2-{N-[ω-(1-Benzylpiperidin-4-yl)alkyl]amino}-6-[(prop-2-yn-1-yl)amino]pyridine-3,5-dicarbonitriles Showing High Affinity for σ1/2 Receptors
Abstract
1. Introduction
 | |||
| Comp. | Ki Human σ1R a (nM) | Ki Rat σ2R b (nM) | Selectivity Index of Rat σ2R/Human σ1R |
|---|---|---|---|
| 1 | 29.2 (23.9; 34.5) | 173 | 5.9 |
| 2 | 7.57 ± 0.59 | 42 ± 8 | 5.6 |
| 3 | 2.97 ± 0.22 | 37 ± 5 | 13 |
| 4 | 3.97 ± 0.66 | 163 ± 50 | 41 |
| 5 | 1.45 ± 0.43 | 389 | 270 |
| 6 | 3.05 ± 1.27 | 129 ± 26 | 42 |
| 7 | 3.09 ± 1.25 | 288 | 93 |
| 8 | 27.3 (21.4; 33.2) | 141 | 5.2 |
| 9 | 7.45 ± 4.32 | 104 ± 22 | 14 |
| 10 | 15.4 (12.3; 18.5) | 39 | 2.5 |
| 11 | 119 (157; 81.4) | 55 | 0.46 |
| 12 | 10.9 ± 1.9 | 393 | 36 |
| NE-100 | 2.00 (1.9; 2.1) | n.d. c | n.d. c |
| PB28 | 1.9 | n.d. c | n.d. c |
2. Results and Discussion
2.1. In Vitro Modulation of σ1R and σ2R by Pyridines of Type I
- The linker between the 1-benzylpiperidine moiety and the pyridine ring plays a crucial role in σ1R affinity, as increasing the length from the amino group in compound 1 (n = 0, Ki = 29.2 nM) to an ethylamino (2: n= 2, Ki = 7.57 ± 0.59 nM), a propylamino (3: n = 3, Ki = 2.97 ± 0.22 nM) and to a butylamino group (4: n = 4, Ki = 3.97 ± 0.66 nM), resulted in increased hσ1R affinity.
- The introduction of a methyl moiety at the N-(prop-2-yn-1-yl)amino substituent at C6 of ligands bearing R1 as H, such as 2, 3, and 4, led to pyridines 5, 6, and 7, significantly increasing their affinity from ligand 2 (Ki = 7.57 ± 0.59 nM) to pyridine 5 (Ki = 1.45 nM) with n = 2 as the linker, but had no remarkable effect on the transition from the ligands 3 and 4 to the pyridines 6 and 7 with n = 3 as the linker. However, the introduction of a methyl substituent for the R2 group in compound 9 (Ki = 7.45 nM) bearing R1 as Ph to yield compound 12 (Ki = 10.9 nM) significantly decreased the affinity.
- The insertion of a phenyl group at C4 of the pyridine ring had no impact on the σ1R affinity for n = 0 as the linker, 1 vs. 8, but significantly decreased the affinity from ligand 3 to compound 9 (n = 3), and from ligand 4 to compound 10 (n = 4). A similar decreasing σ1R affinity effect was induced by the insertion of a phenyl group at C4 on the N-methyl-propynylamine-substituted pyridine 6 (Ki = 3.05 nM) compared to compound 12 (Ki = 10.9 nM) for n = 3 as the linker.
- Finally, compound 11 showed the lowest hσ1R affinity, most likely due to the combination of the sub-optimal distance of the two essential hydrophobic regions (n = 1) and the effects of the N-methyl-propynylamine and the phenyl-substituted pyridine moiety serving as the aromatic hydrophobic region.
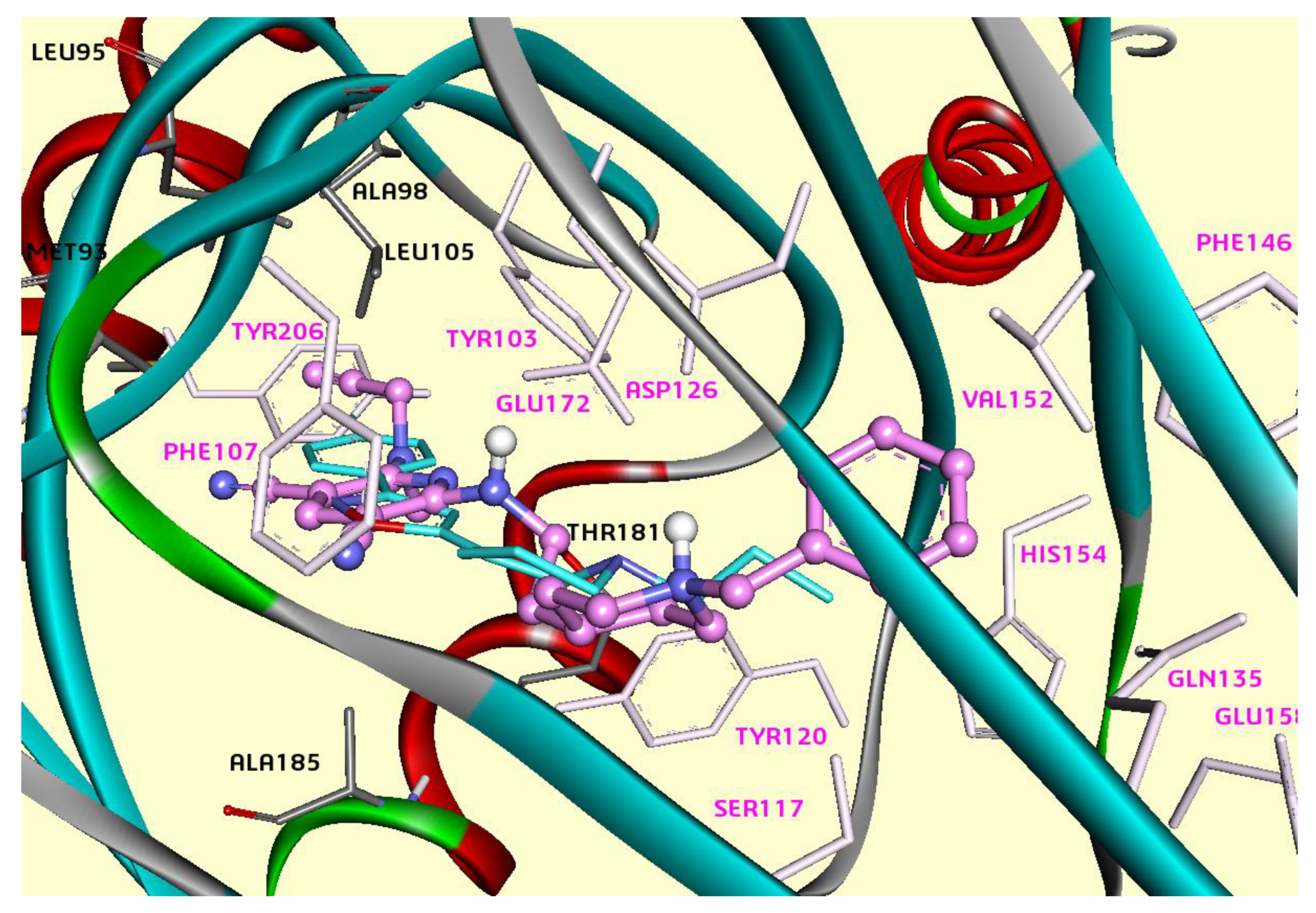
2.2. Molecular Docking of Pyridines 3, 5, 6, 9, 11 and 12 with σ1R and σ2R
2.3. Virtual ADME of Pyridines 3, 5, 6, 9, 11, and 12
3. Conclusions
4. Materials and Methods
4.1. Biological Assays
4.1.1. In Vitro σ1R Competitive Binding Assay
4.1.2. In Vitro σ2R Competitive Binding Assay [38]
4.2. Molecular Simulations
Molecular Modeling
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arena, E.; Dichiara, M.; Floresta, G.; Parenti, C.; Marrazzo, A.; Pittalà, V.; Amata, E.; Prezzavento, O. Novel Sigma-1 Receptor Antagonists: From Opioids to Small Molecules: What is New? Futur. Med. Chem. 2017, 10, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Abate, C.; Niso, M.; Berardi, F. Sigma-2 Receptor: Past, Present and Perspectives on Multiple Therapeutic Exploitations. Futur. Med. Chem. 2018, 10, 1997–2018. [Google Scholar] [CrossRef]
- Brune, S.; Pricl, S.; Wünsch, B. Structure of the σ1 receptor and its ligand binding site. J. Med. Chem. 2013, 56, 9809–9819. [Google Scholar] [CrossRef]
- Amata, E.; Dichiara, M.; Gentile, D.; Marrazzo, A.; Turnaturi, R.; Arena, E.; La Mantia, A.; Tomasello, B.R.; Acquaviva, R.; Di Giacomo, C.; et al. Sigma Receptor Ligands Carrying a Nitric Oxide Donor Nitrate Moiety: Synthesis, In Silico, and Biological Evaluation. ACS Med. Chem. Lett. 2020, 11, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhang, Y.; Huang, Y. Imaging sigma receptors in the brain: New opportunities for diagnosis of Alzheimer’s disease and therapeutic development. Neurosci. Lett. 2018, 691, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Lucke-Wold, B.P.; Mookerjee, S.A.; Cavendish, J.Z.; Robson, M.J.; Scandinaro, A.L.; Matsumoto, R.R. Role of sigma-1 receptors in neurodegenerative diseases. J. Pharmacol. Sci. 2015, 127, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Maurice, T.; Goguadze, N. Role of σ1 Receptors in Learning and Memory and Alzheimer’s Disease-Type Dementia. In Advances in Experimental Medicine and Biology; Smith, S., Su, T.P., Eds.; Springer: Cham, Switzerland, 2017; Volume 964, pp. 213–233. [Google Scholar]
- Meunier, J.; Ieni, J.; Maurice, T. The anti-amnesic and neuroprotective effects of donepezil against amyloid b25-35 peptide-induced toxicity in mice involve an interaction with σ1 receptor, Br. J. Pharmacol. 2006, 149, 998–1012. [Google Scholar]
- Entrena, J.M.; Cobos, E.J.; Nieto, F.R.; Cendán, C.M.; Gris, G.; Del Pozo, E.; Zamanillo, D.; Baeyens, J.M. Sigma-1 receptors are essential for capsaicin-induced mechanical hypersensitivity: Studies with selective sigma-1 ligands and sigma-1 knockout mice. Pain 2009, 143, 252–261. [Google Scholar] [CrossRef]
- Entrena, J.M.; Sánchez-Fernández, C.; Nieto, F.R.; González-Cano, R.; Yeste, S.; Cobos, E.J.; Baeyens, J.M. Sigma-1 Receptor Agonism Promotes Mechanical Allodynia After Priming the Nociceptive System with Capsaicin. Sci. Rep. 2016, 6, 37835. [Google Scholar] [CrossRef]
- Szczepańska, K.; Podlewska, S.; Dichiara, M.; Gentile, D.; Patamia, V.; Rosier, N.; Mönnich, D.; Cantero, M.C.R.; Karcz, T.; Łażewska, D.; et al. Structural and Molecular Insight into Piperazine and Piperidine Derivatives as Histamine H3 and Sigma-1 Receptor Antagonists with Promising Antinociceptive Properties. ACS Chem. Neurosci. 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Dichiara, M.; Artacho-Cordón, A.; Turnaturi, R.; Santos-Caballero, M.; González-Cano, R.; Pasquinucci, L.; Barbaraci, C.; Rodríguez-Gómez, I.; Gómez-Guzmán, M.; Marrazzo, A.; et al. Dual Sigma-1 receptor antagonists and hydrogen sulfide-releasing compounds for pain treatment: Design, synthesis, and pharmacological evaluation. Eur. J. Med. Chem. 2022, 230, 114091. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef] [PubMed]
- Almansa, C.; Vela, J.M. Selective Sigma-1 Receptor Antagonists for The Treatment of Pain. Futur. Med. Chem. 2014, 6, 1179–1199. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.R.; Cendán, C.M.; Sánchez-Fernández, C.; Cobos, E.J.; Entrena, J.M.; Tejada, M.A.; Zamanillo, D.; Vela, J.M.; Baeyens, J.M. Role of Sigma-1 Receptors in Paclitaxel-Induced Neuropathic Pain in Mice. Pain 2012, 13, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Bruna, J.; Velasco, R. Sigma-1 receptor: A new player in neuroprotection against chemotherapy-induced peripheral neuropathy. Neural Regen. Res. 2018, 13, 775–778. [Google Scholar] [CrossRef]
- Prezzavento, O.; Campisi, A.; Parenti, C.; Ronsisvalle, S.; Aricò, G.; Arena, E.; Pistolozzi, M.; Scoto, G.M.; Bertucci, C.; Vanella, A.; et al. Synthesis and Resolution of cis-(±)-Methyl (1R,2S/1S,2R)-2-[(4-Hydroxy-4-phenylpiperidin-1-yl)methyl]-1-(4-methylphenyl)cyclopropanecarboxylate [(±)-PPCC)]: New σ Receptor Ligands with Neuroprotective Effect. J. Med. Chem. 2010, 53, 5881–5885. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, A.; Parenti, C.; Scavo, V.; Ronsisvalle, S.; Scoto, G.M.; Ronsisvalle, G. In vivo evaluation of (+)-MR200 as a new selective sigma ligand modulating MOP, DOP and KOP supraspinal analgesia. Life Sci. 2006, 78, 2449–2453. [Google Scholar] [CrossRef]
- McCracken, K.A.; Bowen, W.D.; Matsumoto, R.R. Novel σ receptor ligands attenuate the locomotor stimulatory effects of cocaine. Eur. J. Pharmacol. 1999, 365, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Mésangeau, C.; Narayanan, S.; Green, A.M.; Shaikh, J.; Kaushal, N.; Viard, E.; Xu, Y.-T.; Fishback, J.A.; Poupaert, J.H.; Matsumoto, R.R.; et al. Conversion of a Highly Selective Sigma-1 Receptor–Ligand to Sigma-2 Receptor Preferring Ligands with Anticocaine Activity. J. Med. Chem. 2008, 51, 1482–1486. [Google Scholar] [CrossRef]
- Lan, Y.; Chen, Y.; Cao, X.; Zhang, J.; Wang, J.; Xu, X.; Qiu, Y.; Zhang, T.; Liu, X.; Liu, B.-F.; et al. Synthesis and Biological Evaluation of Novel Sigma-1 Receptor Antagonists Based on Pyrimidine Scaffold as Agents for Treating Neuropathic Pain. J. Med. Chem. 2014, 57, 10404–10423. [Google Scholar] [CrossRef] [PubMed]
- Utech, T.; Köhler, J.; Wünsch, B. Synthesis of 4-(aminoalkyl) substituted 1,3-dioxanes as potent NMDA and σ receptor antagonists. Eur. J. Med. Chem. 2011, 46, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Shi, M.; Zhang, W.; Zheng, Y.-M.; Xu, Y.-Z.; Shi, J.-J.; Liu, T.; Gunosewoyo, H.; Pang, T.; Gao, Z.-B.; et al. Development of Novel Alkoxyisoxazoles as Sigma-1 Receptor Antagonists with Antinociceptive Efficacy. J. Med. Chem. 2016, 59, 6329–6343. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.L.; Christmann, U.; Fernández, A.; Luengo, M.; Bordas, M.; Enrech, R.; Carro, M.; Pascual, R.; Burgueño, J.; Merlos, M.; et al. Synthesis and Biological Evaluation of a New Series of Hexahydro-2H-pyrano[3,2-c]quinolines as Novel Selective σ1 Receptor Ligands. J. Med. Chem. 2013, 56, 3656–3665. [Google Scholar] [CrossRef]
- Díaz, J.L.; Cuberes, R.; Berrocal, J.; Contijoch, M.; Christmann, U.; Fernández, A.; Port, A.; Holenz, J.; Buschmann, H.; Laggner, C.; et al. Synthesis and Biological Evaluation of the 1-Arylpyrazole Class of σ1 Receptor Antagonists: Identification of 4-{2-[5-Methyl-1-(naphthalen-2-yl)-1H-pyrazol-3-yloxy]ethyl}morpholine (S1RA, E-52862). J. Med. Chem. 2012, 55, 8211–8224. [Google Scholar] [CrossRef]
- Lalut, J.; Santoni, G.; Karila, D.; Lecoutey, C.; Davis, A.; Nachon, F.; Silman, I.; Sussman, J.; Weik, M.; Maurice, T.; et al. Novel multitarget-directed ligands targeting acetylcholinesterase and σ1 receptors as lead compounds for treatment of Alzheimer’s disease: Synthesis, evaluation, and structural characterization of their complexes with acetylcholin-esterase. Eur. J. Med. Chem. 2019, 162, 234–248. [Google Scholar] [CrossRef]
- Vela, J.M.; Merlos, M.; Almansa, C. Investigational sigma-1 receptor antagonists for the treatment of pain. Expert Opin. Investig. Drugs 2015, 24, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Bach, P.; Boström, J.; Brickmann, K.; van Giezen, J.; Hovland, R.; Petersson, A.U.; Ray, A.; Zetterberg, F. A novel series of piperazinyl-pyridine ureas as antagonists of the purinergic P2Y12 receptor. Bioorganic Med. Chem. Lett. 2011, 21, 2877–2881. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Möller, G.; Schepmann, D.; Wünsch, B. Pyridine analogues of spirocyclic σ1 receptor ligands. Bioorganic Med. Chem. 2014, 22, 4277–4284. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Ferorelli, S.; Niso, M.; Lovicario, C.; Infantino, V.; Convertini, P.; Perrone, R.; Berardi, F. 2-Aminopyridine Deriva-tives as Potential σ2 Receptor Antagonists. ChemMedChem 2012, 7, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Entrena, J.M.; Artacho-Cordón, A.; Ravez, S.; Liberelle, M.; Melnyk, P.; Toledano-Pinedo, M.; Almendros, P.; Cobos, E.J.; Marco-Contelles, J. The proof of concept of 2-{3-[N-(1-benzylpiperidin-4-yl)propyl]amino}-6-[N-methyl-N-(prop-2-yn-1-yl)amino]-4-phenylpyridine-3,5-dicarbonitrile for the therapy of neuropathic pain. Bioorg. Chem. 2024, 150, 107537. [Google Scholar] [CrossRef]
- Samadi, A.; Chioua, M.; Bolea, I.; de los Ríos, C.; Iriepa, I.; Moraleda, I.; Bastida, A.; Esteban, G.; Unzeta, M.; Gálvez, E.; et al. Synthesis, biological assessment and molecular modeling of new multipotent MAO and cholinesterase in-hibitors as potential drugs for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2011, 46, 4665–4668. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Aguilera, O.M.; Esteban, G.; Chioua, M.; Nikolic, K.; Agbaba, D.; Moraleda, I.; Iriepa, I.; Soriano, E.; Samadi, A.; Unzeta, M.; et al. Multipotent cholinesterase/monoamine oxidase inhibitors for the treatment of Alzheimer’s disease: Design, synthesis, biochemical evaluation, ADMET, molecular modeling, and QSAR analysis of novel donepezil-pyridyl hybrids. Drug Des. Devel. Ther. 2014, 8, 1893–1910. [Google Scholar] [PubMed]
- Pascual, R.; Almansa, C.; Plata-Salamán, C.; Vela, J.M. A New Pharmacophore Model for the Design of Sigma-1 Ligands Validated on a Large Experimental Dataset. Front. Pharmacol. 2019, 10, 519. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.R.; Zheng, S.; Gurpinar, E.; Koehl, A.; Manglik, A.; Kruse, A.C. Crystal structure of the human σ1 receptor. Nature 2016, 532, 527–530. [Google Scholar] [CrossRef]
- Cheng, Y.; Prusoff, W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [PubMed]
- Maier, C.A.; Wünsch, B. Novel σ Receptor Ligands. Part 2. SAR of Spiro[[2]benzopyran-1,4‘-piperidines] and Spi-ro[[2]benzofuran-1,4‘-piperidines] with Carbon Substituents in Position 3. J. Med. Chem. 2002, 45, 4923–4930. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Aguilera, O.M.; Budni, J.; Mina, F.; Medeiros, E.B.; Deuther-Conrad, W.; Entrena, J.M.; Moraleda, I.; Iriepa, I.; López-Muñoz, F.; Marco-Contelles, J. Contilisant, a tetratarget small molecule for Alzheimer’s disease therapy combining cholinesterase, monoamine oxidase inhibition, and H3R antagonism with S1R agonism profile. J. Med. Chem. 2018, 61, 6937–6943. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Jorgensen, W. Efficient Drug Lead Discovery and Optimization. Acc. Chem. Res. 2009, 42, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Renaudo, A.; L’Hoste, S.; Guizouarn, H.; Borgèse, F.; Soriani, O. Cancer Cell Cycle Modulated by a Functional Coupling between Sigma-1 Receptors and Cl– Channels. J. Biol. Chem. 2007, 282, 2259–2267. [Google Scholar] [CrossRef] [PubMed]
- Mach, R.H.; Smith, C.R.; Childers, S.R. Ibogaine possesses a selective affinity for σ2 receptors. Life Sci. 1995, 57, PL57–PL62. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L., III; MacKerell, A.D., Jr.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository—New features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deuther-Conrad, W.; Schepmann, D.; Iriepa, I.; López-Muñoz, F.; Chioua, M.; Wünsch, B.; Samadi, A.; Marco-Contelles, J. 2-{N-[ω-(1-Benzylpiperidin-4-yl)alkyl]amino}-6-[(prop-2-yn-1-yl)amino]pyridine-3,5-dicarbonitriles Showing High Affinity for σ1/2 Receptors. Int. J. Mol. Sci. 2025, 26, 1266. https://doi.org/10.3390/ijms26031266
Deuther-Conrad W, Schepmann D, Iriepa I, López-Muñoz F, Chioua M, Wünsch B, Samadi A, Marco-Contelles J. 2-{N-[ω-(1-Benzylpiperidin-4-yl)alkyl]amino}-6-[(prop-2-yn-1-yl)amino]pyridine-3,5-dicarbonitriles Showing High Affinity for σ1/2 Receptors. International Journal of Molecular Sciences. 2025; 26(3):1266. https://doi.org/10.3390/ijms26031266
Chicago/Turabian StyleDeuther-Conrad, Winnie, Dirk Schepmann, Isabel Iriepa, Francisco López-Muñoz, Mourad Chioua, Bernhard Wünsch, Abdelouahid Samadi, and José Marco-Contelles. 2025. "2-{N-[ω-(1-Benzylpiperidin-4-yl)alkyl]amino}-6-[(prop-2-yn-1-yl)amino]pyridine-3,5-dicarbonitriles Showing High Affinity for σ1/2 Receptors" International Journal of Molecular Sciences 26, no. 3: 1266. https://doi.org/10.3390/ijms26031266
APA StyleDeuther-Conrad, W., Schepmann, D., Iriepa, I., López-Muñoz, F., Chioua, M., Wünsch, B., Samadi, A., & Marco-Contelles, J. (2025). 2-{N-[ω-(1-Benzylpiperidin-4-yl)alkyl]amino}-6-[(prop-2-yn-1-yl)amino]pyridine-3,5-dicarbonitriles Showing High Affinity for σ1/2 Receptors. International Journal of Molecular Sciences, 26(3), 1266. https://doi.org/10.3390/ijms26031266









