Abstract
Rare genetic movement disorders usually manifest early in life with dystonia, parkinsonism, chorea, or a combination thereof. These are often associated with neurodevelopmental delay, intellectual disability, speech problems, retinal abnormalities, seizures, ataxia, spasticity, or systemic features. Due to their vast number and pheno–genotypic heterogeneity, the diagnosis of these disorders can be challenging. However, recognising their core motor phenomenology as well as clinical, laboratory, and neuroradiological clues can expedite appropriate diagnostic workup, molecular diagnosis, and adequate treatment. In this review, we outline diagnostic clues to rare movement disorders (RMDs), focusing on those that present mainly with dystonia, parkinsonism, or paroxysmal dyskinesia due to genetic causes. Additionally, we provide a decision tree approach linking clinical, genetic, and imaging testing. Finally, we highlight selected RMDs that should not be missed, as they possess established treatments that can hinder their progression, prevent irreversible or life-threatening sequelae and, in certain cases, lead to complete symptom remission.
1. Introduction
Rare movement disorders (RMDs) are genetic syndromes that often result from structural or functional abnormalities of the basal ganglia. They present with hypo- and/or hyperkinetic movements, either isolated or associated with other neurological and systemic manifestations. Although RMDs can also have an acquired aetiology (e.g., antibody-mediated), this review focuses on genetic RMDs according to the Orphanet definition of “rare diseases”, i.e., conditions affecting no more than 1 in 2000 individuals in Europe [1]. Using selected groups of RMDs as exemplars, this review aims to support the recognition of some key clinical features, alongside specific findings from laboratory tests and neuroimaging, that can assist neurologists in establishing an aetiological diagnosis and facilitating the timely initiation of further investigations or dedicated treatments.
Although most of the RMDs discussed in this review are individually very rare, they collectively represent a significant proportion of referrals to movement disorder specialists. Far from being exhaustive, this review focuses on selected monogenic RMDs grouped according to their underlying pathological mechanisms, including brain mineralisation disorders, lysosomal storage disorders, episodic disorders, vitamin deficiency-related disorders, and dopamine responsive disorders. Despite their diverse aetiologies, all of these disorders disrupt the neural pathways that regulate movement control.
2. General Clinical Approach to RMDs
The diagnosis of RMDs is often challenging as different disorders share similar phenotypic manifestations (genotypic heterogeneity), and many RMDs lack pathognomonic features or diagnostic biomarkers [2]. Furthermore, the clinical presentation may differ from the known core disease phenotype (phenotypic heterogeneity) [3]. A movement disorder specialist is often best placed to formally diagnose an RMD, given their clinical expertise and experience in the field [4]. Accordingly, referral to a specialist centre is appropriate once an RMD is suspected in order to expedite diagnosis, ensure a comprehensive diagnostic workup, establish the molecular diagnosis, and initiate specific treatments. Such settings also allow for the creation of a multidisciplinary team that can provide highly specialised input on diagnosis and treatment. However, general neurologists should remain vigilant for certain clinical clues and findings from investigations that can help establish a diagnosis. This is particularly relevant for patients in resource-limited settings, where access to specialist care may be significantly delayed [5]. In such cases, it is essential that a basic neurological history, clinical examination, and initial investigations are conducted to avoid missing time-critical treatments.
The age of symptom onset can be a helpful diagnostic clue, with half of all RMDs manifesting in childhood [6]. Nevertheless, genetic disorders typically associated with a childhood onset may still occasionally present later in life and vice versa. For example, PLA2G6-associated neurodegeneration most often presents with neuroaxonal dystrophies in early childhood but can also sometimes manifest with dystonia–parkinsonism in late adolescence or early adulthood [7,8]. In contrast, while Huntington’s disease usually presents in mid-adulthood with generalised chorea as the core motor feature [9], akinetic–rigid syndrome (Westphal variant) may also occur as a juvenile presentation of the disease [10].
A detailed clinical history is instrumental as an initial step to establish an appropriate differential diagnosis in RMDs. Developmental history and details of perinatal history are important. As most RMDs display a variety of neurological manifestations, some with intellectual impairment and spasticity, cerebral palsy is often a consideration [11]. In such situations, a detailed timeline of events, including the onset and progression of each symptom, can help to determine a progressive course that would favour an RMD or non-progressive sequelae of a perinatal insult in cerebral palsy [12]. Past medical and family history are vital to further indicate a possible diagnosis. For example, a personal or family history of epilepsy, hearing impairment, or myoclonus may suggest a mitochondrial disorder [13,14]. Constructing a good pedigree can assist in identifying a mode of inheritance and refining the differential diagnosis. Notwithstanding, an unremarkable family history should not prevent efforts to establish a molecular diagnosis if there is a high index of suspicion of an RMD, particularly in view of the frequent occurrence of incomplete penetrance in autosomal dominant disorders [15,16], compound heterozygosity in autosomal recessive disorders, and intrafamilial phenotypic heterogeneity [2].
By recognising movement disorders as either “isolated” when occurring alone or “combined” when associated with other movement disorders, non-motor neurological features or systemic manifestations (suggestive of an underlying syndrome) can point towards specific aetiologies [17]. Following the taking of clinical history, a comprehensive clinical examination remains imperative to facilitate an accurate diagnosis of an RMD. For instance, among early-onset dystonia syndromes, the detection of isolated generalised dystonia will first raise the suspicion of TOR1A- or THAP1-related dystonia [18,19], whereas the co-occurrence of myoclonus will suggest mutations in SGCE, ANO3, or KCTD17 [20,21,22]. A thorough examination may also reveal pathognomonic or highly typical signs, such as Kayser–Fleischer rings in Wilson’s disease or the forceful sensory geste so-called “mantis sign” in pantothenate kinase-associated neurodegeneration (PKAN) (Table 1) [23,24].

Table 1.
Clinical cues and helpful investigation findings, suggestive of a specific diagnosis.
Targeted investigations would be contingent on the information gathered and overall suspicions from the clinical history and examination, but may include biochemical tests (Table 1), searching for elevated plasma creatine kinase in neuroacanthocytosis or raised plasma/CSF lactic acid levels in mitochondrial disorders [25,26,27], low and absent plasma ceruloplasmin in Wilson’s disease and aceruloplasminemia, respectively, increased urine copper in Wilson’s disease, raised plasma oxysterols in Niemann–Pick disease type C (NP-C) [28], a reduced CSF-to-blood glucose ratio in GLUT1 deficiency syndrome, and abnormal CSF catecholamine metabolite levels in dopamine synthesis disorders [29].
Neuroimaging may also be pursued and is often crucial in the diagnostic workflow, as there may be particular findings which are highly suggestive of a specific RMD. For instance, low MRI-SWI signal from the basal ganglia and dentate nuclei corresponding to CT hyperdensity implies a brain calcification disorder [30], while if the CT is normal, it suggests iron deposition [31]. The latter can present with a typical pattern, such as the “eye of the tiger” sign that highly suggests but is not pathognomonic of PKAN [32]. The finding of MRI-T1 hyperintensity in the basal ganglia is another example, detected in disorders with manganese deposition in the brain [33].
Genetic testing may be considered based on the clinical history and examination alone or following suggestive investigation findings to reach a molecular diagnosis. A dopamine trial is also recommended for all patients with suspected RMD without a clear diagnosis, as dopa-responsiveness is a very helpful clue to elucidate the correct diagnosis [6].
Figure 1 illustrates a decision-tree approach that integrates clinical, neuroimaging, and laboratory data. It begins with the temporal pattern of motor symptoms and signs (persistent/progressive, episodic/paroxysmal, or showing diurnal variation) and progresses towards genetic testing, guided by neuroradiological and laboratory findings. This structured flowchart aims to support the diagnostic workup of the RMD reviewed in this article.
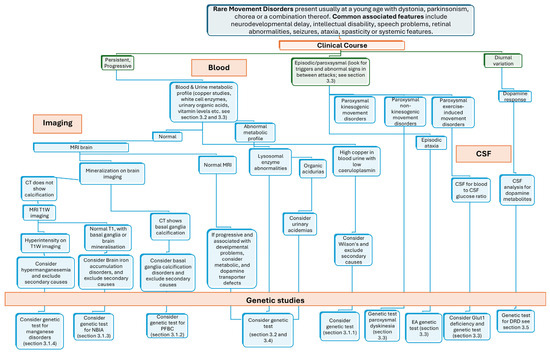
Figure 1.
A decision-tree approach linking clinical, genetic, and imaging testing to facilitate the recognition of RMDs overviewed in this review. Legend: DRD, dopa-responsive dystonia; EA, episodic ataxia; NBIA, neurodegeneration with brain iron accumulation; PFBC, primary familial brain calcification.
3. Overview and Phenotypic Clues of RMD
3.1. Brain Mineralopathies
3.1.1. Copper
Wilson’s disease (WD) is the best-known brain mineralisation disorder and is caused by biallelic variants in ATP7B, which normally encodes a copper-transporting P-type ATPase expressed predominantly in the liver [34]. Defects in the encoded protein lead to reduced biliary excretion, excessive hepatic storage, and plasma accumulation of copper, which ultimately causes copper deposition in other organs, including the nervous system and eyes [35]. Manifestations of WD reflect the distribution of copper pathological deposition, with neurological features encompassing tremor, chorea, dystonia, dysautonomia, seizures, and psychiatric disturbances [36]. Clinical and ophthalmological assessments may importantly reveal Kayser–Fleischer rings, i.e., abnormal copper deposition within the Descemet membrane, which are pathognomonic of the disease [24]. Biochemical testing can be helpful, classically demonstrating low serum ceruloplasmin and copper, as well as high 24 h urinary copper levels [36]. A brain MRI may additionally reveal the “face of the giant panda” sign relating to the midbrain, in which the red nucleus and substantia nigra are surrounded by high T2 signals in the tegmentum [37]. Other possible signs that have been described include the “face of the miniature panda”, “double panda”, “bright claustrum”, “split thalamus”, and “whorl” signs [38,39,40]. WD is a treatable condition, and the outcome is very favourable in treated vs. untreated cases. The best evidence for treatment is by copper chelation, using D-penicillamine and trientine, to increase the urinary excretion of copper [6]. Zinc salts may also be used to prevent copper absorption in the gut, although they should not be used in conjunction with chelating agents [41]. In severe circumstances, liver transplantation may be required [36].
3.1.2. Calcium
Idiopathic (or inherited) basal ganglia calcification (IBGC) disorders are diseases characterised by extensive, bilateral, symmetrical calcification in the basal ganglia that shows a coarse conglomerate pattern on imaging [42]. However, the dentate nuclei, thalamus, brainstem, centrum semiovale, and subcortical white matter may also be involved [43]. These disorders tend to present with a wide range of neurological and psychiatric features (Table 2). Until recently, IBGC was assumed to be inherited in an autosomal dominant fashion, justifying the term “primary familial brain calcification” [44]. However, the widespread availability of next-generation sequencing has also enabled the identification of BGC-related genes with an autosomal recessive pattern of inheritance (Table 2) [30]. The treatment of IBGC disorders is currently supportive and guided toward symptomatic management [44]. Furthermore, it should be noted that calcium deposition in the brain may not always be pathological, especially in the absence of clinical signs and symptoms and other secondary causes need to also be excluded in the differential diagnosis (e.g., hypoparathyroidism, infections of the central nervous system, human immunodeficiency virus, and systemic lupus erythematosus), which may possess more effective treatments [45].

Table 2.
Causative genes associated with primary familial brain calcification disorders and common clinical features.
3.1.3. Iron
Neurodegeneration with brain iron accumulation (NBIA) represents a group of inherited disorders characterised by the abnormal accumulation of iron in the basal ganglia and, in some cases, the dentate nuclei (Table 3) [46]. The majority of these display an autosomal recessive inheritance pattern (PKAN, PLAN, MPAN, CoPAN, aceruloplasminemia, FAHN, KRD/PARK9, or WSS), although autosomal dominant (neuroferritinopathy) and X-linked dominant (BPAN) inheritance patterns have also been described [46,47]. In addition to the classical forms, novel NBIA syndromes have been described in the last decade [48]. There is significant variability in the age of onset between NBIA subtypes [46]. Clinical features of NBIA typically involve a progressive dystonia and dysarthria, spasticity, parkinsonism, neuropsychiatric disturbance, optic atrophy, and retinal degeneration [47]. Although Woodhouse–Sakati syndrome is often grouped with NBIA disorders due to overlapping neuroimaging findings, it is more accurately characterized as a multisystem endocrinopathy. In addition to neurological symptoms, it typically presents with hypogonadism, diabetes mellitus, hypothyroidism, and progressive childhood-onset alopecia [49]. The treatment of NBIA is also largely guided towards symptomatic management, utilising pharmacological agents or, in selected cases, deep brain stimulation [50]. An exception to this is noted for aceruloplasminaemia, for which iron chelation and plasma-derived ceruloplasmin supplementation have proven themselves as effective treatment strategies to diminish central nervous system iron accumulation and prevent/ameliorate associated neurological symptoms [51].

Table 3.
Clinical and specific neuroradiological features of NBIA subtypes.
3.1.4. Manganese
Genetically determined hypermanganesemia, leading to manganese accumulation in the brain, also accounts for a group of RMDs. Mutations in SLC30A10 and SLC39A14 have been recently described to lead to hypermanganesemia [52,53]. A further gene, SLC39A8, has also been linked to manganese homoeostasis; however, it codes for a transporter protein responsible for the cellular uptake of manganese, and hence, mutations lead to decreased manganese levels within the brain [54]. The clinical presentation of these disorders usually accompanies parkinsonism; however, gait impairment and postural instability tend to occur earlier than Parkinson’s Disease [55]. The gait abnormality in hypermanganesemia additionally appears to be characterised by retropulsion, propulsion, freezing, and a characteristic ”cock-walk” (the individual walks on the metatarsophalangeal joints associated with an erect spine and flexed elbows) [56]. Dystonia and neuropsychiatric changes have also been described [55].
The genetic forms of hypermanganesemia can be identified early, presenting in childhood or early adolescence. Patients with SLC30A10 mutations tend to present slightly earlier (often in infancy) than those with SLC39A14 mutations, have slightly higher blood manganese levels and also exhibit manganese content in the muscle [57,58]. MRI is invaluable in manganese deposition disorders, demonstrating hyperintense signal on T1-weighted imaging in the globus pallidi bilaterally [33], whilst T2 sequences show normal signal in these corresponding areas [59]. Additional investigations may also be useful, particularly in differentiating between the genetic forms, as hepatomegaly/cirrhosis with an associated transaminitis or polycythaemia are commonly reported in patients with SLC30A10 mutations, but not SLC39A14 mutations [57]. The treatment of hypermanganesemia is primarily conducted with chelation therapy using intravenous disodium calcium edetate, which increases the urinary excretion of manganese to result in reduced serum levels [60].
3.2. Lysosomal Storage Diseases
Lysosomal storage diseases (LSDs) are inherited metabolic disorders that result in the progressive accumulation of substrates, leading to damage across multiple tissues [61]. These diseases typically manifest in childhood, with the central nervous system frequently being involved [62]. Examples of LSDs include Gaucher’s Disease, Fabry Disease, Niemann–Pick Disease Type C, Neuronal Ceroid Lipofuscinoses, Mucopolysaccharidoses, aspartylglucosaminuria, Salla disease, and Tay–Sachs disease [61]. The clinical presentation of LSDs can vary widely (Table 4), although ataxia is often a common symptom, typically accompanied by dystonia, myoclonus, or a resting tremor [61]. While many patients are managed with supportive care, enzyme replacement therapy has proven to be an effective treatment for certain LSDs, compensating for the defective metabolic process [63,64,65]. Substrate reduction therapy is another potential treatment approach, and it may be more effective when combined with enzyme replacement therapy; however, it is currently approved only for a limited number of LSDs [65,66]. LSDs often result from missense mutations, which lead to abnormal folding of the lysosomal enzymes, preventing their normal function; hence, chaperone therapy denotes another treatment measure by which administered “chaperone” molecules assist the proteins to fold correctly and regain at least part of their catalytic activity [65]. Haematopoietic stem cell transplantation, especially used prior to the development of enzyme replacement therapy [65], allows donor cells to migrate into the recipient’s organs and correct the metabolic defect by locally releasing the missing enzyme. Gene therapy and stop-codon readthrough may be further avenues of treatment to correct the underlying pathological mutation of LSDs, but these require further study to validate their use [65].

Table 4.
Clinical features of lysosomal storage disorders, vitamin deficiency-related disorders, and other metabolic diseases.
3.3. Episodic Movement Disorders
Paroxysmal movement disorders are characterised by episodic involuntary movements and are largely divided into paroxysmal dyskinesia or episodic ataxia (EA) [67]. The pathophysiological framework of paroxysmal movement disorders revolves around abnormalities in ion channels, proteins of the vesical synaptic cycle, or proteins implicated in neuronal energy metabolism [67]. Symptoms often begin in childhood and may improve with age, although with episodic ataxia, symptoms can also worsen [68].
Paroxysmal dyskinesia typically presents with transient episodes of involuntary movements, particularly dystonia and/or chorea, without loss of consciousness [69]. Paroxysmal dyskinesia is further subdivided into paroxysmal kinesigenic dyskinesia (PKD), paroxysmal non-kinesigenic dyskinesia (PNKD) and paroxysmal exercise-induced dyskinesia (PED), dependent on the underlying trigger of the episodes [70]. Mutations in the SLC2A1 gene, which encodes the glucose transporter GLUT1, have been particularly linked to a phenotypic spectrum of isolated PED [71]. The last two decades have additionally provided much greater insight into the aetiology of PKD, establishing associations with mutations in the PRRT2, TMEM151A, and KCNJ10 genes [72,73,74,75]. The PRRT2 gene, which encodes a protein that interacts with the SNAP25 protein that affects presynaptic neurotransmitter release, accounts for the large majority of familial cases of PKD and also a significant proportion of sporadic cases [76,77]. The pathophysiology of PNKD remains unclear, but it is suggested to be linked to mutations in the PNKD gene, as this has been reported in up to 60% of patients [78]. Episodic ataxia (EA), on the other hand, presents with episodes of cerebellar dysfunction, typically accompanied by dystonia, hemiplegia, headaches, or tinnitus [79]. Unlike paroxysmal dyskinesia, EA is subdivided by the underlying genetic abnormality (e.g., KCNA1 mutations in EA1, CACNA1A mutations in EA2), as there is often significant overlap in clinical characteristics between the different subtypes [79]. Examination during the interictal phase of paroxysmal movement disorders is usually normal; however, in EA, nystagmus or myokymia may be present [80]. Requesting video recordings of the episodes is therefore essential to facilitate early diagnosis.
The treatment of paroxysmal movement disorders is primarily focused on managing symptoms and preventing attacks. A combination of supportive measures and therapeutic medications is subsequently used. The avoidance of triggers (e.g., emotional stress, fatigue, and alcohol/caffeine consumption) is pivotal and may be highly effective in preventing attacks [81]. A ketogenic diet is also recommended in PED, as it provides an alternative energy source to bypass the defective GLUT1 protein [82,83]. Potential medications include anticonvulsants, benzodiazepines, levodopa, and acetazolamide. Anticonvulsants are particularly efficacious in PKD, whilst benzodiazepines tend to be more helpful in PNKD [84,85]. In contrast, EA2 responds very well to acetazolamide and 4-aminopyridine [86].
3.4. Vitamin Deficiency-Related Disorders
A genetically determined inability to produce or utilise vitamins can also lead to RMDs. These diseases include biotinidase deficiency (BTD mutations), biotin–thiamine-responsive basal ganglia disease (SLC19A3 mutations), abetalipoproteinemia (MTTP mutations), ataxia with vitamin E deficiency (TTPA mutations), homocystinuria (CBS mutations), cobalamin deficiency, cerebral folate deficiency (FLR1 and SLC46A1 mutations), coenzyme Q10 deficiency, and pyruvate dehydrogenase complex deficiency [87]. Symptoms vary significantly between vitamin deficiency-related disorders, although patients typically present with ataxia, dystonia, parkinsonism, or spasticity (Table 4) [87].
MRI can be useful in these disorders. For instance, biotin–thiamine-responsive basal ganglia disease can show bilateral high T2 signal intensity in the caudate head, putamen, globi pallidi, thalami, infra- and supra-tentorial cortex, brainstem, and cerebellum [88]. However, other disorders, such as cerebral folate deficiency, can appear normal on MRI, although they more typically demonstrate diffuse, leukodystrophy-like, white matter changes [89]. The treatment of vitamin deficiency-related disorders involves replacing the missing or impaired vitamins. Additional dietary measures may also be implemented, such as a ketogenic diet for pyruvate dehydrogenase complex deficiency, which produces a significant improvement in symptoms [6,90].
3.5. Dopamine Responsive Disorders
Dopa-responsive dystonia (DRD) represents a group of disorders, typically manifesting with limb-onset, diurnally fluctuating dystonia (particularly commencing in childhood) that exhibit a robust and sustained response to levodopa treatment [91]. These disorders are often caused by genetic defects in the biosynthesis of dopamine, demonstrating a wide spectrum of clinical manifestations (Table 5). However, DRD has also seldom been associated with conditions that do not affect dopamine biosynthesis pathways (e.g., spinocerebellar ataxia type 3, ataxic telangiectasia, and hereditary spastic paraplegia type II) [91,92]. There has subsequently been a recent suggestion from Lee et al. (2018) for the definition of DRD to be refined to represent a group of non-neurodegenerative conditions with genetic defects involving the nigrostriatal dopaminergic system with cardinal manifestations [93]. They additionally posed a further group of conditions, DRD-plus disorders, which appear similar to DRD but also present with atypical features, such as hypotonia, drowsiness, ptosis, cerebellar dysfunction, infantile onset, developmental delay, psychomotor retardation, and seizures [93]. Finally, a third group, DRD look-alikes, was also suggested, which encompasses neurodegenerative (e.g., ataxia telangiectasia) or non-neurodegenerative (e.g., TOR1A-related dystonia, GLUT1 deficiency syndrome, myoclonus–dystonia) conditions that do not involve the nigrostriatal dopaminergic system, or neurodegenerative disorders that involve the nigrostriatal dopaminergic system (e.g., juvenile Parkinson’s disease, pallidopyramidal syndrome, and spinocerebellar ataxia type 3) [93].

Table 5.
Clinical features and useful biochemical investigations for common disorders associated with a defect in the dopamine synthesis pathway.
4. Further Treatment Considerations
4.1. Conservative Treatments
Education and counselling play a crucial role in the management of RMDs, directing patients and their carers toward reliable web-based resources and support groups that are increasingly accessible. The involvement of multidisciplinary teams, particularly occupational therapists, physiotherapists, and speech and language therapists, provides the opportunity to support and empower patients in adapting their lifestyle around their disorder. As previously outlined, dietary modifications and trigger avoidance can be highly effective and straightforward interventions in certain disorders. However, in refractory cases, clinicians should remain vigilant to the possibility of patient non-compliance to these measures. Additionally, managing patients with RMDs involves screening for known complications associated with the disorder, such as malignancy in ataxic telangiectasia [94].
4.2. Medical Treatments
It is important to recognise that, although various medical (mostly symptomatic) treatments are available for RMDs, clinical trials assessing their efficacy and safety often involve small sample sizes and lack long-term outcome data [6]. Despite these limitations, due to the substantial impact of these disorders on quality of life, it is common practice for clinicians to prescribe such treatments off-label, while closely monitoring for possible therapeutic complications. Furthermore, in the context of RMDs, certain disorders may be highly responsive to specific therapies, potentially leading to complete symptom remission [6]. Therefore, the early recognition of treatable RMDs is crucial for optimising patient outcomes (Table 6).

Table 6.
Treatable rare movement disorders.
4.3. Genetic Testing and Counselling
The early recognition of RMDs can be significantly facilitated by the advances and wide availability of next-generation sequencing, as well as constantly improving pipelines for data analysis, which are increasingly integrated into the standard diagnostic workflow for these conditions [95]. For instance, a recent study involving a wide spectrum of ataxia phenotypes identified a genetic basis in 50% of the cohort using whole-exome sequencing [96]. Moreover, molecular testing plays a crucial role in elucidating the phenotypic heterogeneity of genetic RMDs, as exemplified by GNAO1-related disorders that are now known to present with both infantile dyskinetic encephalopathy and late-onset focal abnormal movements [97,98]. However, both whole-exome and whole-genome sequencing generate vast amounts of data, posing a challenge in determining which identified variants are causally linked to the phenotype under investigation [95]. Automated pipelines, using a series of computational mutation-filtration steps, partly address this challenge, but these depend heavily on online resources and bioinformatics tools, which still have considerable limitations. As a result, several variants of uncertain significance remain, and there is ongoing debate regarding whether these findings should be reported and communicated to patients and their families [95]. The re-analysis of genomic sequencing data at regular intervals will be crucial to address this challenge. In addition, sharing findings from molecular testing within the broader genetics community will help to strengthen genotype–phenotype correlations. This is especially pertinent in light of recent advances in gene therapy, which present a promising therapeutic approach for RMDs, underscored by the initiation of the first clinical trial utilising gene therapy for AADC deficiency [99].
The molecular diagnosis of RMDs has significant implications for genetic counselling, as it allows for a more accurate assessment of the inheritance pattern and potential risk for family members of affected individuals. Identifying the underlying genetic cause can provide crucial information for relatives regarding their own risk of developing the disorder, as well as the likelihood of passing it on to future generations. This information can also guide family planning, including prenatal testing and carrier screening. Additionally, genetic counselling can help families understand the implications of the diagnosis, manage expectations regarding disease progression, and make informed decisions about available treatment options and participation in clinical trials.
5. Conclusions
Although rare by definition, RMDs as a group are often encountered by movement disorder specialists, requiring a detailed clinical history and examination as the first step to narrow down the differential diagnosis. Investigations, including biochemical tests, neuroimaging, and genetic tests, can be crucial in elucidating the etiological diagnosis. Certain RMDs respond very well to specific therapies, sometimes resulting in complete symptom remission, making it essential not to overlook these conditions. Collaborative efforts should continue to enhance the understanding of RMDs, particularly with regard to genotype–phenotype correlations, and further develop RMD patient registries and biobanks to facilitate ongoing research. Advances in dissecting the molecular basis of RMDs offer a promising avenue for treatment with gene therapy, and this may emerge as an effective management option for several of these disorders in the future.
Funding
No specific funding was required for this work.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were generated within this work.
Acknowledgments
We acknowledge the support of the Rare Neurological Diseases panel of EAN in preparing this manuscript. Francesca Magrinelli acknowledges funding through the NIHR UCLH Biomedical Research Centre (BRC) Translational Neuroscience Intermediate Clinical Fellowship (Grant ID: BRC1287/TN/FM/101410), the Edmond J. Safra Movement Disorders Research Career Development Award (Grant ID: MJFF-023893), Parkinson’s UK (Grant ID: G-2401), the American Parkinson Disease Association (Grant ID: 1282403), and the David Pearlman Charitable Foundation. Amit Batla acknowledges funding over the last three years from MRC-NIHR (UKRI CARP award), National Brain Appeal, MSA Trust, UCL Therapeutic Acceleration Support (TAS) Fund Round 10 and the UCL Centre for Equality Research in Brain Sciences. He received speaker honorarium from Ipsen and Omniprex and is a Trustee for Fahr Beyond (UK Registered charity) and Dystonia UK. He received book royalties from the book “Understanding Parkinsonism” (Jaypee brothers 2017). Kailash P. Bhatia has received grant support from the Edmond J. Safra Movement Disorders Research Career Development Award (Grant ID: MJFF-023893), Parkinson’s UK (Grant ID: G-2401), EPSRC, and the David Pearlman Charitable Foundation. He has received a stipend from the International Parkinson and Movement Disorder Society as editor of the Movement Disorders Clinical Practice journal and book royalties from Oxford University Press.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Orphanet: Diseases. Available online: https://www.orpha.net/en/disease (accessed on 27 November 2024).
- Magrinelli, F.; Balint, B.; Bhatia, K.P. Challenges in Clinicogenetic Correlations: One Gene—Many Phenotypes. Mov. Disord. Clin. Pract. 2021, 8, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Gannamani, R.; van der Veen, S.; van Egmond, M.; de Koning, T.J.; Tijssen, M.A.J. Challenges in Clinicogenetic Correlations: One Phenotype—Many Genes. Mov. Disord. Clin. Pract. 2021, 8, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Farbman, E.S. The Case for Subspecialization in Neurology: Movement Disorders. Front. Neurol. 2011, 2, 22. [Google Scholar] [CrossRef]
- Mayor, S. Neurological patients experience long delays to see specialists, finds survey. BMJ 2019, 366, 14605. [Google Scholar] [CrossRef]
- Jinnah, H.A.; Albanese, A.; Bhatia, K.P.; Cardoso, F.; Da Prat, G.; de Koning, T.J.; Espay, A.J.; Fung, V.; Garcia-Ruiz, P.J.; Gershanik, O.; et al. Treatable inherited rare movement disorders. Mov. Disord. 2018, 33, 21–35. [Google Scholar] [CrossRef]
- Khateeb, S.; Flusser, H.; Ofir, R.; Shelef, I.; Narkis, G.; Vardi, G.; Shorer, Z.; Levy, R.; Galil, A.; Elbedour, K.; et al. PLA2G6 mutation underlies infantile neuroaxonal dystrophy. Am. J. Hum. Genet. 2006, 79, 942–948. [Google Scholar] [CrossRef]
- Magrinelli, F.; Mehta, S.; Di Lazzaro, G.; Latorre, A.; Edwards, M.J.; Balint, B.; Basu, P.; Kobylecki, C.; Groppa, S.; Hegde, A.; et al. Dissecting the Phenotype and Genotype of PLA2G6-Related Parkinsonism. Mov. Disord. 2022, 37, 148–161. [Google Scholar] [CrossRef]
- Ajitkumar, A.; Lui, F.; De Jesus, O. Huntington Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK559166/ (accessed on 29 May 2025).
- Schneider, S.A.; Bird, T. Huntington’s Disease, Huntington’s Disease Look-Alikes, and Benign Hereditary Chorea: What’s New? Mov. Disord. Clin. Pract. 2016, 3, 342–354. [Google Scholar] [CrossRef]
- Vitrikas, K.; Dalton, H.; Breish, D. Cerebral Palsy: An Overview. Am. Fam. Physician 2020, 101, 213–220. [Google Scholar]
- Pearson, T.S.; Pons, R.; Ghaoui, R.; Sue, C.M. Genetic mimics of cerebral palsy. Mov. Disord. 2019, 34, 625–636. [Google Scholar] [CrossRef]
- Lopriore, P.; Gomes, F.; Montano, V.; Siciliano, G.; Mancuso, M. Mitochondrial Epilepsy, a Challenge for Neurologists. Int. J. Mol. Sci. 2022, 23, 13216. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, J.L.; Kirse, D.J.; Kearns, D.; Deutsch, R.; Spruijt, L.; Naviaux, R.K. The Otolaryngological Manifestations of Mitochondrial Disease and the Risk of Neurodegeneration With Infection. Arch. Otolaryngol. Neck Surg. 2002, 128, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Otto, P.A.; Horimoto, A.R.V.R. Penetrance rate estimation in autosomal dominant conditions. Genet. Mol. Biol. 2012, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Kingdom, R.; Wright, C.F. Incomplete Penetrance and Variable Expressivity: From Clinical Studies to Population Cohorts. Front. Genet. 2022, 13, 920390. [Google Scholar] [CrossRef]
- Balint, B.; Bhatia, K.P. Isolated and combined dystonia syndromes—An update on new genes and their phenotypes. Eur. J. Neurol. 2015, 22, 610–617. [Google Scholar] [CrossRef]
- Saffari, A.; Lau, T.; Tajsharghi, H.; Karimiani, E.G.; Kariminejad, A.; Efthymiou, S.; Zifarelli, G.; Sultan, T.; Toosi, M.B.; Sedighzadeh, S.; et al. The clinical and genetic spectrum of autosomal-recessive TOR1A-related disorders. Brain 2023, 146, 3273–3288. [Google Scholar] [CrossRef]
- Xiromerisiou, G.; Houlden, H.; Scarmeas, N.; Stamelou, M.; Kara, E.; Hardy, J.; Lees, A.J.; Korlipara, P.; Limousin, P.; Paudel, R.; et al. THAP1 Mutations and Dystonia Phenotypes: Genotype Phenotype Correlations. Mov. Disord. 2012, 27, 1290–1294. [Google Scholar] [CrossRef]
- Raymond, D.; Saunders-Pullman, R.; Ozelius, L. SGCE Myoclonus-Dystonia. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: http://www.ncbi.nlm.nih.gov/books/NBK1414/ (accessed on 23 March 2025).
- Stamelou, M.; Charlesworth, G.; Cordivari, C.; Schneider, S.A.; Kägi, G.; Sheerin, U.-M.; Rubio-Agusti, I.; Batla, A.; Houlden, H.; Wood, N.W.; et al. The phenotypic spectrum of DYT24 due to ANO3 mutations. Mov. Disord. 2014, 29, 928–934. [Google Scholar] [CrossRef]
- Mencacci, N.E.; Rubio-Agusti, I.; Zdebik, A.; Asmus, F.; Ludtmann, M.H.R.; Ryten, M.; Plagnol, V.; Hauser, A.-K.; Bandres-Ciga, S.; Bettencourt, C.; et al. A Missense Mutation in KCTD17 Causes Autosomal Dominant Myoclonus-Dystonia. Am. J. Hum. Genet. 2015, 96, 938–947. [Google Scholar] [CrossRef]
- Martins, J.; Darling, A.; Garrido, C.; Espinós, C.; Martí, M.J.; Dueñas, B.P.; Temudo, T. Sensory Tricks in Pantothenate Kinase-Associated Neurodegeneration: Video-Analysis of 43 Patients. Mov. Disord. Clin. Pract. 2019, 6, 704–707. [Google Scholar] [CrossRef]
- Pandey, N.; Blair, K.; John, S. Kayser-Fleischer Ring. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK459187/ (accessed on 1 August 2024).
- Yiş, U.; Becker, K.; Yılmaz, Ş.; Çırak, S. Acanthocytosis and HyperCKemia. Turk. J. Hematol. 2018, 35, 296–297. [Google Scholar] [CrossRef] [PubMed]
- Chinnery, P.F. Primary Mitochondrial Disorders Overview. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: http://www.ncbi.nlm.nih.gov/books/NBK1224/ (accessed on 28 March 2025).
- Ostojic, S.M. Plasma creatine as a marker of mitochondrial dysfunction. Med. Hypotheses 2018, 113, 52–53. [Google Scholar] [CrossRef]
- Degtyareva, A.V.; Proshlyakova, T.Y.; Gautier, M.S.; Degtyarev, D.N.; Kamenets, E.A.; Baydakova, G.V.; Rebrikov, D.V.; Zakharova, E.Y. Oxysterol/chitotriosidase based selective screening for Niemann-Pick type C in infantile cholestasis syndrome patients. BMC Med. Genet. 2019, 20, 123. [Google Scholar] [CrossRef] [PubMed]
- Leen, W.G.; Wevers, R.A.; Kamsteeg, E.-J.; Scheffer, H.; Verbeek, M.M.; Willemsen, M.A. Cerebrospinal fluid analysis in the workup of GLUT1 deficiency syndrome: A systematic review. JAMA Neurol. 2013, 70, 1440–1444. [Google Scholar] [CrossRef]
- Donzuso, G.; Mostile, G.; Nicoletti, A.; Zappia, M. Basal ganglia calcifications (Fahr’s syndrome): Related conditions and clinical features. Neurol. Sci. 2019, 40, 2251–2263. [Google Scholar] [CrossRef] [PubMed]
- Kruer, M.C.; Boddaert, N.; Schneider, S.A.; Houlden, H.; Bhatia, K.P.; Gregory, A.; Anderson, J.C.; Rooney, W.D.; Hogarth, P.; Hayflick, S.J. Neuroimaging Features of Neurodegeneration with Brain Iron Accumulation. AJNR Am. J. Neuroradiol. 2012, 33, 407–414. [Google Scholar] [CrossRef]
- Chang, C.-L.; Lin, C.-M. Eye-of-the-Tiger sign is not Pathognomonic of Pantothenate Kinase-Associated Neurodegeneration in Adult Cases. Brain Behav. 2011, 1, 55–56. [Google Scholar] [CrossRef]
- Herrero Hernandez, E.; Valentini, M.C.; Discalzi, G. T1-weighted hyperintensity in basal ganglia at brain magnetic resonance imaging: Are different pathologies sharing a common mechanism? Neurotoxicology 2002, 23, 669–674. [Google Scholar] [CrossRef]
- Kumar, M.; Gaharwar, U.; Paul, S.; Poojary, M.; Pandhare, K.; Scaria, V.; Bk, B. WilsonGen a comprehensive clinically annotated genomic variant resource for Wilson’s Disease. Sci. Rep. 2020, 10, 9037. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. Wilson Disease—NIDDK. Available online: https://www.niddk.nih.gov/health-information/liver-disease/wilson-disease (accessed on 1 August 2024).
- Stremmel, W.; Merle, U.; Weiskirchen, R. Clinical features of Wilson disease. Ann. Transl. Med. 2019, 7, S61. [Google Scholar] [CrossRef]
- Gupta, A.; Chakravarthi, S.; Goyal, M.K. ‘Face of giant panda’: A rare imaging sign in Wilson’s disease. QJM Int. J. Med. 2014, 107, 579. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.A.; Markowitz, C.E.; Liebeskind, D.S.; Galetta, S.L. The “double panda sign” in Wilson’s disease. Neurology 2003, 61, 969. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kaur, R. Wilson’s Disease: A Brief Review with Neuroimaging Features. Indian J. Appl. Radiol. 2015, 1, 102. [Google Scholar]
- Rędzia-Ogrodnik, B.; Członkowska, A.; Antos, A.; Bembenek, J.; Kurkowska-Jastrzębska, I.; Przybyłkowski, A.; Skowrońska, M.; Smoliński, Ł.; Litwin, T. Pathognomonic neuroradiological signs in Wilson’s disease—Truth or myth? Park. Relat. Disord. 2023, 107, 105247. [Google Scholar] [CrossRef]
- Avan, A.; Członkowska, A.; Gaskin, S.; Granzotto, A.; Sensi, S.L.; Hoogenraad, T.U. The Role of Zinc in the Treatment of Wilson’s Disease. Int. J. Mol. Sci. 2022, 23, 9316. [Google Scholar] [CrossRef]
- Kıroğlu, Y.; Callı, C.; Karabulut, N.; Oncel, C. Intracranial calcifications on CT. Diagn. Interv. Radiol. Ank. Turk. 2010, 16, 263–269. [Google Scholar] [CrossRef]
- Valdés Hernández, M.d.C.; Maconick, L.C.; Tan, E.M.J.; Wardlaw, J.M. Identification of mineral deposits in the brain on radiological images: A systematic review. Eur. Radiol. 2012, 22, 2371–2381. [Google Scholar] [CrossRef]
- Magrinelli, F.; Jesuthasan, A.; Bhatia, K.P.; Batla, A. Basal ganglia calcification: ‘Fahr’s disease’. Pract. Neurol. 2025. published online first. [Google Scholar] [CrossRef]
- Monfrini, E.; Arienti, F.; Rinchetti, P.; Lotti, F.; Riboldi, G.M. Brain Calcifications: Genetic, Molecular, and Clinical Aspects. Int. J. Mol. Sci. 2023, 24, 8995. [Google Scholar] [CrossRef]
- Batla, A.; Gaddipati, C. Neurodegeneration with Brain Iron Accumulation. Ann. Indian Acad. Neurol. 2019, 22, 267–276. [Google Scholar] [CrossRef]
- Gregory, A.; Hayflick, S. Neurodegeneration with Brain Iron Accumulation Disorders Overview. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: http://www.ncbi.nlm.nih.gov/books/NBK121988/ (accessed on 1 August 2024).
- Gupta, P.R.; Gospe, S.M. Ophthalmic manifestations of MEPAN syndrome. Ophthalmic Genet. 2023, 44, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Bohlega, S.A.; Abusrair, A. Woodhouse-Sakati Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: http://www.ncbi.nlm.nih.gov/books/NBK378974/ (accessed on 21 May 2025).
- Iankova, V.; Karin, I.; Klopstock, T.; Schneider, S.A. Emerging Disease-Modifying Therapies in Neurodegeneration With Brain Iron Accumulation (NBIA) Disorders. Front. Neurol. 2021, 12, 629414. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, H.; Takahashi, Y.; Kamata, T.; Shimizu, H.; Sakai, N.; Gitlin, J.D. Use of desferrioxamine in the treatment of aceruloplasminemia. Ann. Neurol. 1997, 41, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S. Familial manganese-induced neurotoxicity due to mutations in SLC30A10 or SLC39A14. Neurotoxicology 2018, 64, 278–283. [Google Scholar] [CrossRef]
- Tuschl, K.; Meyer, E.; Valdivia, L.E.; Zhao, N.; Dadswell, C.; Abdul-Sada, A.; Hung, C.Y.; Simpson, M.A.; Chong, W.K.; Jacques, T.S.; et al. Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism–dystonia. Nat. Commun. 2016, 7, 11601. [Google Scholar] [CrossRef]
- Choi, E.-K.; Aring, L.; Peng, Y.; Correia, A.B.; Lieberman, A.P.; Iwase, S.; Seo, Y.A. Neuronal SLC39A8 deficiency impairs cerebellar development by altering manganese homeostasis. JCI Insight 2024, 9, e168440. [Google Scholar] [CrossRef]
- Cersosimo, M.G.; Koller, W.C. The diagnosis of manganese-induced parkinsonism. Neurotoxicology 2006, 27, 340–346. [Google Scholar] [CrossRef]
- Kim, H.M.; Hu, S.-C.; Samii, A. Cock-walk. In Encyclopedia of Movement Disorders; Kompoliti, K., Metman, L.V., Eds.; Academic Press: Oxford, UK, 2010; pp. 228–230. ISBN 978-0-12-374105-9. Available online: https://www.sciencedirect.com/science/article/pii/B9780123741059004470 (accessed on 1 August 2024).
- Marti-Sanchez, L.; Ortigoza-Escobar, J.D.; Darling, A.; Villaronga, M.; Baide, H.; Molero-Luis, M.; Batllori, M.; Vanegas, M.I.; Muchart, J.; Aquino, L.; et al. Hypermanganesemia due to mutations in SLC39A14: Further insights into Mn deposition in the central nervous system. Orphanet J. Rare Dis. 2018, 13, 28. [Google Scholar] [CrossRef]
- Quadri, M.; Federico, A.; Zhao, T.; Breedveld, G.J.; Battisti, C.; Delnooz, C.; Severijnen, L.-A.; Di Toro Mammarella, L.; Mignarri, A.; Monti, L.; et al. Mutations in SLC30A10 Cause Parkinsonism and Dystonia with Hypermanganesemia, Polycythemia, and Chronic Liver Disease. Am. J. Hum. Genet. 2012, 90, 467–477. [Google Scholar] [CrossRef]
- Majewski, M.; Piwko, K.; Ordak, M.; Muszynska, E.; Nasierowski, T.; Bujalska-Zadrozny, M. Magnetic Resonance Imaging and Manganism: A Narrative Review and Laboratory Recommendations. J. Clin. Med. 2024, 13, 2823. [Google Scholar] [CrossRef]
- Avila, D.S.; Puntel, R.L.; Aschner, M. Manganese in health and disease. Met. Ions Life Sci. 2013, 13, 199–227. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Fakhari, D.; Hildebrandt, C.; Davis, P.E.; Rodan, L.H.; Anselm, I.; Bodamer, O. The Spectrum of Movement Disorders in Childhood-Onset Lysosomal Storage Diseases. Mov. Disord. Clin. Pract. 2018, 5, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Elendu, C.; Babawale, E.A.; Babarinde, F.O.; Babatunde, O.D.; Chukwu, C.; Chiegboka, S.F.; Shode, O.P.; Ngozi-ibeh, J.K.; Njoku, A.; Ikokwu, M.N.; et al. Neurological manifestations of lysosomal storage diseases. Ann. Med. Surg. 2024, 86, 6619–6635. [Google Scholar] [CrossRef]
- Del Grosso, A.; Parlanti, G.; Mezzena, R.; Cecchini, M. Current treatment options and novel nanotechnology-driven enzyme replacement strategies for lysosomal storage disorders. Adv. Drug Deliv. Rev. 2022, 188, 114464. [Google Scholar] [CrossRef] [PubMed]
- Barton, N.W.; Brady, R.O.; Dambrosia, J.M.; Bisceglie, A.M.D.; Doppelt, S.H.; Hill, S.C.; Mankin, H.J.; Murray, G.J.; Parker, R.I.; Argoff, C.E.; et al. Replacement Therapy for Inherited Enzyme Deficiency—Macrophage-Targeted Glucocerebrosidase for Gaucher’s Disease. N. Engl. J. Med. 1991, 324, 1464–1470. [Google Scholar] [CrossRef]
- Beck, M. Treatment strategies for lysosomal storage disorders. Dev. Med. Child Neurol. 2018, 60, 13–18. [Google Scholar] [CrossRef]
- Coutinho, M.F.; Santos, J.I.; Alves, S. Less Is More: Substrate Reduction Therapy for Lysosomal Storage Disorders. Int. J. Mol. Sci. 2016, 17, 1065. [Google Scholar] [CrossRef]
- Erro, R.; Magrinelli, F.; Bhatia, K.P. Paroxysmal movement disorders: Paroxysmal dyskinesia and episodic ataxia. Handb. Clin. Neurol. 2023, 196, 347–365. [Google Scholar] [CrossRef]
- Harvey, S.; King, M.D.; Gorman, K.M. Paroxysmal Movement Disorders. Front. Neurol. 2021, 12, 659064. [Google Scholar] [CrossRef]
- Magrinelli, F.; Bhatia, K.P. Chapter 10—Paroxysmal movement disorders. In Handbook of Clinical Neurology; Neurologic Channelopathies; Hanna, M.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 203, pp. 145–156. Available online: https://www.sciencedirect.com/science/article/pii/B9780323908207000100 (accessed on 29 May 2025).
- Demirkiran, M.; Jankovic, J. Paroxysmal dyskinesias: Clinical features and classification. Ann. Neurol. 1995, 38, 571–579. [Google Scholar] [CrossRef]
- Gras, D.; Roze, E.; Caillet, S.; Méneret, A.; Doummar, D.; Billette de Villemeur, T.; Vidailhet, M.; Mochel, F. GLUT1 deficiency syndrome: An update. Rev. Neurol. 2014, 170, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, J.; Yang, T.; Xiong, W.; Qin, L.; An, D.; Hu, F.; Zhou, D. Genetic and phenotypic analyses of PRRT2 positive and negative paroxysmal kinesigenic dyskinesia. Ther. Adv. Neurol. Disord. 2024, 17, 17562864231224110. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-J.; Li, H.-F.; Wu, Z.-Y. Paroxysmal Kinesigenic Dyskinesia: Genetics and Pathophysiological Mechanisms. Neurosci. Bull. 2023, 40, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Mounir Alaoui, O.; Charbonneau, P.-F.; Prin, P.; Mongin, M.; Choquer, M.; Damier, P.; Riant, F.; Degos, B. TMEM151A as an alternative to PRRT2 in paroxysmal kinesigenic dyskinesia: About three new cases. Park. Relat. Disord. 2023, 108, 105295. [Google Scholar] [CrossRef]
- Zorzi, G.; Zibordi, F.; Sorrentino, U.; Prokisch, H.; Garavaglia, B.; Zech, M. Potassium Channel Subunit Kir4.1 Mutated in Paroxysmal Kinesigenic Dyskinesia: Screening of an Italian Cohort. Mov. Disord. 2024, 39, 2302–2304. [Google Scholar] [CrossRef]
- De Gusmao, C.M.; Silveira-Moriyama, L. Paroxysmal movement disorders—Practical update on diagnosis and management. Expert Rev. Neurother. 2019, 19, 807–822. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Chen, D.-F.; Ke, H.-Z.; Zhao, S.-Y.; Li, H.-F.; Wu, Z.-Y. Paroxysmal Kinesigenic Dyskinesia Caused by 16p11.2 Microdeletion and Related Clinical Features. Neurol. Genet. 2022, 8, e659. [Google Scholar] [CrossRef]
- Shimojima, K.; Okumura, A.; Natsume, J.; Aiba, K.; Kurahashi, H.; Kubota, T.; Yokochi, K.; Yamamoto, T. Spinocerebellar ataxias type 27 derived from a disruption of the fibroblast growth factor 14 gene with mimicking phenotype of paroxysmal non-kinesigenic dyskinesia. Brain Dev. 2012, 34, 230–233. [Google Scholar] [CrossRef]
- Garone, G.; Capuano, A.; Travaglini, L.; Graziola, F.; Stregapede, F.; Zanni, G.; Vigevano, F.; Bertini, E.; Nicita, F. Clinical and Genetic Overview of Paroxysmal Movement Disorders and Episodic Ataxias. Int. J. Mol. Sci. 2020, 21, 3603. [Google Scholar] [CrossRef]
- de Gusmao, C.M.; Garcia, L.R.; Jesuthasan, A.; Muir, M.; Paciorkowski, A.; Mink, J.W.; Silveira-Moriyama, L. Kinesigenic Triggers in Episodic Ataxia Type 1. Mov. Disord. Clin. Pract. 2020, 7, 723–724. [Google Scholar] [CrossRef]
- Bhatia, K.P. The paroxysmal dyskinesias. J. Neurol. 1999, 246, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Leen, W.G.; Mewasingh, L.; Verbeek, M.M.; Kamsteeg, E.-J.; van de Warrenburg, B.P.; Willemsen, M.A. Movement disorders in GLUT1 deficiency syndrome respond to the modified Atkins diet. Mov. Disord. 2013, 28, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- de Gusmao, C.M.; Peixoto de Barcelos, I.; Pinto, A.L.R.; Silveira-Moriyama, L. Pearls & Oy-sters: Paroxysmal Exercise-Induced Dyskinesias Due to Pyruvate Dehydrogenase Deficiency. Neurology 2023, 101, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Erro, R. Familial Paroxysmal Nonkinesigenic Dyskinesia. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: http://www.ncbi.nlm.nih.gov/books/NBK1221/ (accessed on 28 March 2025).
- Larsh, T. Alternative Medications for Paroxysmal Kinesigenic Dyskinesia (1972). Neurology 2021, 96, 1972. [Google Scholar] [CrossRef]
- Unterberger, I.; Trinka, E. Diagnosis and treatment of paroxysmal dyskinesias revisited. Ther. Adv. Neurol. Disord. 2008, 1, 4–11. [Google Scholar] [CrossRef]
- Sondhi, V.; Sharma, S. Vitamin-Responsive Movement Disorders in Children. Ann. Indian Acad. Neurol. 2020, 23, 325–331. [Google Scholar] [CrossRef]
- Ozand, P.T.; Gascon, G.G.; Al Essa, M.; Joshi, S.; Al Jishi, E.; Bakheet, S.; Al Watban, J.; Al-Kawi, M.Z.; Dabbagh, O. Biotin-responsive basal ganglia disease: A novel entity. Brain J. Neurol. 1998, 121 Pt 7, 1267–1279. [Google Scholar] [CrossRef]
- Manco, C.; Cortese, R.; Alberti, M.; Bianchi, S.; Monti, L.; De Stefano, N.; Battisti, C. FOLR1 Gene Variation With Adult-Onset Cerebral Folate Deficiency and Stable Clinical and MRI Features up to 2 Years. Neurol. Genet. 2023, 9, e200104. [Google Scholar] [CrossRef]
- Sofou, K.; Dahlin, M.; Hallböök, T.; Lindefeldt, M.; Viggedal, G.; Darin, N. Ketogenic diet in pyruvate dehydrogenase complex deficiency: Short- and long-term outcomes. J. Inherit. Metab. Dis. 2017, 40, 237–245. [Google Scholar] [CrossRef]
- Wijemanne, S.; Jankovic, J. Dopa-responsive dystonia—Clinical and genetic heterogeneity. Nat. Rev. Neurol. 2015, 11, 414–424. [Google Scholar] [CrossRef]
- Lee, W.-W.; Jeon, B.S. Clinical Spectrum of Dopa-Responsive Dystonia and Related Disorders. Curr. Neurol. Neurosci. Rep. 2014, 14, 461. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-W.; Jeon, B.; Kim, R. Expanding the Spectrum of Dopa-Responsive Dystonia (DRD) and Proposal for New Definition: DRD, DRD-plus, and DRD Look-alike. J. Korean Med. Sci. 2018, 33, e184. [Google Scholar] [CrossRef] [PubMed]
- Hecht, F.; Hecht, B.K. Cancer in ataxia-telangiectasia patients. Cancer Genet. Cytogenet. 1990, 46, 9–19. [Google Scholar] [CrossRef]
- Zech, M.; Winkelmann, J. Next-generation sequencing and bioinformatics in rare movement disorders. Nat. Rev. Neurol. 2024, 20, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Johnson, A.K.; Nelakuditi, V.; Guidugli, L.; Fischer, D.; Arndt, K.; Ma, L.; Sandford, E.; Shakkottai, V.; Boycott, K.; et al. Targeted exome analysis identifies the genetic basis of disease in over 50% of patients with a wide range of ataxia-related phenotypes. Genet. Med. 2019, 21, 195–206. [Google Scholar] [CrossRef]
- Feng, H.; Sjögren, B.; Karaj, B.; Shaw, V.; Gezer, A.; Neubig, R.R. Movement disorder in GNAO1 encephalopathy associated with gain-of-function mutations. Neurology 2017, 89, 762–770. [Google Scholar] [CrossRef]
- Wirth, T.; Garone, G.; Kurian, M.A.; Piton, A.; Millan, F.; Telegrafi, A.; Drouot, N.; Rudolf, G.; Chelly, J.; Marks, W.; et al. Highlighting the Dystonic Phenotype Related to GNAO1. Mov. Disord. 2022, 37, 1547–1554. [Google Scholar] [CrossRef]
- FDA. FDA Approves First Gene Therapy for Treatment of Aromatic L-Amino Acid Decarboxylase Deficiency. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-treatment-aromatic-l-amino-acid-decarboxylase-deficiency (accessed on 22 March 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).