M.C.; Logue, M.W.; Lyons, M.J.; Franz, C.E.; et al. Alzheimer’s Disease Polygenic Scores Predict Changes in Episodic Memory and Executive Function Across 12 Years in Late Middle Age. J. Int. Neuropsychol. Soc. 2023, 29, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Darst, B.F.; Koscik, R.L.; Racine, A.M.; Oh, J.M.; Krause, R.A.; Carlsson, C.M.; Zetterberg, H.; Blennow, K.; Christian, B.T.; Bendlin, B.B.; et al. Pathway-Specific Polygenic Risk Scores as Predictors of Amyloid-β Deposition and Cognitive Function in a Sample at Increased Risk for Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Vasiljevic, E.; Deming, Y.K.; Jonaitis, E.M.; Koscik, R.L.; Van Hulle, C.A.; Lu, Q.; Carboni, M.; Kollmorgen, G.; Wild, N.; et al. Effect of Pathway-specific Polygenic Risk Scores for Alzheimer’s Disease (AD) on Rate of Change in Cognitive Function and AD-related Biomarkers among Asymptomatic Individuals. J. Alzheimers Dis. 2023, 94, 1587–1605. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.J.; Hill, W.D.; Marioni, R.E.; Davies, G.; Hagenaars, S.P.; Harris, S.E.; Cox, S.R.; Taylor, A.M.; Corley, J.; Pattie, A.; et al. Polygenic predictors of age-related decline in cognitive ability. Mol. Psychiatry 2020, 25, 2584–2598. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; King, A.E.; Bindoff, A.; Summers, M.J.; Vickers, J.C. The role of Alzheimer’s disease polygenic risk scores in changes of cognitive function in older adults: A longitudinal cohort study. Alzheimer’s Dement. 2020, 16, e037853. [Google Scholar] [CrossRef]
- Kauppi, K.; Rönnlund, M.; Adolfsson, N.A.; Pudas, S.; Adolfsson, R. Effects of polygenic risk for Alzheimer’s disease on rate of cognitive decline in normal aging. Transl. Psychiatry 2020, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Tomassen, J.; den Braber, A.; van der Lee, S.J.; Reus, L.M.; Konijnenberg, E.; Carter, S.F.; Yaqub, M.; van Berckel, B.N.M.; Collij, L.E.; Boomsma, D.I.; et al. Amyloid-β and APOE genotype predict memory decline in cognitively unimpaired older individuals independently of Alzheimer’s disease polygenic risk score. BMC Neurol. 2022, 22, 484. [Google Scholar] [CrossRef]
- Ge, T.; Sabuncu, M.R.; Smoller, J.W.; Sperling, R.A.; Mormino, E.C.; Alzheimer’s Disease Neuroimaging Initiative. Dissociable influences of APOE ε4 and polygenic risk of AD dementia on amyloid and cognition. Neurology 2018, 90, e1605–e1612. [Google Scholar] [CrossRef]
- Mormino, E.C.; Sperling, R.A.; Holmes, A.J.; Buckner, R.L.; De Jager, P.L.; Smoller, J.W.; Sabuncu, M.R.; Alzheimer’s Disease Neuroimaging Initiative. Polygenic risk of Alzheimer disease is associated with early- and late-life processes. Neurology 2016, 87, 481–488. [Google Scholar] [CrossRef]
- Pettigrew, C.; Nazarovs, J.; Soldan, A.; Singh, V.; Wang, J.; Hohman, T.; Dumitrescu, L.; Libby, J.; Kunkle, B.; Gross, A.L.; et al. Alzheimer’s disease genetic risk and cognitive reserve in relationship to long-term cognitive trajectories among cognitively normal individuals. Alzheimers Res. Ther. 2023, 15, 66. [Google Scholar] [CrossRef]
- Manzali, S.B.; Yu, E.; Ravona-Springer, R.; Livny, A.; Golan, S.; Ouyang, Y.; Lesman-Segev, O.; Liu, L.; Ganmore, I.; Alkelai, A.; et al. Alzheimer’s Disease Polygenic Risk Score Is Not Associated with Cognitive Decline Among Older Adults with Type 2 Diabetes. Front. Aging Neurosci. 2022, 14, 853695. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.; Burnham, S.C.; Milicic, L.; Savage, G.; Maruff, P.; Lim, Y.Y.; Li, Q.X.; Ames, D.; Masters, C.L.; Rainey-Smith, S.; et al. Utility of an Alzheimer’s Disease Risk-Weighted Polygenic Risk Score for Predicting Rates of Cognitive Decline in Preclinical Alzheimer’s Disease: A Prospective Longitudinal Study. J. Alzheimers Dis. 2018, 66, 1193–1211. [Google Scholar] [CrossRef]
- Rutten-Jacobs, L.C.A.; Tozer, D.J.; Duering, M.; Malik, R.; Dichgans, M.; Markus, H.S.; Traylor, M. Genetic Study of White Matter Integrity in UK Biobank (N=8448) and the Overlap with Stroke, Depression, and Dementia. Stroke 2018, 49, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Craik, F.I.M. Memory, aging and the brain: Old findings and current issues. Aging Brain 2023, 4, 100096. [Google Scholar] [CrossRef] [PubMed]
- Proust-Lima, C.; Amieva, H.; Letenneur, L.; Orgogozo, J.M.; Jacqmin-Gadda, H.; Dartigues, J.F. Gender and education impact on brain aging: A general cognitive factor approach. Psychol. Aging 2008, 23, 608–620. [Google Scholar] [CrossRef] [PubMed]
- de Frias, C.M.; Nilsson, L.G.; Herlitz, A. Sex differences in cognition are stable over a 10-year period in adulthood and old age. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2006, 13, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Wetherell, J.L.; Reynolds, C.A.; Gatz, M.; Pedersen, N.L. Anxiety, cognitive performance, and cognitive decline in normal aging. J. Gerontol. B Psychol. Sci. Soc. Sci. 2002, 57, P246–P255. [Google Scholar] [CrossRef]
- Anstey, K.J.; Ehrenfeld, L.; Mortby, M.E.; Cherbuin, N.; Peters, R.; Kiely, K.M.; Eramudugolla, R.; Huque, M.H. Gender differences in cognitive development in cohorts of young, middle, and older adulthood over 12 years. Dev. Psychol. 2021, 57, 1403–1410. [Google Scholar] [CrossRef]
- Tschanz, J.T.; Corcoran, C.D.; Schwartz, S.; Treiber, K.; Green, R.C.; Norton, M.C.; Mielke, M.M.; Piercy, K.; Steinberg, M.; Rabins, P.V.; et al. Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: The Cache County Dementia Progression study. Am. J. Geriatr. Psychiatry 2011, 19, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Agüero-Torres, H.; Fratiglioni, L.; Guo, Z.; Viitanen, M.; Winblad, B. Prognostic factors in very old demented adults: A seven-year follow-up from a population-based survey in Stockholm. J. Am. Geriatr. Soc. 1998, 46, 444–452. [Google Scholar] [CrossRef]
- Tifratene, K.; Robert, P.; Metelkina, A.; Pradier, C.; Dartigues, J.F. Progression of mild cognitive impairment to dementia due to AD in clinical settings. Neurology 2015, 85, 331–338. [Google Scholar] [CrossRef]
- van Loenhoud, A.C.; van der Flier, W.M.; Wink, A.M.; Dicks, E.; Groot, C.; Twisk, J.; Barkhof, F.; Scheltens, P.; Ossenkoppele, R.; Alzheimer’s Disease Neuroimaging Initiative. Cognitive reserve and clinical progression in Alzheimer disease: A paradoxical relationship. Neurology 2019, 93, e334–e346. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, N.C.; Cribbs, D.H.; Coleman, P.D.; Rogers, J.; Head, E.; Kim, R.; Beach, T.; Miller, C.; Troncoso, J.; Trojanowski, J.Q.; et al. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl. Acad. Sci. USA 2008, 105, 15605–15610. [Google Scholar] [CrossRef]
- Hobel, Z.; Isenberg, A.L.; Raghupathy, D.; Mack, W.; Pa, J.; Alzheimer’s Disease Neuroimaging Initiative and the Australian Imaging Biomarkers and Lifestyle flagship study of ageing. APOEɛ4 Gene Dose and Sex Effects on Alzheimer’s Disease MRI Biomarkers in Older Adults with Mild Cognitive Impairment. J. Alzheimers Dis. 2019, 71, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Vemuri, P.; Rocca, W.A. Clinical epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clin. Epidemiol. 2014, 6, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; Ferreira Santos-Galduróz, R.; Ferri, C.P.; Fernandes Galduróz, J.C. Rate of cognitive decline in relation to sex after 60 years-of-age: A systematic review. Geriatr. Gerontol. Int. 2014, 14, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y. Cognitive reserve: Implications for diagnosis and prevention of Alzheimer’s disease. Curr. Neurol. Neurosci. Rep. 2004, 4, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, T.; McClendon, M.J.; Smyth, K.A.; Ogrocki, P.K. Effects of educational attainment and occupational status on cognitive and functional decline in persons with Alzheimer-type dementia. Int. Psychogeriatr. 2002, 14, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y.; Albert, S.; Tang, M.X.; Tsai, W.Y. Rate of memory decline in AD is related to education and occupation: Cognitive reserve? Neurology 1999, 53, 1942–1947. [Google Scholar] [CrossRef] [PubMed]
- Singh-Manoux, A.; Marmot, M.G.; Glymour, M.; Sabia, S.; Kivimäki, M.; Dugravot, A. Does cognitive reserve shape cognitive decline? Ann. Neurol. 2011, 70, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Soldan, A.; Pettigrew, C.; Cai, Q.; Wang, J.; Wang, M.C.; Moghekar, A.; Miller, M.I.; Albert, M.; BIOCARD Research Team. Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease. Neurobiol. Aging 2017, 60, 164–172. [Google Scholar] [CrossRef]
- Mungas, D.; Early, D.R.; Glymour, M.M.; Zeki Al Hazzouri, A.; Haan, M.N. Education, bilingualism, and cognitive trajectories: Sacramento Area Latino Aging Study (SALSA). Neuropsychology. 2018, 32, 77–88. [Google Scholar] [CrossRef]
- Karlamangla, A.S.; Miller-Martinez, D.; Aneshensel, C.S.; Seeman, T.E.; Wight, R.G.; Chodosh, J. Trajectories of cognitive function in late life in the United States: Demographic and socioeconomic predictors. Am. J. Epidemiol. 2009, 170, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Dardiotis, E.; Kosmidis, M.H.; Yannakoulia, M.; Hadjigeorgiou, G.M.; Scarmeas, N. The Hellenic Longitudinal Investigation of Aging and Diet (HELIAD): Rationale, study design, and cohort description. Neuroepidemiology 2014, 43, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, C.A.; Yannakoulia, M.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Arampatzi, X.; Bougea, A.; Labropoulos, I.; Scarmeas, N. Mediterranean diet and cognitive health: Initial results from the Hellenic longitudinal investigation of ageing and diet. PLoS ONE 2017, 12, e0182048. [Google Scholar] [CrossRef]
- Mourtzi, N.; Charisis, S.; Tsapanou, A.; Ntanasi, E.; Hatzimanolis, A.; Ramirez, A.; Heilmann-Heimbach, S.; Grenier-Boley, B.; Lambert, J.C.; Yannakoulia, M.; et al. Genetic propensity for cerebral amyloidosis and risk of mild cognitive impairment and Alzheimer’s disease within a cognitive reserve framework. Alzheimer’s Dement. 2023, 19, 3794–3805. [Google Scholar] [CrossRef]
- Sampatakakis, S.N.; Mourtzi, N.; Charisis, S.; Mamalaki, E.; Ntanasi, E.; Hatzimanolis, A.; Ramirez, A.; Lambert, J.C.; Yannakoulia, M.; Kosmidis, M.H.; et al. Genetic Predisposition for White Matter Hyperintensities and Risk of Mild Cognitive Impairment and Alzheimer’s Disease: Results from the HELIAD Study. Curr. Issues Mol. Biol. 2024, 46, 934–947. [Google Scholar] [CrossRef]
- Deming, Y.; Li, Z.; Kapoor, M.; Harari, O.; Del-Aguila, J.L.; Black, K.; Carrell, D.; Cai, Y.; Fernandez, M.V.; Budde, J.; et al. Genome-wide association study identifies four novel loci associated with Alzheimer’s endophenotypes and disease modifiers. Acta Neuropathol. 2017, 133, 839–856. [Google Scholar] [CrossRef]
- Sargurupremraj, M.; Suzuki, H.; Jian, X.; Sarnowski, C.; Evans, T.E.; Bis, J.C.; Eiriksdottir, G.; Sakaue, S.; Terzikhan, N.; Habes, M.; et al. Cerebral small vessel disease genomics and its implications across the lifespan. Nat. Commun. 2020, 11, 6285. [Google Scholar] [CrossRef] [PubMed]
- Tsapanou, A.; Mourtzi, N.; Charisis, S.; Hatzimanolis, A.; Ntanasi, E.; Kosmidis, M.H.; Yannakoulia, M.; Hadjigeorgiou, G.; Dardiotis, E.; Sakka, P.; et al. Sleep Polygenic Risk Score Is Associated with Cognitive Changes over Time. Genes 2021, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Kapral, M.K.; Vyas, M.V.; Fang, J.; Yu, A.Y.X. Using Multilevel Models and Generalized Estimating Equation Models to Account for Clustering in Neurology Clinical Research. Neurology 2024, 103, e209947. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Vlahou, C.H.; Kosmidis, M.H.; Dardagani, A.; Tsotsi, S.; Giannakou, M.; Giazkoulidou, A.; Zervoudakis, E.; Pontikakis, N. Development of the Greek Verbal Learning Test: Reliability, Construct Validity, and Normative Standards. Arch. Clin. Neuropsychol. 2012, 28, 52–64. [Google Scholar] [CrossRef]
- Ingram, F.; Soukup, V.M.; Ingram, P.T. The Medical College of Georgia Complex Figures: Reliability and preliminary normative data using an intentional learning paradigm in older adults. J. Int. Neuropsychol. Soc. 1997, 10, 144–146. [Google Scholar]
- Kosmidis, M.H.; Vlahou, C.H.; Panagiotaki, P.; Kiosseoglou, G. The verbal fluency task in the Greek population: Normative data, and clustering and switching strategies. J. Int. Neuropsychol. Soc. 2004, 10, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Tsapkini, K.; Vlahou, C.H.; Potagas, C. Adaptation and validation of standardized aphasia tests in different languages: Lessons from the Boston Diagnostic Aphasia Examination - Short Form in Greek. Behav. Neurol. 2009, 22, 111–119. [Google Scholar] [CrossRef]
- Vlahou, C.H.; Kosmidis, M.H. The Greek trail making test: Preliminary norms for clinical and research use. Psychol. J. Hell Psychol. Soc. 2002, 9, 336–352. (In Greek) [Google Scholar]
- Kosmidis, M.H.; Tsotsi, S.; Karambela, O.; Takou, E.; Vlahou, C.H. Cultural factors influencing performance on visuo- perceptual neuropsychological tasks. Behav. Neurol. 2010, 23, 245–247. [Google Scholar] [CrossRef]
- Bozikas, V.P.; Giazkoulidou, A.; Hatzigeorgiadou, M.; Karavatos, A.; Kosmidis, M.H. Do age and education contribute to performance on the clock drawing test? Normative data for the Greek population. J. Clin. Exp. Neuropsychol. 2008, 30, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Giaglis, G.; Kyriazidou, S.; Paraskevopoulou, E.; Taskos, N.; Kosmidis, M.H. Evaluating premorbid level: Preliminary findings regarding the vulnerability of scores on cognitive measures in patients with MS. J. Int. Neuropsychol. Soc. 2010, 15. [Google Scholar]
- Grove, M.L.; Yu, B.; Cochran, B.J.; Haritunians, T.; Bis, J.C.; Taylor, K.D.; Hansen, M.; Borecki, I.B.; Cupples, L.A.; Fornage, M.; et al. Best Practices and Joint Calling of the HumanExome BeadChip: The CHARGE Consortium. PLoS ONE 2013, 8, e68095. [Google Scholar] [CrossRef]
- Abraham, G.; Qiu, Y.; Inouye, M. FlashPCA2: Principal component analysis of Biobank-scale genotype datasets. Bioinformatics 2017, 33, 2776–2778. [Google Scholar] [CrossRef]
- McCarthy, S.; Das, S.; Kretzschmar, W.; Delaneau, O.; Wood, A.R.; Teumer, A.; Kang, H.M.; Fuchsberger, C.; Danecek, P.; Sharp, K.; et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 2016, 48, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alfoldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Marchini, J.; Howie, B.; Myers, S.; McVean, G.; Donnelly, P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat. Genet. 2007, 39, 906–913. [Google Scholar] [CrossRef]
- Das, S.; Forer, L.; Schönherr, S.; Sidore, C.; Locke, A.E.; Kwong, A.; Vrieze, S.I.; Chew, E.Y.; Levy, S.; McGue, M.; et al. Next-generation genotype imputation service and methods. Nat. Genet. 2016, 48, 1284–1287. [Google Scholar] [CrossRef]
- Wang, K.; Hu, X.; Peng, Y. An Analytical Comparison of the Principal Component Method and the Mixed Effects Model for Association Studies in the Presence of Cryptic Relatedness and Population Stratification. Hum. Hered. 2013, 76, 1–9. [Google Scholar] [CrossRef]
- Reese, S.E.; Archer, K.J.; Therneau, T.M.; Atkinson, E.J.; Vachon, C.M.; De Andrade, M.; Kocher, J.-P.A.; Eckel-Passow, J.E. A new statistic for identifying batch effects in high-throughput genomic data that uses guided principal component analysis. Bioinformatics 2013, 29, 2877–2883. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).