Biotin Induces Inactive Chromosome X Reactivation and Corrects Physiopathological Alterations in Beta-Propeller-Protein-Associated Neurodegeneration
Abstract
1. Introduction
2. Results
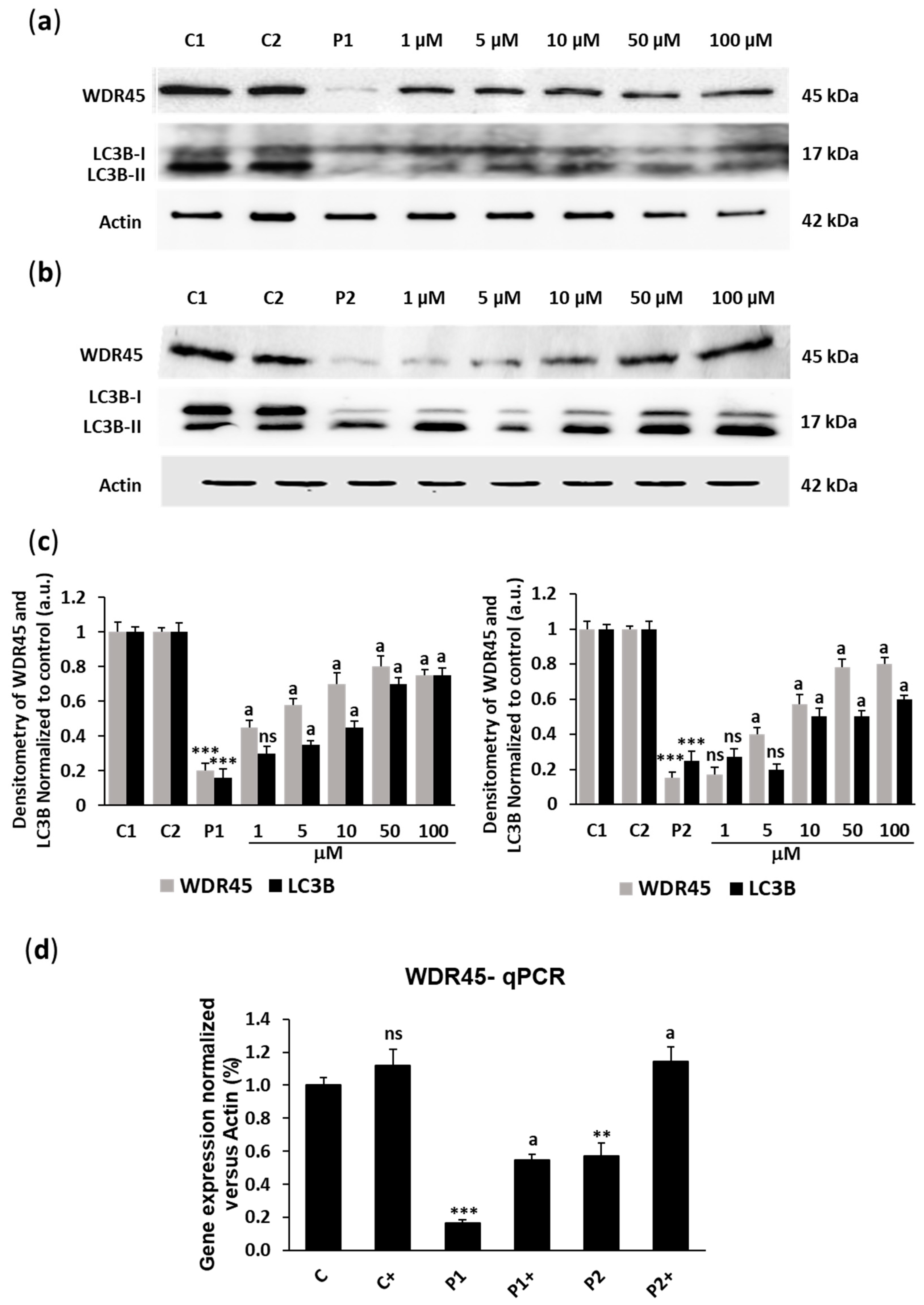
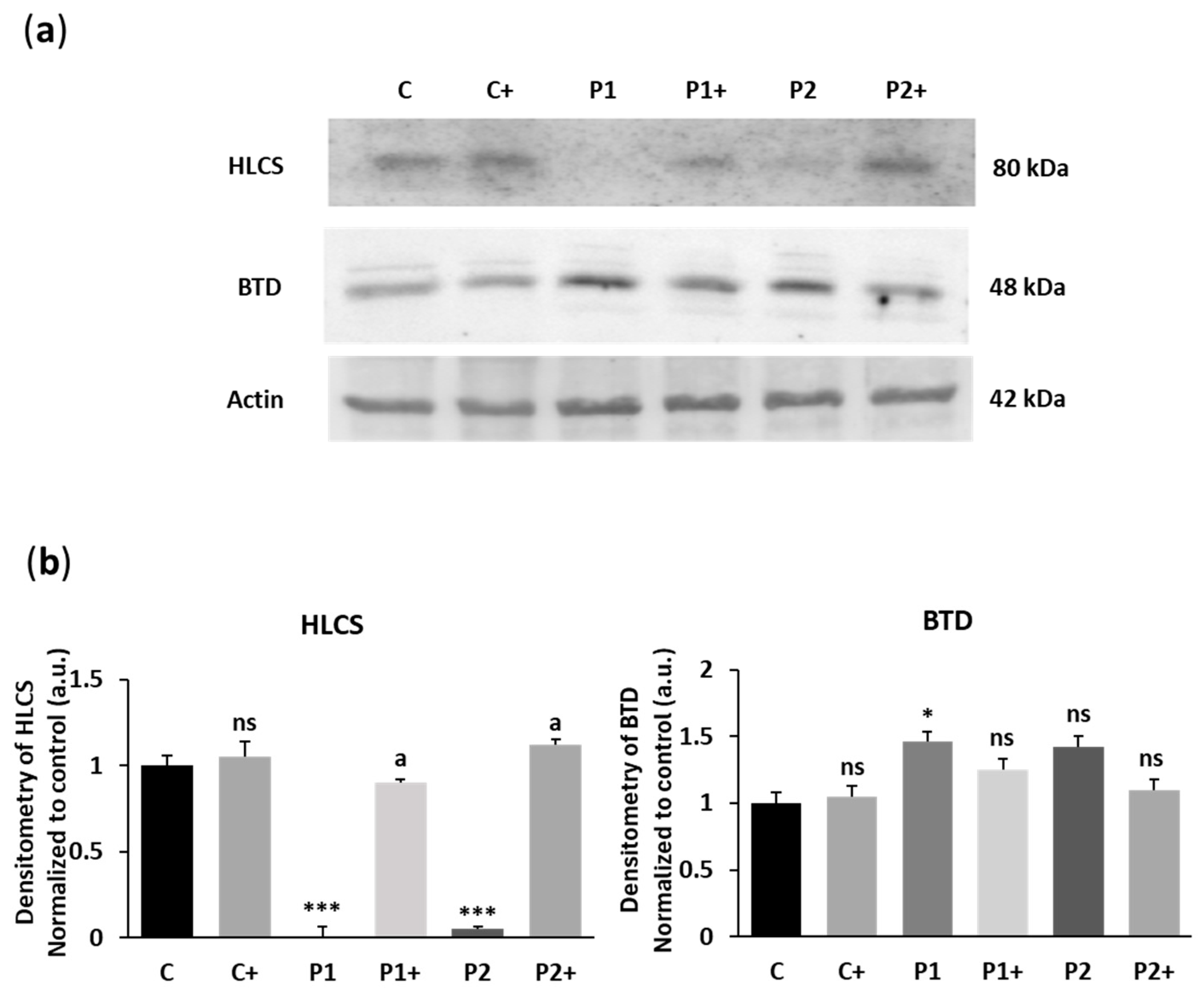
2.1. Biotin Supplementation Increases WDR45 Protein and Transcript Expression Levels in BPAN Fibroblasts
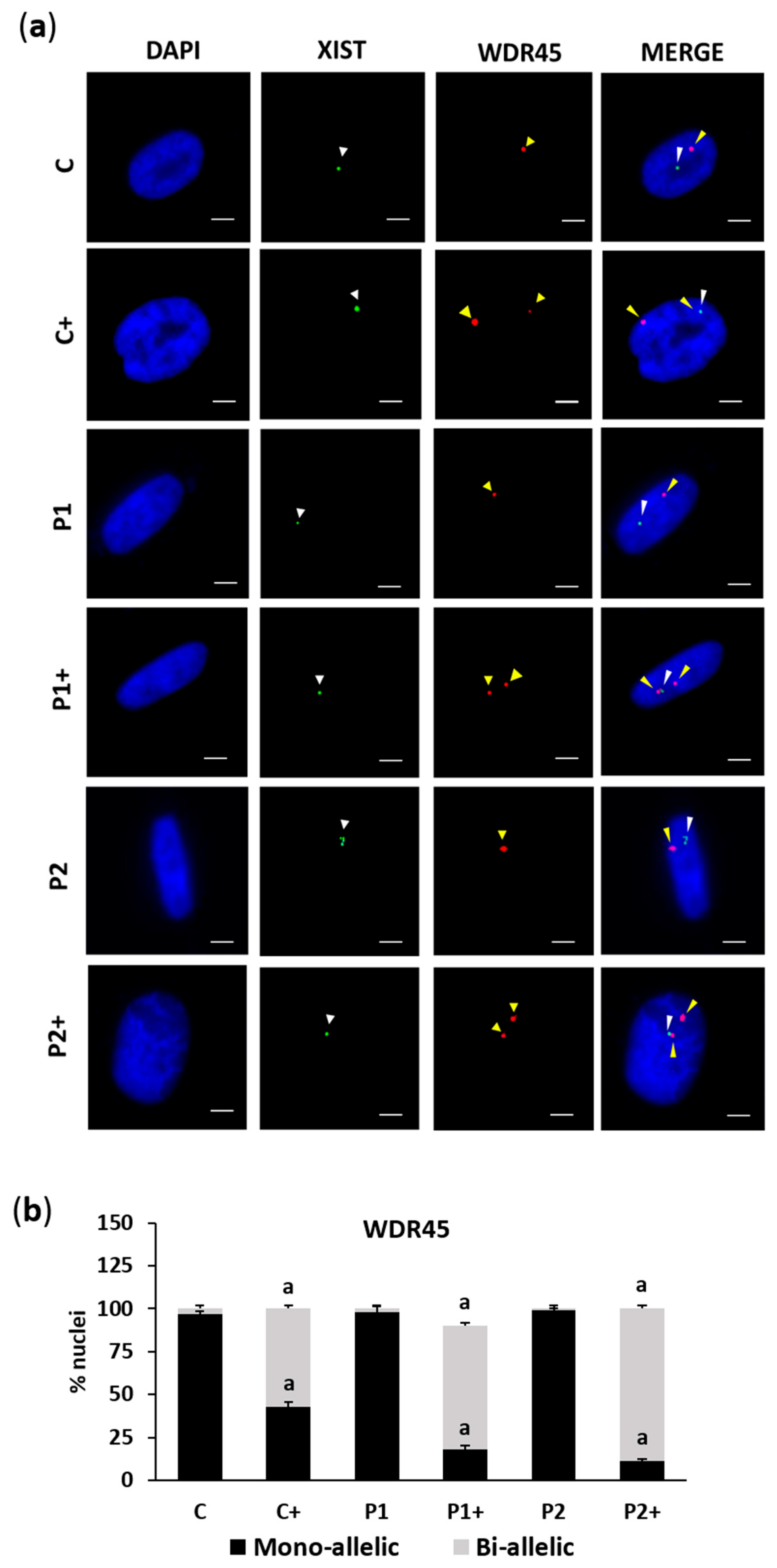
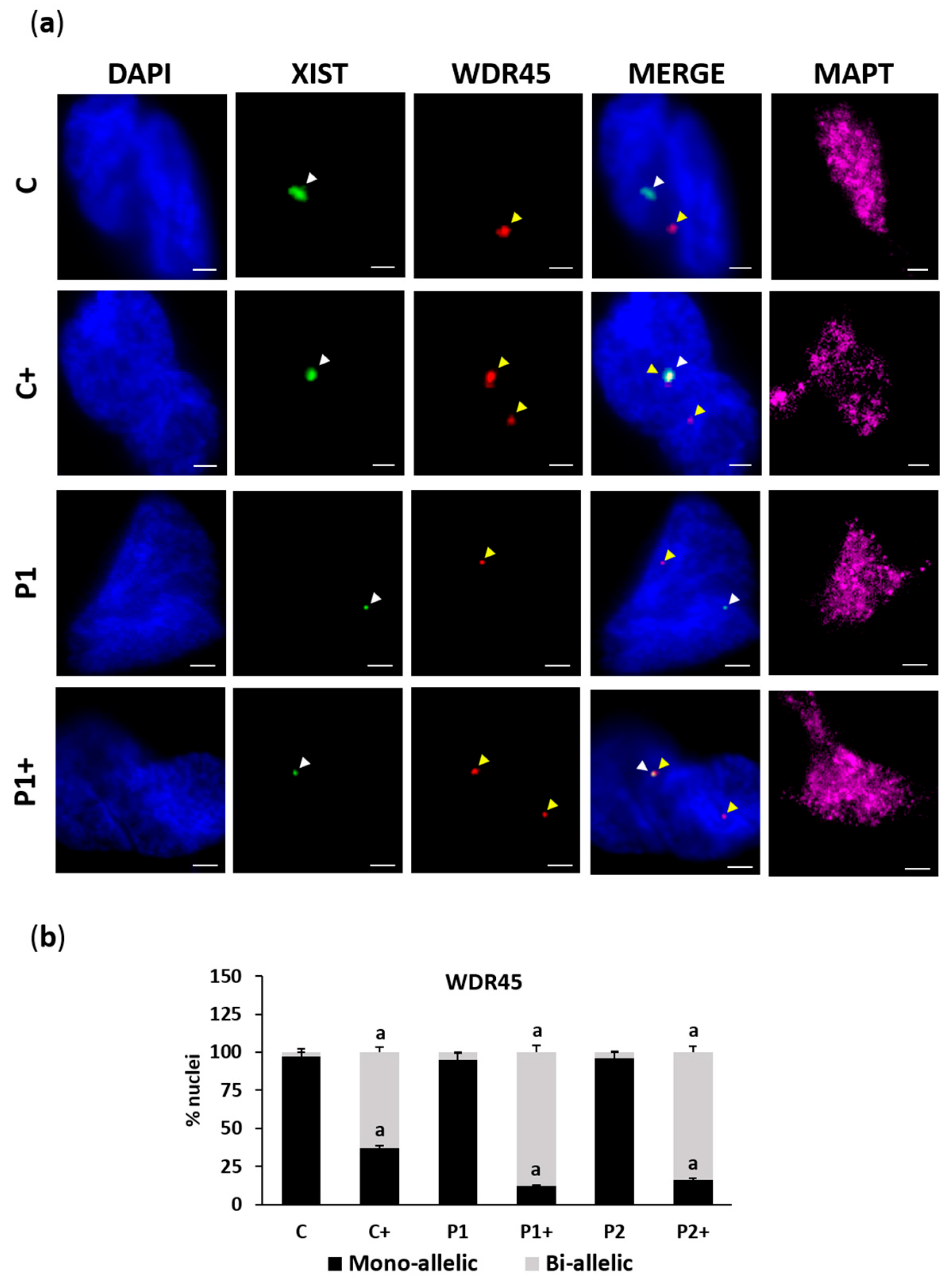
2.2. Biotin Supplementation Induces Inactive Chromosome X Reactivation and Increases WDR45 Transcript Expression in BPAN Fibroblasts
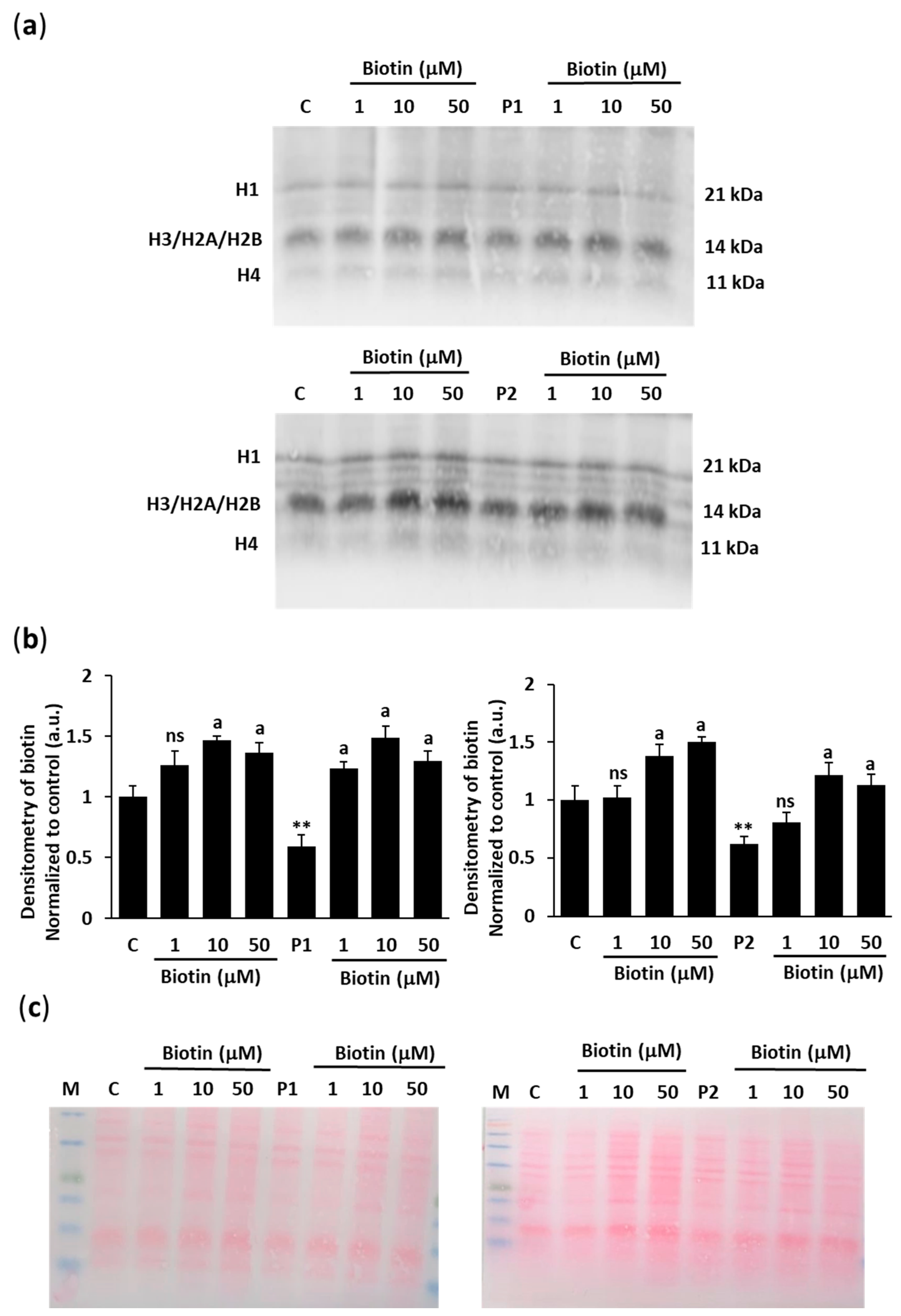
2.3. Biotin Supplementation Increases Histone Biotinylation in BPAN Fibroblasts
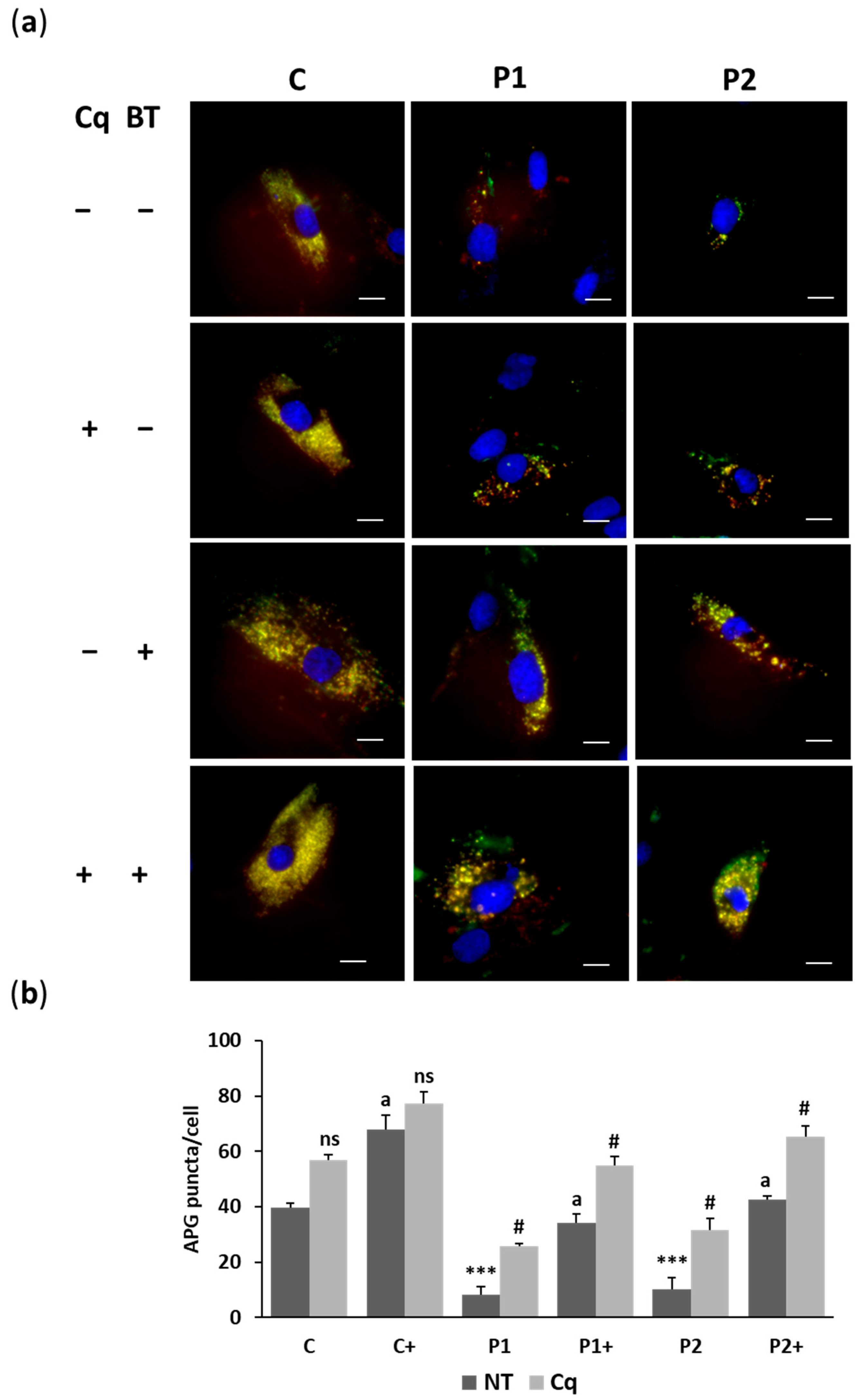
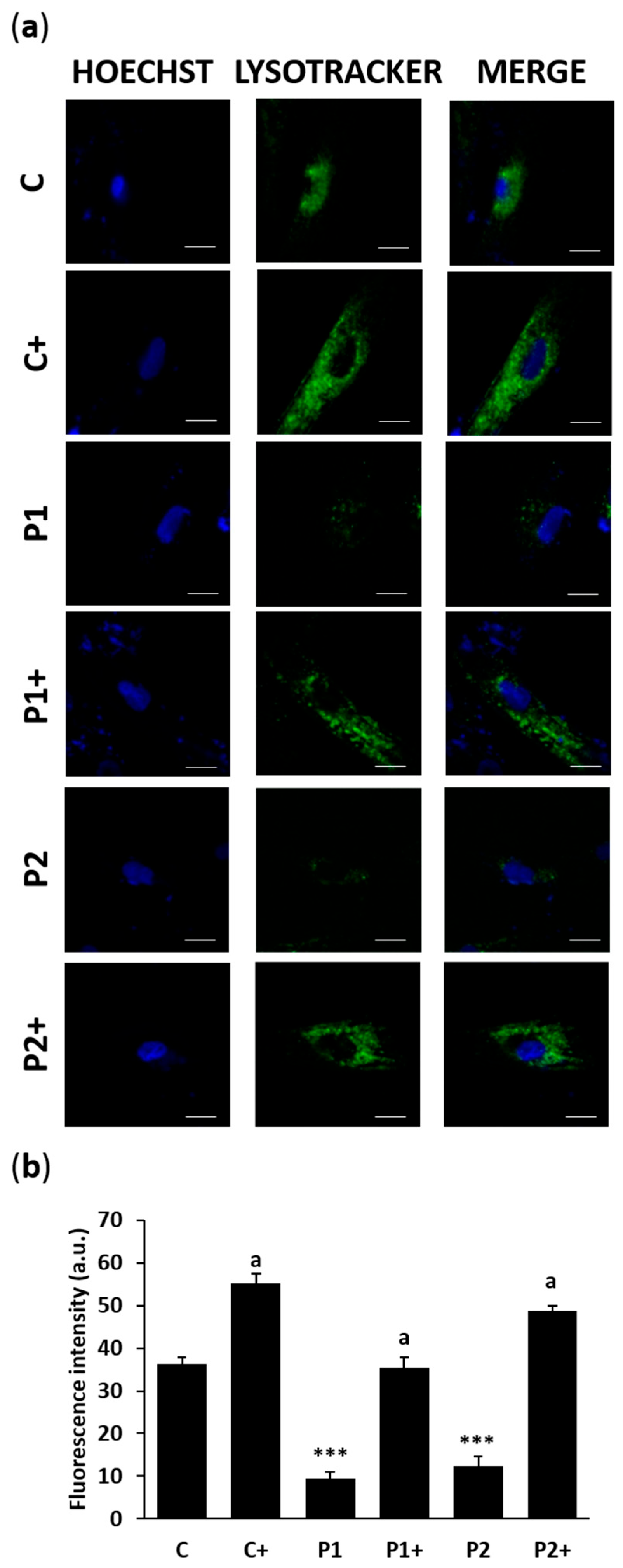
2.4. Biotin Treatment Restores Autophagosome Formation and Lysosome Acidification in BPAN Fibroblasts
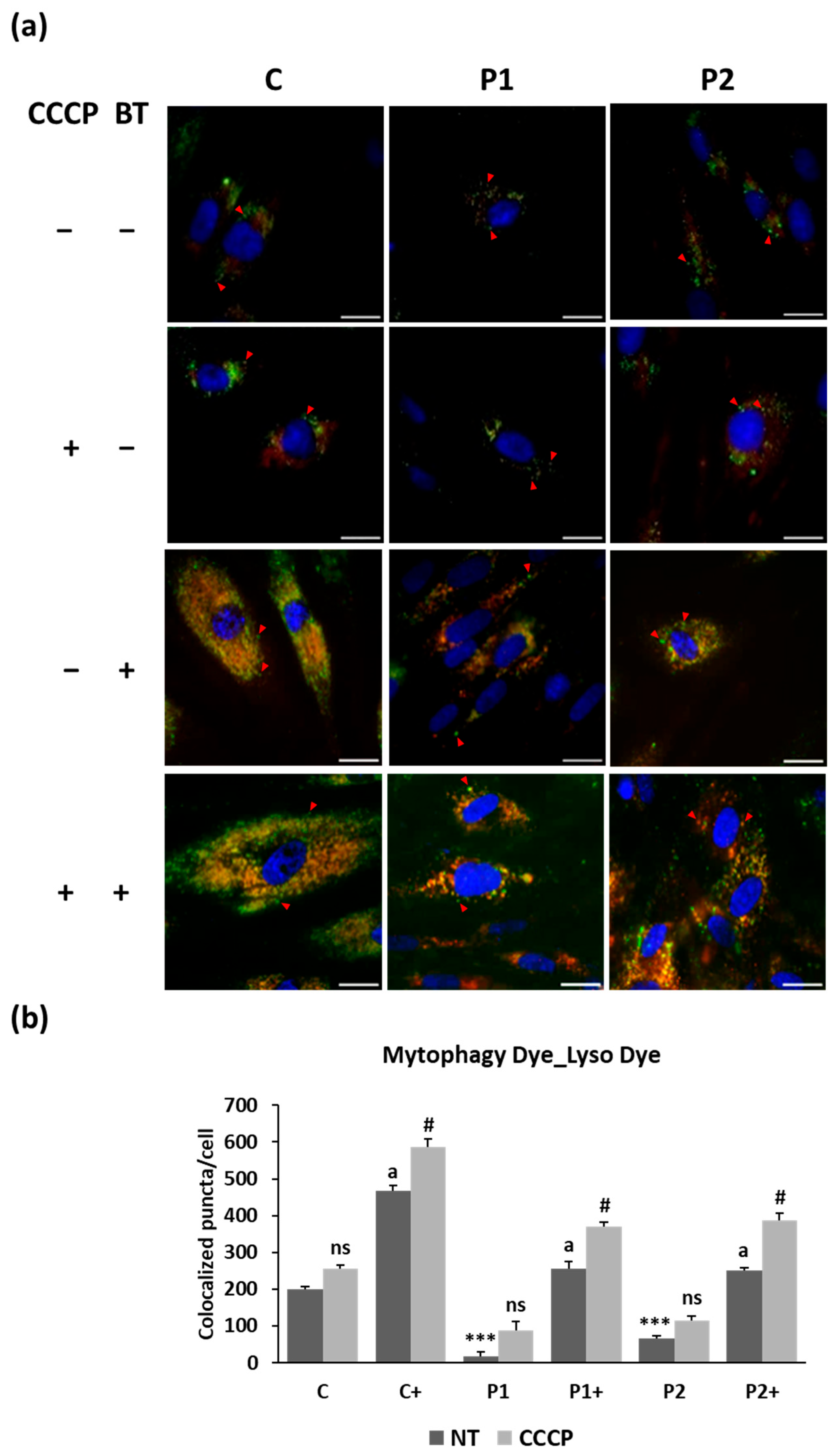
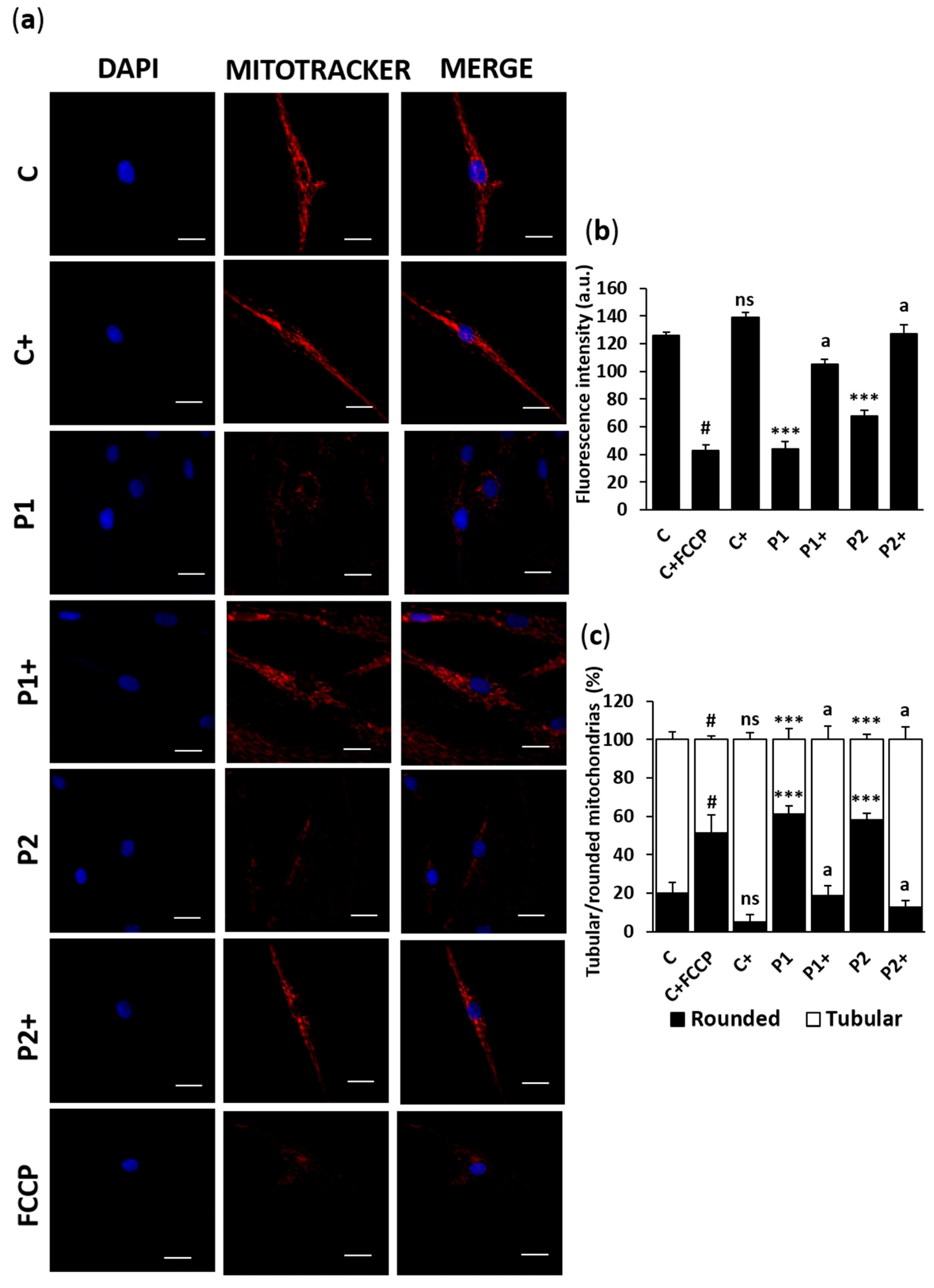
2.5. Biotin Supplementation Improves Mitophagy Activity in BPAN Fibroblasts
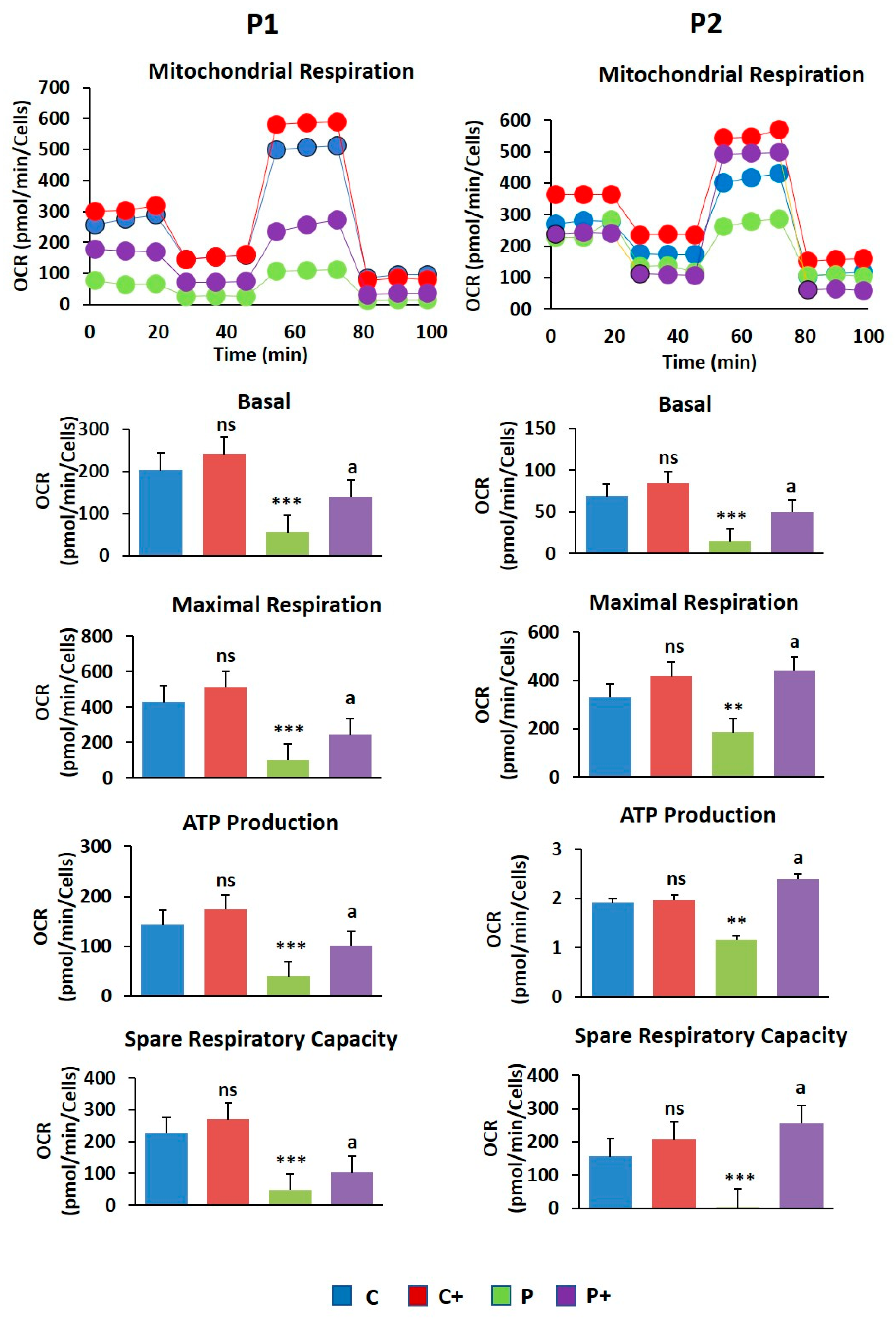
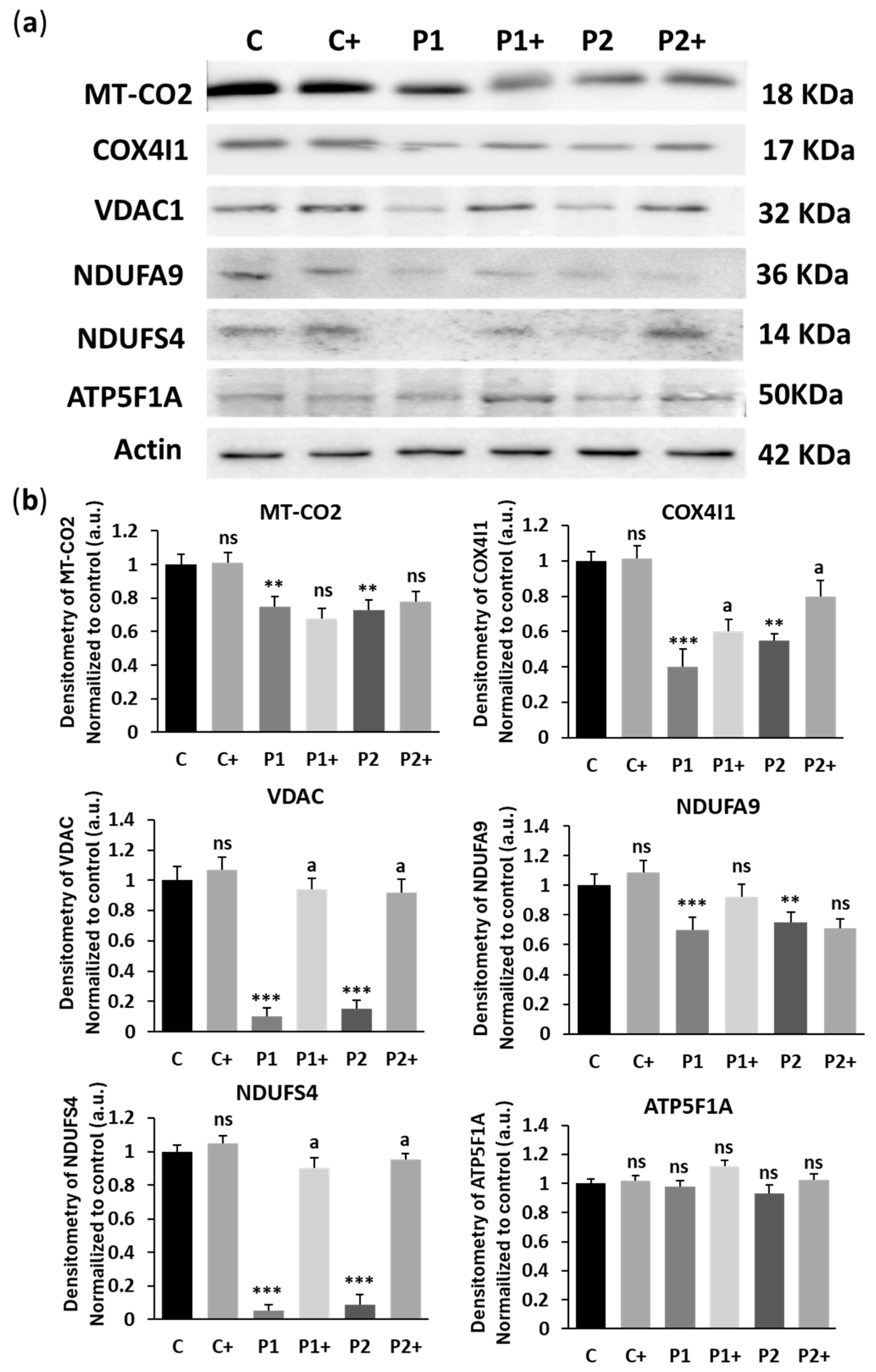
2.6. Biotin Treatment Increases Mitochondrial Bioenergetics in BPAN Fibroblasts
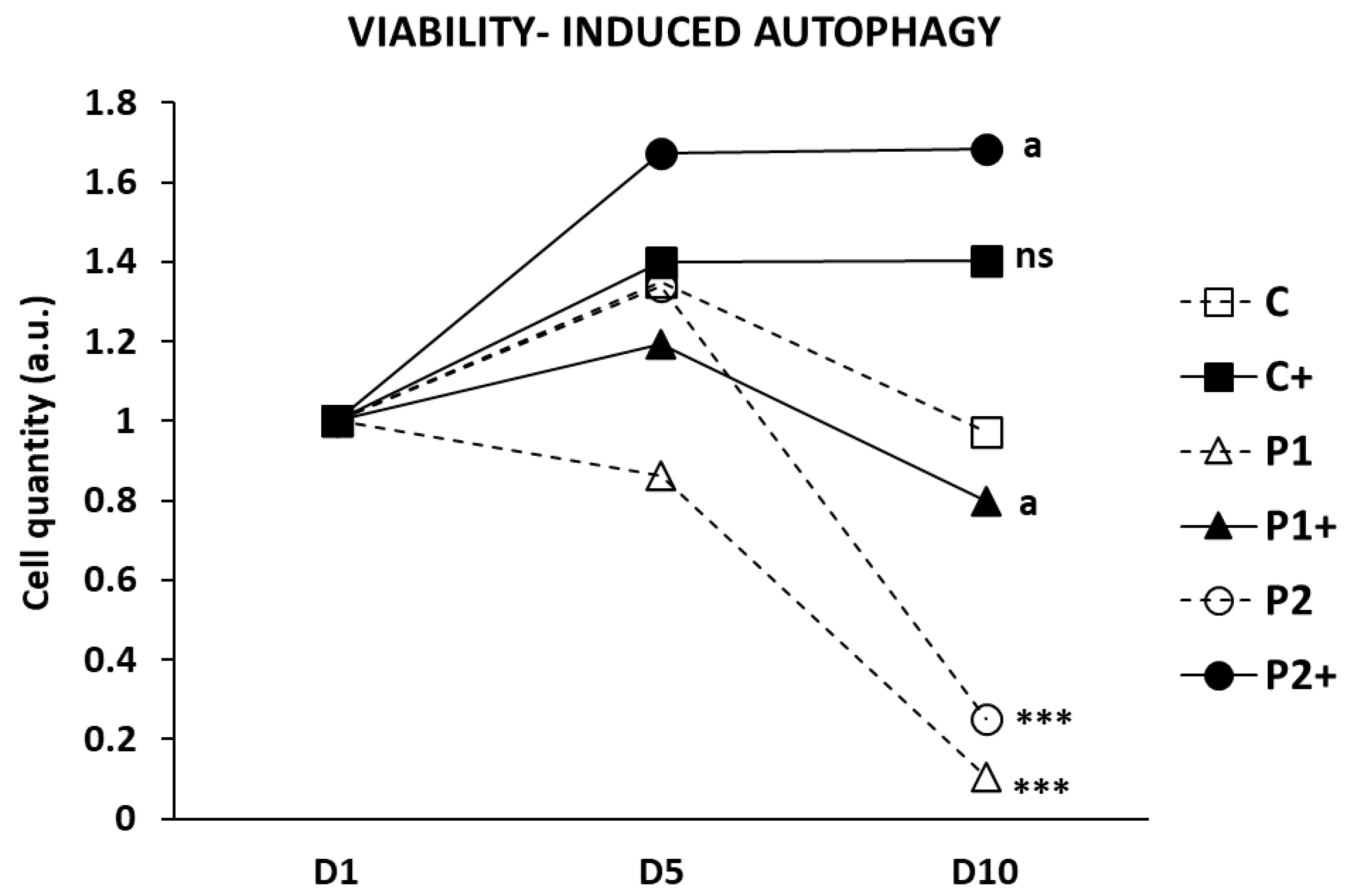
2.7. Biotin Supplementation Increases BPAN Fibroblast Viability in Serum-Free Medium
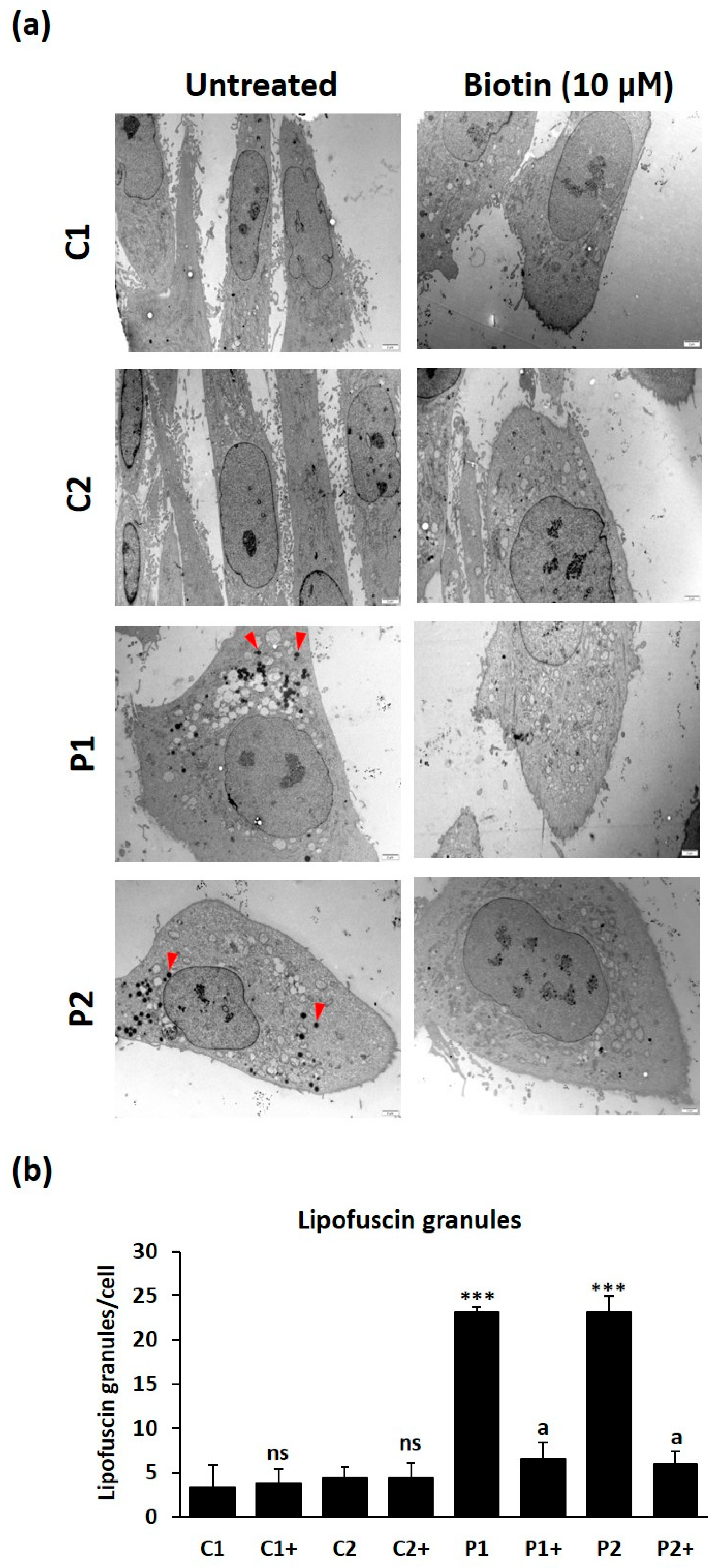
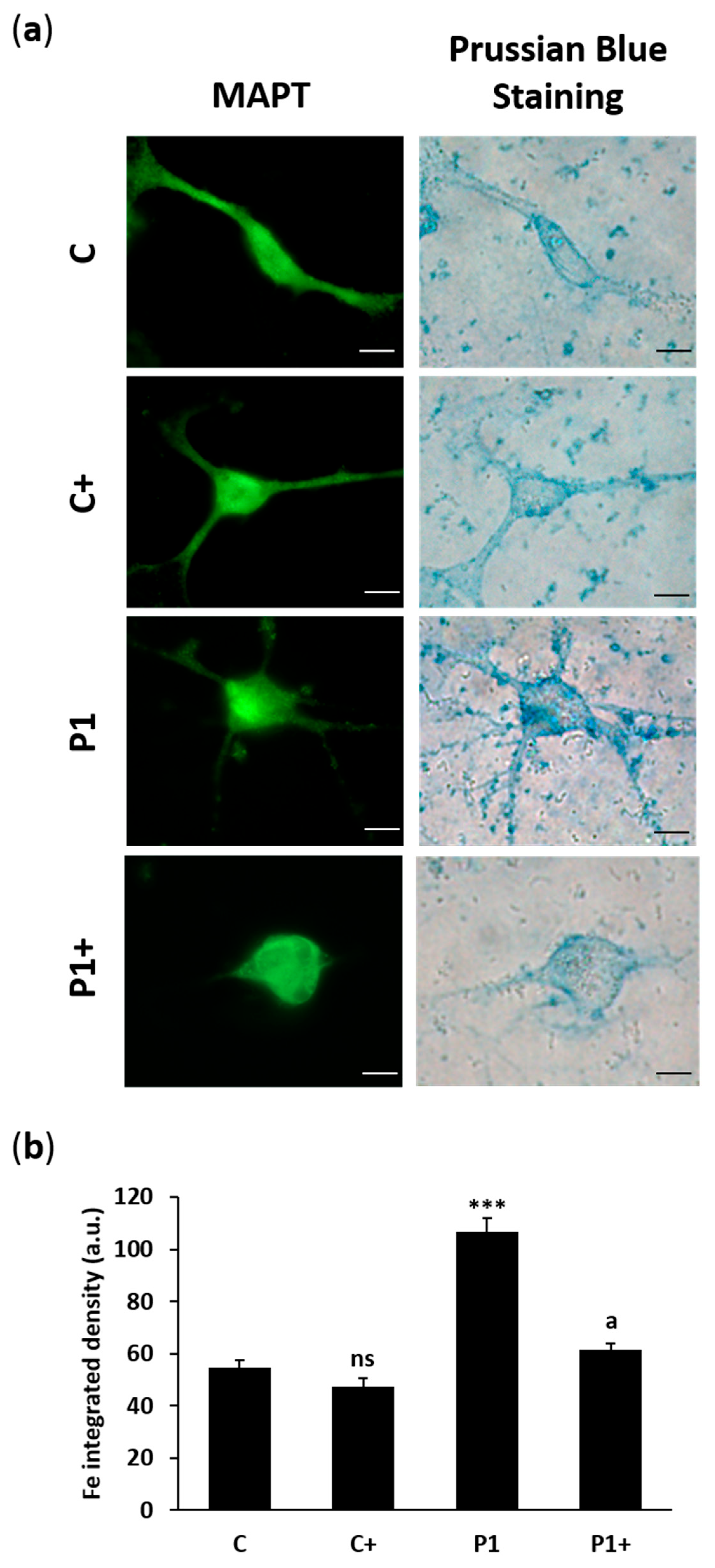
2.8. Biotin Supplementation Prevents Iron and Lipofuscin Accumulation in BPAN Fibroblasts
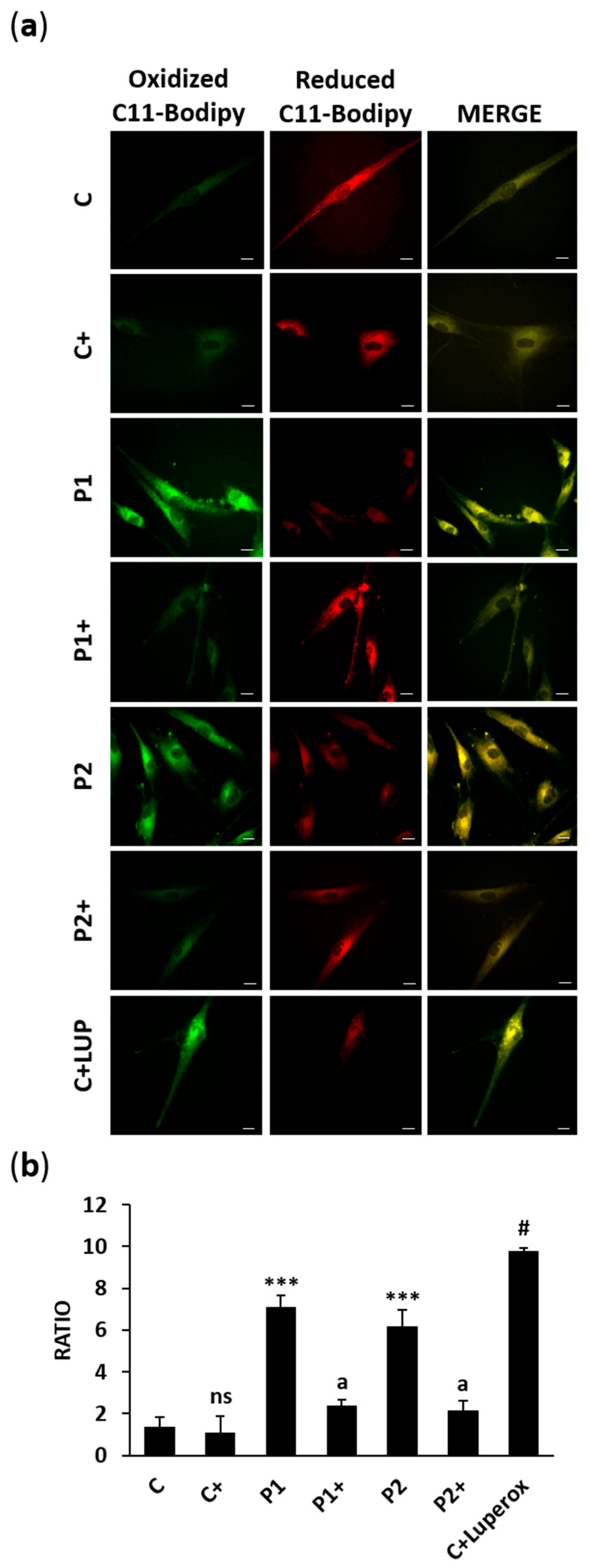
2.9. Biotin Treatment Prevents Lipid Peroxidation in BPAN Fibroblasts
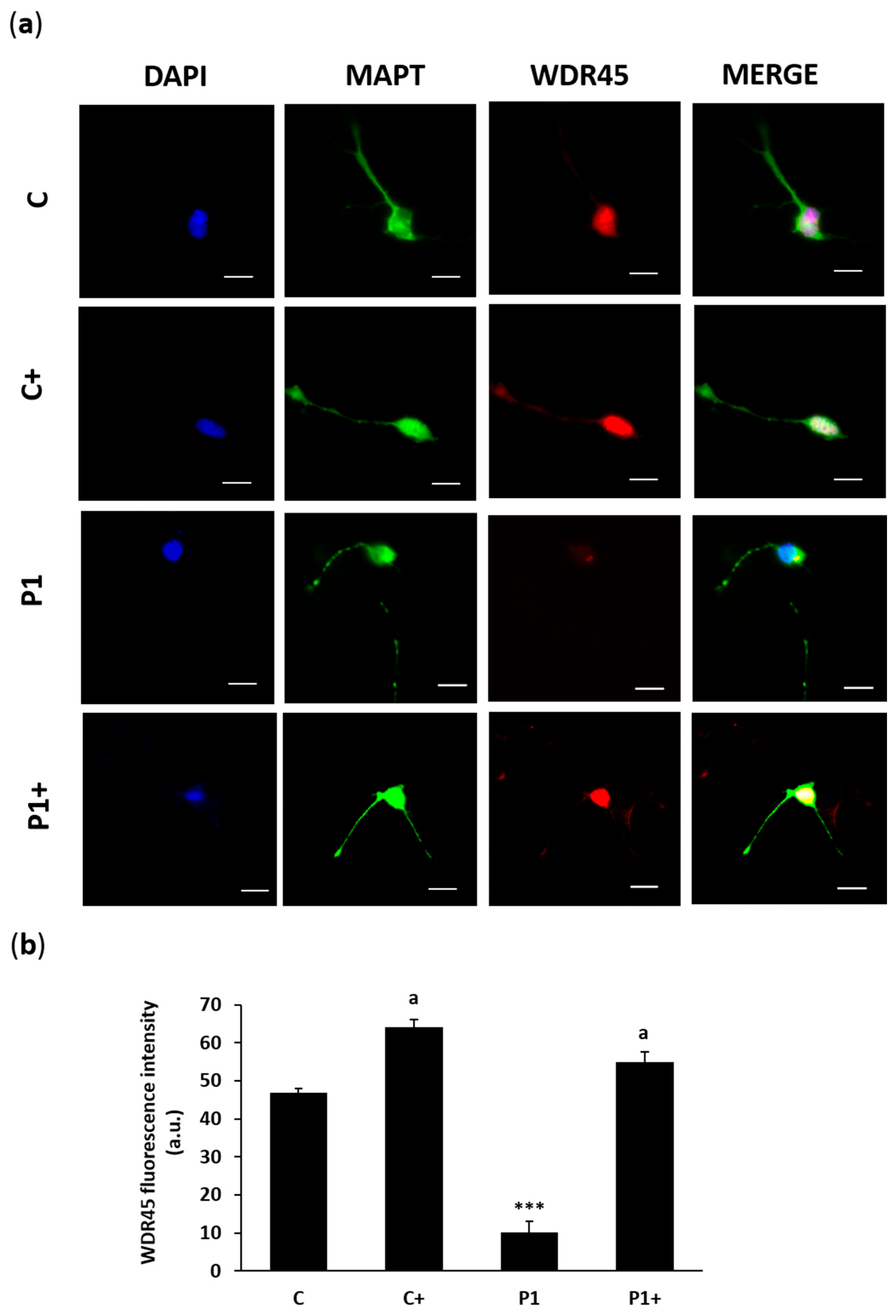
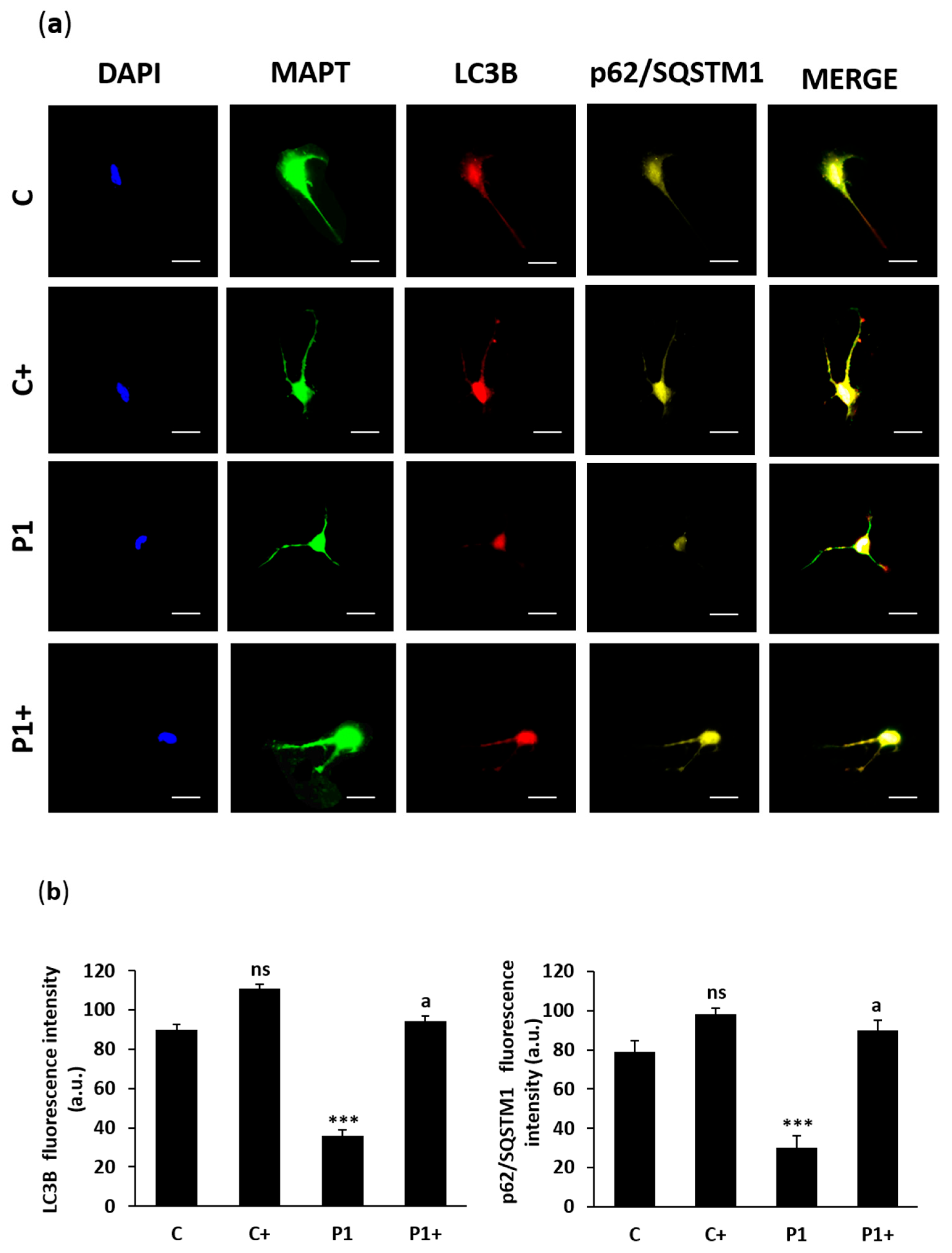
2.10. Effect of Biotin Supplementation on BPAN-Induced Neurons (iNs)
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Patient Cell Culture
4.3. Immunoblotting
4.4. Real-Time Quantitative PCR
4.5. Immunofluorescence Microscopy
4.6. RNA FISH
4.7. Histone Biotinylation
4.8. Tandem Sensor RFP- GFP-LC3B
4.9. Lysotracker Staining
4.10. Mitophagy Assay
4.11. Bioenergetic and Oxidative Stress Analysis
4.12. Analysis of the Mitochondrial Network by Mitotracker Staining
4.13. Cell Viability Assay
4.14. Prussian Blue Staining and ICP-MS
4.15. Labile Iron Pool (LIP) Determination
4.16. Lipofuscin Accumulation
4.17. TEM Analysis
4.18. Lipid Peroxidation
4.19. Direct Reprograming
4.20. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP5F1A | ATP-synthase F1 subunit 1 alpha |
| BPAN | beta-propeller-protein-associated neurodegeneration |
| BTD | biotinidase |
| CCCP | carbonyl cyanide m-chlorophenylhydrazone |
| COX4I1 | cytochrome c oxidase subunit 4I1 |
| Cq | chloroquine |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s modified eagle’s medium |
| ER | endoplasmic reticulum |
| FBS | fetal bovine serum |
| FCCP | carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone |
| FISH | fluorescence in situ hybridization |
| HLCS | holocarboxylase synthetase |
| ICP-MS | inductively coupled plasma mass spectrometry |
| iNs | induced neurons |
| KO | knockout |
| LC3B | microtubule-associated protein 1 light chain 3 beta |
| MAPT | microtubule-associated protein tau |
| MT-CO2 | mitochondrially encoded cytochrome c oxidase II |
| NBIA | neurodegeneration with brain iron accumulation |
| NDUFA9 | NADH:ubiquinone oxidoreductase subunit A9 |
| NDUFS4 | NADH:ubiquinone oxidoreductase subunit S4 |
| PTMs | post-translational modifications |
| p62/SQSTM1 | sequestosome 1 |
| TEM | transmission electron microscope |
| VDAC1 | voltage dependent anion channel 1 |
| WDR45 | WD repeat domain 45 |
| XCI | X-chromosome inactivation |
| Xi | inactive chromosome X |
| Xist | X-inactive-specific transcript |
References
- Ganz, T. Systemic iron homeostasis. Physiol. Rev. 2013, 93, 1721–1741. [Google Scholar] [CrossRef] [PubMed]
- Salvador, G.A. Iron in neuronal function and dysfunction. Biofactors 2010, 36, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.; Hayflick, S. Neurodegeneration with Brain Iron Accumulation Disorders Overview. In GeneReviews® [Internet]; 2013 [Updated 2019]; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mirzaa, G., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Stanga, D.; Zhao, Q.; Milev, M.P.; Saint-Dic, D.; Jimenez-Mallebrera, C.; Sacher, M. TRAPPC11 functions in autophagy by recruiting ATG2B-WIPI4/WDR45 to preautophagosomal membranes. Traffic 2019, 20, 325–345. [Google Scholar] [CrossRef]
- Wan, H.; Wang, Q.; Chen, X.; Zeng, Q.; Shao, Y.; Fang, H.; Liao, X.; Li, H.S.; Liu, M.G.; Xu, T.L.; et al. WDR45 contributes to neurodegeneration through regulation of ER homeostasis and neuronal death. Autophagy 2020, 16, 531–547. [Google Scholar] [CrossRef]
- Haack, T.B.; Hogarth, P.; Gregory, A.; Prokisch, H.; Hayflick, S.J. BPAN: The only X-linked dominant NBIA disorder. Int. Rev. Neurobiol. 2013, 110, 85–90. [Google Scholar] [CrossRef]
- Saitsu, H.; Nishimura, T.; Muramatsu, K.; Kodera, H.; Kumada, S.; Sugai, K.; Kasai-Yoshida, E.; Sawaura, N.; Nishida, H.; Hoshino, A.; et al. De novo mutations in the autophagy gene WDR45 cause static encephalopathy of childhood with neurodegeneration in adulthood. Nat. Genet. 2013, 45, 445–449. [Google Scholar] [CrossRef]
- Cong, Y.; So, V.; Tijssen, M.A.J.; Verbeek, D.S.; Reggiori, F.; Mauthe, M. WDR45, one gene associated with multiple neurodevelopmental disorders. Autophagy 2021, 17, 3908–3923. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Emr, S.D. Autophagy as a regulated pathway of cellular degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef]
- Lee, J.H.; Nam, S.O.; Kim, E.K.; Shin, J.H.; Oh, S.H.; Ryu, D.; Lee, H.E.; Mun, J.Y. Autophagic defects observed in fibroblasts from a patient with beta-propeller protein-associated neurodegeneration. Am. J. Med. Genet. Part A 2021, 185, 3866–3871. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Sun, L.; Miao, G.; Ji, C.; Zhao, H.; Sun, H.; Miao, L.; Yoshii, S.R.; Mizushima, N.; Wang, X.; et al. The autophagy gene Wdr45/Wipi4 regulates learning and memory function and axonal homeostasis. Autophagy 2015, 11, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Ingrassia, R.; Memo, M.; Garavaglia, B. Ferrous Iron Up-regulation in Fibroblasts of Patients with Beta Propeller Protein-Associated Neurodegeneration (BPAN). Front. Genet. 2017, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Seibler, P.; Burbulla, L.F.; Dulovic, M.; Zittel, S.; Heine, J.; Schmidt, T.; Rudolph, F.; Westenberger, A.; Rakovic, A.; Munchau, A.; et al. Iron overload is accompanied by mitochondrial and lysosomal dysfunction in WDR45 mutant cells. Brain 2018, 141, 3052–3064. [Google Scholar] [CrossRef] [PubMed]
- Adang, L.A.; Pizzino, A.; Malhotra, A.; Dubbs, H.; Williams, C.; Sherbini, O.; Anttonen, A.K.; Lesca, G.; Linnankivi, T.; Laurencin, C.; et al. Phenotypic and Imaging Spectrum Associated With WDR45. Pediatr. Neurol. 2020, 109, 56–62. [Google Scholar] [CrossRef]
- Wilson, J.L.; Gregory, A.; Kurian, M.A.; Bushlin, I.; Mochel, F.; Emrick, L.; Adang, L.; Group, B.G.C.A.; Hogarth, P.; Hayflick, S.J. Consensus clinical management guideline for beta-propeller protein-associated neurodegeneration. Dev. Med. Child Neurol. 2021, 63, 1402–1409. [Google Scholar] [CrossRef]
- Long, M.; Abdeen, N.; Geraghty, M.T.; Hogarth, P.; Hayflick, S.; Venkateswaran, S. Novel WDR45 Mutation and Pathognomonic BPAN Imaging in a Young Female With Mild Cognitive Delay. Pediatrics 2015, 136, e714–e717. [Google Scholar] [CrossRef]
- Abidi, A.; Mignon-Ravix, C.; Cacciagli, P.; Girard, N.; Milh, M.; Villard, L. Early-onset epileptic encephalopathy as the initial clinical presentation of WDR45 deletion in a male patient. Eur. J. Hum. Genet. 2016, 24, 615–618. [Google Scholar] [CrossRef]
- Lee, J.H.; Yun, J.Y.; Gregory, A.; Hogarth, P.; Hayflick, S.J. Brain MRI Pattern Recognition in Neurodegeneration With Brain Iron Accumulation. Front. Neurol. 2020, 11, 1024. [Google Scholar] [CrossRef]
- Aring, L.; Choi, E.K.; Kopera, H.; Lanigan, T.; Iwase, S.; Klionsky, D.J.; Seo, Y.A. A neurodegeneration gene, WDR45, links impaired ferritinophagy to iron accumulation. J. Neurochem. 2022, 160, 356–375. [Google Scholar] [CrossRef]
- Suárez-Carrillo, A.; Álvarez-Córdoba, M.; Romero-González, A.; Talaverón-Rey, M.; Povea-Cabello, S.; Cilleros-Holgado, P.; Piñero-Pérez, R.; Reche-López, D.; Gómez-Fernández, D.; Romero-Domínguez, J.M.; et al. Antioxidants Prevent Iron Accumulation and Lipid Peroxidation, but Do Not Correct Autophagy Dysfunction or Mitochondrial Bioenergetics in Cellular Models of BPAN. Int. J. Mol. Sci. 2023, 24, 14576. [Google Scholar] [CrossRef]
- Grimm, N.B.; Lee, J.T. Selective Xi reactivation and alternative methods to restore MECP2 function in Rett syndrome. Trends Genet. 2022, 38, 920–943. [Google Scholar] [CrossRef] [PubMed]
- Ohhata, T.; Senner, C.E.; Hemberger, M.; Wutz, A. Lineage-specific function of the noncoding Tsix RNA for Xist repression and Xi reactivation in mice. Genes Dev. 2011, 25, 1702–1715. [Google Scholar] [CrossRef] [PubMed]
- Migeon, B.R. X-linked diseases: Susceptible females. Genet. Med. 2020, 22, 1156–1174. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Y.I.; Zempleni, J. Epigenetic regulation of chromatin structure and gene function by biotin. J. Nutr. 2006, 136, 1763–1765. [Google Scholar] [CrossRef] [PubMed]
- Hymes, J.; Fleischhauer, K.; Wolf, B. Biotinylation of histones by human serum biotinidase: Assessment of biotinyl-transferase activity in sera from normal individuals and children with biotinidase deficiency. Biochem. Mol. Med. 1995, 56, 76–83. [Google Scholar] [CrossRef]
- Lee, D.Y.; Hayes, J.J.; Pruss, D.; Wolffe, A.P. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell 1993, 72, 73–84. [Google Scholar] [CrossRef]
- Pham, A.D.; Sauer, F. Ubiquitin-activating/conjugating activity of TAFII250, a mediator of activation of gene expression in Drosophila. Science 2000, 289, 2357–2360. [Google Scholar] [CrossRef]
- Sommerville, J.; Baird, J.; Turner, B.M. Histone H4 acetylation and transcription in amphibian chromatin. J. Cell. Biol. 1993, 120, 277–290. [Google Scholar] [CrossRef]
- Kothapalli, N.; Camporeale, G.; Kueh, A.; Chew, Y.C.; Oommen, A.M.; Griffin, J.B.; Zempleni, J. Biological functions of biotinylated histones. J. Nutr. Biochem. 2005, 16, 446–448. [Google Scholar] [CrossRef]
- Wang, S.; Long, H.; Hou, L.; Feng, B.; Ma, Z.; Wu, Y.; Zeng, Y.; Cai, J.; Zhang, D.W.; Zhao, G. The mitophagy pathway and its implications in human diseases. Signal Transduct. Target. Ther. 2023, 8, 304. [Google Scholar] [CrossRef]
- Noguchi, M.; Hirata, N.; Tanaka, T.; Suizu, F.; Nakajima, H.; Chiorini, J.A. Autophagy as a modulator of cell death machinery. Cell Death Dis. 2020, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.Y.; Mitoma, H.; Fatemi, A. Metabolic ataxias. Handb. Clin. Neurol. 2018, 155, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.J.; Kovacs, G.G. Mitochondrial diseases. Handb. Clin. Neurol. 2017, 145, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Loda, A.; Collombet, S.; Heard, E. Gene regulation in time and space during X-chromosome inactivation. Nat. Rev. Mol. Cell Biol. 2022, 23, 231–249. [Google Scholar] [CrossRef]
- Haack, T.B.; Hogarth, P.; Kruer, M.C.; Gregory, A.; Wieland, T.; Schwarzmayr, T.; Graf, E.; Sanford, L.; Meyer, E.; Kara, E.; et al. Exome sequencing reveals de novo WDR45 mutations causing a phenotypically distinct, X-linked dominant form of NBIA. Am. J. Hum. Genet. 2012, 91, 1144–1149. [Google Scholar] [CrossRef]
- Zarate, Y.A.; Jones, J.R.; Jones, M.A.; Millan, F.; Juusola, J.; Vertino-Bell, A.; Schaefer, G.B.; Kruer, M.C. Lessons from a pair of siblings with BPAN. Eur. J. Hum. Genet. 2016, 24, 1080–1083. [Google Scholar] [CrossRef]
- Puck, J.M.; Willard, H.F. X inactivation in females with X-linked disease. N. Engl. J. Med. 1998, 338, 325–328. [Google Scholar] [CrossRef]
- Van den Veyver, I.B. Skewed X inactivation in X-linked disorders. Semin. Reprod. Med. 2001, 19, 183–191. [Google Scholar] [CrossRef]
- Fieremans, N.; Van Esch, H.; Holvoet, M.; Van Goethem, G.; Devriendt, K.; Rosello, M.; Mayo, S.; Martinez, F.; Jhangiani, S.; Muzny, D.M.; et al. Identification of Intellectual Disability Genes in Female Patients with a Skewed X-Inactivation Pattern. Hum. Mutat. 2016, 37, 804–811. [Google Scholar] [CrossRef]
- Hayflick, S.J.; Kruer, M.C.; Gregory, A.; Haack, T.B.; Kurian, M.A.; Houlden, H.H.; Anderson, J.; Boddaert, N.; Sanford, L.; Harik, S.I.; et al. beta-Propeller protein-associated neurodegeneration: A new X-linked dominant disorder with brain iron accumulation. Brain 2013, 136, 1708–1717. [Google Scholar] [CrossRef]
- Nakashima, M.; Takano, K.; Tsuyusaki, Y.; Yoshitomi, S.; Shimono, M.; Aoki, Y.; Kato, M.; Aida, N.; Mizuguchi, T.; Miyatake, S.; et al. WDR45 mutations in three male patients with West syndrome. J. Hum. Genet. 2016, 61, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Zhu, X.; Ou, J.; Lin, L.; Chamberlain, L.; Zhu, L.J.; Wajapeyee, N.; Green, M.R. Genetic and pharmacological reactivation of the mammalian inactive X chromosome. Proc. Natl. Acad. Sci. USA 2014, 111, 12591–12598. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Hiatt, J.B.; Nguyen, D.K.; Ercan, S.; Sturgill, D.; Hillier, L.W.; Schlesinger, F.; Davis, C.A.; Reinke, V.J.; Gingeras, T.R.; et al. Evidence for compensatory upregulation of expressed X-linked genes in mammals, Caenorhabditis elegans and Drosophila melanogaster. Nat. Genet. 2011, 43, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.K.; Disteche, C.M. Dosage compensation of the active X chromosome in mammals. Nat. Genet. 2006, 38, 47–53. [Google Scholar] [CrossRef]
- Marahrens, Y.; Panning, B.; Dausman, J.; Strauss, W.; Jaenisch, R. Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev. 1997, 11, 156–166. [Google Scholar] [CrossRef]
- Yang, L.; Kirby, J.E.; Sunwoo, H.; Lee, J.T. Female mice lacking Xist RNA show partial dosage compensation and survive to term. Genes Dev. 2016, 30, 1747–1760. [Google Scholar] [CrossRef]
- Yang, L.; Yildirim, E.; Kirby, J.E.; Press, W.; Lee, J.T. Widespread organ tolerance to Xist loss and X reactivation except under chronic stress in the gut. Proc. Natl. Acad. Sci. USA 2020, 117, 4262–4272. [Google Scholar] [CrossRef]
- Yildirim, E.; Kirby, J.E.; Brown, D.E.; Mercier, F.E.; Sadreyev, R.I.; Scadden, D.T.; Lee, J.T. Xist RNA is a potent suppressor of hematologic cancer in mice. Cell 2013, 152, 727–742. [Google Scholar] [CrossRef]
- Huret, C.; Ferraye, L.; David, A.; Mohamed, M.; Valentin, N.; Charlotte, F.; Savignac, M.; Goodhardt, M.; Guery, J.C.; Rougeulle, C.; et al. Altered X-chromosome inactivation predisposes to autoimmunity. Sci. Adv. 2024, 10, eadn6537. [Google Scholar] [CrossRef]
- Adrianse, R.L.; Smith, K.; Gatbonton-Schwager, T.; Sripathy, S.P.; Lao, U.; Foss, E.J.; Boers, R.G.; Boers, J.B.; Gribnau, J.; Bedalov, A. Perturbed maintenance of transcriptional repression on the inactive X-chromosome in the mouse brain after Xist deletion. Epigenet. Chromatin 2018, 11, 50. [Google Scholar] [CrossRef]
- Carrette, L.L.G.; Wang, C.Y.; Wei, C.; Press, W.; Ma, W.; Kelleher, R.J., 3rd; Lee, J.T. A mixed modality approach towards Xi reactivation for Rett syndrome and other X-linked disorders. Proc. Natl. Acad. Sci. USA 2018, 115, E668–E675. [Google Scholar] [CrossRef] [PubMed]
- Patrat, C.; Ouimette, J.F.; Rougeulle, C. X chromosome inactivation in human development. Development 2020, 147, dev183095. [Google Scholar] [CrossRef] [PubMed]
- Payer, B.; Lee, J.T. X chromosome dosage compensation: How mammals keep the balance. Annu. Rev. Genet. 2008, 42, 733–772. [Google Scholar] [CrossRef] [PubMed]
- Sahakyan, A.; Plath, K.; Rougeulle, C. Regulation of X-chromosome dosage compensation in human: Mechanisms and model systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160363. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Theunissen, T.W. Modeling X-chromosome inactivation and reactivation during human development. Curr. Opin. Genet. Dev. 2023, 82, 102096. [Google Scholar] [CrossRef]
- Napoletano, F.; Baron, O.; Vandenabeele, P.; Mollereau, B.; Fanto, M. Intersections between Regulated Cell Death and Autophagy. Trends Cell Biol. 2019, 29, 323–338. [Google Scholar] [CrossRef]
- Mollereau, B.; Hayflick, S.J.; Escalante, R.; Mauthe, M.; Papandreou, A.; Iuso, A.; Celle, M.; Aniorte, S.; Issa, A.R.; Lasserre, J.P.; et al. A burning question from the first international BPAN symposium: Is restoration of autophagy a promising therapeutic strategy for BPAN? Autophagy 2023, 19, 3234–3239. [Google Scholar] [CrossRef]
- Millan-Zambrano, G.; Burton, A.; Bannister, A.J.; Schneider, R. Histone post-translational modifications—Cause and consequence of genome function. Nat. Rev. Genet. 2022, 23, 563–580. [Google Scholar] [CrossRef]
- Luger, K.; Dechassa, M.L.; Tremethick, D.J. New insights into nucleosome and chromatin structure: An ordered state or a disordered affair? Nat. Rev. Mol. Cell Biol. 2012, 13, 436–447. [Google Scholar] [CrossRef]
- Lai, W.K.M.; Pugh, B.F. Understanding nucleosome dynamics and their links to gene expression and DNA replication. Nat. Rev. Mol. Cell Biol. 2017, 18, 548–562. [Google Scholar] [CrossRef]
- Turner, B.M. Decoding the nucleosome. Cell 1993, 75, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ramesh, V.; Locasale, J.W. The evolving metabolic landscape of chromatin biology and epigenetics. Nat. Rev. Genet. 2020, 21, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.; Bannister, A.J. Two genomes, one cell: Mitochondrial-nuclear coordination via epigenetic pathways. Mol. Metab. 2020, 38, 100942. [Google Scholar] [CrossRef] [PubMed]
- McMahon, R.J. Biotin in metabolism and molecular biology. Annu. Rev. Nutr. 2002, 22, 221–239. [Google Scholar] [CrossRef]
- Chapman-Smith, A.; Cronan, J.E., Jr. The enzymatic biotinylation of proteins: A post-translational modification of exceptional specificity. Trends Biochem. Sci. 1999, 24, 359–363. [Google Scholar] [CrossRef]
- Leon-Del-Rio, A.; Leclerc, D.; Akerman, B.; Wakamatsu, N.; Gravel, R.A. Isolation of a cDNA encoding human holocarboxylase synthetase by functional complementation of a biotin auxotroph of Escherichia coli. Proc. Natl. Acad. Sci. USA 1995, 92, 4626–4630. [Google Scholar] [CrossRef]
- Suzuki, Y.; Aoki, Y.; Ishida, Y.; Chiba, Y.; Iwamatsu, A.; Kishino, T.; Niikawa, N.; Matsubara, Y.; Narisawa, K. Isolation and characterization of mutations in the human holocarboxylase synthetase cDNA. Nat. Genet. 1994, 8, 122–128. [Google Scholar] [CrossRef]
- Wolf, B.; Grier, R.E.; Allen, R.J.; Goodman, S.I.; Kien, C.L. Biotinidase deficiency: The enzymatic defect in late-onset multiple carboxylase deficiency. Clin. Chim. Acta 1983, 131, 273–281. [Google Scholar] [CrossRef]
- Ingaramo, M.; Beckett, D. Selectivity in post-translational biotin addition to five human carboxylases. J. Biol. Chem. 2012, 287, 1813–1822. [Google Scholar] [CrossRef]
- Choi, S.W.; Friso, S. Epigenetics: A New Bridge between Nutrition and Health. Adv. Nutr. 2010, 1, 8–16. [Google Scholar] [CrossRef]
- Hassan, Y.I.; Zempleni, J. A novel, enigmatic histone modification: Biotinylation of histones by holocarboxylase synthetase. Nutr. Rev. 2008, 66, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Chew, Y.C.; Bao, B.; Pestinger, V.; Wijeratne, S.S. Repression of transposable elements by histone biotinylation. J. Nutr. 2009, 139, 2389–2392. [Google Scholar] [CrossRef] [PubMed]
- Camporeale, G.; Giordano, E.; Rendina, R.; Zempleni, J.; Eissenberg, J.C. Drosophila melanogaster holocarboxylase synthetase is a chromosomal protein required for normal histone biotinylation, gene transcription patterns, lifespan, and heat tolerance. J. Nutr. 2006, 136, 2735–2742. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Healy, S.; Perez-Cadahia, B.; Jia, D.; McDonald, M.K.; Davie, J.R.; Gravel, R.A. Biotin is not a natural histone modification. Biochim. Biophys. Acta 2009, 1789, 719–733. [Google Scholar] [CrossRef]
- Martínez-Rubio, D.; Hinarejos, I.; Sancho, P.; Gorría-Redondo, N.; Bernadó-Fonz, R.; Tello, C.; Marco-Marín, C.; Martí-Carrera, I.; Martínez-González, M.J.; García-Ribes, A.; et al. Mutations, Genes, and Phenotypes Related to Movement Disorders and Ataxias. Int. J. Mol. Sci. 2022, 23, 11847. [Google Scholar] [CrossRef]
- Alvarez-Cordoba, M.; Fernandez Khoury, A.; Villanueva-Paz, M.; Gomez-Navarro, C.; Villalon-Garcia, I.; Suarez-Rivero, J.M.; Povea-Cabello, S.; de la Mata, M.; Cotan, D.; Talaveron-Rey, M.; et al. Pantothenate Rescues Iron Accumulation in Pantothenate Kinase-Associated Neurodegeneration Depending on the Type of Mutation. Mol. Neurobiol. 2019, 56, 3638–3656. [Google Scholar] [CrossRef]
- Yue, M.; Charles Richard, J.L.; Yamada, N.; Ogawa, A.; Ogawa, Y. Quick fluorescent in situ hybridization protocol for Xist RNA combined with immunofluorescence of histone modification in X-chromosome inactivation. J. Vis. Exp. 2014, 93, e52053. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Wang, L.; Hu, X.; Liu, X.; Liu, M.; Cao, Z.; Shangguan, D.; Tan, W. A Cyanine Dye to Probe Mitophagy: Simultaneous Detection of Mitochondria and Autolysosomes in Live Cells. J. Am. Chem. Soc. 2016, 138, 12368–12374. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3B1–A3B3. [Google Scholar] [CrossRef]
- Tarohda, T.; Ishida, Y.; Kawai, K.; Yamamoto, M.; Amano, R. Regional distributions of manganese, iron, copper, and zinc in the brains of 6-hydroxydopamine-induced parkinsonian rats. Anal. Bioanal. Chem. 2005, 383, 224–234. [Google Scholar] [CrossRef]
- Georgakopoulou, E.A.; Tsimaratou, K.; Evangelou, K.; Fernandez Marcos, P.J.; Zoumpourlis, V.; Trougakos, I.P.; Kletsas, D.; Bartek, J.; Serrano, M.; Gorgoulis, V.G. Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging 2013, 5, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Drouin-Ouellet, J.; Pircs, K.; Barker, R.A.; Jakobsson, J.; Parmar, M. Direct Neuronal Reprogramming for Disease Modeling Studies Using Patient-Derived Neurons: What Have We Learned? Front. Neurosci. 2017, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Zufferey, R.; Nagy, D.; Mandel, R.J.; Naldini, L.; Trono, D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 1997, 15, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Paz, M.; Povea-Cabello, S.; Villalon-Garcia, I.; Alvarez-Cordoba, M.; Suarez-Rivero, J.M.; Talaveron-Rey, M.; Jackson, S.; Falcon-Moya, R.; Rodriguez-Moreno, A.; Sanchez-Alcazar, J.A. Parkin-mediated mitophagy and autophagy flux disruption in cellular models of MERRF syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165726. [Google Scholar] [CrossRef]
- Le Boedec, K. Sensitivity and specificity of normality tests and consequences on reference interval accuracy at small sample size: A computer-simulation study. Vet. Clin. Pathol. 2016, 45, 648–656. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reche-López, D.; Romero-González, A.; Álvarez-Córdoba, M.; Suárez-Carrillo, A.; Cilleros-Holgado, P.; Piñero-Pérez, R.; Gómez-Fernández, D.; Romero-Domínguez, J.M.; López-Cabrera, A.; González-Granero, S.; et al. Biotin Induces Inactive Chromosome X Reactivation and Corrects Physiopathological Alterations in Beta-Propeller-Protein-Associated Neurodegeneration. Int. J. Mol. Sci. 2025, 26, 1315. https://doi.org/10.3390/ijms26031315
Reche-López D, Romero-González A, Álvarez-Córdoba M, Suárez-Carrillo A, Cilleros-Holgado P, Piñero-Pérez R, Gómez-Fernández D, Romero-Domínguez JM, López-Cabrera A, González-Granero S, et al. Biotin Induces Inactive Chromosome X Reactivation and Corrects Physiopathological Alterations in Beta-Propeller-Protein-Associated Neurodegeneration. International Journal of Molecular Sciences. 2025; 26(3):1315. https://doi.org/10.3390/ijms26031315
Chicago/Turabian StyleReche-López, Diana, Ana Romero-González, Mónica Álvarez-Córdoba, Alejandra Suárez-Carrillo, Paula Cilleros-Holgado, Rocío Piñero-Pérez, David Gómez-Fernández, José Manuel Romero-Domínguez, Alejandra López-Cabrera, Susana González-Granero, and et al. 2025. "Biotin Induces Inactive Chromosome X Reactivation and Corrects Physiopathological Alterations in Beta-Propeller-Protein-Associated Neurodegeneration" International Journal of Molecular Sciences 26, no. 3: 1315. https://doi.org/10.3390/ijms26031315
APA StyleReche-López, D., Romero-González, A., Álvarez-Córdoba, M., Suárez-Carrillo, A., Cilleros-Holgado, P., Piñero-Pérez, R., Gómez-Fernández, D., Romero-Domínguez, J. M., López-Cabrera, A., González-Granero, S., García-Verdugo, J. M., & Sánchez-Alcázar, J. A. (2025). Biotin Induces Inactive Chromosome X Reactivation and Corrects Physiopathological Alterations in Beta-Propeller-Protein-Associated Neurodegeneration. International Journal of Molecular Sciences, 26(3), 1315. https://doi.org/10.3390/ijms26031315







